Abstract
Dental implant is being considered successful if the patient is pleased with both of its functional and esthetic outcome. As implant complications (such as peri-implantitis, inappropriate implant position, wrong angulation, and implant location too close to anatomical structures) have been frequently encountered in dental practice, therefore, thorough knowledge to manage such complications is the key prerequisite to prevent the failure of implant. The present case report discussed the etiology, diagnosis of early peri-implantitis, and periodontal abscess with their successful management through periodontal regeneration and diode laser-assisted therapy.
Keywords: Early peri-implantitis, implant decontamination, intraoral sinus, laser-assisted curettage, periodontal regenerative therapy
Introduction
The implant complications which may either be related to the treatment plan, procedure, anatomy, or other (iatrogenic) factors are commonly observed and can lead to a number of poor treatment outcome if not recognized and addressed at the earliest.[1] The majority of problems that can arise in an implantology treatment are accidents, complications, or iatrogenic errors and are a consequence of an inadequate indication, poor quality or quantity of bone, an erroneous surgical technique, infections, lack of oral hygiene, smoking habit, systemic diseases that were poorly controlled, et cetera.[2] Therefore, to provide successful functional and esthetic implant treatment outcome, the clinician should have a thorough knowledge of all the aspects of oral implantology which is the key factor not only to prevent or eliminate the risk of complications associated with treatment but also for the management of complications even if they do occur. The aim of the present case report is to discuss the etiology, diagnosis of soft and hard tissue complications observed in an implant with their successful management through diode laser (DL), and periodontal regeneration-assisted therapy.
Case Report
A 35–year-old otherwise healthy female patient referred to the Department of Periodontology with complaint of mild gingival swelling and pus discharge from her right upper front implant 3 weeks after the impression taken for crown with respect to (w.r.t) tooth number (#) 11. Past dental history reported Adin implant 3.75 and 13 mm was placed utilizing conventional flap technique with less primary stability and immediately intraoral periapical (IOPA)-X-ray was taken [Figure 1] followed by gingival former placement 5 months back. Prophylactic antibiotics and anti-inflammatory were prescribed. Removable partial denture was inserted 1 month postoperatively. Approximately, 2–3 months postoperatively, patient complained about gingival irritation and bleeding gums while brushing w.r.t #11; subgingival area was debrided and irrigated with chlorhexidine (CHX) but 2–3 weeks after which occasional gingival bleeding persists and patient ignored further consultation. Final impression was taken for prosthetic rehabilitation at 5-month follow-up. 1–2 days after, patient complained about pain and swelling w.r.t #11. Implant site was thoroughly irrigated and tablet amoxicillin (500 mg) with clavulanic acid (125 mg) twice daily and tablet ibuprofen 600 mg thrice daily for 5–7 days and oral rinsing twice daily with 0.2% CHX rinse for 2 weeks was advised. Pain subsided after medication, so she missed the follow-up visit. Three weeks after, clinician observed swelling with pus discharge through intraoral sinus and immediately referred the case. Intraoral examination revealed mild bleeding on probing; 3–4 mm pocket depth (PD) all around labially positioned implant w.r.t #11, but only on labial surface, 4 mm pocket with 2 mm of clinical attachment loss was present as implant threads were palpable with probe [Figure 2]. Intraoral sinus involved labial attached gingiva [Figure 3] w.r.t #11 but no clinically visible communication was observed between sinus site and labial pocket after probing. Lateral stability was assessed through Misch clinical implant mobility scale [Table 1] and scored 0, after applying approximately 500 g of force with the help of two rigid instruments labiopalatally, mesiodistally, and all around the implant whereas secondary stability was assessed and confirmed using reverse torque analysis test value reported was >20 Ncm. Immediate IOPA X-ray showed mild interproximal alveolar bone resorption [Figure 4]. Immediate adjacent teeth were vital on cold testing with healthy periodontium. On the basis of clinical and radiographic findings, a provisional diagnosis of chronic localized early labial peri-implantitis with periodontal abscess-induced intraoral sinus w.r.t #11 region was made. It was evident that labial plate was probably at high risk of resorption soon in labially positioned implant w.r.t #11. Hence, DL (Biolase)-assisted curettage followed by periodontal regeneration therapeutic approaches was discussed with their pros and cons in detail and patient submitted written signed consent in favor of bone graft with platelet-rich fibrin (PRF)-assisted regeneration.
Figure 1.
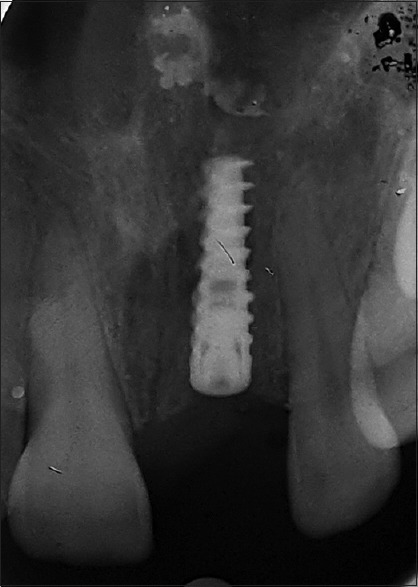
Immediate after implant placement 5 months back, showed residual infection//past incident-associated bone resorption w.r.t #11
Figure 2.
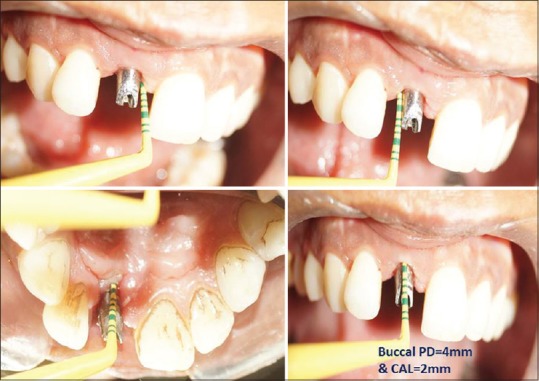
Preoperative clinical probing at mesial, distal, labial, and palatal aspect of implant w.r.t #11 region clockwise
Figure 3.
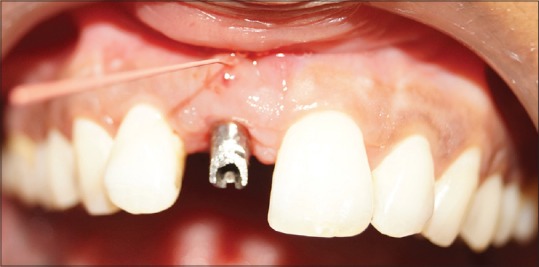
Preoperative intraoral sinus at labial attached gingiva w.r.t #11
Table 1.
Misch clinical implant mobility scale

Figure 4.
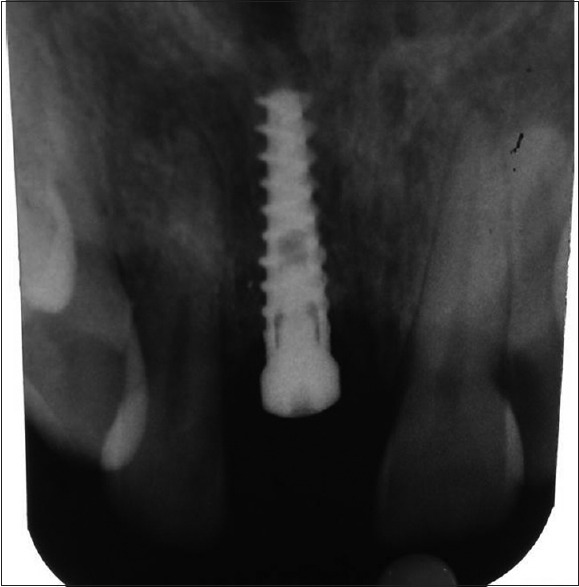
Mild interdental alveolar bone loss observed in intraoral periapical X-ray w.r.t #11 region
On day 1, after phase one therapy; sinus and pockets were irrigated thoroughly with sterile normal saline solution. Biolase DL assisted pocket, and sinus curettage was done w.r.t #11 at 2W for total 15 s at labial sulcus under 0.05 ms pulse interval utilizing 300 μm fiber optic in contact and continuous mode under high power suction, followed by 0.2% CHX irrigation at surgical site. Routine blood and urine investigations were carried out and were within normal range.
At 10th-day postoperative recall visit clinically sinus was healed uneventfully with mild edema w.r.t #11. 3 weeks postoperatively sinus was completely healed along with no sulcular bleeding on probing, and surgery was planned immediately. Local anesthesia was administered under aseptic condition. Just before surgery, 9 ml blood was obtained from antecubital vein and was immediately transferred in 10 ml sterile test tube without any anticoagulant and centrifuged at 2500 rpm for 10 min at room temperature for Choukrone's PRF preparation.[3] The crevicular incision was given w.r.t #21 and 12. Crestal incision was given w.r.t #11 with vertical releasing incision distal to #12. Full-thickness flap was reflected, and early bone dehiscence in the coronal third and transparency of implant through apical two-third labial bone was evident w.r.t #11 [Figure 5]. Recipient bed was prepared in the apical two-third area of labial bone [Figure 6] and surgical site including implant surface was thoroughly decontaminated utilizing implant maintenance plastic curettes followed by irrigation with 0.2% CHX. A portion of prepared PRF was then amalgamated with hydroxyapatite bioactive glass (HABG) graft and placed at recipient site whereas larger volume was compressed between sterile gauze pieces to procure PRF membrane which was further used to stabilize the graft. Flap was approximated utilizing 3–0 black silk suture. Coe-pack was placed and postsurgically, patient was instructed to use (after 24 h) 0.2% CHX rinse twice daily for 4 weeks for 30 s without dilution, but to quit brushing for 7 days at surgical site only and tablet ibuprofen 600 mg sos. Patient's wound healed uneventfully at 14 days postoperatively. Maintenance therapy was given at 6 weeks, 3 and 6 months postoperatively. Crown cementation was done at 3 months postoperatively, as scalloped gingival margins, with good gingival esthetic and healed labial pocket (with gain in 2 mm of clinical attachment with 2 mm of gingival sulcus), with no interproximal bone loss was observed [Figure 7]. Due to unavoidable circumstances, patient missed 9-month follow-up. Clinical healing was excellent with the excellent color match with scalloped margins and thick keratinized gingiva at the surgical site along with labial, mesial and distal probing depth was 2 and 5 mm, respectively, as well as IOPA radiograph showed no interproximal bone loss 6 months postoperatively [Figure 8]. All the parameters reported to be normal at 12 months postoperatively [Figure 9]. A noninvasive, economical parallel profile radiographic (PPRx) technique-assisted IOPA X-ray through lateral view[4] showed labial bone regeneration w.r.t #11 [Figure 10a and b] in comparison to clinical baseline [Figure 5]. Patient is highly pleased and satisfied with the outcome.
Figure 5.
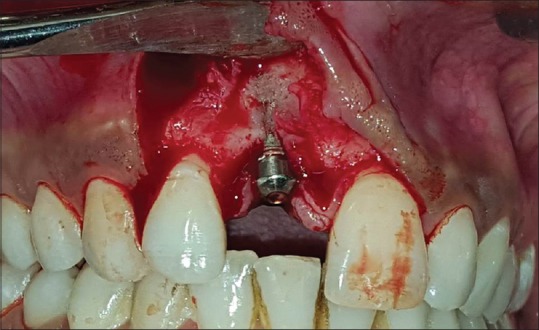
After full thickness flap reflection, early labial bone loss (bone dehiscence) in coronal <1/3rd with transparency of implant through rest of the body of implant along with mild interdental bone loss w.r.t #11 was observed
Figure 6.
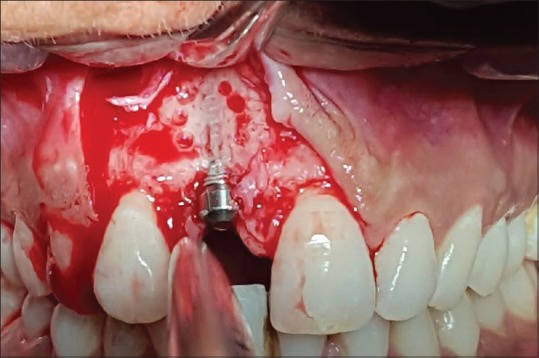
Recipient bed was prepared with surgical round carbide bur under copious irrigation on labial bone w.r.t #11 after apical two-third area debridement showed transparency of implant throughout clearly followed by mechanical and chlorhexidine-assisted decontamination at throughout implant site
Figure 7.
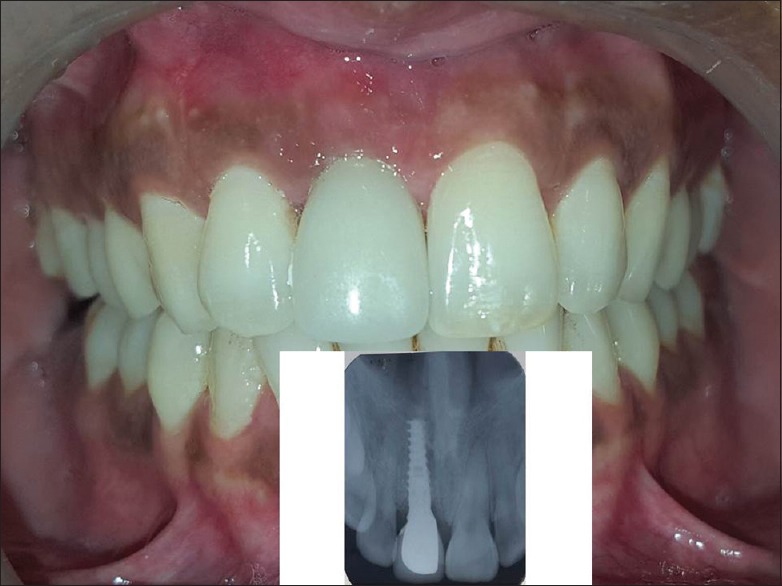
Clinically, gingiva is healthy with the excellent color match, and well-developed scalloped margin and radiograph showed no interproximal bone loss 3 months postoperatively
Figure 8.

Clinical healing was excellent with the excellent color match with scalloped margins and thick keratinized gingiva at surgical site. Clinically labial, mesial and distal, robing depth was 2 and 5 mm, respectively, as well as intraoral periapical radiograph showed no interproximal bone loss 6 months postoperatively
Figure 9.
Distal pocket reduced to 3 mm at 12 months postoperatively whereas rest of the clinical and radiographical parameters remained stationary as observed at 6 months postoperatively
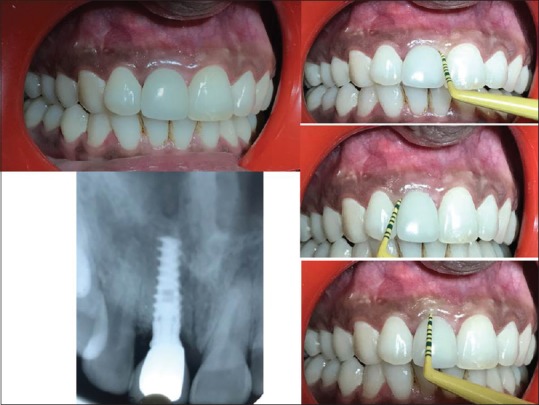
Figure 10.
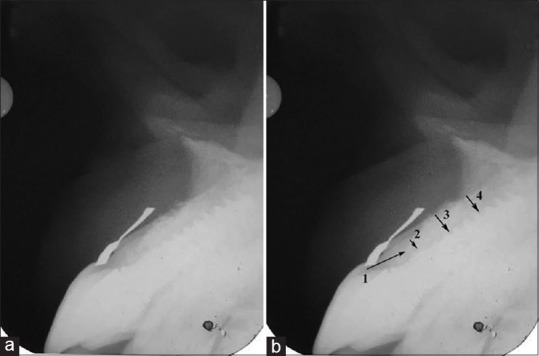
(a) 13 months postoperatively parallel profile radiographic technique-assisted intraoral periapical X-ray showed bone regeneration at labial surface, (b) (1) dentogingival unit, (2) bone thickness at crestal third, (3) midlabial, and (4) at apical labial bone
Discussion
Nowadays, implants are considered most promising and successful treatment for the management of missing teeth. However, to replace the lost tooth in anterior maxilla with an implant is more challenging because of high esthetic, functional, and biological demands.[1] Although multiple factors contribute in implant complications,[1,2] the etiology of periodontal abscess-induced intraoral sinus complication in the present case may be because of impaction of local factors from the pocket area[5] or/and impression material remnants in the subgingival area at the time of impression taken whereas radicular bone resorption (reduction in labial bone thickness) at peri-implant may occur because of an erroneous surgical technique[2]/unintentional clinical mistake of inappropriately positioned implant beyond an acceptable position (<2 mm) labially, that is why labial surface was unable to compensate for the effect of remodeling[6] and undergone resorption throughout the body of implant and left very thin labial plate over the implant surface, which may further come under the influence of mild chronic gingival inflammation in peri-implant site leading to complete bone resorption in less than coronal one-third area of labial bone as well as interproximal site along with sulcus deepening (pocket formation). The diagnosis of periodontal abscess and localized chronic early (Stage I) peri-implantitis was made on the basis of report of Corbet[5] and American academy reports.[7,8]
Although a number of researchers utilized DLs at 1.5W–2.5 W for 10–30 s utilizing 300–2000 μm fibro-optic in continuous mode for curettage and reported the significant clinical outcome.[9] Therefore, the parameters used in the present study were within the prescribed limits for soft tissue curettage. Complete healing of sinus and peri-implant mucosal inflammation (no sulcular bleeding) may occur because laser-assisted sites reported to improve adenosine triphosphate synthesis, fibroblast proliferation, collagen synthesis, phagocytosis of macrophages, and accelerate inflammatory phase of wound healing. All these mechanisms can result in cellular proliferation and acceleration of wound healing process.[10,11] To the best of our knowledge, this was the first report used DL for sinus curettage.
Till date, no universally acceptable standardized protocol of peri-implantitis management is available; majority of treatment comprised of mechanical debridement with or without antimicrobial solution and/or antibiotic agents followed by the surgical (resective/regenerative) intervention whereas to increase the radicular bone thickness-guided bone regeneration was recommended. As nonsurgical mechanical therapy on its own would appear to be insufficient for peri-implantitis treatment,[12] but when used in combination with CHX, it improves the clinical and microbiological parameters, and the addition of local or systemic administration of antibiotics reduces bleeding on probing and probing depth.[13,14] Therefore, for such reasons, surgical access is recommended to achieve complete removal of granulation tissue and to obtain access for the decontamination of the implant surface.[15,16] That's why, the above factors were taken into consideration in the present case, surgical access-assisted mechanical debridement along with the use of 0.2% CHX as irrigant to decontaminate the surgical site including implant surface which may also contribute in the outcome.
Second-generation natural and synthetic resorbable collagen membranes were designed to avoid the need for surgical removal. In spite of promising but variable outcome, collagen membranes are associated with several complications such as early degradation, epithelial down growth along the material, and premature loss, whereas PRF membrane in comparison to collagen membrane offers pleasant alternative due to its cost-effectiveness and relative safety due to autologous nature[17] that may be the reason patient opted PRF.
The present case report showed reduction in probing depth and gain in clinical attachment and bone formation as observed in IOPA X-ray and PPRx; may be attributed because (i) PRF induced the cell proliferation of osteoblast, periodontal ligament cells, and growth factors but suppress the epithelial cell growth,[18] whereas HABG graft that bonds with host bone faster than hydroxyapatite graft and resorbs slowly but completely than bioactive glass which is replaced and remodeled by new bone.[19] The combined effect of PRF and HABG bone graft-assisted therapies in association with thorough mechanical debridement and chemical decontamination of implant with CHX may resulted in excellent outcome which is in consistence with the report of Talrija et al.[20] Ethical concerns restricted the clinician for surgical reentry.
Conclusion
Early recognition and intervention of peri-implant soft and hard tissue complications through minimally invasive nonsurgical therapy and regenerative therapy in association with mechanical as well as chemical decontamination respectively, not only controlled the disease process and its associated complications but also made the environment more conducive for regeneration of lost structure, thereby improving the fate of implant.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Misch K, Wang HL. Implant surgery complications: Etiology and treatment. Implant Dent. 2008;17:159–68. doi: 10.1097/ID.0b013e3181752f61. [DOI] [PubMed] [Google Scholar]
- 2.Angeles Sanchez Garces MA, Escoda-Francoli J, Gay-Escoda C. Implant complications. Implant Dentistry-The Most Promising Discipline of Dentistry. In: Turkyilmaz I, editor. New York, USA: InTech; 2011. pp. 481–8. [Google Scholar]
- 3.Choukroun J, Adda F, Schoeffler C, Vervelle A. Une Opportunite en paro- implantologie: Le PRF. Implantodontie. 2000;42:55–62. [Google Scholar]
- 4.Alpiste-Illueca F. Dimensions of the dentogingival unit in maxillary anterior teeth: A new exploration technique (parallel profile radiograph) Int J Periodontics Restorative Dent. 2004;24:386–96. [PubMed] [Google Scholar]
- 5.Corbet EF. Oral diagnosis and treatment planning: Part 3. Periodontal disease and assessment of risk. Br Dent J. 2012;213:111–21. doi: 10.1038/sj.bdj.2012.666. [DOI] [PubMed] [Google Scholar]
- 6.Grunder U, Gracis S, Capelli M. Influence of the 3-D bone-to-implant relationship on esthetics. Int J Periodontics Restorative Dent. 2005;25:113–9. [PubMed] [Google Scholar]
- 7.American academy of periodontology task force report on the update to the 1999 classification of periodontal diseases and conditions. J Periodontol. 2015;86:835–8. doi: 10.1902/jop.2015.157001. [DOI] [PubMed] [Google Scholar]
- 8.Poli PP, Cicciu M, Beretta M, Maiorana C. Peri-implant mucositis and peri-implantitis: A Current understanding of their diagnosis, clinical implications, and a report of treatment using a combined therapy approach. J Oral Implantol. 2017;43:45–50. doi: 10.1563/aaid-joi-D-16-00082. [DOI] [PubMed] [Google Scholar]
- 9.Qadri T, Javed F, Johannsen G, Gustafsson A. Role of diode lasers (800-980 nm) as adjuncts to scaling and root planing in the treatment of chronic periodontitis: A systematic review. Photomed Laser Surg. 2015;33:568–75. doi: 10.1089/pho.2015.3914. [DOI] [PubMed] [Google Scholar]
- 10.Saperia D, Glassberg E, Lyons RF, Abergel RP, Baneux P, Castel JC, et al. Demonstration of elevated type I and type III procollagen mRNA levels in cutaneous wounds treated with helium-neon laser. Proposed mechanism for enhanced wound healing. Biochem Biophys Res Commun. 1986;138:1123–8. doi: 10.1016/s0006-291x(86)80399-0. [DOI] [PubMed] [Google Scholar]
- 11.Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B. 1999;49:1–7. doi: 10.1016/S1011-1344(98)00219-X. [DOI] [PubMed] [Google Scholar]
- 12.Karring ES, Stavropoulos A, Ellegaard B, Karring T. Treatment of peri-implantitis by the vector system. Clin Oral Implants Res. 2005;16:288–93. doi: 10.1111/j.1600-0501.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 13.Salvi GE, Persson GR, Heitz-Mayfield LJ, Frei M, Lang NP. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: Clinical and radiographic outcomes. Clin Oral Implants Res. 2007;18:281–5. doi: 10.1111/j.1600-0501.2007.01377.x. [DOI] [PubMed] [Google Scholar]
- 14.Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: A literature review. J Clin Periodontol. 2008;35:305–15. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 15.Máximo MB, de Mendonça AC, Renata Santos V, Figueiredo LC, Feres M, Duarte PM, et al. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res. 2009;20:99–108. doi: 10.1111/j.1600-0501.2008.01618.x. [DOI] [PubMed] [Google Scholar]
- 16.de Waal YC, Raghoebar GM, Huddleston Slater JJ, Meijer HJ, Winkel EG, van Winkelhoff AJ, et al. Implant decontamination during surgical peri-implantitis treatment: A randomized, double-blind, placebo-controlled trial. J Clin Periodontol. 2013;40:186–95. doi: 10.1111/jcpe.12034. [DOI] [PubMed] [Google Scholar]
- 17.Sam G, Pillai BR. Evolution of barrier membranes in periodontal regeneration-“Are the third generation membranes really here?”. J Clin Diagn Res. 2014;8:ZE14–7. doi: 10.7860/JCDR/2014/9957.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai CH, Shen SY, Zaho JH, Cahng YC. Platelet rich fibrin modulates cell proliferation of human periodontal related cell in vitro reports. J Dent Sci. 2009;4:130e5. [Google Scholar]
- 19.Debnath T, Chakraborty A, Pal TK. A clinical study on the efficacy of hydroxyapatite – Bioactive glass composite granules in the management of periodontal bony defects. J Indian Soc Periodontol. 2014;18:593–600. doi: 10.4103/0972-124X.142451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talreja PS, Gayathri GV, Mehta DS. Treatment of an early failing implant by guided bone regeneration using resorbable collagen membrane and bioactive glass. J Indian Soc Periodontol. 2013;17:131–6. doi: 10.4103/0972-124X.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]


