Abstract
Purpose:
To compare and evaluate the surface quality of silicone impression materials after ozone water disinfection.
Materials and Methods:
A total of 60 samples were prepared on a stainless steel die (American Dental Association specification no. 19 and International Standard of Organization - 4823). The samples were divided into four groups; each group contains 15 samples. Group A as control, Group B, C, and D disinfected with 2% glutaraldehyde, 5.25% sodium hypochlorite, and ozone water, respectively. The samples were made according to the manufacturer's instructions, and the samples were allowed to set in a thermostatically controlled water bath at 35°C ± 1°C and retrieved after 10 min. The surface qualities of the samples were measured in stereomicroscope with ×20 magnification.
Results:
The data obtained were analyzed using Chi-square test, and the “P” value was calculated. The results showed that there were no differences in the surface quality among the Groups A, C, and D for addition silicone putty and light body and medium body impression materials than the Group B.
Conclusion:
This study concluded that ozone water disinfection showed least changes when compared to 5.25%sodium hypochloride and 2% glutaraldehyde disinfection for addition silicone putty , light body and medium body impression materials.
Keywords: Disinfection, O3 water, silicone impression material, surface quality
Introduction
Contamination of the working atmosphere during the clinical practice or laboratories creates risk to the health of professionals. Cross-infection can be carried directly by blood or saliva and indirectly by the contaminated equipments, surfaces, and airway which could transmit the diseases such as human immunodeficiency virus, hepatitis-B virus, and tuberculosis. According to Miller, a saliva droplet contains more than 50,000 bacteria,[1] with the risk of inducing infections, which can survive on the impression surface and it can be transferred to the stone casts.[2,3,4,5,6] Hence, impression disinfection is now considered as a routine clinical procedure in dental offices and laboratories, and also American Dental Association (ADA) and the Centers for Disease Control recommended to disinfect the impressions to prevent cross-infection.[7,8,9]
Disinfecting solutions should eliminate the pathogens while also adversely not affect the surface quality of the impression materials.[10] Silicone impression materials have gained popularity among dentists because of their excellent physical properties, favorable handling properties, and good patient acceptance.[10] Condensation silicones show dimensional changes after polymerization of the material, which may be caused by their slow setting, or by loss of water and alcohol, as a byproduct of the setting reaction. Addition silicones have insignificant number of byproducts released, which provides a dimensionally stable impression.[11] The impression should be a negative copy of the patient's anatomical structures; from that, the accurate positive replica of the oral structures was established on which the fixed prosthesis was fabricated to achieve a perfect adaptation.
Any dimensional change in the impression causes lack of adaptation in the prosthesis. Clinical success for prosthesis depends on the accurate detail reproduction of impressions because during laboratory procedures, these materials face problems related to contraction and expansion. Therefore, the knowledge of the surface quality of impression materials is important.[12]
Although many studies have advocated various methods of disinfection process, the immersion method is considered more effective among them because the immersion method guarantees that all surfaces of impression and tray are covered with the disinfectant solutions.[13] Aqueous solutions of alcohols, aldehydes, phenolics, biguanides, quaternary ammonium compounds, sodium hypochlorite, glutaraldehyde, povidone-iodine, and chlorhexidine gluconate are the disinfectant solutions that have been recommended to disinfect the dental impressions.[14] Ozone water is also being used as a disinfection solution in various fields of dentistry due to its antimicrobial, disinfectant, biocompatibility, and healing properties. Studies found that ozonated water was highly effective in killing of both Gram-positive and Gram-negative microorganisms.[15] Hence, this study was conducted to evaluate the surface quality of elastomeric impression materials after disinfection with ozone water, 2% glutaraldehyde, and 5.25% sodium hypochlorite.
Materials and Methods
According to International Standard of Organization – 4823, a stainless steel die was prepared with the following dimensions: length – 31 mm, outer and inner diameter – 38 mm and 29.97 mm, and the height of inner diameter – 3 mm. Three horizontal parallel lines x, y, and z (25, 50, 75 μm wide, 25 mm in length) were inscribed between the two vertical lines D1 and D2 (0.075 ± 0.008 mm wide) on the superior surface of the die to determine the surface quality changes. The distance between x and z lines was 5 mm (between each line 2.5 mm spaced apart). The stainless steel ring (6 mm height, 30 mm inner diameter, and 38 mm outer diameter) was placed on the ruled die and fitted around the borders of the die which acts as a mold for the impression material.
The addition silicone putty and light body (Aquasil, Dentsply De Trey, Konstanz, Germany), addition silicone medium body (Aquasil, Dentsply Caulk Milford, Delaware, USA), and condensation silicone putty and light body (Speedex, Coltene/Whaledent, Switzerland) were used. For the disinfection process, 5% sodium hypochlorite (GlaxoSmithKline Pharmaceutical Pvt. Ltd., India), 2% glutaraldehyde (Jalgaon Chemicals Pvt. Ltd., Jalgaon, India), and ozone water were used. Ozone water was produced with a high-voltage electrical discharge at a constant flow rate by the apparatus and was ejected into the diffuser through the output tube. The concentration of ozone used was 10 ppm, and it was generated for 20 min in the apparatus.
The samples were divided into four groups. Five impressions were made in each impression materials. A total of 60 samples were made for this study. The samples which were not subjected to any disinfection procedures were considered as control (Group A), samples subjected to 2% glutaraldehyde disinfection were considered as Group B, samples subjected to 5.25% sodium hypochlorite disinfection were considered as Group C, and samples subjected to ozone water disinfection were considered as Group D. The samples were disinfected with disinfectant solutions for 10 min.
Before making impression, the die was cleaned with ethanol and allowed to dry at room temperature. Single and double-mix techniques were used to make impression within a minute from the start of mixing. A universal 50-ml cartridge dispenser was used to obtain uniform proportions and homogeneity of the material. The impression mold was placed on the ruled block, and a freshly mixed homogeneous impression material was placed in the center of the block and spread to fill the mold. A flat glass slab was placed on the impression mold, and constant pressure was applied on the impression material; the excess material was allowed to extrude. A positive metal-to-metal contact between the mold and test block was achieved by this method. A metal flat weight of 1 kg simulating the operator's finger pressure on a tray was placed over the glass plate. The samples were allowed to set in a thermostatically controlled water bath at 35°C ± 1°C to simulate oral temperature and retrieved after the manufacturer's recommended setting time. After the impression material had set, the sample was removed from the water bath and rinsed for 30 s under tap water; then, subjected to disinfection procedure for 10 min.
The surface qualities of the samples were measured using ×20 stereomicroscope. The surface quality was assessed by examining the detail reproduction of the impression material with rating values [Figure 1].[16,17]
Figure 1.
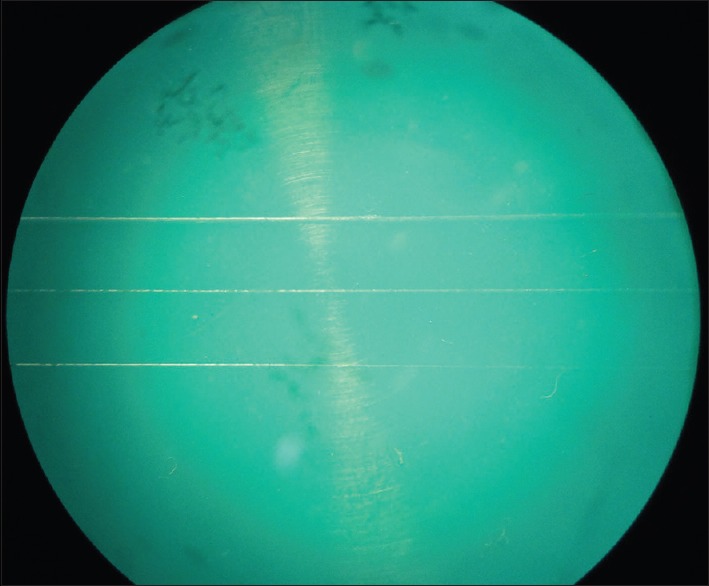
Stereomicroscope image of surface quality
Rating 1 – well-defined sharp detail and continuous line
Rating 2 – continuous line but with some loss of sharpness
Rating 3 – poor detail or loss of continuity of line
Rating 4 – marginally or completely not discernible line.
Results
The surface quality was evaluated in rate scoring system. Hence, the proportions were compared using Chi-square test, and the “P” value was calculated. If P < 0.05, then Fisher's exact Chi-square test was applied to calculate the P value. SPSS version 22.0 (IBM, USA) was used to analyze the data.
Table 1 shows the comparison of proportions of surface quality of addition silicone medium body material after disinfection. The samples in Groups A, C, and D showed more rating - 1 and rating - 2 in x (25 μm) and y (50 μm) lines when compared to Group B.
Table 1.
Comparison of proportions of surface quality between groups in addition silicone medium body material
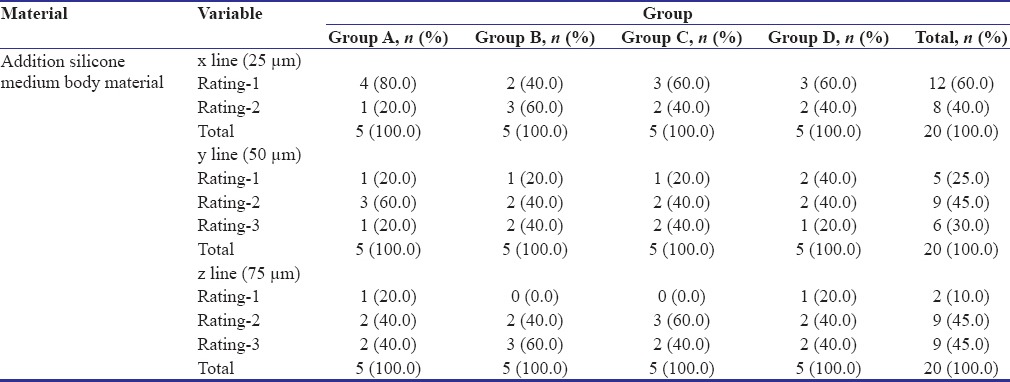
Table 2 shows Fisher's exact test for the proportions of surface quality. The statistical analysis showed that the significant value P for Groups A, B, C, and D in x (25 μm), y (50 μm), and z (75 μm) lines in both addition silicone putty and light body and medium body materials were 0.291, 0.712, 0.805 and 0,921, 0.995, 0.990 respectively [Figure 2]. All the P > 0.05. Hence, there were insignificant surface quality changes in addition silicone putty and light body material in Group A (Control), Group B (2% glutaraldehyde), Group C (5.25% sodium hypochlorite), and Group D (ozone water).
Table 2.
Chi-square test for proportions of surface quality between Groups A, B, C, and D
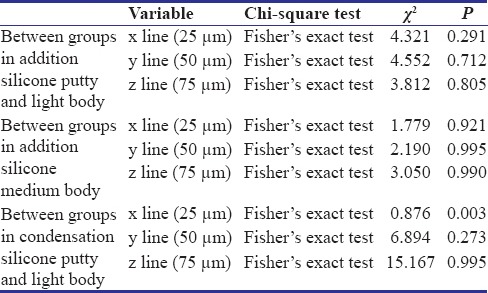
Figure 2.
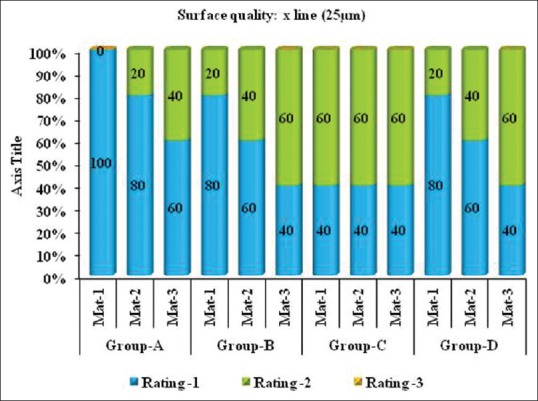
Comparison of surface quality of silicone impression materials after disinfection with 2% glutaraldehyde, 5.25% sodium hypochlorite and ozone water
The statistical analysis for condensation silicone putty and light body material surface quality showed that the significant value P for Groups A, B, C and D in x (25 μm) line was 0.003 and in y (50 μm) and z (75 μm) lines were 0.273 and 0.995, respectively. The significant P < 0.05 in x line. Hence, there were significant changes in surface quality of condensation silicone putty and light body material in Group B (2% glutaraldehyde), Group C (5.25% sodium hypochlorite), and Group D (ozone water) compared to Group A in x (25 μm) line.
Discussion
The disinfection solutions have the capacity to change and alter the surface quality of the impression materials. Hence, it is critical to evaluate the adaptability of the disinfection solution to the respective impression materials. The ADA[18] advocated using the spray disinfectants for disinfecting the impression surface for 10 min in 1:10 dilution of 5.25% (0.525%) sodium hypochlorite in 1988. The ADA recommends that impression materials can be immersed in disinfectant solutions for <30 min. The disinfection process is to eliminate the microorganisms from the surface of the impression without affecting the surface quality of impression.
In this study, 2% glutaraldehyde and 5.25% sodium hypochlorite disinfection solutions and ozone water were used. Sodium hypochlorite is the recommended disinfecting solution for alginate[19] which is also recommended by the Environmental Protection Agency as a good surface disinfectant, nonirritating, and efficient against wide-spectrum microorganisms.[20] Glutaraldehyde provided a broad spectrum of activity against the Gram-negative and Gram-positive organisms, viruses, fungi, and mycobacterium. The previous studies reported that irreversible hydrocolloid impressions, disinfected by 0.5% sodium hypochlorite solutions in 1:10 dilution for 10 min, produced a greater reduction in viable organisms. A pH of 10 was the only level that was consistently effective at decreased immersion times like 3 min or greater.[21]
Ozone application in dentistry has been widely used for the treatment of incipient caries, root canal treatment, periodontal pockets, incomplete wound healing in cases such as ulcerations and herpetic lesions, discolored tooth, peri-implantitis, denture cleaning, and decontamination of toothbrush.[22] Ozone therapy can be defined as a versatile bio-oxidative therapy in which oxygen or ozone is administered through gas or dissolved in water or oil base to obtain therapeutic benefits.[23] During this process, ozone attacks glycoproteins, glycolipids, and other amino acids and inhibits the enzymatic control system of the cell. This results in increase in membrane permeability (the key element of cell viability) leading to immediate function cessation.[24]
Studies also showed that ozone reduced the Candida albicans counts on the removable denture to about 1/10 after 30 min and to 1/103 after 60 min. They suggested that ozone can be used for the of removable prostheses.[23] The results of the study also showed that the Methicillin-resistant Staphylococcus aureus decreased from 3.1 × 103 colony-forming unit (CFU)/mL to 1.0 × 100 CFU/mL after exposure to about 10 ppm of ozone for 10 min in water.[24] Since previous studies showed that ozone water has cleaning and disinfection properties,[25] this study was conducted to evaluate the surface quality of silicone impression materials after disinfection with ozone water.
The statistical analysis showed that samples in Groups A, C, and D showed more Rating 1 (well-defined lines with sharpness) and Rating 2 (well-defined lines with loss of sharpness) in x (25 μm) and y (50 μm) lines when compared to Group B. The samples in Group B showed more Rating 3 (poor detail or loss of continuity of line) in z (75 μm) lines when compared to Groups A, C, and D. All the P > 0.05. Hence, there were no differences in the surface quality among the Groups A (control), C (5.25% sodium hypochlorite), and D (ozone water) as compared to B (2% glutaraldehyde).
The limitations of this study were automix dispensing unit was not used for the mixing of the impression materials. Furthermore, the sample size was limited. To achieve more accurate results, the sample sizes should increase to conclude the effect of disinfection properties of ozone water on impression materials.
Conclusion
Within the limitations of this study, the authors concluded that the ozone water showed comparatively least changes and defined lines when compared to 5.25% sodium hypochlorite, followed by 2% glutaraldehyde. Hence, ozone water can be used as an alternative disinfectant solution for clinical practice.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Miller CH, Cottone JA. The basic principles of infectious diseases as related to dental practice. Dent Clin North Am. 1993;37:1–20. [PubMed] [Google Scholar]
- 2.Powell GL, Runnells RD, Saxon BA, Whisenant BK. The presence and identification of organisms transmitted to dental laboratories. J Prosthet Dent. 1990;64:235–7. doi: 10.1016/0022-3913(90)90185-f. [DOI] [PubMed] [Google Scholar]
- 3.Gerhardt DE, Sydiskis RJ. Impression materials and virus. J Am Dent Assoc. 1991;122:51–4. doi: 10.1016/s0002-8177(91)25016-3. [DOI] [PubMed] [Google Scholar]
- 4.Rice CD, Dykstra MA, Gier RE, Cobb CM. Microbial contamination in four brands of irreversible hydrocolloid impression materials. J Prosthet Dent. 1991;65:419–23. doi: 10.1016/0022-3913(91)90235-o. [DOI] [PubMed] [Google Scholar]
- 5.Samaranayake LP, Hunjan M, Jennings KJ. Carriage of oral flora on irreversible hydrocolloid and elastomeric impression materials. J Prosthet Dent. 1991;65:244–9. doi: 10.1016/0022-3913(91)90169-w. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell DL, Hariri NM, Duncanson MG, Jr, Jacobsen NL, McCallum RE. Quantitative study of bacterial colonization of dental casts. J Prosthet Dent. 1997;78:518–21. doi: 10.1016/s0022-3913(97)70069-6. [DOI] [PubMed] [Google Scholar]
- 7.Recommended infection-control practices for dentistry, 1993. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1993;42:1–12. [PubMed] [Google Scholar]
- 8.Infection control recommendations for the dental office and the dental laboratory. ADA Council on Scientific Affairs and ADA Council on Dental Practice. J Am Dent Assoc. 1996;127:672–80. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- 9.Lepe X, Johnson GH. Accuracy of polyether and addition silicone after long-term immersion disinfection. J Prosthet Dent. 1997;78:245–9. doi: 10.1016/s0022-3913(97)70021-0. [DOI] [PubMed] [Google Scholar]
- 10.Sinobad T, Obradović-Djuricić K, Nikolić Z, Dodić S, Lazić V, Sinobad V, et al. The effect of disinfectants on dimensional stability of addition and condensation silicone impressions. Vojnosanit Pregl. 2014;71:251–8. doi: 10.2298/vsp120709037s. [DOI] [PubMed] [Google Scholar]
- 11.McCabe J, Walls A. Oxford, UK: Blackwell Pub.; 2008. Applied Dental Materials; pp. 167–72. [Google Scholar]
- 12.Graziela P, Ana Paula TB, Pedro Guedes P. The influence of disinfectant agents on the dimensional stability of elastomeric impression materials and surface durability of odontological gympsun. PGR Postgrad Rev Fac Odontol Sao Jose Dos Campos. 2002;5:12–20. [Google Scholar]
- 13.Ahila SC, Subramaniam E. Comparative evaluation of dimensional stability and surface quality of gypsum casts retrieved from disinfected addition silicone impressions at various time intervals: An in vitro study. J Dent Oral Hyg. 2012;4:34–43. [Google Scholar]
- 14.Amalan A, Ginjupalli K, Upadhya N. Evaluation of properties of irreversible hydrocolloid impression materials mixed with disinfectant liquids. Dent Res J (Isfahan) 2013;10:65–73. doi: 10.4103/1735-3327.111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagayoshi M, Fukuizumi T, Kitamura C, Yano J, Terashita M, Nishihara T, et al. Efficacy of ozone on survival and permeability of oral microorganisms. Oral Microbiol Immunol. 2004;19:240–6. doi: 10.1111/j.1399-302X.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- 16.Morrow RM, Brown CE, Jr, Stansbury BE, DeLorimier JA, Powell JM, Rudd KD. Compatibility of alginate impression materials and dental stones. J Prosthet Dent. 1971;25:556–66. doi: 10.1016/0022-3913(71)90214-9. [DOI] [PubMed] [Google Scholar]
- 17.Amin WM, Al-Ali MH, Al Tarawneh SK, Taha ST, Saleh MW, Ereifij N, et al. The effects of disinfectants on dimensional accuracy and surface quality of impression materials and gypsum casts. J Clin Med Res. 2009;1:81–9. doi: 10.4021/jocmr2009.04.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ADA Council on Dental Materials, Instruments and Equipment. Infection control recommendations for the dental office and the dental laboratory. J Am Dent Assoc. 1988;11:241–8. doi: 10.14219/jada.archive.1988.0341. [DOI] [PubMed] [Google Scholar]
- 19.Gladwin M, Bagby M. 1st ed. Philadelphia: Lippincott Williams and Wilkins; 2000. Disinfection of impressions, dentures and other appliances and materials Clinical Aspects of Dental Materials; pp. 262–7. [Google Scholar]
- 20.Washington, D.C: United States Environmental Protection Agency; 1991. United States Environmental Protection Agency. R. E. D. Facts ” Sodium and Calcium Hypochlorite Salts, Office of Pesticides and Toxic Substances. [Google Scholar]
- 21.Schwartz RS, Hensley DH, Bradley DV Jr. Immersion disinfection of irreversible hydrocolloid impression in pH-adjusted sodium hypochlorite. Part 1: Microbiology. Int J Prosthodont. 1996;9:217–22. [PubMed] [Google Scholar]
- 22.Gopalakrishnan S, Parthiban S. Ozone – A new revolution in dentistry. J. Bio Innov. 2012;1:58–69. [Google Scholar]
- 23.Azarpazhooh A, Limeback H. The application of ozone in dentistry: A systematic review of literature. J Dent. 2008;36:104–16. doi: 10.1016/j.jdent.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Murakami H, Sakuma S, Nakamura K, Ito Y, Hattori M, Asai A, et al. Disinfection of removable dentures using ozone. Dent Mater J. 1996;15:220–5. doi: 10.4012/dmj.15.220. [DOI] [PubMed] [Google Scholar]
- 25.Murakami H, Mizuguchi M, Hattori M, Ito Y, Kawai T, Hasegawa J, et al. Effect of denture cleaner using ozone against methicillin-resistant Staphylococcus aureus and E. coli T1 phage. Dent Mater J. 2002;21:53–60. doi: 10.4012/dmj.21.53. [DOI] [PubMed] [Google Scholar]


