Abstract
We assess annual costs of screening provision activities implemented by 23 of the Centers for Disease Control and Prevention’s Colorectal Cancer Control Program (CRCCP) grantees and report differences in costs between colonoscopy and FOBT/FIT-based screening programs. We analysed annual cost data for the first three years of the CRCCP (July 2009–June 2011) for each screening provision activity and categorized them into clinical and non-clinical screening provision activities. The largest cost components for both colonoscopy and FOBT/FIT-based programs were screening and diagnostic services, program management, and data collection and tracking. During the first 3 years of the CRCCP, the average annual clinical cost for screening and diagnostic services per person served was $1150 for colonoscopy programs, compared to $304 for FIT/FOBT-based programs. Overall, FOBT/FIT-based programs appear to have slightly higher non-clinical costs per person served (average $1018; median $838) than colonoscopy programs (average $980; median $686). Colonoscopy-based CRCCP programs have higher clinical costs than FOBT/FIT-based programs during the 3-year study timeframe (translating into fewer people screened). Non-clinical costs for both approaches are similar and substantial. Future studies of the cost-effectiveness of colorectal cancer screening initiatives should consider both clinical and non-clinical costs.
Keywords: Screening cost, Activity-based costing, Economic evaluation, Colorectal cancer screening
1. Introduction
Colorectal cancer (CRC) poses a significant health burden in the United States as it accounts for approximately 8 percent of all new cancer cases and nearly 9 percent of all cancer deaths annually (U.S. Cancer Statistics Working Group, 2015). The United States Preventive Services Task Force recommends CRC screening for average-risk individuals aged 50–74 years (Whitlock, Lin, Liles, Beil, & Fu, 2008) using guaiac based fecal occult blood test (FOBT), fecal immunochemical test (FIT), sigmoidoscopy or colonoscopy. FOBTs and FITs (hereafter referred to as FOBT/FIT) are recommended annually; sigmoidoscopies are recommended every five years in combination with fecal testing every three years; and colonoscopies are recommended once every ten years (National Governors Association Center for Best Practices, 2008; Rex et al., 2009; Smith et al., 2015; Winawer et al., 2003).
Despite the availability of multiple screening tests for prevention and early detection of CRC, the use of CRC screening tests remains suboptimal (Centers for Disease Control and Prevention, 2013; Sabatino, White, Thompson, & Klabunde, 2015). In an effort to increase screening rates, the Centers for Disease Control and Prevention (CDC) established the Colorectal Cancer Control Program (CRCCP), a six-year initiative beginning in 2009. Details on the CRCCP are provided elsewhere (Tangka & Subramanian, under review). Briefly, the CRCCP-funded 29 grantees with several programs choosing endoscopic tests, mostly colonoscopy, with others selecting FOBT/FIT based tests. This difference in screening modality across grantee programs provides a natural experiment to assess differences in the cost of implementing and providing CRC screening in the CRCCP using endoscopy versus FOBT/FIT based tests.
Although both FOBT/FIT and endoscopy-based screening tests are cost-effective approaches to screen for CRC (Pignone, Russell, & Wagner, 2005; Vijan et al., 2007; Zauber et al., 2007), there are some variations in guideline recommendations due to the differences in test characteristics (National Governors Association Center for Best Practices, 2008; Rex et al., 2009; Smith et al., 2015; Winawer et al., 2003). Endoscopic tests allow for prevention via identification and removal of precancerous polyps as well as the detection of cancer, while FOBT/FIT tests are much less sensitive in detecting polyps and do not allow for removal of precancerous polyps unless a follow-up colonoscopy is conducted following positive test results (Smith et al., 2015). In addition, although no guidelines have considered cost-effectiveness in developing recommendations, independent analyses have shown that under certain circumstances, the use of FOBT may provide better value than colonoscopy (Fisher, Fikry, & Troxel, 2006; Subramanian, Bobashev, & Morris, 2010). Therefore, there is an ongoing need to systematically assess potential cost differences between the CRC screening modalities.
In this study we assess the differences in clinical and non-clinical screening provision costs incurred by colonoscopy-based and FOBT/FIT-based programs during the first 3 years of the CRCCP program. No prior study has addressed potential variation in the non-clinical cost of managing and operating programs using different CRC screening modalities. Analysis of the non-clinical costs of CRCCP implementation offers real-world estimates pooled across multiple public health programs. Although the primary focus of this study is on the non-clinical programmatic costs, we also report the costs of screening and diagnostic services. The findings from this study provide an economic evidence-base to inform future program funding and resource allocation to scale up public health CRC screening programs to achieve the National Colorectal Cancer Roundtable targeted screening rate of 80% by 2018 (National Colorectal Cancer Roundtable, n.d.).
2. Methods
2.1. Conceptual framework
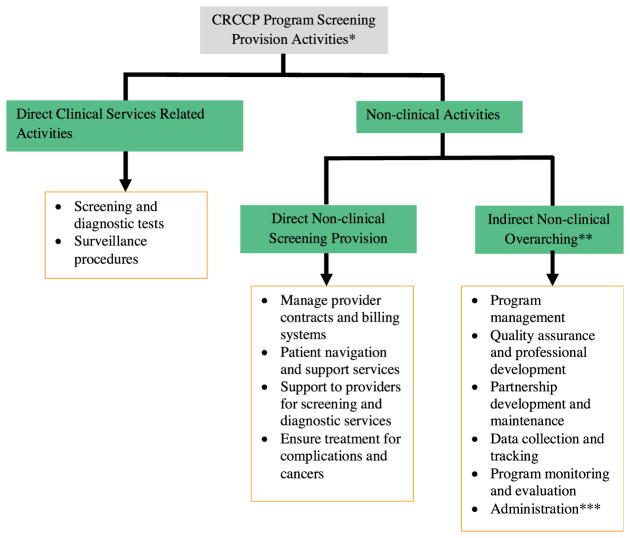
To systematically compare the colonoscopy and FOBT/FIT programs, we categorized cost into direct clinical, direct non-clinical, and indirect non-clinical costs. Key components of these cost categories included the following:
Direct clinical services-related activities—provision of screening tests, diagnostic services (diagnostic colonoscopy after positive FOBT or FIT), and surveillance procedures (follow up procedures after polyp or cancer diagnosis for individuals requiring surveillance);
Direct non-clinical screening provision activities—managing provider contracts, billing systems and other procedures, providing patient navigation and support services, providing operations support to providers for screening and diagnostic services, and ensuring appropriate treatment for complications and cancers (programs do not finance any required treatments); and
Indirect non-clinical overarching activities—program management, program monitoring and evaluation, and administration.
The details on the program components and the specific activities performed by the CRCCP grantees are shown in Appendix A, Fig. A1.
2.2. Data collection process
We used a pre-tested and validated web-based cost assessment tool (CAT) to collect cost and resource use data annually from all CRCCP-funded grantees during the first three years of the program (July 2009–June 2011). The CAT is based on well-established methods of collecting cost data for program evaluation; details on developing, testing and evaluating the CAT have been published previously (Drummond, Schulpher, Torrance, O’Brien, & Stoddard, 2005; Salome, French, Miller, & McLellan, 2003; Subramanian, Ekwueme, Gardner, & Trogdon, 2009). All grantees were trained to input data into the web-based CAT and were also provided with a user’s guide and technical assistance to ensure standardized reporting. Grantees reported the following information annually: staff salaries, roles and percent time spent on the CRCCP; types of screening promotion and screening provision activities performed; costs of materials, contracts, and consultants; and costs of overhead and administration. We asked grantees to indicate funding amounts supporting their CRCCP from the CDC and from other sources, such as the state, as well as to provide in-kind costs regarding labor, materials, and contracts.
We collected data on direct clinical, direct non-clinical, and indirect non-clinical costs. Patient navigation was not collected as a separate activity until year 2; some year 1 patient navigation costs may have been reported under other activities but since the average start-up time to begin screening was 9 months, only a small amount of expenditure was incurred for these activities in year 1. We collected information in the CAT to allow us to separate out the proportion of these overarching activities that supported screening promotion and screening provision activities. Promotion activities and cost are summarized in a companion manuscript (Tangka et al., 2016). Each year we prepared summaries of the CAT for each grantee to review for accuracy and approve. In a few instances, programs were unable to separate costs into the specific activities and these costs are reported as ‘other costs.’
In addition to the cost data, the grantees submitted detailed person-level data on screening and surveillance services provided by the grantee programs. Clinical activities funded directly by CDC were reported using the Colorectal Cancer Clinical Data Elements (CCDEs) and those funded through other sources were reported in the CAT using the same standardized definitions. The data elements include type of screening test, proportion receiving a diagnostic follow-up procedure and procedure type, polyps identified and cancers detected. Details on the CCDEs and definitions used for the data elements have been reported previously (Seeff & Rohan, 2013).
2.3. Analytic framework and approach
We present details on cost and resource use stratified by programs that provided colonoscopies versus FOBT/FIT-based testing. All the programs offered colonoscopy for diagnostic follow-up after a positive FOBT/FIT result. Several programs offered colonoscopy screening for increased risk individuals as recommended by guidelines and some programs offered stool tests as an alternative to colonoscopy (Rex et al., 2009; Smith et al., 2015). We classified colonoscopy programs as those programs that provided more than 85% of their screens using colonoscopy or sigmoidoscopy, and classified FOBT/FIT program similarly. We excluded 5 programs from the analysis as they offered mixed screening or switched modalities during the first three years of the CRCCP and one program did not report cost for screening tests during the study period.
To compare across the 14 colonoscopy and 9 FOBT/FIT-based programs we provide descriptive statistics on the number of screens provided, diagnostic follow-up tests and polyps or cancers identified. We report mean and median costs for each screening provision activity stratified by type of screening program. The costs are also reported in the broad categories of direct non-clinical screening provision activities, indirect non-clinical overarching activities and clinical services-related activities. Median costs are presented along with the average as there are large variations in the costs reported across the programs. In-kind contributions are included in all estimates. We also provide the proportion of in-kind contributions made to the grantee program by activity. To assess potential variation across the program years, we show annual costs by activity and, finally, we compare the average costs and cost per person served for each activity between the colonoscopy and FOBT/FIT-based programs. All costs are reported from a program perspective and have not been adjusted for cost of living differences. Past analysis has shown that adjustments using regional cost-of-living index do not adequately control for differences (Subramanian, Ekwueme, Gardner, Bapat, & Kramer, 2008). Furthermore, the geographic distribution of the programs is not substantially different. On a per person basis, we would consider even a $20 difference in specific costs of program activities as meaningful (even if not statistically significant) as this would result in substantial cost for programs serving a large volume of individuals. For example, if 1000 individuals are screened, the difference in cost would be $20,000.
3. Results
Table 1 provides descriptive statistics on the clinical services provided through the colonoscopy and FOBT/FIT-based programs. On average, the FOBT/FIT-based programs screened more individuals than colonoscopy programs (mean of 1471 versus 879) but the median values were similar. Diagnostic follow-up tests were much higher in the FOBT/FIT-based group; this is expected as colonoscopy follow-up would be required for all persons with a positive initial screening test. The surveillance colonoscopies were provided to a large proportion of individuals in colonoscopy programs compared to the FOBT/FIT-based programs (891 individuals versus 429). The number of polyps identified was also substantially higher in the colonoscopy programs with 3899 polyps compared to 983 polyps for the FOBT/FIT-based programs. Overall, 48 colorectal cancers were identified in the colonoscopy group and 32 cancers in the FOBT/FIT-based program.
Table 1.
Comparison of CRCCP Screening Tests, Diagnostic Services, and Cancers Detected, 2009–2011.
| Colonoscopy Programs | FOBT/FIT-based Programs | |
|---|---|---|
| Number of programs (N) | 14 | 9 |
| Total individuals screened (N)a | 12,309 | 13,243 |
| Mean per program (median) | 879 (801) | 1471 (811) |
| Total screening tests (N) | 12,407 | 13,327 |
| Mean per program (median) | 886 (806) | 1,481 (811) |
| Total diagnostic follow-up tests (N) | 346 | 841 |
| Mean per program (median) | 25 (17) | 93 (25) |
| Under surveillance (N)b | 891 | 429 |
| Mean per program (median) | 64 (21) | 48 (15) |
| Polyps (N) | 3,899 | 983 |
| Mean per program (median) | 279 (176) | 109 (36) |
| Cancers detected (N) | 48 | 32 |
| Mean per program (median) | 3 (2) | 4 (2) |
CRCCP Colorectal Cancer Control Program; FOBT fecal occult blood test; FIT fecal immunochemical test.
Total of unduplicated individuals screened per year.
Total number of individuals undergoing surveillance.
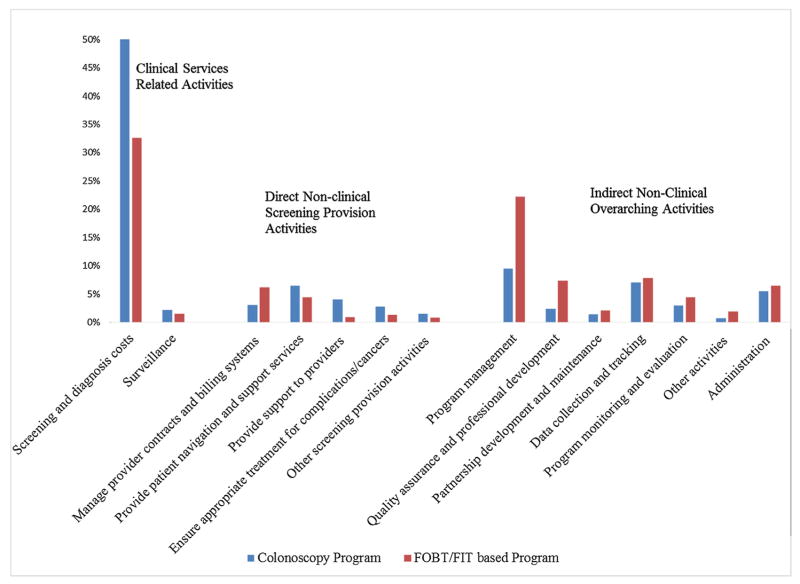
Fig. 1 provides the percent distribution of cost across all activities performed by the colonoscopy and FOBT/FIT-based programs. (Appendix A, Table A1 presents the average and median costs along with the proportion of in-kind contributions for each activity.) The largest cost components for both types of programs were clinical services related to screening and diagnostic services, followed by program management and then data collection and tracking. Total screening and diagnostic costs were 1.9 times higher for the colonoscopy programs compared to FOBT/FIT-based programs (average of $754,228 compared to $405,791) (Appendix A, Table A1). There was also a large difference in mean program management costs, but the median costs were similar indicating that this variation is likely due to a few outliers and not a systematic difference between the groups. The mean expenditure on quality assurance and professional development was 2.5 times higher for the FOBT/FIT-based programs, but again, comparison based on the median showed difference was reduced to 1.4 times higher (median of $39,158 versus $27,326). There were also differences for direct non-clinical screening provision activities, with 1.6 times higher expenditure incurred by FOBT/FIT-based programs for provider contract management (mean of $76,495 compared to $46,625 for colonoscopy-based programs) but colonoscopy programs had higher mean expenditure for all other direct non-clinical screening provision activities (although there is variation in the median costs). Patient navigation and provider support cost estimates indicate extensive variation across grantee programs, but cost related to ensuring treatment for complications and cancers was 2.6 times higher for colonoscopy programs. The proportion of in-kind contributions varied across program activities for both groups and there were no consistent patterns.
Fig. 1.
Percent Distribution of Costs for Program Activities by Screening Tests.
FOBT fecal occult blood test; FIT fecal immunochemical test
Note: Other activities include costs that could not be separated into specific activities
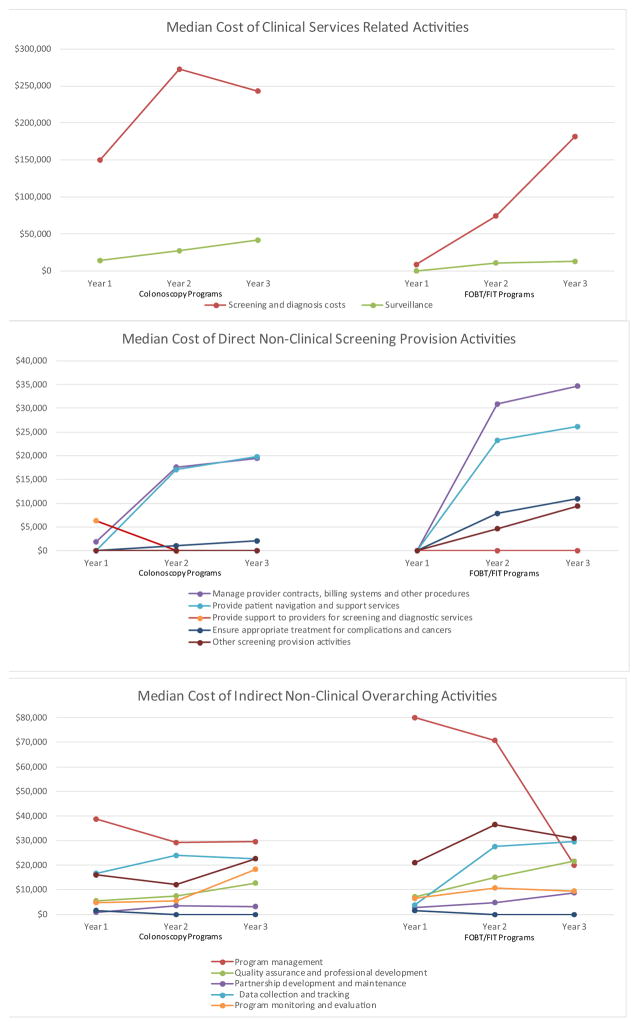
Fig. 2 presents the median cost for each of the three program years separately to identify patterns in the distribution of expenditure over time. Both screening and surveillance costs were higher in years 2 and 3 compared to year 1 due to the increase in individuals screened over time. The number of individuals screened across the FOBT/FIT-based programs in the first year was 2365 and this increased to 6197 in the third year. For colonoscopy programs the screening numbers were 2723 and 4700 in the first and third years (data not shown in figure). For both colonoscopy and FOBT/FIT-based programs, the direct non-clinical screening provision costs increased over time, again likely reflecting the increase in individuals screened. Program management costs for FOBT/FIT-based programs declined steeply over the three year period while expenditures for other activities (for example, data collection and tracking, administration) generally showed less variation.
Fig. 2.
Median Cost by Activity for Each of the Three Years of the CRCCP.
Note: Patient navigation cost only collected in year 2 and year 3. Other activities include costs that could not be separated into specific activities. The number of individuals screened in the FOBT/FIT based programs in first year was 2,365 and this increased to 6,197 in the third year. Correspondingly, for colonoscopy programs the screening numbers were 2,723 and 4,700.
CRCCP Colorectal Cancer Control Program; FOBT fecal occult blood test; FIT fecal immunochemical test
Table 2 presents the mean cost per person served by the programs with median cost reported in brackets. Clinical cost for screening and diagnostic services was $1150 on average for colonoscopy programs and $304 for FOBT/FIT-based programs, again showing variation across programs. Surveillance cost per person served was variable with an average cost of $1131 and $588 for colonoscopy and FOBT/FIT-based programs, respectively. The average per person direct non-clinical cost was $363 for colonoscopy programs and $225 for FOBT/FIT-based programs. The median costs of the programs were closer in range, indicating substantial variation across the colonoscopy programs; overall, the median costs were lower for colonoscopy programs compared to the FOBT/FIT-based programs ($211 versus $247). The indirect non-clinical overarching costs also showed variation, with a total mean non-clinical cost (direct and indirect) of about $1000 for both types of programs; however, median costs were lower for the colonoscopy programs. The cost of provider contract management was higher for FOBT/FIT-based programs while the cost of patient navigation was higher for colonoscopy programs. The largest per person indirect non-clinical cost by specific activity was program management; mean management cost was $188 for colonoscopy programs and $265 for FOBT/FIT-based programs (median values were similar).
Table 2.
Mean Cost (with median) per Person Served by the Programs.
| Colonoscopy Programs ($) | FOBT/FIT-based Programs ($) | |
|---|---|---|
| Clinical Services Related Activities | 1,634 (1,876) | 630 (618) |
| Screening and diagnosis costs | 1,150 (853) | 304 (275) |
| Surveillance (only programs with surveillance) | 1,131 (1,056) | 588 (517) |
| Direct Non-Clinical Screening Provision Activities | 363 (211) | 225 (247) |
| Manage provider contracts and billing systems | 62 (52) | 102 (77) |
| Provide patient navigation and support services | 101 (47) | 68 (36) |
| Provide support to providers for screening and diagnostic services | 75 (11) | 17 (1) |
| Ensure appropriate treatment for complications and cancers | 47 (17) | 22 (4) |
| Other screening provision activities | 78 (3) | 17 (2) |
| Indirect Non-Clinical Overarching Activities | 617 (475) | 793 (591) |
| Program management | 188 (150) | 265 (146) |
| Quality assurance and professional development | 62 (32) | 158 (42) |
| Partnership development and maintenance | 45 (17) | 31 (18) |
| Data collection and tracking | 111 (99) | 135 (30) |
| Program monitoring and evaluation | 83 (48) | 73 (31) |
| Other activities | 10 (1) | 31 (8) |
| Administration | 118 (96) | 99 (93) |
Note: FOBT fecal occult blood test; FIT fecal immunochemical test. Other activities include costs that could not be separated into specific activities.
4. Discussion
We compared the clinical and non-clinical costs of 14 colonoscopy and 9 FOBT/FIT-based programs that were funded by the CRCCP. On average, about $1000 per person was expended on direct and indirect non-clinical activities. Although the median non-clinical costs are somewhat lower, they are still substantial at about $700–$800 per person. A key finding from this study is that CRC programs incur substantial non-clinical costs that should be taken into account when planning future programs.
Additionally, the clinical cost of colonoscopy is almost four times the cost of FOBT/FIT per person when screening and diagnostic follow up tests are taken into account. Therefore, programs that use colonoscopy will only be able to screen about one-fourth the number of individuals during the early years of the program. As the colonoscopy screening interval is every 10 years compared to every year for FOBT/FIT, the numbers screened will converge over time but the initial screen will be delayed in the colonoscopy versus FOBT/FIT programs. When the goal is to offer first-time screening to a large cohort of individuals over a short period of time, FOBT/FIT tests would be the preferred approach.
The indirect overarching component (which is included in the total non-clinical cost) was about $475–$793 per person served for both types of programs. These costs are likely to decrease if programs expand to cover a large cohort of individuals as economies of scale are achieved. Additionally, previous research has shown that there are substantial fixed and start-up costs involved with program operations (Ekwueme et al., 2014; Subramanian et al., 2013; Trogdon, Ekwueme, Subramanian, & Crouse, 2014). Therefore, the indirect overarching cost should decline on a per person basis as more individuals are screened, but it is unclear to what extent these costs are fixed versus semi-variable.
Surveillance cost, which is an expenditure related to colonoscopy, showed large variation with the mean of about $600 and $1100 per person, respectfully, for the FOBT/FIT-based and colonoscopy programs. This wide variation in the unit cost of tests and procedures between grantees was also reported in prior analysis of CDC’s Colorectal Cancer Screening Demonstration Program (CRCSDP) and variation in clinical costs were also present in screening programs for breast and cervical cancer (Subramanian et al., 2008; Tangka et al., 2013). In the CRCSDP, the cost of FOBT screening ranged from $48 to $149 and colonoscopy screening ranged from $654 to $1600 per patient. Although grantees are required to reimburse for clinical services within Medicare rates, the actual costs of the clinical services are highly dependent on the ability to negotiate payment rates with providers. Therefore, the actual cost of the clinical services is dependent on provider supply, anticipated screening volume, and other factors specific to a given setting.
The strength of the present cost analysis is that we were able to systematically collect and quantify resources, and analyze expenditures from 23 CRCCP programs. We used consistent definitions for activities and a pre-tested data collection tool. Despite these methodological advantages there are several potential limitations. First, in the real world setting, programs may provide more than one type of screening test as they need to accommodate patient preferences and also follow guideline recommendations for screening individuals at increased and high risk. Second, we use program year to assess potential year-to-year variation, but programs generally operate on a continuous basis and therefore screening tests could be performed in one year while diagnostic follow-up and treatment, if required, could be provided in the subsequent year. Therefore, classification of costs and screens into specific time periods are not always accurate. Likewise, the study assesses cost per year and does not account for cost per patient over an extended period of time to compare long-term cost of colonoscopy versus FOBT/FIT-based programs. We report cost for only the first three years of the CRCCP and there could have been changes in the program costs after the data collection time period. Third, we report average and median costs to account for variation across programs but the differences in grantees across the two groups of screening programs, colonoscopy versus FOBT/FIT-based, could still influence the costs reported. Future research should systematically assess the factors that can explain the differences in cost of specific program activities across CRC screening programs.
5. Conclusions and lessons learned
Our analysis of the activity-based cost data from the first three years of the CRCCP reveal that the choice of FOBT/FIT versus colonoscopy will significantly impact the timeliness of the initial screen offered as a much larger number of individuals can be screened quicker with lower cost FOBT/FIT than colonoscopy. In addition, CRC screening programs incur substantial non-clinical costs, regardless of whether the program is colonoscopy or FOBT/FIT-based. Future studies of the cost-effectiveness of CRC screening programs should consider both these clinical and non-clinical costs in planning program implementation.
Acknowledgments
RTI International was supported by Centers for Disease Control and Prevention Contract Number 200-2008-27958, Task Order 01.
Abbreviations
- CAT
cost assessment tool
- CCDE
Colorectal Cancer Clinical Data Elements
- CDC
Centers for Disease Control and Prevention
- CRC
colorectal cancer
- CRCCP
Colorectal Cancer Control Program
- CRCSDP
Colorectal Cancer Screening Demonstration Program
- FIT
fecal immunochemical test
- FOBT
fecal occult blood test
Appendix A
Fig. A1.
CRCCP Screening Provision Program Components and Specific Activities*.
CRCCP Colorectal Cancer Control Program
* Screening promotion activities are reported in a companion manuscript (20)
** Overarching component supports screening provision and screening promotion activities; the costs of the overarching component assigned to screening provision are reported in this study.
*** For example, support activities such as information management.
Table A1.
Mean, Median, and in-Kind Cost for Program Activities by Screening Tests.
| Colonoscopy Programs | FOBT/FIT-based Programs. | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean (Median) of Years 1–3 | % in-kind costs | Mean (Median) of Years 1–3 | % in-kind costs | |||
| Clinical Services Related Activities | ||||||
| Screening and diagnosis costs | $754,228 | (700,213) | 18% | $405,791 | (252,358) | 23% |
| Surveillance (only programs with surveillance) | $76,745 | (63,309) | 0% | $34,726 | (41,371) | 8% |
| Direct Non-Clinical Screening Provision Activities | ||||||
| Manage provider contracts, billing systems and other procedures | $46,625 | (46,250) | 5% | $76,495 | (68,824) | 4% |
| Provide patient navigation and support services | $97,393 | (35,061) | 14% | $54,747 | (49,290) | 1% |
| Provide support to providers for screening and diagnostic services | $60,079 | (9708) | 36% | $11,159 | (1315) | 0% |
| Ensure appropriate treatment for complications and cancers | $41,026 | (21,118) | 2% | $15,893 | (13,493) | 16% |
| Other screening provision activities | $22,305 | (2667) | 6% | $9,945 | (1276) | 1% |
| Indirect Non-Clinical Overarching Activities | ||||||
| Program management | $142,231 | (123,403) | 18% | $275,863 | (129,547) | 9% |
| Quality assurance and professional development | $35,149 | (27,326) | 6% | $90,760 | (39,158) | 52% |
| Partnership development and maintenance | $21,663 | (17,407) | 6% | $26,607 | (20,924) | 0% |
| Data collection and tracking | $105,079 | (75,265) | 2% | $97,063 | (83,968) | 4% |
| Program monitoring and evaluation | $44,281 | (36,684) | 2% | $54,836 | (26,214) | 14% |
| Other activities | $11,146 | (1141) | 62% | $23,571 | (5340) | 4% |
| Administration | $82,001 | (44,574) | 15% | $80,793 | (82,662) | 0% |
FOBT fecal occult blood test; FIT fecal immunochemical test. Note: Other activities include costs that could not be separated into specific activities.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Centers for Disease Control and Prevention. Vital signs: Colorectal cancer screening test use—United States, 2012. MMWR. Morbidity and Mortality Weekly Report. 2013;62:881–888. [PMC free article] [PubMed] [Google Scholar]
- Drummond M, Schulpher M, Torrance G, O’Brien B, Stoddard G. Methods for the economic evaluation of health care programmes. Oxford, England: Oxford University Press; 2005. [Google Scholar]
- Ekwueme DU, Subramanian S, Trogdon JG, Miller JW, Royalty JE, Li C, et al. Cost of services provided by the national breast and cervical cancer early detection program. Cancer. 2014;120(Suppl 16):2604–2611. doi: 10.1002/cncr.28816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JA, Fikry C, Troxel AB. Cutting cost and increasing access to colorectal cancer screening: Another approach to following the guidelines. Cancer Epidemiology, Biomarkers and Prevention. 2006;15:108–113. doi: 10.1158/1055-9965.EPI-05-0198. [DOI] [PubMed] [Google Scholar]
- National Colorectal Cancer Roundtable. Tools & resources—80% [screening rate] by 2018. National Colorectal Cancer Roundtable; n.d. Accessed from http://nccrt.org/tools/80-percent-by-2018. [Google Scholar]
- National Governors Association Center for Best Practices. State strategies for curbing colorectal cancer. Washington, DC: National Governors Association Center for Best Practices; 2008. [Accessed October 22, 2008]. from http://www.nga.org/files/live/sites/NGA/files/pdf/0806CURBCANCER.PDF. [Google Scholar]
- Pignone M, Russell L, Wagner J. Economic models of colorectal cancer screening in average-risk adults: workshop summary. Publishing; Washington, DC: 2005. [PubMed] [Google Scholar]
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] American Journal of Gastroenterology. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use—United States, 2013. MMWR. Morbidity and Mortality Weekly Report. 2015;64:464–468. [PMC free article] [PubMed] [Google Scholar]
- Salome HJ, French MT, Miller M, McLellan AT. Estimating the client costs of addiction treatment: First findings from the client drug abuse treatment cost analysis program (Client DATCAP) Drug and Alcohol Dependence. 2003;71:195–206. doi: 10.1016/s0376-8716(03)00133-9. [DOI] [PubMed] [Google Scholar]
- Seeff LC, Rohan EA. Lessons learned from the CDC’s colorectal cancer screening demonstration program. Cancer. 2013;119(Suppl 15):2817–2819. doi: 10.1002/cncr.28165. [DOI] [PubMed] [Google Scholar]
- Smith RA, Manassaram-Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, et al. Cancer screening in the United States, 2015: A review of current American cancer society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians. 2015;65:30–54. doi: 10.3322/caac.21261. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Ekwueme DU, Gardner JG, Bapat B, Kramer C. Identifying and controlling for program-level differences in comparative cost analysis: Lessons from the economic evaluation of the National Breast and Cervical Cancer Early Detection Program. Evaluation and Program Planning. 2008;31:136–144. doi: 10.1016/j.evalprogplan.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Ekwueme DU, Gardner JG, Trogdon J. Developing and testing a cost-assessment tool for cancer screening programs. American Journal of Preventive Medicine. 2009;37:242–247. doi: 10.1016/j.amepre.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Bobashev G, Morris RJ. When budgets are tight: There are better options than colonoscopies for colorectal cancer screening. Health Affairs. 2010;29:1734–1740. doi: 10.1377/hlthaff.2008.0898. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Tangka FK, Hoover S, Beebe MC, DeGroff A, Royalty J, et al. Costs of planning and implementing the CDC’s colorectal cancer screening demonstration program. Cancer. 2013;119(Suppl 15):2855–2862. doi: 10.1002/cncr.28158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangka F, Subramanian S. Importance of implementation economics for program planning–the case for colorectal cancer control program. doi: 10.1016/j.evalprogplan.2016.11.007. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangka FK, Subramanian S, Beebe MC, Hoover S, Royalty J, Seeff LC. Clinical costs of colorectal cancer screening in 5 federally funded demonstration programs. Cancer. 2013;119(Suppl 15):286. doi: 10.1002/cncr.28154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangka FKL, Subramanian S, Hoover S, Royalty J, Joseph K, DeGroff A. Costs of promoting cancer screening: Evidence from CDC’s colorectal cancer control program (CRCCP) Preventing Chronic Disease. 2016 doi: 10.1016/j.evalprogplan.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trogdon JG, Ekwueme DU, Subramanian S, Crouse W. Economies of scale in federally-funded state-organized public health programs: Results from the National Breast and Cervical Cancer Early Detection Programs. Health Care Management Science. 2014;17:321–330. doi: 10.1007/s10729-013-9261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group. United States cancer statistics: 1999–2012 incidence and mortality. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2015. [Accessed March 6, 2016]. from www.cdc.gov/uscs. [Google Scholar]
- Vijan S, Hwang I, Inadomi J, Wong RK, Choi JR, Napierkowski J, et al. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. American Journal of Gastroenterology. 2007;102:380–390. doi: 10.1111/j.1572-0241.2006.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: A targeted, updated systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: Clinical guidelines and rationale-update based on new evidence. Gastroenterology. 2003;124:544–560. doi: 10.1053/gast.2003.50044. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Lansdorp-Vogelaar I, Wilschut J, Knudsen AB, van Ballegooijen M, Kuntz KM. Cost-effectiveness of DNA stool testing to screen for colorectal cancer: report to AHRQ and CMS from the Cancer Intervention and Surveillance Modeling Network (CISNET) for MISCAN and SimCRC models. Rockville, MD: Agency for Healthcare Research and Quality; 2007. [Accessed June 1, 2015]. from https://www.cms.hhs.gov/mcd/viewtechassess.asp?from2=viewtechassess.asp&id=212. [PubMed] [Google Scholar]