Abstract
Vectors based on adeno-associated virus serotype 9 (AAV9) efficiently transduce cardiomyocytes in both rodents and large animal models upon either systemic or regional vector delivery. In this chapter, we describe the most widely used production and purification method of AAV9. This production approach does not depend on the use of a helpervirus but instead on transient transfection of HEK293T cells with a plasmid containing the recombinant AAV genome and a second plasmid encoding the AAV9 capsid proteins, the AAV Rep proteins and the adenoviral helper functions. The recombinant AAV is then purified by iodixanol density gradient centrifugation. This chapter also describes in detail the characterization and quality control methods required for assuring high quality vector preparations, which is of particular importance for experiments in large animal models.
Keywords: Adeno-associated virus, Serotype, AAV, AAV9, Cardiotropic, Gene therapy, AAV production, AAV characterization
1 Introduction
Adeno-associated virus (AAV) is a small, non-enveloped virus with a single-stranded DNA genome that is flanked by two inverted terminal repeats (ITRs). It has originally been isolated from an adenoviral preparation [1] and is a “defective” virus in the sense that it requires co-infection with a helpervirus, such as adenovirus or herpesvirus, for productive replication [1].
In part due to its nonpathogenic nature, its limited immunogenicity and its ability to trigger long-term gene expression in postmitotic tissues, even in the absence of genome integration, AAV has been recognized as one of the most promising gene delivery vehicles. Not only has it often become the tool of choice in preclinical animal models, but as of June 2015, 117 clinical trials using AAV have either been completed or are in progress. Moreover, in 2012, treatment of lipoprotein-lipase deficiency has been approved for clinical use in Europe, the first approval of a gene therapeutic treatment in the Western World [2].
The collection of AAV serotypes and variants show broad but distinct tissue and cell tropism. For cardiac gene delivery, the AAV serotype 9 (AAV9) has clearly emerged as the most potent serotype, at least in rodents, when delivered systemically [3] and in pigs (personal communication: Dr. Roger Hajjar, Icahn School of Medicine) and possibly in dogs [4, 5] when delivered regionally.
In this chapter, we describe the production and detailed characterization of AAV9 vectors at a scale that is sufficient for both small and large animal experiments.
The AAV vector genome consists of two genes, the Rep gene, which encodes for the Rep proteins, which are involved in DNA replication and encapsidation of the viral DNA into preformed capsids. The viral capsid is composed of 60 copies of the capsid proteins VP1, VP2, and VP3 that are encoded by the Cap gene. Using an alternative reading frame, the Cap gene also encodes the so-called assembly-activating protein (AAP), which facilitates capsid assembly [6].
For recombinant AAV (rAAV) production, the ITRs are the only cis elements required, whereas the Rep proteins, the serotype-specific capsid proteins, AAP and all the helpervirus functions can be provided in trans. One of the drawbacks of AAV is its limited packaging capacity of ~5 kb. But for most applications this is not an issue because the median size of human proteins is 375 amino acids [7].
For small to medium scale AAV production (1012–1013 vector genome containing particles), AAV is generally produced by transfection of HEK293 cells. The plasmids used are a so-called cisplasmid, which contains the transgene (or elements regulating host gene expression, e.g., shRNAs) flanked by the ITRs, and plasmids encoding Rep, Cap, AAP and the necessary (most often adenoviral) helpervirus proteins [8]. In its simplest form, which is the method preferred in our laboratory, Rep, Cap, AAP, and all the adenoviral helper functions are provided on a single plasmid [9].
For AAV9, the virus is harvested 72 h post-transfection from both the cell culture medium as well as the transfected cells. In our laboratory, and many others, the virus is then purified by Iodixanol gradient centrifugation.
Whereas many laboratories only characterize their virus by determining viral titers by qPCR, our experience is that, especially for large animal studies, a more thorough virus characterization that includes SDS-PAGE (to determine virus purity and total viral capsid titers), qPCR (to determine viral genome titers), alkaline agarose gel electrophoresis (to ensure genome integrity and/or viral genome titer) and negative-staining electron microscopy (to determine the percentage of empty particles) is warranted.
2 Materials
2.1 Plasmid Production
Cis plasmid containing the rAAV genome to be packaged.
pDG9 helper plasmid (AAV9 capsid sequence cloned into the SwaI/ClaI digested pDG (Plasmidfactory, Bielefeld, Germany)).
SURE2 (Agilent Technologies, Santa Clara, CA) or Stbl3 (Life Technologies, Norwalk, CT) competent bacteria for transformation with cis-plasmids.
QIAfilter Plasmid Maxi Kit (Qiagen, Germantown, Maryland).
SmaI (or the isoschizomer XmaI) restriction enzyme.
2.2 Cell Culture
HEK 293T/ 17 cells (ATCC, Manassas, VA).
DMEM 4.5 g/l glucose, + l-glutamate + sodium pyruvate.
Fetal bovine serum.
10× penicillin/streptomycin.
Trypsin 0.025 %.
PBS without magnesium and calcium.
225 cm2 tissue culture flask.
2.3 Transfection
Vented or non-vented tissue culture triple flask(s).
50 µg cis plasmid and 150 µg helper plasmid per triple flask to be transfected.
Linear, 25 kDa polyethylenimine (PEI) (Polysciences Inc. Warrington, PA).
250 ml sterile filter (0.22 µm) bottles.
2.4 Harvesting and Processing
200 ml polypropylene conical centrifuge tubes.
50 ml polypropylene conical tubes.
Lysis buffer: 150 mM NaCl, 50 mM Tris–HCl, pH 8.5.
Sorvall RC-6+ centrifuge.
SH-3000BK, F14-6x-250y, and F13-14x50cy rotors.
Universal Nuclease for Cell Lysis (Thermo Fisher, Waltham, MA) (see also Note 1).
Ammonium sulfate.
2.5 Preparation of Ultracentrifugation Gradients
Optiprep (60 % iodixanol).
5× Optiprep dilution buffer “ODB”: 5× PBS, 5 mM magnesium chloride, 12.5 mM potassium chloride.
Iodixanol Gradient Layer Solutions (see Table 1).
Ti70 rotor (Beckman Coulter, Indianapolis, IN).
OptiSeal polypropylene 26 × 77 mm ultracentrifuge tubes (37.4 ml, Beckman Coulter, Indianapolis, IN).
5 ml syringe and 18 gauge needles.
Table 1.
Components to prepare iodixanol gradient layer solutions sufficient for one complete (8 tubes) Ti70 rotor
| Iodixanol gradient layer (%) |
Optiprep (ml) | 5 M NaCl (ml) | 5× ODB (ml) | Water (ml) | Total for 1 full rotor (ml) |
|---|---|---|---|---|---|
| 15 | 16.1 | 12.8 | 12.8 | 22.5 | 64.2 |
| 25 | 18.0 | 0 | 8.6 | 16.5 | 43.1 |
| 40 | 23.5 | 0 | 7.0 | 4.7 | 35.2 |
| 60 | 35.2 | 0 | 0 | 0 | 35.2 |
2.6 Dialysis
10 mm flat width, 12–14 kDa MWCO, regenerated cellulose dialysis Tubing (Spectrum Labs, Piscataway, NJ).
Dialysis tubing closures.
Lactated Ringer’s Solution.
0.22 µm sterile syringe filter.
Large sterile bottle or container.
Orbital shaker.
2.7 Quantitative Real Time PCR
Real time PCR machine.
Real time PCR tubes and caps compatible with specific machine.
Real time PCR Master Mix SYBR Advantage (Clontech, Mountain View, CA).
Forward and reverse primers that anneal perfectly to both the reference standard and the AAV sample to be quantified (see also Note 2).
AAV2 Reference Standard Material (ATCC VR1616, Manassas, VA) or calibrated “home-made” AAV reference standard (see Note 3).
2.8 Capsid Particle Content and Purity
Equipment and reagents for SDS-PAGE gel electrophoresis.
6× SDS-PAGE loading buffer: 0.8 g SDS, 5 ml 1 M Tris–HCl pH 6.8, 5 ml glycerol, 5 mg bromophenol blue. Store in aliquots at −20 °C. After thawing, add 50 µl beta-mercaptoethanol per 1 ml of 6× loading buffer immediately prior to adding the buffer to the viral or BSA samples.
BSA protein standard (Pierce, Thermo Fisher, Waltham, MA).
Pre-Soak buffer: 50 % methanol, 10 % acetic acid.
Staining buffer: 50 % methanol, 10 % acetic acid, 0.003 % Coomassie Brilliant Blue R-250.
Destaining buffer: 40 % methanol, 8 % acetic acid.
Odyssey infrared scanner (Li-Cor, Lincoln, NE).
2.9 Electron Microscopy
Carbon Coated Copper EM Grids.
Dumont inverse, anti-capillary tweezers.
Uranyl acetate 2 % solution.
Chromatography paper.
Transmission electron microscope.
2.10 Alkaline Gel
“Submarine style” electrophoresis apparatus and power supply.
Electrophoresis grade agarose.
50× Alkaline Gel Buffer: 2.5 M sodium hydroxide, 50 mM EDTA.
Alkaline Gel loading dye: 4× Alkaline Gel Buffer, 1.2 % SDS, 20 % Glycerol, 0.01 % Xylene Cyanol.
DNA Mass Ladder.
Gel Red (Biotium, Fremont, CA) or equivalent dye (e.g., SYBR Gold, (Thermo Fisher, Waltham, MA)) that allows the sensitive detection of single stranded DNA.
3 Methods
3.1 Plasmid Cloning and Production
To prevent the potential loss of intact ITRs all our cis plasmids are cloned and maintained in either SURE2 or Stbl3 cells and grown at 30–32 °C (see Note 4). pDG9 can be maintained in a regular E. coli strain such as DH5α and can be grown at 37 °C. We routinely purify our plasmids with QIAFilter Plasmid Maxi Kit. For the production of the cis plasmid, one 2 l Erlenmeyer flask with 600 ml Luria–Bertani medium should produce enough plasmid for ten triple flasks. Since the amount of pDG9 needed for the same number of triple flasks is three times the amount of the cis plasmid, we grow the bacterial cultures in three 2 l flasks with 600 ml Luria-Bertani medium each.
To confirm that the majority of the cis-plasmid contains two intact ITRs we digest the cis plasmid with SmaI (or XmaI) restriction enzyme and analyze the digested DNA on an agarose gel.
3.2 Cell Maintenance (See Note 5)
HEK293T are maintained in DMEM supplemented with 10 % FBS and 1× Penicillin/Streptomycin (“medium”) for all growth and passaging steps unless otherwise noted. For continuous culture, the cells are grown to approximately 70–80 % confluency and then split 1:10 or 1:20 to be ready to be split again 3 days or 4 days later, respectively.
To split cells from a 225 cm2 flask, gently remove the culture medium, add 20 ml PBS without Mg/Ca to cover the surface and gently rock the flask to completely cover the cells. Remove the PBS and add 5 ml 0.025 % Trypsin to the side of the flask, not directly onto the cells. Spread over the cells by gently “rocking” the flask several times. Incubate at 37 °C for 1–2 min. Then rock the flask to completely dislodge the cells. Pipet 25 ml media onto the cells and pipet up and down to prepare a homogenous cell suspension. Add 50 ml of a 1:10 or 1:20 dilution of cell suspension in fresh medium to a new flask (see also Note 6).
3.3 Transfection
To prepare the PEI solution for transfection add 250 mg PEI powder to 200 ml sterile, deionized water and stir with magnetic stir bar. Adjust pH to 1.9 by addition of 10 N hydrochloric acid. Stir overnight to assure complete dissolution of the PEI. The next day, add dropwise 1 N sodium hydroxide to adjust the pH to 4.5. Then add water to 250 ml and sterile filter with a 0.22 µm filter bottle. Store aliquots at −80 °C. Once thawed, the solutions can be stored at 4 °C for up to 2 months.
The day before transfection, detach cells with Trypsin and split either ~1:4 or 1:6 (see Note 7) into triple flasks. One confluent 225 cm2 flask is sufficient to seed two triple flasks (total area 1000 cm2) at a 1:4 dilution. Prior to seeding the triple flasks, remove a sufficient amount of cells to seed a 225 cm2 flask for the continuation of cell passaging.
The morning after seeding the triple flasks, check the flasks for cell confluency. If ~70 % confluent, proceed to transfection immediately. If the confluency is less than ~70 %, perform the transfection later in the day.
For each triple flask to be transfected, warm 90 ml DMEM (2 % FBS, 1× Pen/Strep) to 37 °C.
Prepare the transfection mix by adding in the following order to 20 ml room temperature DMEM (no FBS, no Pen/Strep): 50 µg cis plasmid and 150 µg pDG9 (briefly vortex) and 700 µl PEI solution (pH 4.5). Vortex for 10–20 s and incubate at room temperature for 15 min.
Add the transfection mix to the pre-warmed 2 % FBS medium and mix by swirling.
Gently remove the media from the cells in the triple flasks and replace with transfection mix media.
Grow the transfected rAAV producing cells for 3 days, then harvest.
3.4 Harvesting and Processing
3.4.1 Preparation of Cell Lysate
Detach the cells by tapping the flask vigorously and transfer the solution to a sterile 200 ml conical flask. Pellet the cells by centrifugation at 1000 × g for 15 min in a Sorvall RC-6+ centrifuge and SH-3000BK swinging bucket rotor.
Collect the cell culture supernatant in a sterile bottle for later processing.
Gently add 10–15 ml PBS to the side of the conical tube. Swirl gently to dislodge, but not break apart, the pellet. Transfer the pellet to a 50 ml conical tube. Multiple pellets can be combined into one 50 ml tube for further processing. If cells remain in the large conical, use the PBS to rinse the cells off the large tube and transfer to the small tube.
Pellet the cells by centrifugation in a Sorvall RC-6+ centrifuge and SH-3000BK rotor at 1000 × g for 10 min. Pour the supernatant into the bottle containing the cell culture supernatant (see Note 8). Proceed with media to step 1 of Subheading 3.4.2. At this step, the cell culture supernatant can be frozen at −80 °C to be processed later.
Resuspend the cell pellet in 10 ml lysis buffer (see Note 9).
To lyse the cells, the cell suspension is repeatedly frozen and thawed. The cell suspension can either be frozen by putting it into a −80 °C freezer or on dry ice. The cell suspension is thawed in a 37 °C water bath followed by brief vortexing. This freeze/thaw cycle is repeated twice. Aggregation of cellular debris during the freeze–thaw cycles is normal. Any of the freezing steps can be used as a stopping point.
To digest genomic DNA and non-encapsidated viral DNA and cellular RNA add 2 µl (10 U/µl) of Universal Nuclease to the thawed, crude lysate and incubate for 30 min at room temperature (see also Note 1).
Centrifuge the crude lysate in a Sorvall RC-6+ centrifuge and F13-14x50cy fixed angle rotor at 5000 × g for 20 min and transfer the supernatant to a new tube (discard the pellet). The new tube is ready for Iodixanol gradient ultracentrifugation. It can be stored at −80 °C at this point.
3.4.2 Processing of the Cell Culture Supernatant
If the cell culture supernatant was frozen (Subheading 3.2), thaw it at 37 °C, but do not incubate longer than necessary. Per 100 ml of supernatant add 31.3 g ammonium sulfate and shake for 1–2 min to dissolve completely the ammonium sulfate. Incubate the mixture on ice for at least 30 min (see Note 10).
To pellet the virus precipitate, centrifuge in a Sorvall RC-6+ centrifuge and an F14-6x250y fixed angle rotor at 8300 × g for 30 min. Because the pellet may be loose, pour off the supernatant as soon as possible after centrifugation. THE SUPERNATANT CANNOT BE STERILIZED WITH BLEACH. INSTEAD, THE SUPERNATANT MUST BE AUTOCLAVED AND THEN BE DISCARDED. Ammonium Sulfate is incompatible with bleach, and treatment of the supernatant with bleach would result in the production of TOXIC GASES AND EXPLOSIVE COMPOUNDS.
The pellet will accumulate on the side of the centrifuge tube. The pellet is resuspended with a pipette in 10 ml Lysis Buffer (see Note 9). The resuspension is complete when there is no remaining pellet on the wall of the tube and the solution is homogeneous without any remaining “chunks”.
Add 2 µl (20 U) of Universal Nuclease for Cell Lysis (see Note 1) and incubate 30 min at room temperature (or according to manufacturer’s instructions if using an alternative nuclease). The virus suspension is now ready for iodixanol gradient purification of the virus.
3.5 Preparation of Ultracentrifugation Gradients
3.5.1 First Gradient
Pipet 9.5 ml AAV cell lysate or virus suspension from cell culture supernatant into OptiSeal ultracentrifuge tubes. Try not to introduce bubbles.
Insert a Pasteur pipet into the tube with the tip centered at the bottom (see Note 11).
To form the gradient, underlay the viral solution in this order by slowly adding to the top of the Pasteur pipette: (a) 7.3 ml 15 % iodixanol, (b) 5 ml 25 % iodixanol, (c) 4 ml 40 % iodixanol, and (d) 4 ml 60 % iodixanol. Before adding the next solution, allow all of the previous solution to reach the bottom of the tube.
Carefully remove the Pasteur pipet without disturbing the gradient.
If at this point the tube is not completely filled, add any leftover AAV crude lysate, virus suspension from the cell culture supernatant or lysis buffer to the top of the tube to fill the tube up to the neck. Any bubbles must be removed prior to centrifugation to prevent the collapse of the tubes during centrifugation. To remove potential bubbles slightly squeeze the tube until the bubbles rise above the neck of the tube and remove them with a pipet. Seal the tube by firmly inserting the plastic stopper.
Use a permanent marker to draw a line at the 40–60 % interface. The interfaces are easy to see before centrifugation but more difficult to visualize after centrifugation.
Centrifuge at (350,333 × g) in a Ti70 rotor for 1 h (see Note 12).
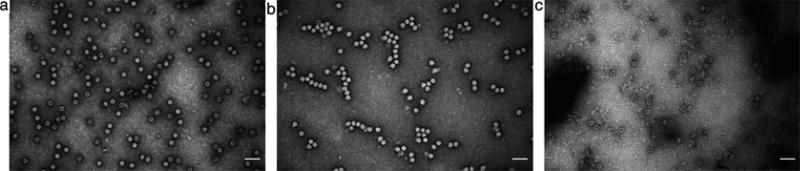
The majority of AAV particles will be located at the 60–40 % iodixanol interface. The virus can be collected by inserting an 18-gauge needle (connected to a 5 ml syringe) just below the interface and slowly withdrawing 3 ml (These 3 ml will consist of approximately the top 1 ml of the 60 % and the bottom 2 ml of the 40 % iodixanol solution). Alternatively, if no second gradient is performed to concentrate further the virus, it is preferable to collect fractions from the bottom (see below: Subheading 3.5.2, steps 5 and 6). Using this approach will yield virus preparation with less empty particles (see Fig. 2).
Determine the AAV titer/yield by qPCR (see below Subheading 3.7). If the viral titers are too low, combine the virus from multiple tubes and proceed to second gradient.
Fig. 2.
Electron microscopy images of AAV. (a) Virus collected after the first ultracentrifugation gradient; (b) Fraction 4 collected from the second ultracentrifugation gradient; (c) Fraction 7 collected from the second ultracentrifugation gradient. Viral capsids containing a viral genome appear as homogeneously white hexagons, while empty capsids show as hexagons with a white rim but a dark center (a) ~60 % genome containing particles, (b) >98 % genome containing particles, (c) ~5 % genome containing particles
3.5.2 Second Gradient
For a second round of iodixanol gradient purification (see Note 13), dilute the harvested AAV/iodixanol solution in 1× ODB such that the percentage of iodixanol is lower than 25 %. For this calculation, the AAV/iodixanol harvested from the first gradient is assumed to be 47 % (1 ml 60 % + 2 ml 40 %).
Add 22 ml of the AAV/iodixanol dilution into an OptiSeal ultracentrifuge tube.
This solution is then underlaid sequentially with 4 ml 40 % iodixanol and 60 % iodixanol using the technique described above.
Centrifuge the tubes at 69,000 rpm (350,333 × g) in a Ti70 rotor for 2 h.
Collect 1.25 ml fractions from the bottom of the tube by creating a needle hole at the bottom of the tube, and letting the contents drip into Eppendorf tubes pre-marked at the 1.25 ml level. This can be done by covering the top opening of the tube with a finger to create a vacuum to prevent flow, then releasing the finger when the appropriate fraction tube is placed under the bottom hole.
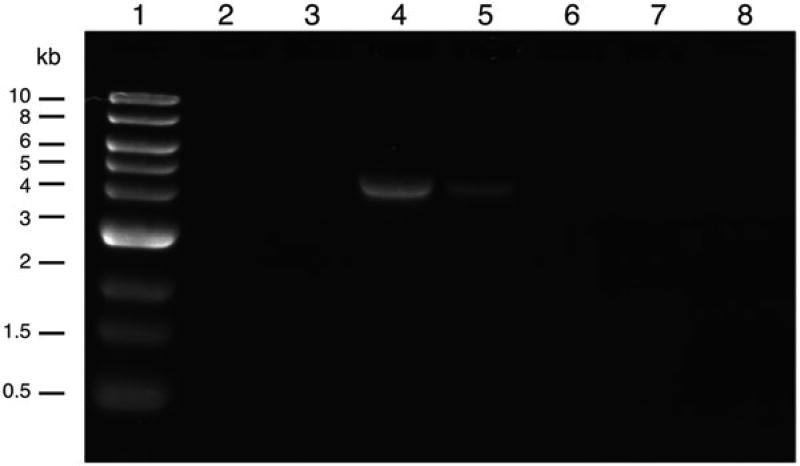
Run an alkaline gel of the fractions to identify the fractions containing the majority of full-length viral genomes and combine those fractions.
3.6 Dialysis
Soak dialysis tubing in lactated Ringer’s solution overnight.
The next morning, squeeze out any lactated Ringer’s solution left in the tubing, clamp one side of the tubing with a dialysis clip, pipet the AAV/Iodixanol into the tubing, and clamp the other end with a dialysis clip. To allow the easy recovery of the virus, leave at least 2 in. of tubing hanging outside one of the clamped ends of the tubing.
Place the closed dialysis tubing in a large sterile bottle with at least 100 dialysate volumes of lactated Ringer’s solution.
Agitate the dialysis solution gently for 1–2 h on an orbital shaker platform, and replace the lactated Ringer’s solution.
Agitate the dialysis solution for an additional 4–5 h, and replace with fresh lactated Ringer’s solution.
-
Shake gently overnight. Remove the clamp on the side with additional tubing and collect the contents of the tubing in a 50 ml tube. Filter through a 0.22 µm sterile syringe filter. Aliquot and store at −80 °C (see Note 14).
AAV Vector Titration and Quality Control (See Note 15)
3.7 Viral Titer Determination by Quantitative Real-Time PCR (qPCR)
Prepare a 2× master mix of reagents (2× SYBR Green, primers, water, ROX passive reference dye) that will be enough for duplicates of the following samples: four dilutions of standards (see Note 2), two dilutions of AAV sample, and one notemplate control. Add 8 µl of master mix and 2 µl virus dilutions to each tube for a final reaction volume of 10 µl.
Thaw an aliquot of the ATCC AAV reference virus (or a well characterized in-house produced AAV standard) as well as an aliquot of the AAV to be quantified (see Note 3).
Prepare a standard curve of the reference virus by diluting the virus stock: 1:1000; 1:10,000; 1:100,000; and 1:1,000,000.
Dilute the AAV sample 1:10,000 and 1:100,000.
Perform the PCR according to the manufacturer’s instructions for the PCR reagents and instrument manufacturer’s instructions.
Calculate the viral titer based on the standard curve and dilution of virus sample using the qPCR machine software (see Note 3).
3.8 Assessment of the Purity of Vector Preparations and Determination of Total Viral Capsid Titers
Prepare 10 % polyacrylamide gels and buffers and assemble apparatus for running SDS-PAGE.
Dilute BSA standard solution to concentrations of 200, 100, 50, 25 ng/µl, then mix 10 µl of each with 20 µl water and 6 µl 6× SDS-PAGE Loading Buffer.
Mix 30 µl of AAV sample with 6 µl 6× SDS-PAGE Loading Buffer.
Heat the viral samples at 95 °C for 5 min and the BSA standards at 95 °C for 3 min (BSA partially degrades at boiling times longer than 3 min).
Load the samples into the wells and run at 125 V until the dye line reaches the bottom of the gel, usually 2–3 h.
Remove the gel from the electrophoresis plate assembly and soak in Pre-Staining Solution for 10 min while gently agitating on a rocking platform.
Immerse the gel in Staining Solution and rock gently overnight in an airtight container.
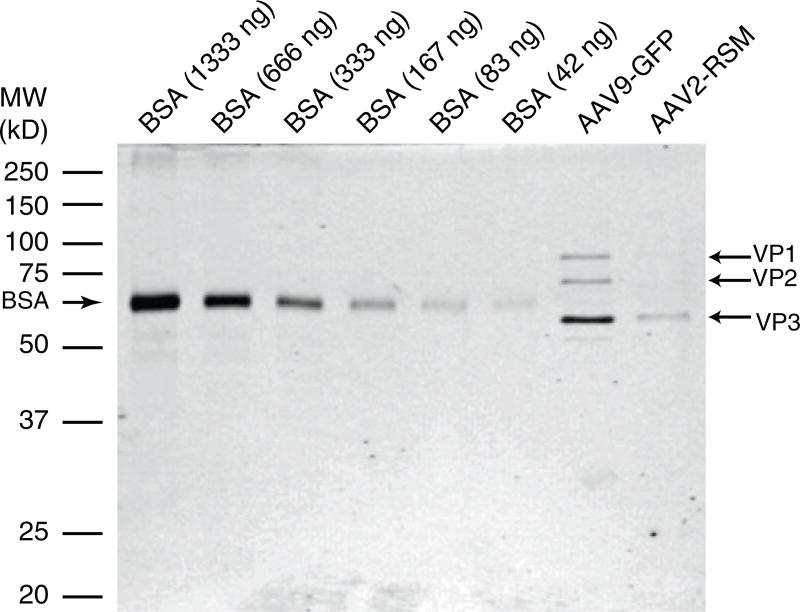
The following day, replace the staining solution with destaining solution and add a rolled up paper towel to one side of the container. Gently rock until the destaining solution and/or the paper towel becomes soaked with stain. Then replace the spent destaining solution with fresh destaining solution and clean paper towel. Repeat until the gel shows little to no background staining (see Fig. 1).
Wash the gel by gently rocking it for 5 min in water. Repeat this wash step once with fresh water.
Scan the gel on the LI-COR Odyssey Infrared Scanner or equivalent fluorescence scanner. If no fluorescence scanner is available, the gel can be scanned with a regular scanner, although this appears to modestly reduce the accuracy of the assay.
Quantify the relative intensity of the BSA and AAV VP3 bands with the LI-COR software or freely available Image J (http://imagej.nih.gov/ij/).
Plot the band intensity (arbitrary units [au]) vs. ng of BSA loaded. Use a linear regression to obtain an equation that correlates ng of protein to band intensity. Use this equation to calculate the ng of protein in the VP3 band of the AAV sample.
The total viral particle (vp) titer can be calculated with the following formula: vp/µl = ng VP3/4.987 × 10−9 /µl AAV sample loaded per lane (see Note 16).
Fig. 1.
Representative gel for assessment of capsid content and purity. From left to right : BioRad Precision Plus Dual Color Ladder, BSA 1,333 ng, BSA 666 ng, BSA 333 ng, BSA 167 ng, BSA 83 ng, BSA 42 ng, AAV9-GFP (1.32×10 vg), AAV2-RSM (2.41×10 vg)
3.9 Determination of Percent Genome Containing AAV Particles by Electron Microscopy
Pick up an EM grid with a Dumont Anti-Capillary Reverse (self-closing) tweezers and set down on the bench with the shiny side of the grid facing up.
Pipet 5 µl of AAV sample onto the grid and allow it to dry by evaporation. This may take 30–60 min.
Wash the grid by pipetting, drop by drop, about 200 µl water onto the grid.
Wick excess water by slowly placing chromatography paper vertically next to the grid.
Pipet 5 µl 2 % uranyl acetate solution onto the grid. Incubate for 5 min (see Note 17), and then wick off as above. Let the grid dry.
Visualize AAV particles with a transmission electron microscope at 50,000-fold magnification. Viral capsid containing a viral genome will appear as homogeneously white hexagons, while empty capsids show as hexagons with a white rim but a dark center (see Fig. 2).
Randomly count at least 100 particles to determine approximate percentage of genome-containing vs. empty AAV particles.
3.10 Alkaline Gel Electrophoresis to Determine Vector Genome Integrity (and Viral Genome Titers)
Prepare the alkaline agarose gel by adding 1 g agarose to 98 ml water and microwave to dissolve. Let the solution cool until it can be handled without gloves, then add 2 ml 50× Alkaline Electrophoresis Buffer, swirl to mix, pour to cast the gel.
Place the gel into an electrophoresis apparatus, fill with 1× Alkaline Running Buffer, and place everything in a cold room.
Dilute DNA ladder (500 ng) to 25 µl with water.
Mix 25 µl AAV sample or diluted DNA ladder with 8.5 µl 4× alkaline sample loading buffer, heat to 95 °C for 3 min, then cool on ice prior to loading on the gel.
Load the gel and run overnight at 20 V in the cold room using a dedicated power supply (see Note 18).
Remove the gel from the electrophoresis apparatus, place the gel in a container and cover it with 0.1 M Tris–HCl pH 8.5 and rock gently for 1 h.
Discard the buffer and replace with 4× GelRed or SYBR Gold in 0.1 M NaCl and rock protected from light for 2 h.
Rinse the gel briefly in tap water.
Visualize the gel with a UV transilluminator and capture a digital picture (see Fig. 3 and Note 19).
Fig. 3.
Alkaline gel of fractions collected from an iodixanol gradient. Lane 1: 1 kb DNA ladder (500 ng). Lanes 2 – 8, 20 µl of fractions 2–8 of a first iodixanol gradient of AAV9-LMNA. The capsid protein content in fractions 5 and 6 is higher than in fraction 4, indicating that fraction 5 and especially fraction 6 contain empty capsids (SDS-PAGE, not shown)
4 Notes
Alternative nucleases that digest double DNA, single-stranded DNA and RNA can also be used, for instance benzonase (Sigma-Aldrich, St. Louis, MO), to achieve the same result. If benzonase is used, add 2 µl 2.5 M MgCl2 and 2 µl benzonase (1500 U/µl) to 10 ml crude lysate and incubate 1 h at 37 °C.
Extensively characterized, AAV2 and AAV8 reference standard material can be obtained from ATCC (cat. nos. VR-1616 and VR-1816, respectively). The broad use of these reference standards should facilitate the reproducibility of titer determinations among different laboratories. For economic reasons, it might be preferable to employ the ATCC standard viruses to prepare a thoroughly characterized in house reference virus preparation. It needs to be pointed out; however, unless ITR-specific primers are used [12], the reference standards can only be used for the titration of AAVs that share other common sequence elements with the AAV standards.
If an in house reference standard is used that is single-stranded, and the virus to be titered is self-complementary, the final titer should be divided by 2. In this context, it also needs to be pointed out that the accurate titer determination of double-stranded AAVs has additional pitfalls, see [13].
For further information on designing and constructing cis plasmids, see Gray [10].
HEK293T cells are a human cell line, and all work must be performed in accordance with Biosafety Level 2 (BSL2) regulations. This includes the use of a BSL2 laminar flow tissue culture hood. Adeno-associated viruses are BSL1, but all work with AAV should be performed under sterile conditions.
293T cells can be passaged for about 1–2 months after thawing the initial vial, as long as they are regularly split at least twice a week.
Split cells depending on confluency. If the flask is very confluent, split 1:6, if less confluent 1:4. The goal is to achieve approximately 70 % cell confluency the next day.
Because this PBS pellet wash solution can contain AAV, we routinely combine it with the cell culture supernatant.
The maximum volume of crude lysate that can be loaded in a single ultracentrifugation tube is 10 ml. However, the cell pellets from up to three triple flasks can be combined and resuspended in 10 ml lysis buffer. After freeze/thaw and clearing of the lysate the combined lysate can be loaded into a single tube. Similarly, it is possible to resuspend the pellets of precipitated AAV from cell culture supernatant of up to three triple flasks.
Although incubation on ice for as little as 30 min is sufficient for virus precipitation, keeping the ammonium sulfate/cell culture supernatant mixture for several days at 4 °C will not affect the quality of the virus. However, once the virus precipitate has been spun down, the pellet should be resuspended and loaded onto a gradient the same day. Resuspending the pellet and freezing for a later gradient is not recommended as aggregation appears upon thawing.
The creation of multiple iodixanol gradients can be facilitated by using a multichannel peristaltic pump (available, for instance, from Watson-Marlow; Paramus, NJ) to assemble several gradients simultaneously. With such a system the different iodixanol solutions can be delivered to multiple tubes at the same time, which reduces the number of necessary pipetting steps. Using this method, up to four centrifugations (with Ti70 rotors, holding 8 tubes each) can be performed in 1 day.
If a different rotor is used, the speed and run times have to be adjusted using the k-factors of the Ti70 rotor the 69,000 rpm (k = 44.9) and the respective k-factor of the alternate rotor at a given speed. A convenient tool for this calculation can be found at: https://www.beckmancoulter.com
Calculate whether the AAV titer is sufficiently high for the planned experiments. Especially for in vivo experiments, when small injection volumes are necessary, it might be necessary to concentrate further the virus. In addition to concentrating the virus, if the virus was collected as a single fraction during the first gradient purification, this second ultracentrifugation gradient also allows the removal of empty capsids that may have been collected together with the genome-containing AAV particles from the first gradient.
For long-term storage, AAV is preferably stored at −80 °C. However, AAVs are extraordinarily stable; recent reports have shown no loss of activity after 1 week of storage at room temperature [11]. This can have important implications for the shipping of AAVs. In particular, the quality of the virus is likely unaffected if all dry ice has evaporated due to unanticipated delays in the delivery of the virus. Because of the danger of damaging the AAV genome, never expose AAV to UV light (such as in a bio-safety cabinet).
The quality of AAVs purchased from academic vector cores or commercial vendors can vary significantly. This is especially true for double-stranded AAVs or AAVs with a genome size approaching the maximum packaging capacity of the AAV capsid. Therefore, we recommend a thorough in-house characterization before performing any experiments.
The calculation of capsid concentration is based on the molecular weight of VP3 (calculated from its amino acid composition), which is 60,063 Da. Because each capsid contains 50 VP3 subunits, the combined molecular weight of VP3 subunits in a single capsid is approximately 3 MDa, and each capsid contains 4.9868 × 10−18 g of VP3 [14]. Dividing the amount of VP3 (in g) by 4.9868 × 10−18 g will yield the number of viral particles.
The incubation time is critical. Shorter incubation times will cause empty viral particles to appear as particles with encapsidated genomes.
Moving the power supply frequently from the cold room to room temperature will damage the power supply due to water condensation.
Alkaline gel electrophoresis can also be used to determine AAV titers. For this, a dilution series of a mass DNA ladder, or dilutions of a linear DNA fragment of known concentration and, preferably, of similar size to the AAV genome (e.g., the cis plasmid digested with SmaI if the only SmaI sites are in the viral ITRs) will be loaded onto the same gel as the AAV samples to be quantified. Quantification can then be achieved with the freely available ImageJ (http://imagej.nih.gov/ij/) or similar program. This method is especially useful if the AAV samples to be quantified do not share sequence elements with the reference standard and for double-stranded AAVs.
Acknowledgments
This work is supported by NIH P50 HL112324, R01 HL119046, R01 HL117505, R01 HL128099, R01 HL129814, R01 HL131404 and Trans- Atlantic Network of Excellence grants 13CVD01 and 14CVD03 from the Leducq Foundation. We would also like to acknowledge the Gene Therapy Resource Program (GTRP) of the National Heart, Lung, and Blood Institute, National Institutes of Health for providing some of the gene vectors used in these studies.
References
- 1.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 2.Buning H. Gene therapy enters the pharma market: the short story of a long journey. EMBO Mol Med. 2013;5(1):1–3. doi: 10.1002/emmm.201202291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 4.Moulay G, Ohtani T, Ogut O, Guenzel A, Behfar A, Zakeri R, Haines P, Storlie J, Bowen L, Pham L, Kaye D, Sandhu G, O’Connor M, Russell S, Redfield M. Cardiac AAV9 gene delivery strategies in adult canines: assessment by long-term serial SPECT imaging of sodium iodide symporter expression. Mol Ther. 2015;23(7):1211–1221. doi: 10.1038/mt.2015.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woitek F, Zentilin L, Hoffman NE, Powers JC, Ottiger I, Parikh S, Kulczycki AM, Hurst M, Ring N, Wang T, Shaikh F, Gross P, Singh H, Kolpakov MA, Linke A, Houser SR, Rizzo V, Sabri A, Madesh M, Giacca M, Recchia FA. Intracoronary cytoprotective gene therapy: a study of VEGF-B167 in a preclinical animal model of dilated cardiomyopathy. J Am Coll Cardiol. 2015;66(2):139–153. doi: 10.1016/j.jacc.2015.04.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonntag F, Schmidt K, Kleinschmidt JA. A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc Natl Acad Sci U S A. 2010;107(22):10220–10225. doi: 10.1073/pnas.1001673107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brocchieri L, Karlin S. Protein length in eukaryotic and prokaryotic proteomes. Nucleic Acids Res. 2005;33(10):3390–3400. doi: 10.1093/nar/gki615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amiss TJ, Samulski RJ. Methods for adeno-associated virus-mediated gene transfer into muscle. Methods Mol Biol. 2001;175:455–469. doi: 10.1385/1-59259-235-X:455. [DOI] [PubMed] [Google Scholar]
- 9.Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adenoassociated virus vectors. Hum Gene Ther. 1998;9(18):2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 10.Gray JT, Zolotukhin S. Design and construction of functional AAV vectors. Methods Mol Biol. 2011;807:25–46. doi: 10.1007/978-1-61779-370-7_2. [DOI] [PubMed] [Google Scholar]
- 11.Gruntman AM, Su L, Su Q, Gao G, Mueller C, Flotte TR. Stability and compatibility of recombinant adeno-associated virus under conditions commonly encountered in human gene therapy trials. Hum Gene Ther Methods. 2015;26(2):71–76. doi: 10.1089/hgtb.2015.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I, Nitschko H, Busch U, Sing A, Ehrhardt A, Baiker A. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods. 2012;23(1):18–28. doi: 10.1089/hgtb.2011.034. [DOI] [PubMed] [Google Scholar]
- 13.Fagone P, Wright JF, Nathwani AC, Nienhuis AW, Davidoff AM, Gray JT. Systemic errors in quantitative polymerase chain reaction titration of self-complementary adeno-associated viral vectors and improved alternative methods. Hum Gene Ther Methods. 2012;23(1):1–7. doi: 10.1089/hgtb.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohlbrenner E, Henckaerts E, Rapti K, Gordon RE, Linden RM, Hajjar RJ, Weber T. Quantification of AAV particle titers by infrared fluorescence scanning of Coomassie-stained sodium dodecyl sulfate-polyacrylamide gels. Hum Gene Ther Methods. 2012;23(3):198–203. doi: 10.1089/hgtb.2012.049. [DOI] [PMC free article] [PubMed] [Google Scholar]