Abstract
Polyploidy can lead to aneuploidy and tumorigenesis. Here, we report that the Hippo pathway effector Yap promotes the diploid-polyploid conversion and polyploid cell growth through the Akt-Skp2 axis. Yap strongly induces the acetyltransferase p300-mediated acetylation of the E3 ligase Skp2 via Akt signaling. Acetylated Skp2 is exclusively localized to the cytosol, which causes hyper-accumulation of the cyclin-dependent kinase inhibitor p27, leading to mitotic arrest and subsequently cell polyploidy. Additionally, the pro-apoptotic factors FoxO1/3 are overly degraded by acetylated Skp2, resulting in polyploid cell division, genomic instability and oncogenesis. Importantly, the depletion or inactivation of Akt or Skp2 abrogated Hippo signal deficiency-induced liver tumorigenesis, indicating their epistatic interaction. Thus, we conclude that Hippo-Yap signaling suppresses cell polyploidy and oncogenesis through Skp2.
Keywords: Hippo, Yap, Skp2, p27, polyploidy, tumorigenesis
Introduction
Polyploidy is a state in which cells possess more than two sets of homologous chromosomes. Although it is less frequently found in animals, some tissues, including the liver, have a high percentage of polyploid cells. Polyploid hepatocytes undergo ploidy reversal to specifically generate unique hepatocytes with different mixtures of chromosomes (Duncan, 2013; Gentric and Desdouets, 2014; Pandit et al., 2013). This genetic diversity may be an adaptive mechanism, serving as a means for the selection of hepatocytes most resistant to xenobiotic or nutritional injury. Gene redundancy shields polyploids from the deleterious effects of mutations (Duncan et al., 2009; Duncan et al., 2010). However, polyploid cells precede aneuploid cells that give rise to increased genomic instability and tumor progression (Davoli and de Lange, 2011; Ganem and Pellman, 2007; Gordon et al., 2012). Consistently, two-thirds partial hepatectomy (PH)-induced liver regeneration results in increased cell polyploidy and causes the normally quiescent polyploid hepatocytes to undergo cell cycle re-entry and division, accelerating the ability of oncogenes to induce hepatocellular carcinoma (HCC) (Beer et al., 2004). Polyploid cells are normally arrested in the G1 phase of the cell cycle, thus preventing genomic instability, aneuploidy, and tumorigenesis. Thus, it is of particular interest to determine the mechanisms regulating polyploid formation and polyploid cell division.
Cell polyploidy can result from cell fusion or abnormal cell division, including endoreduplication, mitotic slippage and cytokinesis failure (Pandit et al., 2013). Cytokinesis failure and mitotic slippage events have a pivotal role in establishing hepatocyte polyploidy (Celton-Morizur et al., 2009; Hsu et al., 2016; Pandit et al., 2012). Skp2 is a major cytokinetic regulator and a F-box protein that targets p27 for ubiquitination and subsequent degradation to promote cell cycle progression (Carrano et al., 1999; Nakayama et al., 2000; Nakayama et al., 2004). Skp2-null mice develop a phenotype of polyploidy and centrosome amplification in the liver (Kossatz et al., 2004; Nakayama et al., 2004; Serres et al., 2012). Elevated levels of p27 in the S and G2/M phases upon the depletion of Skp2 cause cytokinesis failure and mitotic slippage events. These phenotypes are completely rescued by the concomitant deletion of p27 (Nakayama et al., 2004). Recent studies revealed that Skp2 stability, subcellular localization, and activity are regulated by its phosphorylation and acetylation (Chan et al., 2012; Gao et al., 2009; Inuzuka et al., 2012; Lin et al., 2009). Skp2 expression is highly upregulated in a variety of human cancers (Calvisi et al., 2009; Lee et al., 2015; Lin et al., 2010; Wang et al., 2010; Zhao et al., 2013). These results indicate that certain signaling may be required for the regulation of Skp2 function in controlling cell polyploidy.
The Hippo signaling pathway is a critical regulator of stem cell self-renewal, tissue regeneration and organ size (Johnson and Halder, 2014; Pan, 2010; Yu et al., 2015). Central to this pathway is a kinase cascade formed by mammalian sterile20 kinases Mst1 and Mst2 (Mst1/2), a scaffolding protein Salvador/WW45 (Sav), a NDR family kinases large tumor suppressor 1 (Lats1) and Lats2, and an adaptor protein Mob1. Mst1/2 phosphorylates and activates Lats1/2-Mob1, which then phosphorylates the yes-associated protein (Yap) or WW domain-containing transcription regulator protein 1 (Taz). Phospho-Yap/Taz is either degraded or sequestered in the cytoplasm by 14-3-3 protein. When the Hippo pathway is inactivated, Yap/Taz translocates to the nucleus and forms a functional hybrid transcriptional factor with TEA domain family members (TEADs) to turn on pro-proliferative and pro-survival genes, enabling cell proliferation. The Hippo pathway also mediates crosstalk with other signaling pathways including the phosphoinositol-3-kinase (PI3K)-Akt pathway (Dupont et al., 2011; Heallen et al., 2011; Tumaneng et al., 2012; Yu et al., 2012). Genetic defects in this pathway in mice lead to tissue overgrowth and cancer development in multiple organs (Camargo et al., 2007; Dong et al., 2007; Zhou et al., 2009). A recent study showed that Hippo signaling is highly activated in polyploid cells (Ganem et al., 2014). p53 is noramlly required for the induction of cellular senescence to limit polyploid cell growth (Davoli et al., 2010; Fujiwara et al., 2005; Kurinna et al., 2013). Lats2 kinase of the Hippo signaling pathway was reported to stabilize p53 by inhibiting murine double minute 2 (Mdm2) (Aylon et al., 2006; Iida et al., 2004), which resulted in tetraploid cell cycle arrest. Thus, polyploidy status might trigger Lats2 kinase to activate p53 to prevent polyploidy cell proliferation (Ganem et al., 2014). Interestingly, Yap overexpression dramatically increases hepatocyte polyploidy, suggesting that Yap is a downstream effector of Lats2 kinase in ploidy regulation (Ganem et al., 2014). Recent studies have shown that Yap is phosphorylated by Cyclin-dependent kinase 1 (CDK1) and that this mitotic phosphorylation of Yap is required for the activation of the spindle checkpoint in immortalized epithelial cells (Yang et al., 2015; Yang et al., 2013). However, the mechanism by which Yap determines cell ploidy and chromosomal stability remains unclear. Genetic evidence in animals supporting the central role of Hippo signaling in polyploidy formation and subsequent neoplastic transformation is still largely lacking.
Results
Loss of Hippo signaling promotes hepatocyte polyploidy
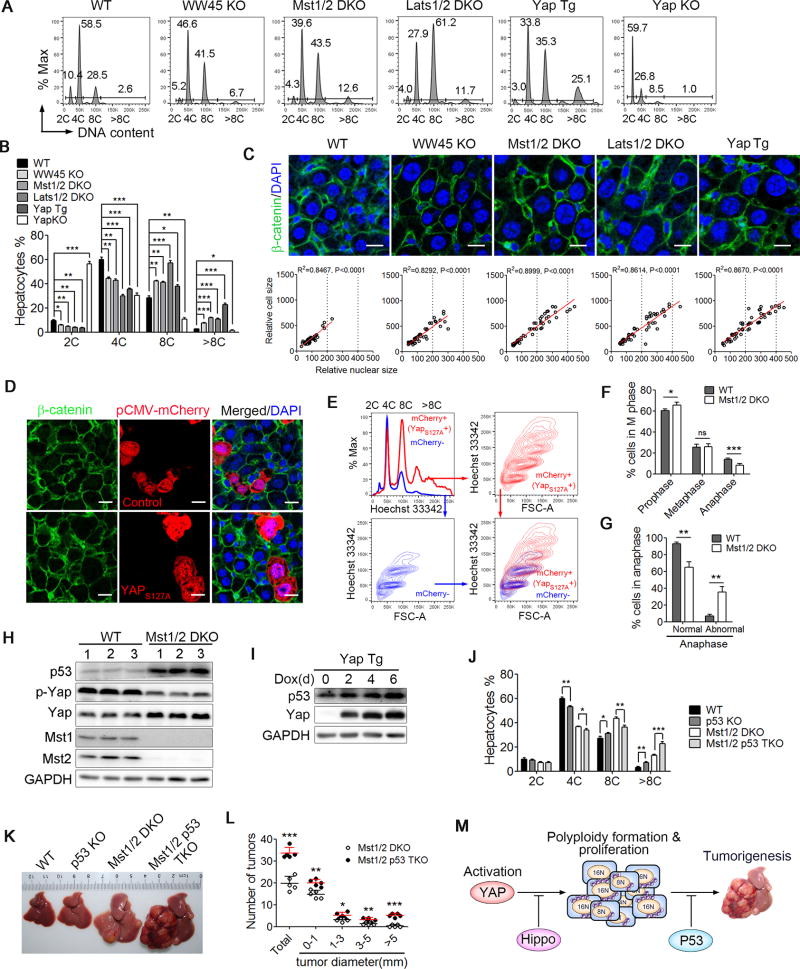
Hepatocytes are either mononucleated or binucleated, and each nucleus is diploid, tetraploid, octaploid, or higher, which makes the liver a valuable organ in which to study cell ploidy regulation. To characterize the function of Hippo signaling on hepatocyte ploidy, we determined the hepatocyte ploidy and nuclear size in liver tissues isolated from various mouse strains with a liver-specific mutation for Hippo signaling components. Compared to wild-type (WT) mice, hepatocytes in the livers of WW45f/fAlb-Cre (WW45 KO), Mst1f/fMst2f/fAlb-Cre (Mst1/2 DKO), Lats1f/fLats2f/fAlb-Cre (Lats1/2 DKO) or doxycycline-inducible active Yap (S127A) transgenic (Yap Tg) mice exhibited markedly enlarged nuclear size and increased cell polyploidy, whereas Yapf/fAlb-Cre (Yap KO) animals showed smaller nuclear size and reduced cell polyploidy in the liver (Figures 1A, 1B, S1A and S1B). In addition, we found that cells of different genotypes with larger nuclei have larger cell sizes (Figure 1C). Furthermore, immunofluorescent staining and flow cytometric analysis clearly showed that Yap transgenic hepatocytes had an increased DNA content and greater cell size (Figures 1D and 1E). These results demonstrated that Hippo signaling plays a critical role in ploidy regulation.
Figure 1. Yap activation increases hepatocyte polyploidy and synergizes with p53 inactivation to enhance liver tumorigenesis.
(A and B) Fluorescence-activated cell sorting (FACS) analysis (A) and the DNA content quantification (B) of polyploid hepatocytes from wild-type (WT), WW45f/fAlb-Cre (WW45 KO), Mst1f/fMst2f/fAlb-Cre (Mst1/2 DKO), Lats1f/fLats2f/fAlb-Cre (Lats1/2 DKO), Yap (S127A) transgenic (Yap Tg) and Yapf/fAlb-Cre (Yap KO) mice (n = 3). 2C, 4C, 8C and >8C DNA content, corresponding to diploid, tetraploid, octaploid and higher polyploid hepatocytes, respectively.
(C) Hepatocytes in liver sections from the indicated genotypes were labeled with DAPI and an antibody against β-catenin. The areas of the cell (cell size) and the DAPI positive compartment (nucleus) were imaged with a Zeiss LSM 780 (upper panel) and quantified using ImageJ software (lower panel). Scale bars: 20 µm.
(D and E) The correlation of hepatocyte size and ploidy status from wild-type mice after the hydrodynamic delivery of pCMV-mCherry control or pCMV-mCherry-YapS127A was assessed by immunofluorescent staining (D) and FACS (E) approaches. Scale bars: 20 µm.
(F and G) The quantification of the percentage of cells at the different mitotic phases (F) and the percentage of abnormal anaphase cells (G) according H&E and immunohistochemistry (IHC) staining for pHH3 in liver sections.
(H) Immunoblot analysis of p53, phosphorylated (p-) Yap, Yap, Mst1, Mst2 and GAPDH in WT or Mst1/2 DKO liver tissues.
(I) Immunoblot analysis of p53, Yap and GAPDH in WT or Yap Tg liver tissues.
(J) The DNA content quantification of polyploid hepatocytes from WT, Mst1/2 DKO p53KO or Mst1/2 p53 TKO mice using FACS analysis (n = 3).
(K) A representative liver picture and the liver-to-body weight ratios (n = 5) of 2 month old WT, Mst1/2 DKO p53KO or Mst1/2 p53 TKO mice.
(K and L) A representative liver picture (K) and the quantification of the size and number of liver tumors (n = 5) (L) of 4.5 month old WT, Mst1/2 DKO p53KO or Mst1/2 p53 TKO mice.
(M) A proposed working model for Hippo signaling decreases hepatocyte polyploidy and synergizes with p53 to inhibit liver tumorigenesis.
Data were assessed by Student’s t-test and represented as mean ± SD ns, no significant, *p < 0.05, **p < 0.01, ***p < 0.001 compared between indicated groups. See also Figure S1.
We also found that the loss of Mst1/2 or the overexpression of Yap induced supernumerary centrosomes and abnormal mitotic spindle formation in dividing hepatocytes (Figures S1C and S1D). In addition, during the regeneration process after PH, more dividing cells at prophase and metaphase were found in Mst1/2 DKO livers than in WT livers (Figures 1F and S1E). Furthermore, fewer cells but with a higher incidence of abnormal anaphase were found in Mst1/2 DKO livers, indicating that cytokinesis failure occurs in Mst1/2 DKO livers (Figure 1G). These data demonstrated that Hippo signaling is important for ensuring accurate centrosome duplication and chromosome segregation to maintain genome stability.
p53 is normally required to induce G1 arrest and cellular senescence in response to tetraploidy or missegregated chromosomes. Lats2 kinase of Hippo signaling pathway was previously reported to activate and stabilize p53 (Aylon et al., 2006). Surprisingly, we found that the p53 protein levels were dramatically increased in the livers of WW45KO, Mst1/2 DKO, Lats1/2 DKO and Yap Tg mice compared with those in WT livers (Figures 1H, 1I, S1F and S1G). We speculated that the highly increased p53 protein levels might be the result of a potent negative feedback loop in response to increased cell polyploidy upon the disruption of Hippo signals. Indeed, the loss of p53 in Mst1/2 DKO (Mst1/2 p53 TKO) mice led to a larger nuclei size and higher polyploidy numbers than those in their Mst1/2 DKO littermates (Figures 1J, S1H and S1I). Mst1/2 p53 TKO mice also exhibited increased ratios of liver/body weight and accelerated liver tumor formation compared with WT, p53 KO or Mst1/2 DKO mice (Figures 1K 1L and S1J). Taken together, these results indicated that Yap activation increases hepatocyte polyploidy and synergizes with p53 inactivation to enhance liver tumorigenesis (Figure 1M).
Hippo signal deficiency induces polyploidy via p27
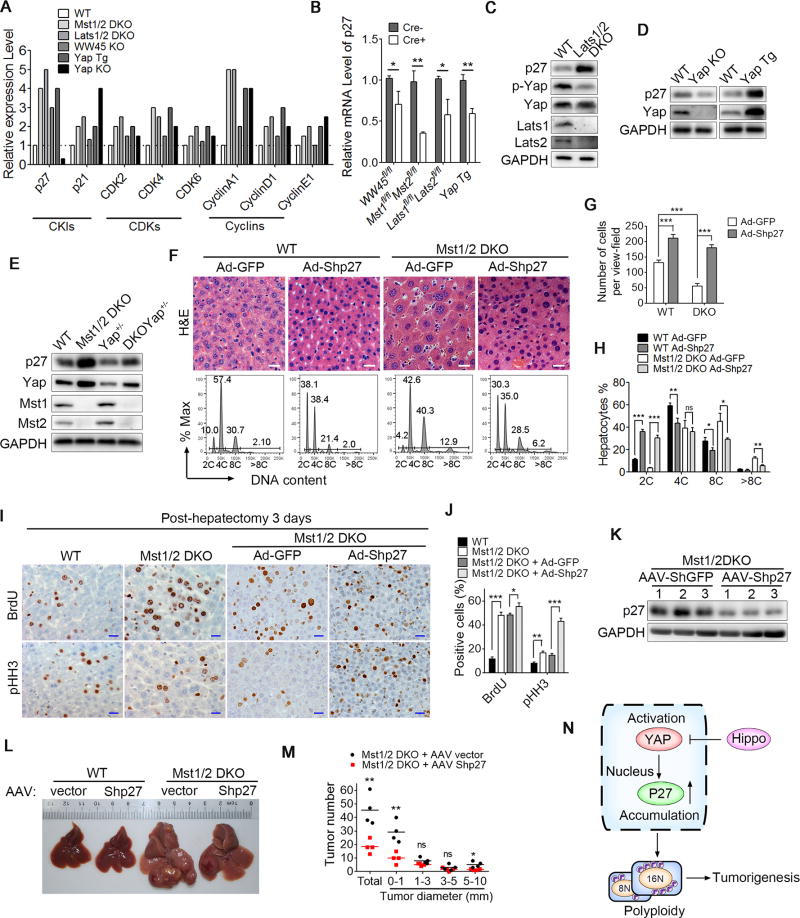
Polyploidy may occur due to cell fusion or abnormal cell division. To identify the potential cell cycle regulators that induce hepatocyte polyploidy from various mouse strains with liver-specific mutations of the Hippo signaling pathway, we analyzed the protein expression profile of cell cycle related proteins, i.e. CKIs (p27 and p21), CDKs (CDK2, CDK4 and CDK6) and cyclins (CyclinA1, CyclinD1 and CyclinE1). We found that only the expression level of p27 was consistently increased in primary hepatocytes from WW45 KO, Mst1/2 DKO, Lats1/2 DKO and Yap(S127A) Tg mice, but it was decreased in hepatocytes from Yap KO animals (Figures 2A, S2A and S2B), indicating that p27 might be a direct target downstream of the Hippo signaling pathway. Interestingly, unlike its protein levels, the p27 mRNA level was reduced in Hippo signaling deficient livers suggesting that the Hippo signaling might regulate the protein stability of p27 (Figure 2B). The positive correlation of p27 levels with Yap activity was further confirmed in primary mouse embryonic fibroblasts (MEFs) isolated from WT, Lats1/2 DKO, Yap(S127A) Tg or Yap KO mice (Figures 2C–2E), and one Yap allele deletion in Mst1/2 DKO liver (Mst1/2 DKO Yap+/−) was sufficient to reduce the level of the p27 protein to the level in normal WT hepatocytes (Figures 2E and S2C). These data indicated that Hippo signaling controls the protein level of p27.
Figure 2. Loss of Hippo signaling resulted in the accumulation of p27 leading to polyploidy.
(A) The quantification of the relative protein expression levels of cell cycle related proteins p27, p21, CDK2, CDK4, CDK6, Cyclin A1, Cyclin D1 and Cyclin E1 in livers from the indicated mouse strains with a liver-specific mutation of the Hippo signaling components.
(B) Quantitative PCR analysis of the p27 mRNA expression in hepatocytes from the indicated liver-specific mutant mice.
(C) Immunoblot analysis of p27, p-Yap, Yap, Lats1, Lats2 and GAPDH in WT or Lats1/2 DKO MEFs.
(D) Immunoblot analysis of p27, Yap and GAPDH in WT, Yap Tg or Yap KO control liver tissues.
(E) Immunoblot analysis of p27, Yap, Mst1, Mst2 and GAPDH in WT, Mst1/2 DKO, Yap+/flAlb-Cre (YAP+/−) or Mst1f/fMst2f/fYap+/flAlb-Cre (DKO Yap+/−) liver tissues.
(F–H) H&E staining of liver sections (F, upper panel) and the quantification of cell number per viewfield (G) or the DNA content quantification (F, lower panel and H) of polyploid hepatocytes by FACS from WT or Mst1/2 DKO mice infected with either adenovirus expressing a GFP control vector (Ad-GFP) or p27-knock-down shRNA (Ad-Shp27) as indicated (n = 3). Scale bars: 20 µm.
(I and J) IHC staining of BrdU or pHH3 in the liver sections from WT, Mst1/2 DKO, or Mst1/2 DKO mice infected with either Ad-GFP or Ad-Shp27 as indicated after partial hepatectomy (I). The bar graph shows the quantifications of BrdU- or pHH3-positive cells in the livers (n = 3) (J). Scale bars: 20 µm.
(K) Immunoblot analysis of the p27 levels in the livers from Mst1/2 DKO mice infected with adeno-associated virus (AAV) expressing control GFP or p27 shRNA.
(L and M) A representative liver picture (L) and the quantification of the size and number of liver tumors in 4.5 month old Mst1/2 DKO mice (n = 4) infected with AAV-GFP or AAV-Shp27.
(N) A proposed working model for Yap activation promoting nuclear accumulation of p27 resulted in increased polyploidy and tumor formation.
Data were assessed by Student’s t-test and represented as mean ± SD *p < 0.05, **p < 0.01, ***p < 0.001 compared between the indicated groups. See also Figure S2.
Previous studies showed that elevated p27 could cause a failure to enter mitosis and thereby induce polyploidy (Kossatz et al., 2004; Nakayama et al., 2004). We next sought to determine whether the p27 hyper-accumulation is responsible for increased polyploidy in hepatocytes with a Hippo signaling deficiency. Indeed, the ablation of p27 using adenoviral shRNA (Ad-Shp27) (Figures S2D and S2E) in Hippo signaling deficient livers or MEF cells resulted in decreased hepatocyte polyploidy and smaller nuclear size (Figures 2F–2H and S2F–S2G). To determine whether the hyper-accumulation of p27 results in a failure to enter mitosis, we assessed the DNA synthesis rate and mitosis events in Mst1/2 deficient hepatocytes after a partial hepatectomy. Compared to the WT liver, the Mst1/2 DKO liver had a dramatically higher number (approximately 4-fold) of BrdU-positive (DNA synthesis phase) hepatocytes but a moderately higher level (approximately 2-fold) of pHH3-positive (mitosis phase) hepatocytes, indicating that a high fraction of polyploid cells in Mst1/2 DKO liver were arrested in mitosis, whereas the knockdown of p27 in Mst1/2 DKO livers slightly increased the number of BrdU-positive hepatocytes but dramatically increased pHH3-positive hepatocytes. This finding indicated that more cells entered mitosis for proliferation and division (Figures 2I and 2J). Furthermore, we knocked down p27 expression in WT and Mst1/2 DKO livers using adeno-associated virus (AAV), which can have a long-term effect of up to 6 months. We found that knocking down p27 in Hippo-deficient livers resulted in decreased cell polyploidy and significantly reduced the number and volume of tumor size at 5 weeks post-infection with AAC p27 shRNA (Figures 2K–2M and S2H). We found similar results in Mst1f/fMst2f/fAlb-Cre mice with one p27 allele deletion (Mst1/2 DKO p27+/−) (Figures S2I–S2N). We further observed much lower incidences of abnormal anaphase cells in the livers of Mst1/2 DKO p27+/− mice than in Mst1/2 DKO livers, indicating that p27 downregulation restored cellular cytokinesis to normal levels in Mst1/2 DKO livers (Figures S2K–S2L). As the mitosis of polyploid cells leads to genomic instability and a higher incidence of cancer formation, it is not surprising that we observed that the loss of p27 resulted in a lower incidence and delayed tumor formation in Hippo signal-deficient livers by reducing cell polyploidy in the context of a much higher fraction of polyploid cells in Mst1/2 DKO liver tissues, although p27 downregulation increased the cell mitosis and proliferation of diploid cells (Figures 2L–2M and S2M–S2N). These results indicated that the Hippo signaling pathway limits polyploidy formation and prevents tumor formation, at least in part, through the downregulation of p27 (Figure 2N).
Hippo signaling deficiency enhances the cytoplasmic retention of Skp2
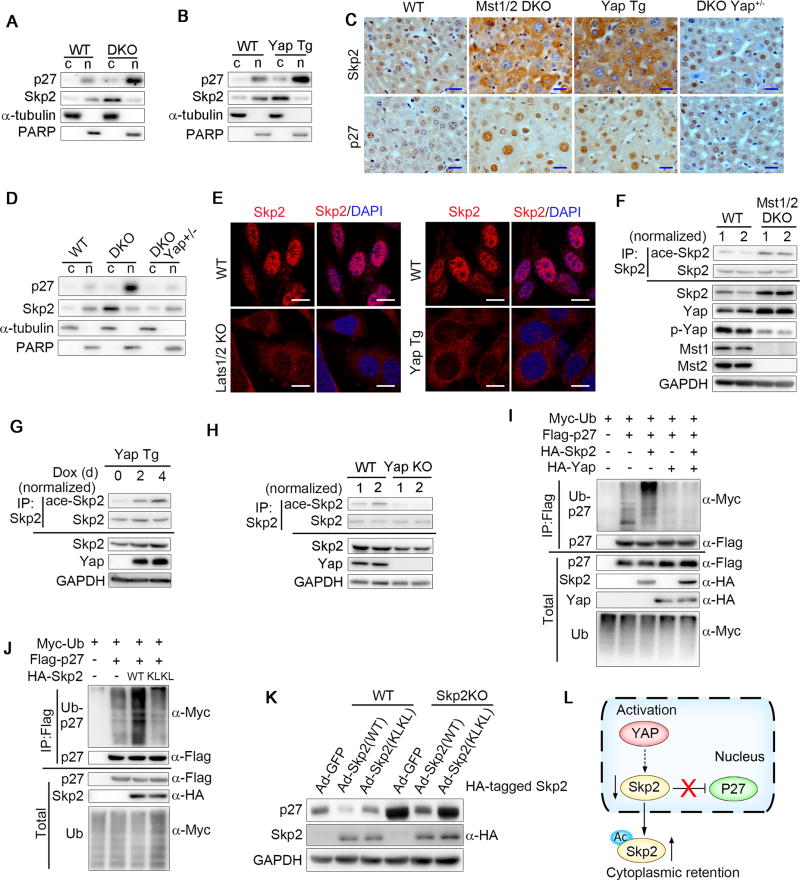
Previous studies showed that S-phase kinase-associated protein 2 (Skp2) in the nuclear compartment is required for ubiquitin-mediated p27 degradation. We measured the levels of Skp2 and p27 in whole cell lysates and the cytoplasmic and nuclear fractions from WT, Mst1/2 DKO or Yap Tg hepatocytes and found that the protein levels of Skp2 and p27 were increased in whole-cell lysates of Mst1/2 DKO or Yap Tg hepatocytes compared with those in WT cells (Figures S3A and S3B). However, these proteins were present in distinct subcellular locations (Figures 3A and 3B). The cytoplasmic retention of Skp2 in Mst1/2 DKO or Yap Tg livers was further confirmed by IHC staining (Figures 3C and 3D) and was observed in primary MEFs isolated from Lats1/2 DKO or Yap Tg mice (Figure 3E) and a HepG2 cell line overexpressing Yap (Figure S3C). Furthermore, the loss of one allele of Yap in Mst1/2 DKO hepatocytes restored the nuclear localization of Skp2 and thereby reducing the p27 levels (Figures 3C and 3D). These data suggested that loss of Hippo signaling resulted in the cytoplasmic retention of Skp2, leading to the nuclear accumulation of p27. Previous studies showed that the acetylation of Skp2 promotes its translocation from the nuclei to the cytosol (Inuzuka et al., 2012). In line with its sub-cellular localization, Skp2 acetylation levels were greatly increased in Mst1/2 DKO and Yap Tg hepatocytes, and attenuated in Yap KO hepatocytes (Figures 3F–3H). In addition, p27 ubiquitination was remarkably attenuated in cells overexpressing Yap or an acetylation-mimetic mutant Skp2 (KLKL) which was mainly located in the cytosol (Figures 3I and 3J). Consistently, the p27 levels were greatly reduced in Skp2 KO livers infected with adenoviruses expressing wild-type Skp2 (WT), but only slightly reduced in Skp2 KO liver infected with acetylation-mimetic mutant Skp2 (Ad-Skp2 (KLKL)) (Figure 3K). These results indicated that Hippo signaling regulates p27 stability through modulating Skp2 acetylation and sub-cellular localization (Figure 3L).
Figure 3. Loss of Hippo signaling enhances the cytoplasmic retention of Skp2.
(A and B) Immunoblot analysis of p27, Skp2, α-tubulin or PARP in the cytoplasmic (c) and nuclear (n) fractions of WT, Mst1/2 DKO (A) or Yap Tg (B) liver tissues.
(C and D) IHC staining (C) or immunoblot analysis of the cell fractions (D) of Skp2 and p27 in liver tissues from WT, Mst1/2 DKO, Mst1f/fMst2f/fYap+/flAlb-Cre (DKO Yap+/−) or Yap Tg mice. Scale bars: 20 µm.
(E) Immunofluorescent staining of Skp2 (red) and the nuclear counterstain (DAPI, blue) in primary MEFs isolated from WT, Lats1/2 KO (left panel) or Yap Tg (right panel) mice. Scale bars: 10 µm.
(F–H) Immunoblot analysis of acetylated (ace-) Skp2, Skp2,p-Yap, Yap, Mst1, Mst2 and GAPDH in the immunoprecipitates or total lysates of hepatocytes isolated from WT, Mst1/2 DKO (F), Yap Tg (G), or Yap KO (H) mice. For the detection of ace-Skp2, the loading of the immunoprecipitates was normalized according to the levels of total Skp2.
(I and J) Immunoassay assessing the ubiquitination of p27 (detected with anti-Myc) in the lysates of 293T cells expressing various combinations of Myc-tagged ubiquitin, Flag-tagged p27, HA-tagged Yap and HA-tagged WT Skp2 (I) or an acetylation-mimetic (KLKL) mutant form of Skp2 (J) as indicated.
(K) Immunoblot analysis of Skp2, p27 and GAPDH in liver tissues from WT and Skp2 KO mice infected with Ad-GFP, Ad-Skp2 (WT) or Ad-Skp2 (KLKL).
(L) A proposed working model for Yap activation regulating p27 stability through modulating Skp2 acetylation and sub-cellular localization.
See also Figure S3.
Yap promotes Skp2 cytoplasmic retention and hepatocyte polyploidy via the Akt-p300 axis
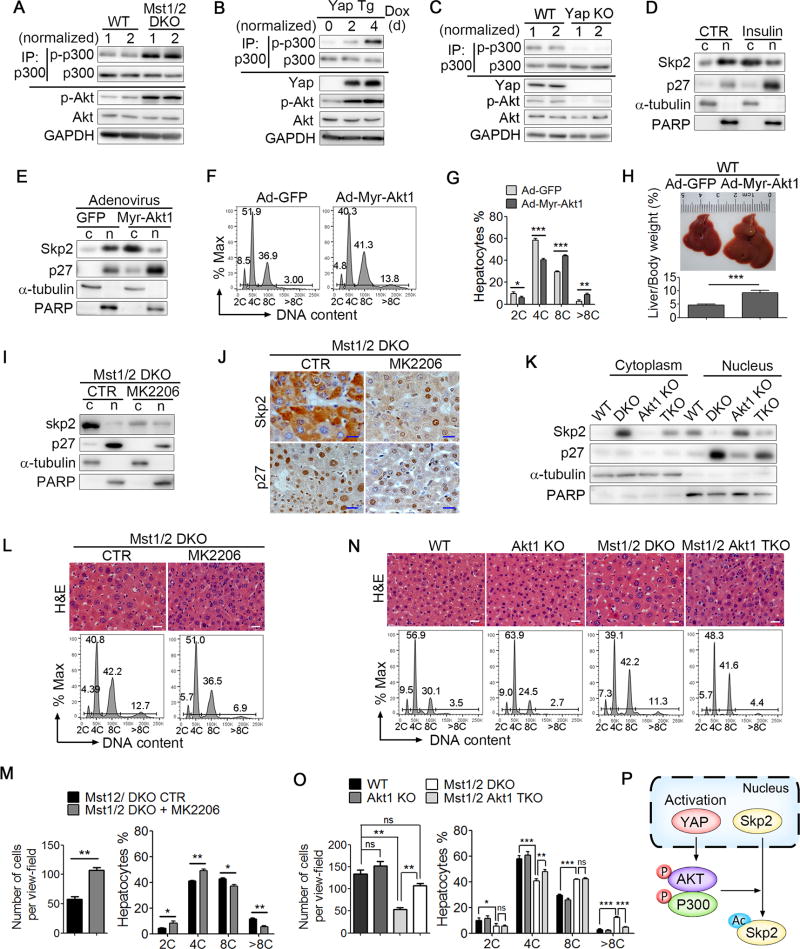
It has been reported that Yap activates the PI3K-Akt pathway (Tumaneng et al., 2012). Akt can phosphorylate and activate the acetyltransferases p300 to enhance Skp2 acetylation at both K68 and K71, which promotes Skp2 cytoplasmic translocation and protein stability (Inuzuka et al., 2012). Indeed, the phosphorylation levels of Akt and p300 were attenuated in Yap KO cells, but greatly increased in WW45 KO, Mst1/2 DKO, Lats1/2 DKO and Yap Tg hepatocytes (Figures 4A–4C and S4A–S4B), in which enhanced acetylation and cytoplasmic retention of Skp2 was found (Figure 3F–3G). A previous study showed that insulin signal activates the PI3K/Akt pathway to promote the hepatocyte tetraploidization process (Celton-Morizur et al., 2009). Interestingly, we found that insulin treatment resulted in increased cytoplasmic Skp2 and nuclear p27 levels in the liver cells, suggesting that insulin-mediated Akt activation may induce hepatocyte polyploidy by promoting the cytoplasmic retention of Skp2 (Figures 4D, S4C and S4D). To further validate this speculation, we infected WT mice with adenovirus expressing a constitutively active myristoylated Akt1 (Ad-Myr-Akt1) or a control Ad-GFP. To avoid fatty liver induced by myr-Akt overexpression (Ono et al., 2003), the virus was titrated and a moderate titer of the virus was used. Compared with control animals infected with Ad-GFP, mice infected with Ad-Myr-Akt1 exhibited enhanced acetylation and cytoplasmic retention of Skp2 in hepatocytes and increased hepatocyte polyploidy and liver mass (Figures 4E–4H and S4E). Furthermore, treatment with the Akt inhibitor MK2206 or the genetic disruption of Akt1 reduced the protein levels of total and cytoplasmic Skp2 and nuclear p27 in Mst1/2 DKO liver cells (Figures 4I–4K and S4F–S4G), resulting in significantly reduced hepatocyte polyploidy in Mst1/2 DKO mice (Figures 4L–4O). Taken together, these data indicated that Hippo signaling controls cell polyploidy through Akt-Skp2 signaling (Figure 4P).
Figure 4. Yap regulates Skp2 cytoplasmic retention via Akt-p300 axis.
(A–C) Immunoblot analysis of p-p300, p300, p-Akt, Akt, Yap or GAPDH in the immunoprecipitates or total lysates of hepatocytes isolated from WT, Mst1/2 DKO (A), Yap Tg (B) or Yap KO (C) mice. For the detection of the p-p300 levels, the loading of the immunoprecipitates was normalized according to the levels of total p300.
(D and E) Immunoblot analysis of p27, Skp2, α-tubulin or PARP in the cytoplasmic (c) and nuclear (n) fractions of liver cells isolated from mice treated with vehicle or insulin (D) or mice infected with Ad-GFP or Ad-Myr-Akt1 (active Akt1) (E).
(F and G) FACS analysis (F) and the DNA content quantification (G) of ploidy hepatocytes from WT mice infected with Ad-GFP or Ad-Myr-Akt1 (n = 3).
(H) A representative liver picture and the liver-to-body weight ratios of WT mice (n = 5) infected with Ad-GFP or Ad-Myr-Akt1.
(I and J) Immunoblot analysis of the cell fractions (I) or IHC staining (J) of Skp2 and p27 from liver tissues isolated from Mst1/2 DKO mice treated with vehicle or the Akt inhibitor MK2206. Scale bars: 20 µm.
(K) Immunoblot analysis of Skp2, p27, α-tubulin or PARP in the cytoplasmic and nuclear fractions of liver tissues from WT, Akt1 KO, Mst1/2 DKO or Mst1f/fMst2f/fAkt1 KO Alb-Cre (TKO) mice.
(L–O) H&E staining of liver sections (L and N, upper panel), the quantification of cell number per viewfield (M and O) or the DNA content quantification by FACS (L and N, lower panel, M and O) of polyploid hepatocytes from Mst1/2 DKO mice treated with the Akt inhibitor MK2206 (n = 3) (L and M) or from WT, Akt1 KO, Mst1/2 DKO or Mst1/2 Akt1 TKO mice (n = 3) (N and O). Scale bars: 20 µm.
(P) A proposed working model for Yap activation modulating Skp2 acetylation and sub-cellular localization via Akt-P300 signaling.
Data were assessed by Student’s t-test and represented as mean ± SD ns, no significant (p > 0.05), *p < 0.05; **p < 0.01; ***p < 0.001 compared between the indicated groups. See also Figure S4.
Cytoplasmic Skp2 potentiates polyploidy cell proliferation and division
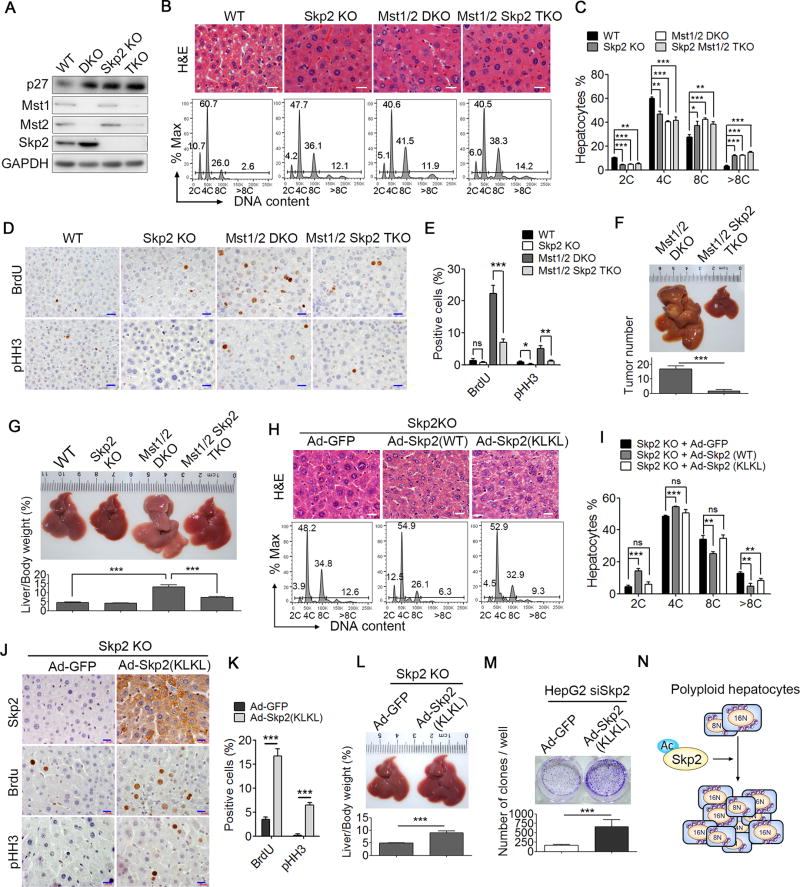
To further determine whether the cytoplasmic retention of Skp2 is responsible for the high cell polyploidy profiles in Hippo-deficient livers, we knocked out Skp2 in Mst1/2 or WW45 KO mice. We found that the loss of Skp2 in Mst1/2-DKO or WW45 KO livers resulted in a slight increase in the p27 protein levels (Figures 5A and S5A) but had no significant effect on the cell polyploidy profiles (Figures 5B, 5C, S5B and S5C). Interestingly, the loss of Skp2 in Mst1/2 or WW45 KO mice resulted in a dramatically decreased hepatocyte proliferation rate, a smaller liver mass, lower liver-to-body weight ratios and fewer liver tumors (Figures 5F–5I and S5F–S5I). In contrast, the overexpression of WT Skp2, which is mainly located in the nucleus, dramatically decreased the level of nuclear p27 and resulted in significantly lower numbers of polyploid cells in both WT and Skp2 KO livers. However, the overexpression of activated Skp2 (KLKL), which is mainly located in the cytoplasm, resulted in only a slightly reduced p27 level (Figure 3K) and thus had no significant effect on the cell polyploidy profiles (Figures 5D, 5E, S5D and S5E). Based on these observations, we speculated that cytoplasmic Skp2 in Hippo signal deficient cells might also promote polyploidy liver cell division in addition to inducing p27-mediated cell polyploidy. Indeed, Skp2 KO mice infected with Ad-Skp2 (KLKL) exhibited a significantly increased number of BrdU-positive hepatocytes and pHH3-positive hepatocytes indicating that more cells enter mitosis for proliferation and division (Figures 5J and 5K). Consistently, Skp2 KO mice infected with Ad-Skp2 (KLKL) had a larger liver mass and greater liver/body weight ratios than control mice that received Ad-GFP (Figure 5L). Furthermore, the expression of Ad-Skp2 (KLKL) in HepG2 cells pretreated with Skp2siRNA (siSkp2) significantly increased its colony formation rate compared to Ad-GFP infected control cells (Figure 5M). These results suggested that cytoplasmic Skp2 promotes polyploidy cell proliferation and division (Figure 5N).
Figure 5. Cytoplasmic Skp2 promotes polyploid cell division.
(A) Immunoblot analysis of p27, Skp2, Mst1, Mst2 and GAPDH in liver lysates of WT, Skp2 KO, Mst1/2 DKO, Mst1f/fMst2f/fSkp2 KO Alb-Cre (Mst1/2 Skp2 TKO) mice.
(B and C) H&E staining of the liver sections (B, upper panel) and the DNA content quantification of hepatocytes of the indicated mice using FACS (n = 3) (B, lower panel, and C). Scale bars: 20 µm.
(D and E) IHC staining (D) and the quantification (E) of BrdU- or pHH3-positive cells in the liver sections from the indicated mice. Scale bars: 20 µm.
(F and G) A representative liver picture and the liver-to-body weight ratios (n = 5, 3 months old, F) and the quantification of liver tumors (n = 5, 5 months old, G) of the indicated mice.
(H and I) H&E staining of the liver sections from Skp2 KO mice infected with Ad-GFP, Ad-Skp2 (WT) or Ad-Skp2 (KLKL) (H, upper panel) and the DNA content quantification of hepatocytes from the indicated mice using FACS (n = 3) (H, lower panel and I). Scale bars: 20 µm.
(J and K) IHC staining of BrdU or pHH3 (J) and the quantification of BrdU- or pHH3-positive cells (K) in liver sections from Skp2 KO mice infected with adenovirus Ad-GFP or Ad-Skp2 (KLKL) (n = 3). Scale bars: 20 µm.
(L) The representative liver sizes and liver-to-body weight ratios (n = 5) of Skp2 KO mice infected with Ad-GFP or AD-Skp2 (KLKL).
(M) A representative image of the clonogenic assay and the quantification of clones per well for Skp2-knockedg down HepG2 cells infected with Ad-GFP or Ad-Skp2 (KLKL).
(N) A proposed working model for acetylated Skp2 promoting polyploidy cell proliferation.
Data were assessed by Student’s t-test and represented as mean ± SD ns, no significant (p > 0.05), *p < 0.05, **p < 0.01, ***p < 0.001 compared between the indicated groups. See also Figure S5.
Cytoplasmic Skp2 degrades FoxOs to enhance polyploid cell proliferation and division
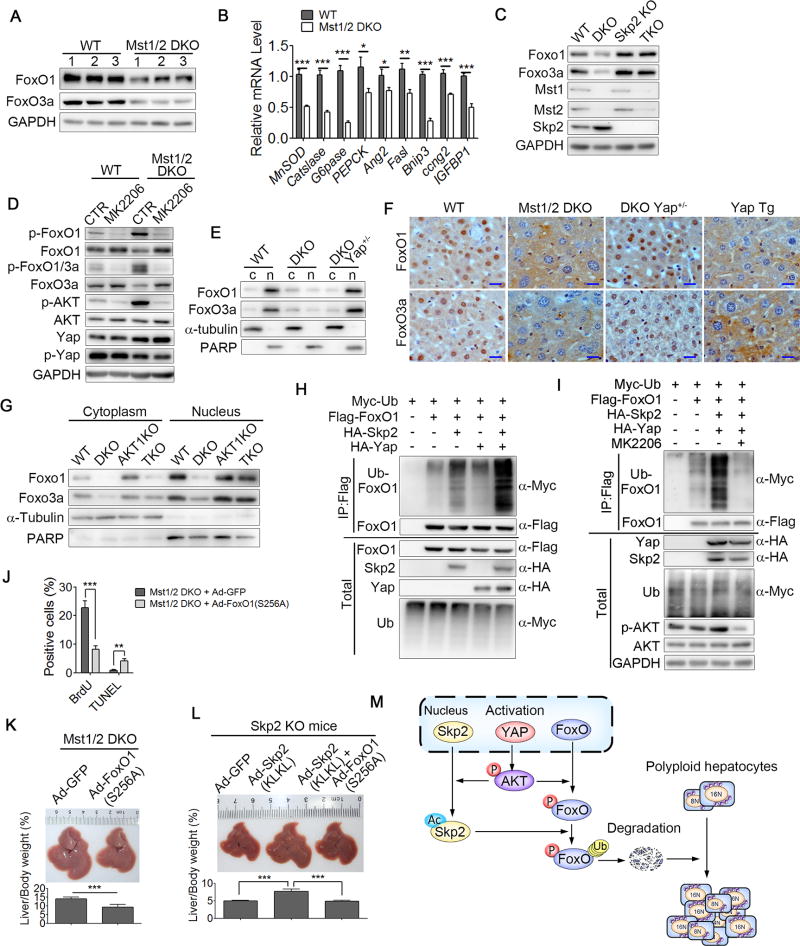
The data above suggested that cytoplasmic Skp2 might have other downstream effectors to regulate cell proliferation and liver size. Previous studies have shown that in contrast to nuclear Skp2 which targets nuclear p27 as shown above, cytoplasmic Skp2 has a distinct set of substrates, including forkhead box O (FoxO) transcription factors which are important pro-apoptotic transcription factors that block cellular proliferation, promote cell death and drives cells into a quiescent state (Huang et al., 2005). Indeed, we found that, compared to WT controls, the total protein levels of FoxO1/3 and the mRNA expression levels of the FoxO1/3 target genes, such as MnSOD, Catslase, G6pase, PEPCK, Ang2, Fasl, Bnip3, ccng2 and IGFBP1, were dramatically reduced in Mst1/2 DKO and Yap Tg livers, indicating the attenuated function of FoxO1/3 in Hippo deficient livers (Figures 6A, 6B, S6A and S6B). Consistently, the genetic disruption of Skp2 increased the protein levels of FoxO1 and FoxO3a in both WT and Mst1/2 DKO mouse livers (Figure 6C). Previous studies established the mechanism of FoxO inhibition in which Akt kinases phosphorylate FoxO1 at Thr24, Ser256, and Ser319 or FoxO3 at Thr32 and Ser253, thereby increasing their association with 14-3-3 proteins (Tran et al., 2003). This, in turn, results in the translocation of FoxO proteins from the nucleus to the cytoplasm, leading to their transcriptional inactivation. In fact, we found that the increased phosphorylation levels of FoxO1(S256, T24) and FoxO3a(T32) correlated with enhanced Akt activity in Mst1/2 DKO and Yap Tg liver lysates (Figures 6D and S6C). Sub-cellular fractions immunoblotting or immunohistochemistry staining assays further confirmed that, compared to WT controls, the FoxO1/3 protein levels were dramatically reduced in the nuclear fractions of Mst1/2 DKO or Yap Tg liver cells (Figure 6E, F and S6D), and the total protein level and subcelluar distribution of FoxO1/3 were restored to relatively normal levels in Mst1/2 DKO Yap+/− liver cells (Figures 6E and 6F). Consistently, inhibition or genetic disruption of Akt reduced the FoxO1/3 phosphorylation levels and restored FoxO1/3 protein levels and subcellular distribution in Mst1/2 DKO and Yap Tg livers (Figures 6D, 6G and S6C). Furthermore, Skp2-mediated FoxO ubiquitination was remarkably enhanced in cells overexpressing Yap while Akt inhibition attenuated this effect (Figures 6H and 6I). Based on these results, we speculated that cytoplasmic Skp2 mediated-FoxO1/3 degradation might be required for Hippo signal deficient polyploid cell survival and proliferation. To examine this possibility, we reintroduced the non-degradable form of FoxO1(S256A) in Mst1/2 DKO or Yap Tg livers using adenoviral vectors (Ad-FoxO1(S256A)). Importantly, Ad-FoxO1(S256A) greatly blocked cell proliferation, enhanced cell apoptosis and reduced liver mass compared to those in control mice receiving Ad-GFP (Figures 6J, 6K and S6E). Notably, Ad-FoxO1(S256A) abrogated the Ad-Skp2 (KLKL)-driven Skp2 deficient liver overgrowth (Figure 6L). These data indicated that the loss of Hippo signaling promotes polyploid cell division and oncogenesis, at least in part, through Skp2-mediated FoxO1/3 degradation (Figure 6M).
Figure 6. Yap promotes polyploid cell division via Skp2-mediated FoxO degradation.
(A and B) Immunoblot analysis of FoxO1, FoxO3a and GAPDH (A) and quantitative PCR analysis of FoxO target genes (B) in liver of WT or Mst1/2 DKO mice.
(C) Immunoblot analysis of FoxO1, FoxO3a, Mst1, Mst2, Skp2 and GAPDH in WT, Mst1/2 DKO, Skp2 KO or Mst1f/fMst2f/fSkp2 KO Alb-Cre (TKO) liver lysates.
(D) Immunoblot analysis of p-FoxO1 (S256), p-FoxO1/3 (T24/T32), p-Akt, p-Yap, FoxO1, FoxO3a, Akt, Yap and GAPDH in liver lysates of WT or Mst1/2 DKO mice treated with vehicle control (CTR) or Akt inhibitor MK2206.
(E and F) Immunoblot analysis of the cell fractions (E) or IHC staining (F) of FoxO1 or FoxO3a of liver cells from WT, Mst1/2 DKO, Mst1f/fMst2f/fYAP+/flAlb-Cre (DKO Yap+/−) or Yap Tg mice. Scale bars: 20 µm.
(G) Immunoblot analysis of FoxO1, FoxO3a, α-tubulin or PARP in the cytoplasmic and nuclear fractions of WT, Mst1/2 DKO, Akt1 KO or Mst1/2 Akt1 TKO liver tissues.
(H and I) Immunoassay of the ubiquitination of FoxO1 (detected with anti-Myc) in the lysates of 293T cells expressing various combinations of Myc-tagged ubiquitin, Flag-tagged FoxO1, HA-tagged Yap and HA-tagged Skp2 (H) with or without Akt inhibitor MK2206 treatment
(J) Quantification of BrdU or TUNEL-positive cells in the liver sections from Mst1/2 DKO mice infected with Ad-GFP or Ad-Foxo1(S256A) (n = 3).
(K and L) The representative liver sizes and liver-to-body weight ratios of Mst1/2 DKO infected with adenovirus Ad-GFP or Ad-FoxO1(S256A) (n = 5) (K) or Skp2 KO mice infected with adenovirus Ad-GFP, Ad-FoxO1(S256A), Ad-Skp2(KLKL) or Ad-FoxO1(S256A) plus Ad-Skp2(KLKL) (n = 5) (L).
(M) A proposed working model for Yap promoting polyploid cell division via Skp2-mediated FoxO degradation.
Data were assessed by Student’s t-test and represented as mean ± SD *p < 0.05, **p < 0.01, ***p < 0.001 compared between the indicated groups.
See also Figure S6.
Yap-Akt-Skp2 signaling is implicated in human HCC development
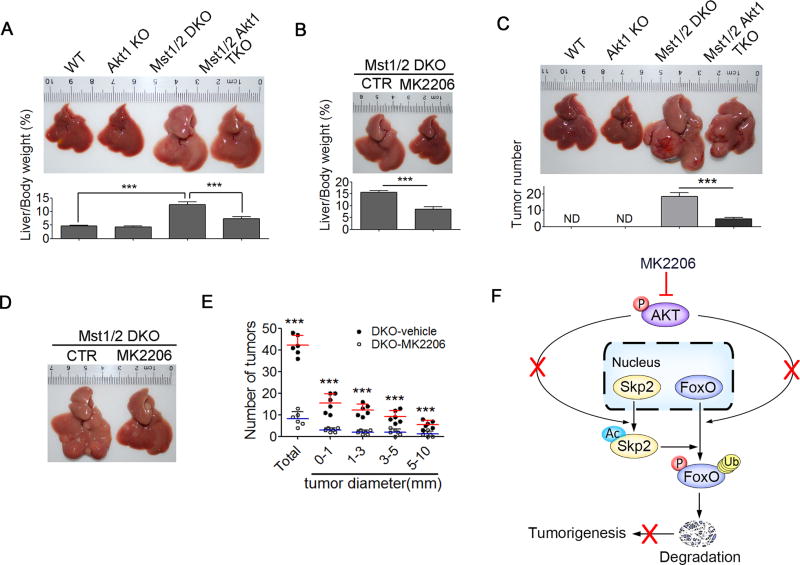
The data above demonstrated that the loss of Hippo signaling in the liver resulted in the cytoplasmic retention of both Skp2 and FoxO through the activation of Akt, which promotes Skp2-mediated FoxO degradation, thereby enhancing p27-induced polyploid cell division, genomic instability and oncogenesis. Importantly, the inhibition or genetic disruption of Akt resulted in significantly reduced p27 levels, hepatocyte polyploidy and decreased liver-to-body weight ratios for Mst1/2 DKO mice (Figures 4I–4O, 7A and 7B). We observed that the loss of Akt1 in Mst1/2 DKO mice resulted in a significantly lower incidence of HCC in old age (Figure 7C). Consistently, Mst1/2 DKO mice that have been treated with the Akt inhibitor MK2206 exhibited a significant decrease in the size and number of tumors, which correlated with the reduced cytoplasmic Skp2 levels in liver tissues compared with control animals (Figures 7D–7F, S4E and S4F). We then examined 60 pairs of liver-derived tumorous (T) and adjacent non-tumorous (N) tissues. Yap activation was associated with decreased FoxO1/3, increased Skp2 acetylation and Akt phosphorylation in tumorous tissues compared with those in adjacent noncancerous tissues (Figures 8A–8C and S7A). These results suggested that Yap-Akt-Skp2 signaling is associated with human HCC development.
Figure 7. Disruption of Akt function attenuates liver tumor formation in Mst1/2 DKO mice.
(A and B) Representative liver pictures and the liver-to-body weight ratios of Mst1/2 DKO mice treated with the Akt inhibitor MK2206 (n = 5) (A) or of WT, Akt1 KO, Mst1/2 DKO, Mst1/2 Akt1 TKO mice (n = 5) (B).
(C) A representative liver picture and the quantification of tumor number from WT, Akt1 KO, Mst1/2 DKO, Mst1/2 Akt1 TKO mice (n = 6).
(D and E) A representative liver picture and the quantification of the size and number of liver tumors (n = 6) of 5 month old Mst1/2 DKO mice treated with vehicle or the Akt inhibitor MK2206.
(F) A proposed working model for the disruption of Akt function attenuating liver tumor formation in Mst1/2 DKO mice.
Data were assessed by Student’s t-test and represented as mean ± SD, ***p < 0.001 compared between the indicated groups. ND, non-detectable.
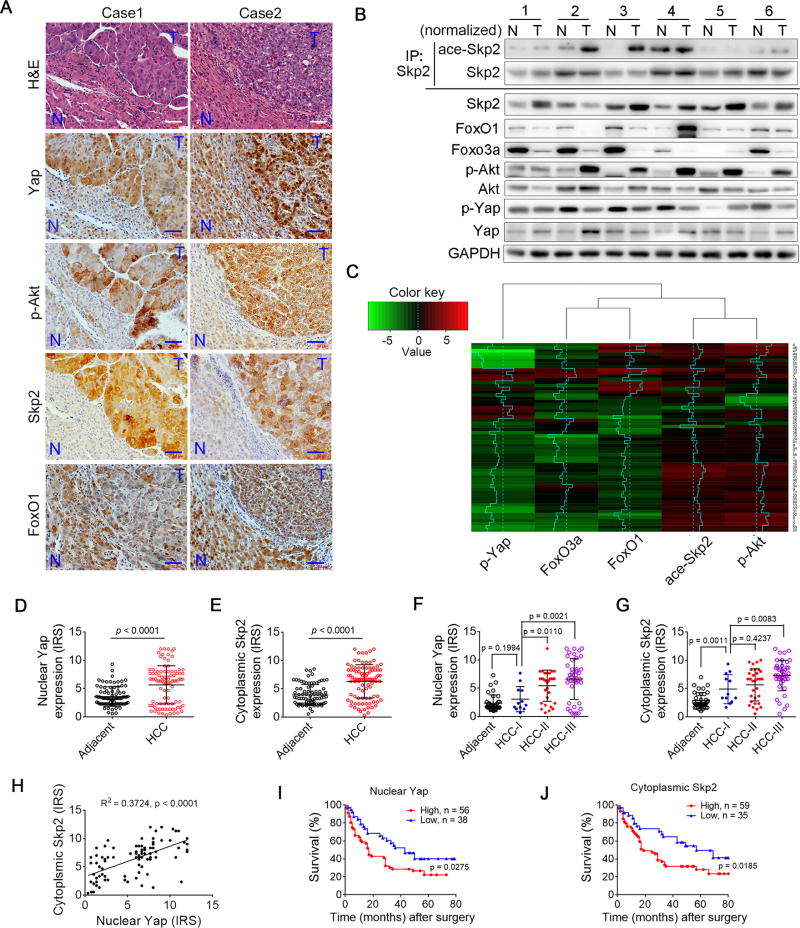
Figure 8. Yap-Akt-Skp2 signal is implicated in human HCC progress.
(A) A representative image of H&E staining and the IHC analysis of p-Yap, p-Akt, Skp2 or FoxO1 in the liver sections of adjacent non-tumorous livers (N) or HCC tissues (T) isolated from one patient. Scale bars: 50 µm.
(B and C) Western blot analysis of ace-Skp2, Skp2, FoxO1, FoxO3a, p-Akt, Akt, p-Yap, Yap and GAPDH in HCC tissue (T) and adjacent non-tumorous liver tissue (N) isolated from one patient. Six representative paired samples are shown (B). See Supplementary Figure S7A for the remaining 54 paired samples. For the detection of ace-Skp2, the loading of immunoprecipitates was normalized according to the levels of total Skp2. The intensities of the immunoblot bands were quantified using the ImageJsoftware. A heatmap representation of the ratio of the relative expression of the proteins p-Yap, Foxo3a, Foxo1, ace-Skp2 or p-Akt in the T and N samples from one patient. Clustering was performed by using Pearson correlation metric and centroid linkage (C).
(D–G) Scatter plot analysis of the immunoreactive score (IRS) of the nuclear Yap (D and F) or the cytoplasmic skp2 (E and G) inpaired liver cancer and adjacent non-cancer paraffin tissue sections or in different tumor stages from the tissue microarray of human liver cancer. IRS scores of 0–1 indicate negative; scores of 2–3 indicate mild; scores of 4–8 indicate moderate; scores of 9–12 indicate strongly positive. A total of 94 paired samples are shown. The data were assessed by Student’s t-test and are represented as the mean ± SD
(H) The IRS of nuclear Yap and the cytoplasmic Skp2 of liver cancer sections from the tissue microarray of human liver cancer was plotted and assessed using a linear regression t-test.
(I and J) Kaplan-meier plot of overall survival of patients with HCC stratified by high IRS (>6) or low IRS (<6) of nuclear Yap (I) or cytoplasmic Skp2 (J) expression levels. A log-rank test is used for statistical analysis. See also Figure S7.
The examination of the tissue microarray of human liver tumors and adjacent non-tumorous tissues (n = 94) by IHC staining revealed that the expression levels of Yap and Skp2 were higher in cancer tissues than in adjacent noncancerous liver tissues (Figure S7B). Nuclear Yap expression and cytoplasmic Skp2 expression were primarily increased in cancer tissues compared to those in adjacent noncancerous tissues and associated with advanced tumor stages (Table S1; Figures 8D–8G). Furthermore, increased nuclear Yap expression in cancer tissues was associated with enhanced cytoplasmic Skp2 expression (Figure 8H, R2 = 0.3724, p < 0.0001). Although there were no significant association between Yap or Skp2 expression and sex or age (Table S1), there was a positive correlation between the nuclear Yap expression or cytoplasmic Skp2 expression and pathological grade (Table S1). In additions, the Kaplan-Meier survival analysis revealed that the survival time for patients with high nuclear Yap expression was obviously shorter than those with low Yap expression (p = 0.0275). Similarly, the cytoplasmic Skp2 expression level had a negative correlation with survival (p = 0.0185). Thus, we concluded that there is a close connection between these two proteins in human HCC development and that the inhibition of Akt activity may be a promising therapeutic strategy to treat HCC resulting from the loss of Hippo signaling.
Discussion
How diploid organisms develop polyploid cells remains largely unknown. Herein, we report that Hippo signaling maintains liver cell ploidy in a p53-independent manner. We found that Hippo signal deficiency or Yap activation turn on Akt signaling, thereby activating the acetyltransferase p300 to promote Skp2 acetylation, stabilization and cytoplasmic retention, which results in p27 hyper-accumulation and induces cell polyploidy. As a result of Skp2 cytoplasmic retention, the pro-apoptotic factors FoxO1/3 are overly degraded and thereby promote polyploid cell division, genomic instability and oncogenesis. Moreover, the inhibition or genetic disruption of Akt or p27 reduces cell polyploidy and oncogenesis, whereas the depletion of Skp2 has no significant effect on Mst1/2-null cell polyploidy but does prevent polyploid cell division and abrogates liver overgrowth and tumorigenesis. These data indicate that Hippo-Skp2 signaling prevents genomic instability through two possible mechanisms: maintaining Skp2-mediated p27 degradation in the nuclei to limit polyploidy formation or preventing Skp2-mediated FoxO1/3 degradation in the cytosol to block polyploidy cell division, thereby limiting the risk of genomic instability, aneuploidy and tumorigenesis.
Our current study demonstrates that the sustained activation of Yap overrides the p27-mediated checkpoint, at least in part, through the degradation of FoxO family proteins, allowing polyploid cells to proliferate inappropriately with mitotic defects and resulting in centrosome amplification, genomic instability and cell oncogenesis. In fact, p27 plays a dual role in the regulation of the cell cycle and genomic stability in the mouse liver (Carrano et al., 1999; Kossatz et al., 2004; Nakayama et al., 2004; Serres et al., 2012). For cell cycle control, elevated p27 arrests the cell cycle via the inhibition of CDKs to prevent cell division. In contrast, increased p27 results in mitotic defects and promotes endoreduplication cycles to induce cell polyploidy and genomic instability in mouse livers, while a loss of p27 reduces cell polyploidy, thereby maintaining genomic stability. In addition, p27 has long been known to be an assembly factor for cyclin D/Cdk4 complexes (LaBaer et al., 1997). Thus, elevated p27 in polyploid cells might increase Cdk4 activity and promote cell divison, aneuploidy and genomic instability, thus contributing to the development of cancer. In the context of the much higher fraction of polyploid cells in Mst1/2 DKO liver tissues, it is not surprising to observe that the loss of p27 in Hippo-deficient livers resulted in decreased cell polyploidy and a significantly reduced number and volume of tumor size. Thus, these data support a key role of p27 expression in the tumorigenesis of polyploid organs, such as livers with deregulated Hippo signaling.
Previous studies have shown that tetraploid cells arrest their cell cycle in a p53-dependent manner. Hippo tumor suppressor pathway Lats1/2 kinases were shown to induce tetraploid cell cycle arrest by preventing Mdm2-mediated p53 degradation (Aylon et al., 2006; Iida et al., 2004; McPherson et al., 2004). However, we found that Yap overexpression or the deletion of Yap inhibitory components such as WW45, Mst1/2 and Lats1/2 in mouse livers results in highly increased p53 expression and activity. Moreover, the combined losses of Hippo signaling and p53 lead to greatly increased polyploidy with multiple nuclei and results in a higher incidence and earlier onset of liver tumors. Thus, the increased p53-mediated response might be a potent negative feedback loop in response to increased cell polyploidy upon the disruption of Hippo signals. Alternatively, other effectors downstream of Yap that positively augment the p53 response possibly exist. These results suggested that Hippo signaling might play a dual role in p53 regulation, positively as a blockage of Mdm2-mediated p53 degradation by Lats1/2 but negatively as an inhibitor of the Yap-induced p53 response. It will be of particular interest to determine how Yap regulates p53 activity in future studies.
In addition, a previous study showed that tetraploid cells have lower Rho activity, which is mainly due to increased Rac activation in the presence of excess microtubules nucleated by extra centrosomes (Ganem et al., 2014). Restoring Rho activity enables the cell to bypass G1 arrest. Consistently, enhanced cell-matrix adhesion, which activates Rho, is reported to reduce the G1 arrest of tetraploid cells. Interestingly, our previous work demonstrated that the Hippo kinases Mst1 and Mst2 are required for Rac activation (Geng et al., 2015). Thus, whether the reintroduction of active Rac induces the G1 arrest of tetraploid cells is an interesting open question. Moreover, a previous study reported that Mst1 limits the kinase activity of aurora B to promote stable kinetochore-microtubule attachment (Oh et al., 2010). Thus, there might be other effectors downstream of Hippo signaling that mediate polyploidy. In conclusion, our results reveal that the modulation of the Hippo signaling pathway orients hepatocytes into a specific cell cycle program, leading to the generation of diploid or polyploid cells. It is of interest to determine whether Hippo signaling is also involved in the regulation of other polyploid cell types such as megakaryocyte polyploidization.
Supplementary Material
Significance.
p53 is required for the induction of cell senescence to limit the proliferation of polyploid cells. We found that Hippo signal deficiency or Yap activation in mouse livers result in polyploid formation and polyploid cell growth. The combined loss of Hippo signals and p53 lead to greatly increased polyploidy and result in a higher incidence and earlier onset of liver tumors. We revealed that Yap induces cell polyploidy through the Skp2-mediated ubiquitin-proteasome pathway. Importantly, the deregulation of the Hippo-Yap-Skp2 axis is found in a substantial fraction of human hepatocellular carcinomas. Thus, Hippo-Yap signaling acts as an alternative polyploid checkpoint, together with p53, to synergistically restrain polyploid cell division, thus limiting the risk of genomic instability, aneuploidy, and tumorigenesis.
Acknowledgments
The Yap (S127A) transgenic mice were kindly provided by Dr. Fernando Camargo from Harvard Medical School, Boston, MA. D.Z. and L.C. were supported by the National Natural Science Foundation of China (31625010, U1505224 and J1310027 to D.Z.; 81372617, 81422018 and U1405225 to L.C.; 81472229 to L.H.), the National Basic Research Program (973) of China (2015CB910502 to L.C.), the Fundamental Research Funds for the Central Universities of China-Xiamen University (20720140551 to L.C. and 2013121034 and 20720140537 to D.Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions
D.Z. and L.C. conceived the project with the input from D.G., L.Z., Q.W., X.D. and K.-H.L. S.Z., Q.C., Q.L., Y.L., X.S., L.H., S.J., C.L., J.G., W.Z., Z.L., Y.Z., D.G. and Q.L. performed experimental biological research. Z.-Y.Y provided human HCC samples. R.-L.J., K.N., K.-I.N., Z.L. and L.Z. provided mutant mice. L.C. and D.Z. co-wrote the paper. All authors edited the manuscript.
Competing interests: The authors declare no competing financial interests.
References
- Aylon Y, Michael D, Shmueli A, Yabuta N, Nojima H, Oren M. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes & development. 2006;20:2687–2700. doi: 10.1101/gad.1447006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer S, Zetterberg A, Ihrie RA, McTaggart RA, Yang Q, Bradon N, Arvanitis C, Attardi LD, Feng S, Ruebner B, et al. Developmental context determines latency of MYC-induced tumorigenesis. PLoS biology. 2004;2:e332. doi: 10.1371/journal.pbio.0020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvisi DF, Ladu S, Pinna F, Frau M, Tomasi ML, Sini M, Simile MM, Bonelli P, Muroni MR, Seddaiu MA, et al. SKP2 and CKS1 promote degradation of cell cycle regulators and are associated with hepatocellular carcinoma prognosis. Gastroenterology. 2009;137:1816–1826. doi: 10.1053/j.gastro.2009.08.005. e1811–1810. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Current biology : CB. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature cell biology. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Celton-Morizur S, Merlen G, Couton D, Margall-Ducos G, Desdouets C. The insulin/Akt pathway controls a specific cell division program that leads to generation of binucleated tetraploid liver cells in rodents. The Journal of clinical investigation. 2009;119:1880–1887. doi: 10.1172/JCI38677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng Z, Huang HY, Tsai KK, Flores LG, Shao Y, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis. Cell. 2012;149:1098–1111. doi: 10.1016/j.cell.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoli T, de Lange T. The causes and consequences of polyploidy in normal development and cancer. Annual review of cell and developmental biology. 2011;27:585–610. doi: 10.1146/annurev-cellbio-092910-154234. [DOI] [PubMed] [Google Scholar]
- Davoli T, Denchi EL, de Lange T. Persistent telomere damage induces bypass of mitosis and tetraploidy. Cell. 2010;141:81–93. doi: 10.1016/j.cell.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW. Aneuploidy, polyploidy and ploidy reversal in the liver. Seminars in cell & developmental biology. 2013;24:347–356. doi: 10.1016/j.semcdb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Duncan AW, Hickey RD, Paulk NK, Culberson AJ, Olson SB, Finegold MJ, Grompe M. Ploidy reductions in murine fusion-derived hepatocytes. PLoS genetics. 2009;5:e1000385. doi: 10.1371/journal.pgen.1000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AW, Taylor MH, Hickey RD, Hanlon Newell AE, Lenzi ML, Olson SB, Finegold MJ, Grompe M. The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature. 2010;467:707–710. doi: 10.1038/nature09414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–1047. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Cornils H, Chiu SY, O'Rourke KP, Arnaud J, Yimlamai D, Thery M, Camargo FD, Pellman D. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell. 2014;158:833–848. doi: 10.1016/j.cell.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Pellman D. Limiting the proliferation of polyploid cells. Cell. 2007;131:437–440. doi: 10.1016/j.cell.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nature cell biology. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, Hong L, Xie C, Li X, Zhao H, et al. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nature immunology. 2015;16:1142–1152. doi: 10.1038/ni.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentric G, Desdouets C. Polyploidization in liver tissue. The American journal of pathology. 2014;184:322–331. doi: 10.1016/j.ajpath.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nature reviews Genetics. 2012;13:189–203. doi: 10.1038/nrg3123. [DOI] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SH, Delgado ER, Otero PA, Teng KY, Kutay H, Meehan KM, Moroney JB, Monga JK, Hand NJ, Friedman JR, et al. MicroRNA-122 Regulates Polyploidization in the Murine Liver. Hepatology. 2016 doi: 10.1002/hep.28573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida S, Hirota T, Morisaki T, Marumoto T, Hara T, Kuninaka S, Honda S, Kosai K, Kawasuji M, Pallas DC, et al. Tumor suppressor WARTS ensures genomic integrity by regulating both mitotic progression and G1 tetraploidy checkpoint function. Oncogene. 2004;23:5266–5274. doi: 10.1038/sj.onc.1207623. [DOI] [PubMed] [Google Scholar]
- Inuzuka H, Gao D, Finley LW, Yang W, Wan L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, et al. Acetylation-dependent regulation of Skp2 function. Cell. 2012;150:179–193. doi: 10.1016/j.cell.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nature reviews Drug discovery. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossatz U, Dietrich N, Zender L, Buer J, Manns MP, Malek NP. Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes & development. 2004;18:2602–2607. doi: 10.1101/gad.321004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurinna S, Stratton SA, Coban Z, Schumacher JM, Grompe M, Duncan AW, Barton MC. p53 regulates a mitotic transcription program and determines ploidy in normal mouse liver. Hepatology. 2013;57:2004–2013. doi: 10.1002/hep.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of CDK inhibitors. Genes & development. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Lee SW, Li CF, Jin G, Cai Z, Han F, Chan CH, Yang WL, Li BK, Rezaeian AH, Li HY, et al. Skp2-dependent ubiquitination and activation of LKB1 is essential for cancer cell survival under energy stress. Molecular cell. 2015;57:1022–1033. doi: 10.1016/j.molcel.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nature cell biology. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson JP, Tamblyn L, Elia A, Migon E, Shehabeldin A, Matysiak-Zablocki E, Lemmers B, Salmena L, Hakem A, Fish J, et al. Lats2/Kpm is required for embryonic development, proliferation control and genomic integrity. The EMBO journal. 2004;23:3677–3688. doi: 10.1038/sj.emboj.7600371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. The EMBO journal. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Nagahama H, Minamishima YA, Miyake S, Ishida N, Hatakeyama S, Kitagawa M, Iemura S, Natsume T, Nakayama KI. Skp2-mediated degradation of p27 regulates progression into mitosis. Developmental cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- Oh HJ, Kim MJ, Song SJ, Kim T, Lee D, Kwon SH, Choi EJ, Lim DS. MST1 limits the kinase activity of aurora B to promote stable kinetochore-microtubule attachment. Current biology : CB. 2010;20:416–422. doi: 10.1016/j.cub.2009.12.054. [DOI] [PubMed] [Google Scholar]
- Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, Anai M, Ogihara T, Fujishiro M, Viana AY, et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes. 2003;52:2905–2913. doi: 10.2337/diabetes.52.12.2905. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Developmental cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit SK, Westendorp B, de Bruin A. Physiological significance of polyploidization in mammalian cells. Trends in cell biology. 2013;23:556–566. doi: 10.1016/j.tcb.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Pandit SK, Westendorp B, Nantasanti S, van Liere E, Tooten PC, Cornelissen PW, Toussaint MJ, Lamers WH, de Bruin A. E2F8 is essential for polyploidization in mammalian cells. Nature cell biology. 2012;14:1181–1191. doi: 10.1038/ncb2585. [DOI] [PubMed] [Google Scholar]
- Serres MP, Kossatz U, Chi Y, Roberts JM, Malek NP, Besson A. p27(Kip1) controls cytokinesis via the regulation of citron kinase activation. The Journal of clinical investigation. 2012;122:844–858. doi: 10.1172/JCI60376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Science's STKE : signal transduction knowledge environment. 2003;2003:RE5. doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo FD, Guan KL. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nature cell biology. 2012;14:1322–1329. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J, Sellers RS, Nakayama K, Nakayama KI, Cobrinik D, et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nature genetics. 2010;42:83–88. doi: 10.1038/ng.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang L, Chen X, Chen Y, Dong J. Oncoprotein YAP regulates the spindle checkpoint activation in a mitotic phosphorylation-dependent manner through up-regulation of BubR1. The Journal of biological chemistry. 2015;290:6191–6202. doi: 10.1074/jbc.M114.624411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang L, Liu M, Chong R, Ding SJ, Chen Y, Dong J. CDK1 Phosphorylation of YAP Promotes Mitotic Defects and Cell Motility and Is Essential for Neoplastic Transformation. Cancer research. 2013;73:6722–6733. doi: 10.1158/0008-5472.CAN-13-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Bauzon F, Fu H, Lu Z, Cui J, Nakayama K, Nakayama KI, Locker J, Zhu L. Skp2 deletion unmasks a p27 safeguard that blocks tumorigenesis in the absence of pRb and p53 tumor suppressors. Cancer cell. 2013;24:645–659. doi: 10.1016/j.ccr.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.