Abstract
Advances in the care of preterm infants have improved survival of infants born at earlier gestational ages. Yet, these infants remain at risk for the chronic lung disease of infancy, bronchopulmonary dysplasia (BPD), which results in prolonged need for supplemental oxygen, recurrent respiratory exacerbations, and exercise intolerance. Recent investigations have highlighted the important contribution of the developing pulmonary circulation to lung development, demonstrating that these infants are also at risk for pulmonary vascular disease (PVD), including pulmonary hypertension (PH) and pulmonary vascular abnormalities, which contributes significantly to morbidity and mortality. In the past few years, several epidemiological studies have delineated the incidence of PH in preterm infants and the impact on outcomes. However, these studies have also highlighted gaps in our understanding of PVD in BPD, including universally accepted definitions, approaches to diagnosis and treatment, and patient outcomes. Associated pulmonary vascular and cardiac abnormalities are increasingly recognized complications contributing to PH in these infants, but incidence of these lesions and degree of contribution to disease remains unknown. Therapeutic strategies for PVD in BPD are largely untested, but recent evidence presents the rationale for the approach to diagnosis and treatment of BPD infants with PH that can be evaluated in future studies.
Keywords: bronchopulmonary dysplasia, pulmonary vascular disease, pulmonary hypertension, echocardiogram, inhaled nitric oxide
Introduction
Bronchopulmonary dysplasia (BPD) is the chronic lung disease that occurs in preterm infants who require mechanical ventilation and oxygen therapy for postnatal respiratory distress.1 BPD is characterized by persistent pulmonary disease with a prolonged need for supplemental oxygen, recurrent respiratory exacerbations with frequent emergency department visits hospitalizations,2,3 exercise intolerance, and associated respiratory problems that can extend into adulthood.4 Improved respiratory support strategies, antenatal steroids, surfactant therapy and other advances in clinical care have decreased the risk for death and the development of BPD in larger preterm infants. These strategies have also increased the survival of infants born earlier in gestation who then have high risk to develop BPD. Thus, the incidence of BPD has remained relatively stable over the past decade.5,6 BPD in this “new” era most often occurs in infants born at 24–28 weeks post menstrual age (PMA), weighing ≤ 1000g, who have less severe acute respiratory symptoms and require less respiratory support than BPD patients in the era when the disease was first described.7,8
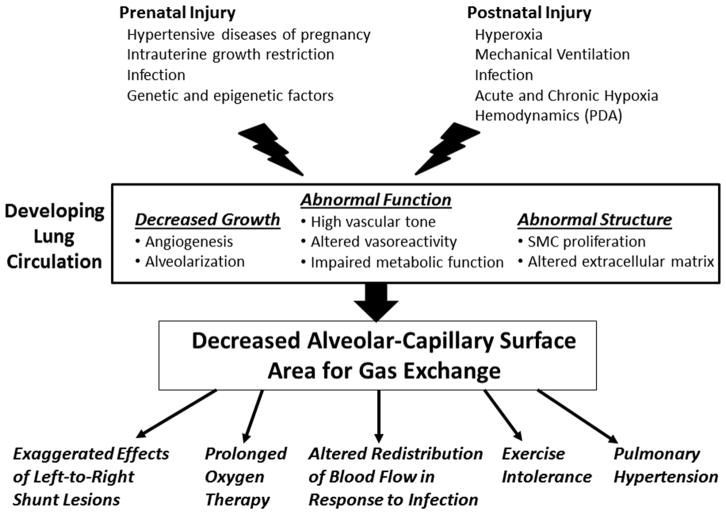
Maternal, genetic, and environmental factors can lead to early injury of the developing lung which impairs angiogenesis and alveolarization, resulting in simplification of the distal lung airspace and clinical manifestations of BPD. The impairments of the developing pulmonary vasculature may result in significant pulmonary vascular disease (PVD) (Figure 1). In its most severe form, PVD results in pulmonary hypertension (PH).9–12 The impact of clinically apparent PH in the “new BPD” era suggest that morbidity13–17 and late mortality is high, with up to 48% mortality 2 years after diagnosis of PH.18
Figure 1.
Schematic illustrating the components contributing to pulmonary vascular disease in bronchopulmonary dysplasia and the clinical manifestations that result. PDA: patent ductus arteriosis, SMC: smooth muscle cells.
Prospective studies have demonstrated that PH is common in BPD impacting 16–25% of infants.15–17 Greater recognition of PH and its impact in this population has led to widespread treatment with pulmonary vasodilator medications. However, there are limited prospective data examining the impact of these medications on pulmonary vascular development as well as the clinical effectiveness of such medications. Thus, there is a need for greater insights into basic mechanisms of BPD and standardized clinical criteria for identifying at risk infants in order to develop better strategies for the prevention, monitoring, and treatment of preterm newborns. This review will present recent studies examining the role of the pulmonary circulation in the pathogenesis of BPD and PVD, the epidemiological studies of PH in BPD, and the rationale for the clinical approach to diagnosis and treatment of PH in BPD.
Pathogenesis of Pulmonary Vascular Disease in BPD
Pulmonary vascular growth is a dynamic process, beginning in the embryonic period and continuing throughout gestation and during postnatal life. Premature birth exposes the developing lung to an environment that can impair the normal developmental process. Histologically, the lung of preterm infants have revealed reduced numbers of both alveoli and intra-acinar arteries, leading to the description of BPD as an arrest of lung development. The pathogenesis of PVD in BPD is multifactorial, resulting from complex interactions between maternal, genetic and epigenetic susceptibility, and environmental factors (both prenatal and postnatal), including hyperoxia, hypoxia, hemodynamic stress, infection, inflammation, and others. Clinical studies strongly suggest these complex interactions contribute to alter normal growth factor expression and signaling pathways, leading to impaired growth, structure and function of the developing pulmonary circulation after premature birth (Figure 1).10,19–27
Disruption of vascular growth and signaling may contribute to impaired lung structure,28–32 leading to a marked reduction in alveolar-capillary surface area.33–35 Abnormal growth of the pulmonary circulation in BPD is characterized by decreased vascular branching, an altered pattern of vascular distribution within the lung interstitium, and persistent intrapulmonary venous anastomoses,31,36–39 which collectively contribute to the symptoms of BPD.40 Several recent studies have shown pre-eclampsia is a risk factor for BPD and PVD,41 possibly through disruption of these vascular signaling pathways.42–45 Intrauterine growth restriction is another condition that has been associated with disruption of vascular signaling46 and an increased risk for BPD and PH in preterm infants.47
The reduction in vessel number resulting from impaired vascular growth coupled with alveolar hypoxia, hyperoxia or hemodynamic stress may worsen pulmonary arterial structural remodeling in the preterm infant. Endothelial injury due to these environmental stressors27,48,49 induces the media of small pulmonary arteries to undergo smooth muscle cell proliferation, precocious maturation of immature mesenchymal cells into mature smooth muscle cells, and incorporate fibroblasts/myofibroblasts into the vessel wall.50 Smooth muscle proliferation also extends abnormally into the smaller peripheral arteries.9 Such pulmonary artery remodeling and disruption of angiogenic factor expression as has been demonstrated in autopsy data from infants dying with BPD,35,51,52 could lead to high pulmonary vascular resistance (PVR) due to narrowing of the vessel diameter and decreased vascular compliance, especially in response to respiratory infections. Persistent abnormalities of pulmonary vascular growth and/or failure of the lung vasculature to “catch-up” to infants born at term may contribute to PVD that becomes increasing symptomatic later in life.53,54
Diagnosis and Epidemiology of Pulmonary Vascular Disease in Preterm Infants
PH and cor pulmonale, resulting from extreme forms of PVD are recognized factors associated with high mortality in preterm infants with BPD.18,55,56 In the past decade, patients with BPD and PH had a reported survival rates of 52% two years after the diagnosis of PH.18 The increasing recognition of PVD and its association with poor outcomes in preterm infants born at earlier gestational ages led to renewed interest determining the incidence of PH in new era BPD and identifying high risk infants as early as possible. However, the lack of a data-derived definition of PH in this population and reliable diagnostic assessment measures to diagnose PH has limited study in this area.
Cardiac catheterization remains the gold standard for diagnosis of PH, but its invasive nature has restricted its application to those infants at highest risk. Non-invasive assessment via echocardiography, despite its significant limitations,14 has become the most often used tool for screening and diagnosis of PH in preterm infants. The most objective measure of PH by echocardiogram is the estimated right ventricular systolic pressure (RVSP) derived from the tricuspid regurgitant jet velocity (TRJV).57–60 Applied from adult criteria, a threshold of RVSP > 35 mm Hg (TRJV > 3 m/s) to define PH has been used. Due to relatively low blood pressures in preterm infants, estimated pulmonary pressures >50% of the systemic pressure has also been used to define PH and appears to correlate better with measurements performed during cardiac catheterization.14,61 Earlier studies of BPD infants with known or suspected PH revealed a measureable TRJV in only 31–61% of these infants.14–16,62 More recent studies that have screened large groups of preterm infants have found that a measureable TRJV is more rare (6–10%)16,17 and was the determining factor to diagnose PH in a very small proportion of infants. Further, the standards for determining the quality of the TRJV used to estimate RVSP and diagnose PH have not been thoroughly reported in some recent studies.15,16,61,63
Yet, the lack of a measureable TRJV does not preclude the presence of PVD or PH. Qualitative echocardiogram findings to diagnose PH, including right atrial enlargement, right ventricular hypertrophy, right ventricular dilation, pulmonary artery dilation, and interventricular septal flattening have correlated with diagnosis of PH by cardiac catheterization. Septal flattening appears to be among the more sensitive of these measures, but is vulnerable to interobserver reliability.
The right ventricular myocardial performance index (MPI; also known as the Tei index) has been used as a surrogate for increased PVR in BPD, and has been shown to remain elevated in infants with BPD compared to preterm infants without BPD.64 Another study demonstrated that respiratory failure requiring mechanical ventilation from multiple etiologies, including BPD, was associated with significantly increased right ventricular MPI compared to controls,65 suggesting that MPI may be a surrogate for worse respiratory disease. Further studies are needed to determine whether high right ventricular MPI is associated with BPD and/or PH.
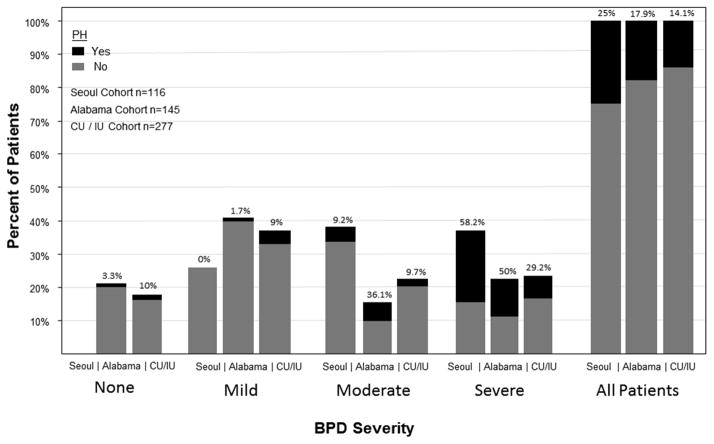
In the past few years, several studies have attempted to determine the incidence of PH among preterm infants using echocardiograms as the screening tool, albeit with slightly different criteria to diagnose PH (Figure 2)15–17,66 The first of these was a retrospective review at a single center in South Korea identifying 116 preterm infants born at <32 weeks postmenstrual age and diagnosed with BPD.15 Echocardiograms performed after 2 months of age at the discretion of the primary caregivers were used to determine PH, but the number and timing of echocardiograms performed were not reported. PH criteria were based on clinical interpretation of echocardiograms and included a TRJV ≥3 m/s in the absence of pulmonary stenosis, and/or a flat or left-deviated interventricular septal configuration with RVH and chamber dilation. These authors identified 29 (25%) of BPD infants with PH at a median postnatal age of 65 days, and the diagnosis of PH was significantly correlated with severity of BPD (Figure 2). All patients diagnosed with PH were reported to have some degree of tricuspid regurgitation (trivial in 27.6%, mild in 44.8%, and moderate 27.6%), but the proportion meeting PH threshold was not reported.
Figure 2.
The incidence of PH according to the degree of BPD severity. Incidence of PH among preterm infants from three studies, Seoul,15 Alabama,16 and Colorado/Indiana.17 The percentage of patients listed on the Y-axis represents the proportion of patients in each study in whom BPD status was ascertained. Please see text for inclusion criteria of each of the studies. BPD severity was based on NIH criteria.38 Physiologic assessment of oxygenation to determine BPD status94 was applied in the Alabama and CU/IU cohorts. The Seoul cohort did not include preterm infants without BPD. Numbers above the bars indicate the percentage of patients with PH.
The second study was a prospective analysis of preterm infants at the University of Alabama at Birmingham (USA) with birth-weight < 1000g who survived to 28 days.16 All patients underwent echocardiogram between 4–6 weeks of age and subsequent echocardiograms were performed if severe lung disease or clinical signs suggestive of right-sided heart failure were present. PH was diagnosed if at least one of the following findings was present: 1) right ventricular hypertrophy, 2) flattening of interventricular septum, 3) presence of tricuspid regurgitation in the absence of pulmonary stenosis, and 4) elevated right ventricular pressures as estimated by Doppler studies of tricuspid regurgitation jet (although the threshold for elevated pressure was not provided). Of 145 eligible patients, 9 (6.2%) were identified by the initial echocardiogram. Another 17 (11.7%) infants were diagnosed by subsequent echocardiograms; median age of diagnosis was 112 days. Of the patients diagnosed with PH, tricuspid regurgitation was identified in 58%, an elevated estimated systolic pulmonary pressure in 69%, right ventricular hypertrophy in 42%, and a flattened interventricular septum in 54%. Fifty-eight percent of infants had persistent signs of PH at NICU discharge, and 3 patients died from complications of PH.
The third study was a prospective evaluation at hospitals affiliated with two academic centers in the US. Two hundred seventy-seven preterm infants with gestational age at birth <34 weeks and birthweights between 500–1250g were screened with echocardiograms at 7 days of age and 36 weeks PMA.17 All echocardiograms were interpreted by a single cardiologist, but this this study employed 3 different a priori criteria for PH to determine which was most closely associated with outcome. The most liberal criteria which were met by any of the following findings: an estimated right ventricular systolic pressure (RVSP) > 40 mm Hg, RVSP/systemic systolic blood pressure (sBP) > 0.5, any cardiac shunt with bidirectional or right-to-left flow, or any degree of ventricular septal wall flattening were most similar to the two studies described above. According to these criteria, the incidence of PH was 14% at 36 weeks PMA, with the highest incidence of PH (29%) occurring in infants with severe BPD, but relatively consistent rate of PH (about 10%) in infants with none, mild, and moderate BPD. PH diagnosed by these criteria was associated with greater mortality and increased duration of respiratory support after adjustment for gestational age at birth, BPD status, and other clinical factors. Applying alternate, more strict criteria for PH, dramatically reduced the incidence of PH (3.3% for criteria only using TRJV based threshold or reversal of cardiac shunt, and 4.7% for criteria that included, TRJV, reversal of shunt, or moderate to severe septal flattening).
A fourth prospectively conducted study at Women & Infants Hospital of Rhode Island (USA) enrolled 120 infants born <28 weeks PMA who underwent echocardiogram screening at 10–14 days of age and 36 weeks PMA.66 They employed more strict criteria for PH (estimated systolic pulmonary artery pressure to systemic systolic artery pressure ratio > 0.5 or moderate or severe interventricular septal flattening). Using these criteria, they found an incidence of PH of 8% at 36 weeks PMA which is comparable the incidence of 4.7% found in the study by Mourani et al. using the similar criteria.17
Aligning similar criteria for PH, these studies reveal comparable rates of PH in patients diagnosed with BPD, with the majority of PH patients having severe BPD. It should be noted that PH can be identified as a “late” finding, with 65% of BPD infants with PH being diagnosed after having a “normal” echocardiogram between 4 to 6 weeks of age.16 With the exception of the study by Mirza,66 these studies employed liberal criteria for PH, only requiring any evidence of septal flattening, and one only requiring the presence of a TRJ without applying threshold for pressure estimates.16 Yet, using liberal criteria for PH, these studies and others have reported higher death rates and increased morbidities in preterm infants with BPD and PH compared to those without PH.15–17,61,63,67 These data suggest that routine screening of preterm infants near term, especially those with more severe BPD will result in identification of substantial number of infants with PH who are at risk for increased morbidity and mortality. These data may further provide useful prognostic information for parents and caregivers and may allow the opportunity for early intervention.
Despite its usefulness for diagnostic screening, echocardiograms still have limitations that may preclude diagnosis of PH, fail to accurately diagnose the severity of PH14 in those identified by echocardiogram, or fail to identify additional cardiovascular abnormalities that contribute to PH. Thus, we recommend cardiac catheterization (Table 1) for preterm infants with the following: 1) persistent signs of severe cardiorespiratory disease or clinical deterioration not directly explained by other diagnostic evaluations; 2) suspected of having significant PH despite optimal management of their lung disease and associated morbidities; 3) chronic vasodilator therapy is being considered; 4) unexplained, recurrent pulmonary edema. The goals of cardiac catheterization are to: assess the severity of PH, exclude or document the severity of associated anatomic cardiac and vascular lesions that may be amenable to intervention during catheterization, and to assess pulmonary vascular reactivity in patients who fail to respond to oxygen therapy alone.
Table 1.
Approach to Pulmonary Hypertension in BPD
| Screening echocardiograms for: |
| Severe BPD at 36 weeks |
| Infants with prolonged ventilator and/or oxygen requirements |
| Cyanotic episodes |
| Marked hypercarbia |
| Persistent pulmonary edema, diuretic dependence |
| Poor growth, IUGR, oligohydramnios |
| General evaluation and treatment for factors contributing to persistent respiratory disease and PH |
| Ensure adequate oxygenation (awake, asleep, feeds) |
| Assess the adequacy of ventilation |
| Chronic aspiration (barium swallow, swallowing study, pH probe, impedance study) |
| Structural airway disease: malacia, subglottic stenosis |
| Optimal treatment of reactive airways disease |
| Neurological abnormalities: hydrocephalus |
| Ensure optimal nutrition |
| Consider cardiac catheterization when work-up fails to reveal a clear etiology for poor clinical status or when optimal management of these factors fail to achieve clinical improvement |
| Assess severity of PH |
| Anatomic heart disease/shunt lesions |
| Structural vascular abnormalities (eg, arterial stenosis, pulmonary venous obstruction, systemic to pulmonary collateral vessels, others) |
| Catheter-based interventions |
| Assess cardiac function (LV diastolic dysfunction) |
| Acute vasoreactivity/hypoxia testing for selection of chronic therapy |
BPD: bronchopulmonary dysplasia, IUGR: intrautuerine growth restrcition, PH: pulmonary hypertension, LV left ventricular
Risk factors for PVD and PH in Preterm Infants
Past clinical studies have suggested that sustained elevations of pulmonary artery pressure as assessed by serial echocardiograms may be associated with increased risk for BPD,64,68 supporting the hypothesis that PH in premature newborns may be an early clinical marker for predicting BPD. Early echocardiographic signs of pulmonary vascular disease in preterm infants have now been associated with increased risk for both BPD and late PH as well as with prolonged oxygen treatment.17,66 Sustained evidence of elevated right ventricular pressure through the first week after birth may reflect early pulmonary vascular injury that increases risk for BPD. Whether these changes are secondary to delayed transition to extrauterine life, injury due to excessive hemodynamic stress from PDA or other shunts as has been previously reported,69–71 or other forms of vascular injury remains to be determined. Understanding the drivers behind these early vascular changes will be crucial to developing novel intervention strategies to prevent both BPD and PH in these infants.
In addition to early hemodynamic indicators of PVD, clinical factors associated with late PH in most studies include lower gestational age, birth weight, and longer periods of respiratory support. Patent ductus arteriosus,15 infection,15 oligohydramnios,72 small for gestational age16,47 and low birth weight z-score17 have also been identified as risk factors for PH in infants born preterm. Further examination of clinical factors associated with PH, including prenatal risks, along with translational investigations and rigorous screening of infants will help elucidate how these clinical factors impair normal pulmonary vascular development and lead to BPD and PVD.
Other Pulmonary Vascular and Cardiac Abnormalities Associated with BPD
While pulmonary hypertension is an extreme form of PVD in preterm infants, other cardiac and pulmonary vascular abnormalities may contribute to increased PVR and exacerbate PH in these infants (Table 2). Several of these abnormalities may be detected by echocardiogram and others may require alternate imaging such CT, MRI, and/or cardiac catheterization to detect. Echocardiographic assessment of anatomic cardiac disease, especially shunt lesions, is an important aspect of cardiovascular evaluation of BPD infants. Excessive left-to-right flow through cardiac level shunts can lead to pulmonary artery remodeling and increased PVR. Unfortunately, the degree of shunt flow cannot always be accurately estimated by echocardiogram and may require cardiac catheterization to quantify the degree of shunt. Other pulmonary vascular abnormalities such as systemic to pulmonary collaterals or pulmonary venous stenosis can occasionally be detected with echocardiogram, but often require cardiac catheterization to identify. However, these abnormalities may be amenable to treatment intervention during the catheterization, such as coiling or balloon dilation, respectively.73 Impaired cardiac function may result from pulmonary hypertension (right sided dysfunction) or may contribute to it (left sided dysfunction). Preterm birth has been associated with alterations in RV structure and reduced function in young adults, suggesting that the heart in addition to the vasculature may be susceptible to perinatal events.74 Decreased shortening fraction, left ventricular ejection fraction, or signs of left ventricular hypertrophy may be signs of left ventricular dysfunction, which may require catheterization for confirmation.14,75,76 Diastolic function may also lead to elevated PVR, but is difficult to ascertain by echocardiogram, and often requires catheterization for diagnosis as well.75 In one study, the combined modality of CT scans (21 patients) and catheterizations (14 patients) identified cardiovascular anomalies in 19 of 29 BPD infants with PH, including systemic to pulmonary collaterals (n = 9), pulmonary vein stenosis (n = 7), atrial septal defects (n = 5), and PDA (n = 9).77 MRI of the chest may provide an additional modality for evaluating pulmonary vascular function and hemodynamics. Phase-contrast flow measurements via MRI could estimate pulmonary artery velocities and pulmonary arterial pressure. These techniques have been applied in other forms of PH, detecting changes of PVD prior to symptoms.78–82
Table 2.
Factors Contributing to Increased PVR in PH
| Pulmonary Vascular Disease | Cardiac Disease | Lung Disease |
|---|---|---|
| High tone and reactivity | RV dysfunction | Hypoxemia |
| Hypertensive vascular remodeling | Impaired LV contractility | Hyperinflation |
| Decreased vascular growth | LV diastolic dysfunction | Atelectasis |
| Systemic-to-pulmonary collateral vessels | left-to right-shunt lesions | Hypercarbia |
| Pulmonary vein stenosis |
RV: right ventricular; LV: left ventricular
BPD lung disease can also increase PVR exacerbating PVD and contribute to PH. Air trapping and hyperinflation from airways disease can over stretch small pulmonary arteries increasing PVR. Conversely, atelectasis constrains pulmonary vessels also increasing PVR. Acute episodes of hypoxia and hypercarbia can induce pulmonary artery vasoconstriction, especially within remodeled/dysmorphic vessels.
Treatment of Pulmonary Hypertension in BPD
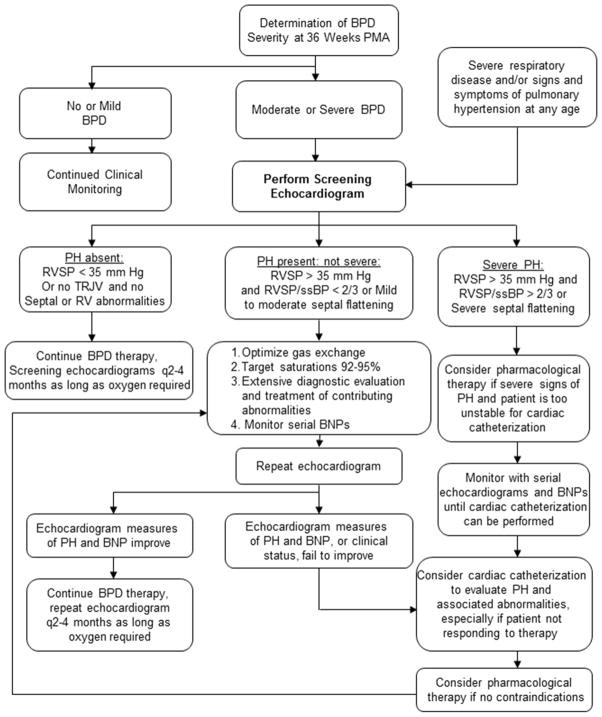
Despite recent evidence that PH is more common in preterm infants than previously thought and is associated with increased morbidity and mortality, proven strategies to improve outcomes are lacking. Several studies have shown improvement in the severity of PH with various strategies,13,15,83 but controlled trials of PH treatment in BPD have not been performed. As pointed out earlier, animal and clinical data suggest that contributions from impaired pulmonary vascular development, cardiac abnormalities and dysfunction, and parenchymal lung disease all contribute to elevated PVR and PH in preterm infants with BPD and may contribute to evolving PVD or time. Therefore, targeted strategies to optimize these areas form the approach to PH therapy in BPD infants (Table 1, Figure 3).
Figure 3.
Proposed clinical approach to screening, evaluation, and monitoring of pulmonary hypertension in bronchopulmonary dysplasia. Adapted from Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: physiology, diagnosis, and treatment. In: Abman SH, ed. Bronchopulmonary Dysplasia. New York: Informa; 2010:347–363; with permission.
95 BNP: brain natriuretic protein; BPD: bronchopulmonary dysplasia; PMA: postmenstrual age; PH: pulmonary hypertension; RVSP: right ventricular systolic pressure; TRJV: tricuspid regurgitant jet velocity; ssBP: systemic systolic blood pressure; RV: right ventricle.
Since a higher rate of PH has been found in those with more severe lung disease, it seems reasonable to aggressively target factors contributing to lung disease, including episodes of hypoxia, ventilatory insufficiency, bronchoconstriction, chronic reflux and aspiration, and upper and lower airway obstruction.40 Immuno-prophylaxis against respiratory infections including respiratory syncytial virus is also an important strategy.
The goal to wean severe BPD infants from respiratory support in preparation for discharge from the NICU should be carefully weighed against the risks for intermittent hypoxia and hypercarbia which can trigger vasoconstriction and further vascular remodeling. Assessments of oxygenation during feeding and sleep should be performed before weaning infants completely from supplemental oxygen. Targeting oxygen saturations to 92–95% should be sufficient to prevent the adverse effects of hypoxia in most infants without increasing the risk of additional lung injury or other systemic effects of hyperoxia. Chronic mechanical ventilation support should be considered for infants who are failing to wean from noninvasive positive pressure support or for infants with frequent episodes of hypoxia and/or impaired ventilation or with inadequate growth velocity despite supplemental oxygen.
As noted above, identifying anatomic cardiovascular abnormalities (Table 2) which contribute to increased PVR and PH and could benefit from specific targeted interventions are important to rule out before considering chronic vasodilator therapy because vasodilators could result in adverse clinical effects in the presence of these lesions. Although the use of vasodilators targeted at pulmonary arterial structural remodeling and vasoreactivity is a rational approach, none of the medications approved for adult use are approved for children in the United States. Given the complex maturational influences related to lung vascular development in preterm infants,84,85 special consideration should be given to use of these medications in infants and children relative to adults (Figure 3). Additionally, use of systemic vasodilators (delivered via the bloodstream) may worsen ventilation/perfusion mismatch and oxygenation in BPD infants.
Inhaled nitric oxide (iNO) is approved for treatment of persistent pulmonary hypertension of the newborn and is the drug with the most safety data available in preterm infants, having been used in several clinical trials for the prevention of BPD.86–88 While the studies performed do date do not justify routine use of iNO for BPD, prolonged iNO therapy for PH may be considered for hospitalized infants who are ventilated or by nasal cannula delivery.89,90 In such patients, dose response studies performed during cardiac catheterization may help to optimize the level of iNO therapy. The acute hemodynamic response to iNO in patients with BPD and PH has been shown to be greater than to calcium channel blockers91 with doses as low as 2 ppm demonstrating improvements of pulmonary pressure. iNO is easiest to deliver via mechanical ventilation and available data suggests that it can be safely administered for prolonged periods. If PH improves in response to iNO, it can gradually be weaned off over days to weeks with careful monitoring prior to discontinuation of mechanical ventilation. For infants in whom iNO fails to sufficiently improve PH as the sole agent, or for those whose clinical setting and needs (upon extubation from mechanical ventilation) may benefit from alternate vasodilator therapy due to limitations with iNO administration, a second-line agent such as sildenafil may be added.
Sildenafil, a selective type 5 phosphodiesterase inhibitor, is approved by the federal drug administration (FDA) for the treatment of PH in adults, but has had large appeal for use in infants and children due the ease of administration in comparison to other PH medications. It has been used extensively off-label for the treatment of PH in BPD based on several case reports and a couple of retrospective studies suggesting safety with potential efficacy.13,83 A recent pilot trial of sildenafil did not improve short-term respiratory outcomes (development of BPD) of extremely preterm infants, but further studies are warranted to determine whether treatment could mitigate development of severe PVD. In 2012, the FDA issued a recommendation against the use of sildenafil in children ages 1–17 (http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm317743.htm). The decision was based on reports of increasing mortality with increasing sildenafil doses in a long-term clinical trial in pediatric patients with PH secondary to idiopathic pulmonary arterial hypertension and congenital heart disease.92 In 2014, the FDA issued a clarification of the statement (http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm391152.htm) stating there may be situations in which the benefit-risk profile of sildenafil may be acceptable in individual children, where treatment options are limited and close monitoring can be provided. Thus, careful consideration and extreme caution should be exercised when contemplating sildenafil treatment for infants and children. Other vasodilators including prostacyclin analogs, endothelin receptor antagonists, and soluble guanylate cyclase modulators could be considered for use in BPD infants with PH, but there are very limited data available examining the effectiveness and safety of these medications for this population.
Currently, there is limited evidence on how long to continue these therapies if initiated. Often, many infants are discharged from the neonatal intensive care unit on these medications and followed-up by pediatric pulmonologists, cardiologists, or centers focusing on pulmonary hypertension. If pulmonary hypertension gradually resolves with lung growth as expected, the medications may be either gradually tapered off, or the infant allowed to outgrow the dose before discontinuation of the drugs one by one (usually the most “invasive” or least effective medication is weaned off first).
BPD infants treated with PH medication require close monitoring of pulmonary and hemodynamic status. Objective assessments of oxygenation should be performed when weaning infants from oxygen therapy or when infants develop respiratory infection. Serial echocardiograms, which should be obtained at least every 2–4 weeks with the acute initiation of therapy and at 4–6 month intervals with stable disease is recommended. Brain natriuretic peptide (BNP) may augment monitoring,93 but evidence does not support its use in the absence of echocardiography and other monitoring modalities. Acute worsening of PH or failure to respond to therapy may reflect several factors, including late pulmonary vascular anatomic abnormalities. Repeat diagnostic procedures, including cardiac catheterization may be indicated for these patients (Figure 3). We recommend weaning medications with serial normal or near-normal echocardiogram findings over weeks with careful monitoring of pulmonary function and growth velocities.
Conclusion
PVD in preterm infants with BPD is characterized by altered lung vascular development, growth, structure, or function, which precedes the onset of measureable PH. PVD due to disruption of normal pulmonary vascular development in association with preterm birth is an important determinant of the pathobiology of BPD and contributes significantly to morbidity and mortality. Exposure to adverse stimuli during the antenatal and/or early postnatal periods impair normal pulmonary vascular development and creates and imbalance between risk and resiliency factors. Recent studies have revealed the magnitude of PH in preterm infants, but many aspects of PVD remain understudied, and ongoing investigations continue to explore risk factors, mechanisms of disease, and long term outcomes. Prospective studies are needed to definitively establish standardized clinical criteria for PVD and PH in BPD, and to determine the best methods for early diagnosis, risk stratification, and disease monitoring. Larger collaborative studies and improved clinical infrastructure to conduct these important investigations will provide answers to these critical questions.
Key points.
Pulmonary vascular disease (PVD) and cardiovascular abnormalities are an increasingly recognized component of bronchopulmonary dysplasia (BPD) and contributes significantly to morbidity and mortality.
Complex interactions between antenatal and postnatal factors contribute to impair normal pulmonary vascular signaling pathways, leading to altered growth, structure, and function of the developing pulmonary circulation after preterm birth.
The impairments of the developing pulmonary vasculature may result prolonged oxygen requirements, exaggerated effects of anatomical shunt lesions, exercise intolerance, altered pulmonary blood flow distribution in response to acute respiratory infections, and ultimately PH.
Several studies utilizing echocardiogram to screen preterm infants have determined the incidence of PH in BPD to be 16–25%, yet some cardiovascular abnormalities may be missed with echocardiogram and require cardiac catheterization.
Further studies are needed to determine the risk factors, mechanisms of disease, and long term outcomes, and to better define the clinical approach and treatment of PVD in BPD.
Acknowledgments
Funding source:
This work was supported in part by grants from the NHLBI HL085703 (P.M.M. and S.H.A.), HL068702 (S.H.A.), and U01 HL102235 (S.H.A.).
Footnotes
Disclosures:
The authors do not have any relationships to disclose.
The authors have no significant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967 Feb 16;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Furman L, Baley J, Borawski-Clark E, Aucott S, Hack M. Hospitalization as a measure of morbidity among very low birth weight infants with chronic lung disease. J Pediatr. 1996 Apr;128(4):447–452. doi: 10.1016/s0022-3476(96)70353-0. [DOI] [PubMed] [Google Scholar]
- 3.Smith VC, Zupancic JA, McCormick MC, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004 Jun;144(6):799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007 Nov 8;357(19):1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 5.Smith VC, Zupancic JA, McCormick MC, et al. Trends in severe bronchopulmonary dysplasia rates between 1994 and 2002. J Pediatr. 2005 Apr;146(4):469–473. doi: 10.1016/j.jpeds.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 6.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bancalari E, Gonzalez A. Clinical course and lung function abnormalities during development of neonatal chronic lung disease. In: Bland R, Coalson J, editors. Chronic lung disease in early infancy. New York: Marcel Dekker; 2000. pp. 41–64. [Google Scholar]
- 8.Charafeddine L, D’Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics. 1999 Apr;103(4 Pt 1):759–765. doi: 10.1542/peds.103.4.759. [DOI] [PubMed] [Google Scholar]
- 9.Hislop AA, Haworth SG. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol. 1990;9(3):152–161. doi: 10.1002/ppul.1950090306. [DOI] [PubMed] [Google Scholar]
- 10.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis”. Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 1):1755–1756. doi: 10.1164/ajrccm.164.10.2109111c. [DOI] [PubMed] [Google Scholar]
- 11.Abman SH. The dysmorphic pulmonary circulation in bronchopulmonary dysplasia: a growing story. Am J Respir Crit Care Med. 2008 Jul 15;178(2):114–115. doi: 10.1164/rccm.200804-629ED. [DOI] [PubMed] [Google Scholar]
- 12.Jobe AH. An Unknown: Lung Growth and Development after Very Preterm Birth. Am J Respir Crit Care Med. 2002;166(12):1529–1530. doi: 10.1164/rccm.2209012. [DOI] [PubMed] [Google Scholar]
- 13.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of Long-Term Sildenafil Treatment for Pulmonary Hypertension in Infants with Chronic Lung Disease. J Pediatr. 2009;154(3):379–384. e372. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical Utility of Echocardiography for the Diagnosis and Management of Pulmonary Vascular Disease in Young Children With Chronic Lung Disease. Pediatrics. 2008;121(2):317–325. doi: 10.1542/peds.2007-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010 Mar;40(3):131–136. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhat R, Salas AA, Foster C, Carlo WA, Ambalavanan N. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012 Mar;129(3):e682–689. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mourani PM, Sontag MK, Younoszai A, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015 Jan 1;191(1):87–95. doi: 10.1164/rccm.201409-1594OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary Artery Hypertension in Formerly Premature Infants With Bronchopulmonary Dysplasia: Clinical Features and Outcomes in the Surfactant Era. Pediatrics. 2007;120(6):1260–1269. doi: 10.1542/peds.2007-0971. [DOI] [PubMed] [Google Scholar]
- 19.Lassus P, Turanlahti M, Heikkila P, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 1):1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 20.D’Angio CT, Maniscalco WM. The role of vascular growth factors in hyperoxia-induced injury to the developing lung. Front Biosci. 2002 Jul 1;7:d1609–1623. doi: 10.2741/A865. [DOI] [PubMed] [Google Scholar]
- 21.Kumar VH, Ryan RM. Growth factors in the fetal and neonatal lung. Front Biosci. 2004 Jan 1;9:464–480. doi: 10.2741/1245. [DOI] [PubMed] [Google Scholar]
- 22.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007 May 15;175(10):978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002 Apr;282(4):L811–823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- 24.Bhandari V, Bizzarro MJ, Shetty A, et al. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006 Jun;117(6):1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 25.Bhandari V, Gruen JR. The genetics of bronchopulmonary dysplasia. Semin Perinatol. 2006 Aug;30(4):185–191. doi: 10.1053/j.semperi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. 2010;661:323–335. doi: 10.1007/978-1-60761-500-2_21. [DOI] [PubMed] [Google Scholar]
- 27.Cornfield DN. Developmental regulation of oxygen sensing and ion channels in the pulmonary vasculature. Adv Exp Med Biol. 2010;661:201–220. doi: 10.1007/978-1-60761-500-2_13. [DOI] [PubMed] [Google Scholar]
- 28.Jakkula M, Le Cras TD, Gebb S, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000 Sep;279(3):L600–607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 29.Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005 Oct 18;112(16):2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 30.Galambos C, Ng YS, Ali A, et al. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol. 2002 Aug;27(2):194–203. doi: 10.1165/ajrcmb.27.2.4703. [DOI] [PubMed] [Google Scholar]
- 31.De Paepe ME, Mao Q, Powell J, et al. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med. 2006 Jan 15;173(2):204–211. doi: 10.1164/rccm.200506-927OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janer J, Andersson S, Kajantie E, Lassus P. Endostatin concentration in cord plasma predicts the development of bronchopulmonary dysplasia in very low birth weight infants. Pediatrics. 2009;123(4):1142–1146. doi: 10.1542/peds.2008-1339. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell SH, Teague WG. Reduced gas transfer at rest and during exercise in school-age survivors of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1998 May;157(5 Pt 1):1406–1412. doi: 10.1164/ajrccm.157.5.9605025. [DOI] [PubMed] [Google Scholar]
- 34.Hakulinen AL, Jarvenpaa AL, Turpeinen M, Sovijarvi A. Diffusing capacity of the lung in school-aged children born very preterm, with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 1996 Jun;21(6):353–360. doi: 10.1002/(SICI)1099-0496(199606)21:6<353::AID-PPUL2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.Allen J, Zwerdling R, Ehrenkranz R, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003 Aug 1;168(3):356–396. doi: 10.1164/rccm.168.3.356. [DOI] [PubMed] [Google Scholar]
- 36.Coalson J. Pathology of new bronchopulmonary dysplasia. Seminars in Neonatology. 2003;8(1):73–81. doi: 10.1016/s1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 37.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998 Jul;29(7):710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 38.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001 Jun;163(7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 39.Galambos C, Sims-Lucas S, Abman SH. Histologic evidence of intrapulmonary anastomoses by three-dimensional reconstruction in severe bronchopulmonary dysplasia. Annals of the American Thoracic Society. 2013 Oct;10(5):474–481. doi: 10.1513/AnnalsATS.201305-124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mourani PM, Abman SH. Pulmonary Vascular Disease in Bronchopulmonary Dysplasia: Physiology, Diagnosis, and Treatment. In: Abman SH, editor. Bronchopulmonary Dysplasia. New York: Informa Healthcare; 2010. pp. 347–363. [Google Scholar]
- 41.Hansen AR, Barnes CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr. 2010 Apr;156(4):532–536. doi: 10.1016/j.jpeds.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Foidart JM, Schaaps JP, Chantraine F, Munaut C, Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia--a step forward but not the definitive answer. J Reprod Immunol. 2009 Nov;82(2):106–111. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Lapaire O, Shennan A, Stepan H. The preeclampsia biomarkers soluble fms-like tyrosine kinase-1 and placental growth factor: current knowledge, clinical implications and future application. Eur J Obstet Gynecol Reprod Biol. 2010 Aug;151(2):122–129. doi: 10.1016/j.ejogrb.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH. Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012 Jan 1;302(1):L36–46. doi: 10.1152/ajplung.00294.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li F, Hagaman JR, Kim HS, et al. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J Am Soc Nephrol. 2012 Apr;23(4):652–660. doi: 10.1681/ASN.2011040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozance PJ, Seedorf GJ, Brown A, et al. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011 Dec;301(6):L860–871. doi: 10.1152/ajplung.00197.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Check J, Gotteiner N, Liu X, et al. Fetal growth restriction and pulmonary hypertension in premature infants with bronchopulmonary dysplasia. J Perinatol. 2013 Jan 17; doi: 10.1038/jp.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts RJ, Weesner KM, Bucher JR. Oxygen-induced alterations in lung vascular development in the newborn rat. Pediatr Res. 1983 May;17(5):368–375. doi: 10.1203/00006450-198305000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Nozik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Adv Exp Med Biol. 2007;618:101–112. doi: 10.1007/978-0-387-75434-5_8. [DOI] [PubMed] [Google Scholar]
- 50.Jones R, Zapol WM, Reid L. Pulmonary artery remodeling and pulmonary hypertension after exposure to hyperoxia for 7 days. A morphometric and hemodynamic study. Am J Pathol. 1984 Nov;117(2):273–285. [PMC free article] [PubMed] [Google Scholar]
- 51.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 1):1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 52.De Paepe ME, Greco D, Mao Q. Angiogenesis-related gene expression profiling in ventilated preterm human lungs. Exp Lung Res. 2010 Sep;36(7):399–410. doi: 10.3109/01902141003714031. [DOI] [PubMed] [Google Scholar]
- 53.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O’Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011 Jun;178(6):2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong PM, Lees AN, Louw J, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008 Aug;32(2):321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 55.Walther FJ, Benders MJ, Leighton JO. Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics. 1992 Dec;90(6):899–904. [PubMed] [Google Scholar]
- 56.Fouron JC, Le Guennec JC, Villemant D, Perreault G, Davignon A. Value of echocardiography in assessing the outcome of bronchopulmonary dysplasia of the newborn. Pediatrics. 1980 Mar;65(3):529–535. [PubMed] [Google Scholar]
- 57.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984 Oct;70(4):657–662. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 58.Berger M, Haimowitz A, Van Tosh A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. J Am Coll Cardiol. 1985 Aug;6(2):359–365. doi: 10.1016/s0735-1097(85)80172-8. [DOI] [PubMed] [Google Scholar]
- 59.Currie PJ, Seward JB, Chan KL, et al. Continuous wave Doppler determination of right ventricular pressure: a simultaneous Doppler-catheterization study in 127 patients. J Am Coll Cardiol. 1985 Oct;6(4):750–756. doi: 10.1016/s0735-1097(85)80477-0. [DOI] [PubMed] [Google Scholar]
- 60.Skjaerpe T, Hatle L. Noninvasive estimation of systolic pressure in the right ventricle in patients with tricuspid regurgitation. Eur Heart J. 1986 Aug;7(8):704–710. doi: 10.1093/oxfordjournals.eurheartj.a062126. [DOI] [PubMed] [Google Scholar]
- 61.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011 Oct;31(10):635–640. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 62.Benatar A, Clarke J, Silverman M. Pulmonary hypertension in infants with chronic lung disease: non-invasive evaluation and short term effect of oxygen treatment. Arch Dis Child Fetal Neonatal Ed. 1995 Jan;72(1):F14–19. doi: 10.1136/fn.72.1.f14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101(1):40–46. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 64.Czernik C, Rhode S, Metze B, Schmalisch G, Buhrer C. Persistently elevated right ventricular index of myocardial performance in preterm infants with incipient bronchopulmonary dysplasia. PLoS One. 2012;7(6):e38352. doi: 10.1371/journal.pone.0038352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kobr J, Fremuth J, Pizingerova K, et al. Repeated bedside echocardiography in children with respiratory failure. Cardiovasc Ultrasound. 2011;9:14. doi: 10.1186/1476-7120-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirza H, Ziegler J, Ford S, Padbury J, Tucker R, Laptook A. Pulmonary hypertension in preterm infants: prevalence and association with bronchopulmonary dysplasia. J Pediatr. 2014 Nov;165(5):909–914. e901. doi: 10.1016/j.jpeds.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 67.Stuart BD, Sekar P, Coulson JD, Choi SE, McGrath-Morrow SA, Collaco JM. Health-care utilization and respiratory morbidities in preterm infants with pulmonary hypertension. J Perinatol. 2013 Jan 17; doi: 10.1038/jp.2012.170. [DOI] [PubMed] [Google Scholar]
- 68.Skinner JR, Boys RJ, Hunter S, Hey EN. Pulmonary and systemic arterial pressure in hyaline membrane disease. Arch Dis Child. 1992 Apr;67(4 Spec):366–373. doi: 10.1136/adc.67.4_spec_no.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brown ER, Stark A, Sosenko I, Lawson EE, Avery ME. Bronchopulmonary dysplasia: possible relationship to pulmonary edema. J Pediatr. 1978 Jun;92(6):982–984. doi: 10.1016/s0022-3476(78)80382-5. [DOI] [PubMed] [Google Scholar]
- 70.Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G. Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr. 1995 Apr;126(4):605–610. doi: 10.1016/s0022-3476(95)70362-4. [DOI] [PubMed] [Google Scholar]
- 71.Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr. 1996 Apr;128(4):470–478. doi: 10.1016/s0022-3476(96)70356-6. [DOI] [PubMed] [Google Scholar]
- 72.Kim D-H, Kim H-S, Choi CW, Kim E-K, Kim BI, Choi J-H. Risk Factors for Pulmonary Artery Hypertension in Preterm Infants with Moderate or Severe Bronchopulmonary Dysplasia. Neonatology. 2012;101(1):40–46. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 73.Drossner DM, Kim DW, Maher KO, Mahle WT. Pulmonary vein stenosis: prematurity and associated conditions. Pediatrics. 2008 Sep;122(3):e656–661. doi: 10.1542/peds.2008-0075. [DOI] [PubMed] [Google Scholar]
- 74.Lewandowski AJ, Bradlow WM, Augustine D, et al. Right ventricular systolic dysfunction in young adults born preterm. Circulation. 2013 Aug 13;128(7):713–720. doi: 10.1161/CIRCULATIONAHA.113.002583. [DOI] [PubMed] [Google Scholar]
- 75.Mourani PM, Ivy DD, Rosenberg AA, Fagan TE, Abman SH. Left ventricular diastolic dysfunction in bronchopulmonary dysplasia. J Pediatr. 2008 Feb;152(2):291–293. doi: 10.1016/j.jpeds.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yates AR, Welty SE, Gest AL, Cua CL. Myocardial Tissue Doppler Changes in Patients with Bronchopulmonary Dysplasia. The Journal of Pediatrics. 2008;152(6):766–770. e761. doi: 10.1016/j.jpeds.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 77.Del Cerro MJ, Sabate Rotes A, Carton A, et al. Pulmonary hypertension in bronchopulmonary dysplasia: Clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014 Jan;49(1):49–59. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 78.Vonk-Noordegraaf A, van Wolferen SA, Marcus JT, et al. Noninvasive assessment and monitoring of the pulmonary circulation. Eur Respir J. 2005 Apr;25(4):758–766. doi: 10.1183/09031936.05.00122104. [DOI] [PubMed] [Google Scholar]
- 79.Ley S, Mereles D, Risse F, et al. Quantitative 3D pulmonary MR-perfusion in patients with pulmonary arterial hypertension: correlation with invasive pressure measurements. Eur J Radiol. 2007 Feb;61(2):251–255. doi: 10.1016/j.ejrad.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 80.Okajima Y, Ohno Y, Washko GR, Hatabu H. Assessment of pulmonary hypertension what CT and MRI can provide. Academic radiology. 2011 Apr;18(4):437–453. doi: 10.1016/j.acra.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Skrok J, Shehata ML, Mathai S, et al. Pulmonary arterial hypertension: MR imaging-derived first-pass bolus kinetic parameters are biomarkers for pulmonary hemodynamics, cardiac function, and ventricular remodeling. Radiology. 2012 Jun;263(3):678–687. doi: 10.1148/radiol.12111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ley S, Mereles D, Puderbach M, et al. Value of MR phase-contrast flow measurements for functional assessment of pulmonary arterial hypertension. European radiology. 2007 Jul;17(7):1892–1897. doi: 10.1007/s00330-006-0559-9. [DOI] [PubMed] [Google Scholar]
- 83.Nyp M, Sandritter T, Poppinga N, Simon C, Truog WE. Sildenafil citrate, bronchopulmonary dysplasia and disordered pulmonary gas exchange: any benefits? J Perinatol. 2012 Jan;32(1):64–69. doi: 10.1038/jp.2011.131. [DOI] [PubMed] [Google Scholar]
- 84.Robbins IM, Moore TM, Blaisdell CJ, Abman SH. Improving outcomes for pulmonary vascular disease. Am J Respir Crit Care Med. 2012 May 1;185(9):1015–1020. doi: 10.1164/rccm.201201-0049WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abman SH, Kinsella JP, Rosenzweig EB, et al. Implications of the FDA Warning Against the Use of Sildenafil for the Treatment of Pediatric Pulmonary Hypertension. Am J Respir Crit Care Med. 2012 Dec 6; doi: 10.1164/rccm.201210-1928PP. [DOI] [PubMed] [Google Scholar]
- 86.Ballard RA, Truog WE, Cnaan A, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006 Jul 27;355(4):343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 87.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003 Nov 27;349(22):2099–2107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 88.Kinsella JP, Cutter GR, Walsh WF, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006 Jul 27;355(4):354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 89.Channick RN, Newhart JW, Johnson FW, et al. Pulsed delivery of inhaled nitric oxide to patients with primary pulmonary hypertension: an ambulatory delivery system and initial clinical tests. Chest. 1996 Jun;109(6):1545–1549. doi: 10.1378/chest.109.6.1545. [DOI] [PubMed] [Google Scholar]
- 90.Ivy DD, Parker D, Doran A, Kinsella JP, Abman SH. Acute hemodynamic effects and home therapy using a novel pulsed nasal nitric oxide delivery system in children and young adults with pulmonary hypertension. Am J Cardiol. 2003 Oct 1;92(7):886–890. doi: 10.1016/s0002-9149(03)00910-x. [DOI] [PubMed] [Google Scholar]
- 91.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004 Nov 1;170(9):1006–1013. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 92.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation. 2012 Jan 17;125(2):324–334. doi: 10.1161/CIRCULATIONAHA.110.016667. [DOI] [PubMed] [Google Scholar]
- 93.Ambalavanan N, Mourani P. Pulmonary hypertension in bronchopulmonary dysplasia. Birth defects research. Part A, Clinical and molecular teratology. 2014 Mar;100(3):240–246. doi: 10.1002/bdra.23241. [DOI] [PubMed] [Google Scholar]
- 94.Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003 Sep;23(6):451–456. doi: 10.1038/sj.jp.7210963. [DOI] [PubMed] [Google Scholar]
- 95.Mourani PM, Abman SH. Pulmonary vascular disease in bronchopulmonary dysplasia: physiology, diagnosis, and treatment. In: Abman SH, editor. Bronchopulmonary Dysplasia. New York: Informa; 2010. pp. 347–363. [Google Scholar]