Abstract
Radiotherapy is one of the most common treatments for head and neck cancers, with an almost obligate side effect of altered taste (Conger AD. 1973. Loss and recovery of taste acuity in patients irradiated to the oral cavity. Radiat Res. 53:338–347.). In mice, targeted irradiation of the head and neck causes transient repression of proliferation of basal epithelial cells responsible for taste cell replacement, leading to a temporary depletion of taste sensory cells within taste buds, including Type II taste cells involved in detection of sweet stimuli (Nguyen HM, Reyland ME, Barlow LA. 2012. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 32:3474–3484.). These findings suggest that irradiation may elevate sucrose detection thresholds, peaking at 7 days postirradiation when loss of Type II cells is greatest. To test this hypothesis, sucrose detection thresholds (concentration detected in 50% of presentations) were measured in mice for 15 days after treatment of: 1) irradiation while anesthetized, 2) anesthetic alone, or 3) saline. Mice were trained to distinguish water from several concentrations of sucrose. Mice were irradiated with one 8 Gy dose (RADSOURCE-2000 X-ray Irradiator) to the nose and mouth while under 2,2,2-tribromethanol anesthesia (Avertin). Unexpectedly, mice given anesthesia showed a small elevation in sucrose thresholds compared to saline-injected mice, but irradiated mice show significantly elevated sucrose thresholds compared to either control group, an effect that peaked at 6–8 days postirradiation. The timing of loss and recovery of sucrose sensitivity generally coincides with the reported maximal reduction and recovery of Type II taste cells (Nguyen HM, Reyland ME, Barlow LA. 2012. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 32:3474–3484.). Thus, even a single dose of irradiation can significantly alter detection of carbohydrates, an important consideration for patients undergoing radiotherapy.
Keywords: anesthetic, radiation, sucrose, taste
Introduction
Radiotherapy for cancer has been in use for well over a century (Wehner et al. 1899; Bryant 1919). An almost obligate side effect observed among radiation patients undergoing head and/or neck irradiation is an altered sense of taste (Lindemann 1949; Mossman and Henkin 1978; Epstein et al. 1999; Sandow et al. 2006). Quantitative investigations of these side effects reveal a severely altered sense of taste for all basic tastes (Conger 1973; Mossman and Henkin 1978). One of the first quantitative investigations found that good tasters (high sensitivity) showed greater losses in taste sensitivity after radiotherapy than patients who already had poor taste sensitivity (Conger 1973). Taste complications are reported as some of the most troubling side effects of radiotherapy, impacting the quality of patient care (Rose-Ped et al. 2002). Some loss of taste sensitivity may be due to xerostomia because of damage to salivary glands (Lindemann 1949; Conger 1973; Mossman and Henkin 1978; Dhanani and Jiang 2012). It is also possible that irradiation-induced losses in taste sensitivity may be due to postirradiation changes in the cellular populations of taste buds.
Taste buds are composed primarily of 3 types of taste sensory cells (TSCs): Type I, Type II and Type III (Roper 1989). Type I TSCs are thought to be supporting cells which may be involved in salt taste (Vandenbeuch et al. 2008). Sweet, bitter, and umami tastes are detected by Type II TSCs (Tomchik et al. 2007), and sour taste is detected by Type III TSCs (Ishimaru et al. 2006). The prototypical sweet taste is elicited by sucrose, and is detected by the T1R2+T1R3 heterodimer, expressed in Type II TSCs (Nelson et al. 2001). Each type of TSC has a relatively short life span. For example, Type II TSCs have a half-life of 8–12 days before dying and being replaced with younger TSCs (Perea-Martinez et al. 2013). The need for cellular replacement is met by proliferating cells in a basal layer just ventral to taste buds (Okubo et al. 2009), but these proliferating cells are susceptible to factors such as chemotherapy drugs (Mukherjee and Delay 2011) or irradiation which can damage or kill cells engaged in the cell cycle. Nguyen et al. (2012) found that an 8 Gy dose of radiation caused a transient repression of proliferation of basal epithelial cells from day 2–4 postirradiation, interrupting the flow of new cells which normally would replace mature taste cells reaching the end of their life span. This transient interruption of new cell supply causes a transient but delayed reduction in Type II TSCs in circumvallate taste buds that lasts until proliferation reinitiates and a wave of new, mature TSCs are integrated into taste buds such that normal Type II TSC number is attained by 10 days after irradiation.
Using this model, we postulated that a disturbance of the cellular aspects of the taste system of this magnitude and duration should induce a transient disruption in taste function. To test this hypothesis, we investigated the effects of irradiation on sweet taste of mice using an operant discrimination assay to see if the extent and timing of behavioral effects of radiotherapy matched those of the documented cellular effects. We hypothesized that disruptions in taste function would coincide with the temporal pattern described by Nguyen et al. (2012). That is, the greatest disruption to sucrose sensitivity should occur around 7 days postirradiation when the number of Type II cells is significantly reduced, followed by recovery of sensitivity as Type II cell complement recovers. To test this hypothesis, sucrose detection thresholds were tested in mice that were irradiated or not irradiated.
Methods
Animals
The subjects of this study were 13 male 8-week-old C57BL/6J (Stock No: 000664; https://www.jax.org/strain/000664) mice obtained from Jackson Laboratory. All mice were housed in groups of 3–4 per cage. The mice were kept on a 22-hour water deprivation schedule, and given ad libitum access to Purina Mouse Chow RMH 3000. All procedures were approved by the University of Vermont IACUC under protocol 10–065.
Apparatus
Computer-controlled gustometers (Knosys Inc.) were used to test sucrose thresholds (Brosvic and Slotnick 1986; Mukherjee and Delay 2011). The gustometers consisted of a chamber 17 cm high, 12 cm long, and 12 cm wide with a removable side door. A fan was mounted in the ceiling for positive pressure airflow into the operant chamber. At one end of the chamber, a 1 cm circular opening was centered 2 cm above the floor. Taste stimuli were delivered via a stainless steel lick spout (outer diameter [O.D.]: 3.4 mm, inner diameter [I.D.]: 2.7 mm) that was accessible through the opening. This lick spout had 9 smaller, stainless steel capillary tubes (22 ga., O.D.: 0.715 mm, I.D.: 0.507 mm) within it, all of which were recessed 2 mm from the tip of the lick spout. Eight of the capillary tubes delivered stimulus solutions to the end of the lick spout and the ninth delivered water reinforcement. Each tube was connected by C-flex capillary tubing (ID: 0.031 in; #06424-60; Cole-Parmer) to a 3 mL syringe barrel in which a stimulus solution was stored. All syringe barrels were mounted 7.5 cm above the lick spout on racks facing away from the operant chamber, which minimized visual cues. Pinch Valves (P/N 075P2-S1013; Bio-Chem Fluidics Inc.) kept the capillary tubing from dispensing water or sucrose solution until opened by the computer. Although these valves are designed to operate quietly, an independent solenoid was used to further mask the opening and closing of the individual pinch valves. Olfactory cueing was minimized as much as possible by the ceiling fan blowing air through the chamber and out of the lick spout hole, the small sample sizes, and the recessed delivery tubes within the lick spout.
When the mouse made contact with the lick spout, a circuit was completed with a stainless steel grate on the floor of the cage, allowing a 60 µA current to pass through the circuit. The stainless steel grate was placed to ensure the mouse could only lick while standing on it. Above the lick spout was a Piezo buzzer (Jameco Electronics) which produced a continuous 2.9 kHz tone inside the testing chamber at 80–90 dB when activated.
Irradiation procedure
Mice (n = 6) assigned to the irradiation condition were anesthetized with 2,2,2-tribromoethanol (TBE), commonly known as Avertin (intraperitoneal [IP], 250 mg/kg; Prod. Num. T48405, Sigma-Aldrich). Three control mice were anesthetized with TBE and 4 control mice were given an equal volume injection of saline (IP, 0.9%, Hospira Inc.). Once a mouse was anesthetized with TBE, it was inserted head-first into a plastic 50 mL centrifuge tube with the tip of the beveled end cut to create a 1 cm diameter opening to allow the mouse to breathe normally. The space behind the mouse was packed with gauze and the cap for the tube was screwed on to ensure the mouse’s head remained in position. Lead shielding was wrapped around the tube in a manner that left only the head and neck exposed to radiation (see Nguyen et al. (2012)). The single 8 Gy dose of X-ray irradiation was performed in a Rad Source model RS2000 irradiator with a 0.3 mm copper filter and X-ray tube settings of 160 kVp and 24 mA (Rad Source Technologies). Calibration of the irradiator was verified by Radiation Safety Office at the University of Vermont.
General procedure
Thresholds were tested using well established methods (Stapleton et al. 2002; Delay et al. 2006; Mukherjee and Delay 2013). After training to lick the lick spout to obtain water, mice were then trained to discriminate between sucrose and water solutions using a discrete trial procedure. Each trial began with the presentation of 7 µL of the taste stimulus to the end of the lick tube, followed by a 2-s period (response interval) during which the mouse had to identify the stimulus and modify its behavior, and ended with the response consequence. In the initial 1.6 s of the response interval, the mouse had to determine if the taste stimulus was an S+ (water) or an S– (sucrose) solution. Lick responses during the last 0.4 s of the response interval determined which of 4 possible responses, each associated with a specific consequence, was emitted by the mouse. If the taste stimulus was water, the mouse had to lick the tube during the last 0.4 s of the 2-s period to receive an additional 10 µL water reinforcement (correct detection or hit), but if the mouse did not lick during the last 0.4 s (an incorrect response or miss), an 85+/−5 dB tone punisher was presented simultaneously with a time out period, both lasting 10 s. If the test stimulus was a sucrose solution, and the mouse did not lick during the last 0.4 s of the response interval, the tone and time out were avoided and a correct response (correct rejection) was registered but if it licked during the last 0.4 s (false alarm), then the tone and the timeout were presented. At the end of each trial, a 10 s intertrial interval occurred before the mouse encountered a variable ratio 18 schedule in which the mouse had to lick the spout a random number (computer determined) of times between 3 and 33. Once this schedule was met, a 5 µL water solution was presented to cleanse its palate and to encourage continued licking. A second variable ratio 18 schedule then had to be completed to initiate the next trial. A test session ended after the animal completed 150 trials or an hour had elapsed, whichever occurred first. Mice began discrimination training with 300 mM sucrose and water solutions. After 1–2 days of training, the next lower concentration of sucrose was added. This process was continued until the final range of 0.1, 2.5, 25, 50, 100, and 175 mM sucrose solutions was presented. Concentrations were chosen based on a log scale from reported sucrose thresholds in mice of around 2.5 mM (Delay et al. 2006). The order of sucrose concentration presentations followed a Latin square procedure. Sucrose trials were interspersed with water trials such that sucrose solutions were presented on an average of 50% of the trials during a session.
Once the mice were trained and their threshold estimates were stable, they were tested for 3 sessions to determine the pretreatment threshold for each mouse. Pairs of mice were then matched for their threshold estimates and randomly assigned to one of the control groups or the irradiation group. All mice were taken off water deprivation after their last session prior to their assigned treatment condition and returned to their schedule 24 h after treatment. Threshold testing restarted 48 h after their assigned treatment and continued daily for 14 days.
Statistical methods
One IR-treated mouse appeared unable to reliably detect even the highest concentration of sucrose after treatment. Since post-treatment thresholds could not be estimated, it was dropped from the experiment and the size of the irradiation group was reduced to n = 5 for all analyses. The data were evaluated with analysis of variance (ANOVA) procedures to determine if irradiation affected sucrose thresholds by evaluating treatment groups (3 levels) as a between subject variable and days (14 levels) as a repeated measures variable. Treatment groups included mice injected with saline, those anesthetized with TBE, and those anesthetized and exposed to 8 Gy irradiation. Sucrose thresholds served as the primary dependent variable and were determined daily for each mouse throughout the experiment. A threshold was defined as the lowest concentration of sucrose that a mouse identified 50% of the time. Since the group sizes were relatively small and mice showed some differences in skill levels, differences between pre- and post-treatment thresholds of each mouse were also evaluated. Difference scores were computed by subtracting the mean of 3 pretreatment thresholds from the daily post-treatment threshold values. Simple effects tests with type I error rates corrected were used to examine group differences each day postirradiation (Howell 2016), followed by post hoc testing with alpha corrected to p < 0.05 using Sidak corrections. Because group sizes were small and response variance of the irradiated group was greater than the other 2 groups, additional analyses using Kruskal–Wallis and Friedman nonparametric procedures were applied but they revealed nearly identical findings. Consequently, only the parametric statistics are reported here. Finally, to examine potential changes in response patterns, the proportion of hits and false alarms were computed against the number of S+ or S− trials, respectively, presented in each test session. Data for each measure were analyzed with separate ANOVA procedures for mixed designs. All statistical tests were performed with SPSS version 24.0 (IBM Software) and the graph made with GraphPad Prism 7 (GraphPad Software Inc.).
Results
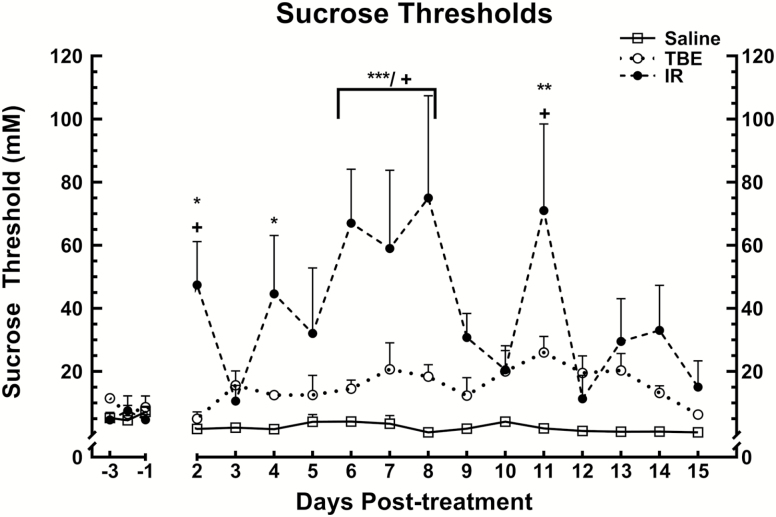
There were no significant group differences in pretreatment sucrose thresholds. Mice in the saline group had sucrose thresholds consistently around 1–3 mM over the course of the study. These thresholds, and the pretreatment thresholds for all of the mice, are comparable to sucrose thresholds reported for C57BL/6J mice (Delay et al. 2006; Mukherjee et al. 2013) and Sprague-Dawley rats (Sclafani and Nissenbaum 1987; Bachmanov et al. 2001; Stapleton et al. 2002).The mixed ANOVA of the postirradiation sucrose thresholds found a significant main effect for treatment condition, F(2, 44) = 7.676, P < 0.0005. Neither the main effect of days post-treatment nor the interaction between days and groups was significant. Simple effects tests revealed that TBE-treated mice showed a small but significant increase in thresholds compared to saline-treated mice, F(1, 22) = 19.523, P < 0.0005. However, irradiated mice had significantly higher thresholds than either saline-treated or TBE-treated mice, F(1, 29) = 41.881, P < 0.0005 and F(1, 26) = 8.648, P = 0.007, respectively. The data were then partitioned to compare the thresholds of all 3 groups each day post-treatment using simple effects tests followed by post hoc tests (Howell 2016). The simple effects tests found significant group effects, F(2, 137) ≥ 3.321, P < 0.039 or less, on days 2, 4, 6, 7, 8, and 11 post-treatment. Alpha-corrected t-tests revealed that the irradiation treated mice had significantly higher sucrose thresholds on days 2, 6, 7, 8, and 11 post-treatment when compared to saline- and TBE-injected animals (P < 0.05 or less, Figure 1; Table 1). These differences were greatest 8 days post-treatment (saline group [mean threshold ± SEM]: 0.67 ± 0.35 mM; TBE group: 18.32 ± 3.82 mM; irradiation group: 75 ± 32.44 mM sucrose). The irradiation-treated mice also showed significantly increased thresholds when compared to the saline (but not TBE) animals 4 days post-treatment (P < 0.05, Figure 1; Table 1).
Figure 1.
Sucrose thresholds after treatment with saline, TBE or 8 Gr irradiation. The x axis represents days relative to treatment, with the day of treatment being zero. The y axis of the graph is the threshold of the mice (mM sucrose). The group of mice that received radiation treatment had significantly higher thresholds (P < 0.05) than saline controls on days 2, 4, 6, 7, 8, and 11 post-treatment. The irradiation group also had significantly higher thresholds (P < 0.05) than TBE mice on days 2, 6, 7, 8, and 11 post-treatment. There were no significant differences between the saline and anesthetic-only groups, however the anesthesia alone appeared to cause a minor, but sustained increase in thresholds. * represents significant comparisons between irradiation and saline groups, + significant comparisons between irradiation and TBE groups. * or + P < 0.05; **P < 0.01; ***P < 0.001.
Table 1.
Summary of alpha levels of post hoc tests comparing thresholds of treatment group over days
| Day | Radiation vs. Saline | Radiation vs. Avertin | Avertin vs. Saline |
|---|---|---|---|
| -3 | P = 0.998 | P = 0.886 | P = 0.888 |
| -2 | P = 0.907 | P = 0.951 | P = 0.907 |
| -1 | P = 0.742 | P = 0.851 | P = 0.908 |
| 2 | P = 0.023* | P = 0.048* | P = 0.908 |
| 3 | P = 0.621 | P = 0.726 | P = 0.470 |
| 4 | P = 0.014* | P = 0.085 | P = 0.593 |
| 5 | P = 0.098 | P = 0.085 | P = 0.593 |
| 6 | P = 0.004* | P = 0.032* | P = 0.563 |
| 7 | P = 0.001* | P = 0.028* | P = 0.358 |
| 8 | P = 0.004* | P = 0.029* | P = 0.653 |
| 9 | P = 0.098 | P = 0.252 | P = 0.663 |
| 10 | P = 0.123 | P = 0.476 | P = 0.472 |
| 11 | P < 0.0005* | P = 0.035* | P = 0.224 |
| 12 | P = 0.931 | P = 0.911 | P = 0.976 |
*Significant difference.
When evaluating post-pre-differences, the mixed ANOVA found a significant main effect for treatment condition, F(2, 38) = 9.597, P < 0.0005. The saline-injected mice showed little change in threshold estimates post-treatment. Compared to the saline group, the TBE mice showed a small but significant elevation in post-pre-difference after their treatment, F(1, 14) = 16.309, P = 0.001. ANOVAs also found that the irradiation group had significantly greater increases in thresholds than both control groups (saline; F(1, 26) = 27.699, P < 0.0005; TBE group F(1, 22) = 10.058, P = 0.004). Simple effect testing of treatment condition by day revealed significant group differences in thresholds on post-treatment days 2, 4, 6, 7, 8, and 11, F(2, 11) ≥ 3.097, P < 0.049 (Table 2). Again, these differences were greatest 8 days post-treatment (saline group [mean difference score ± SEM]: −4.976 ± 13.764 mM; TBE group: 9.444 ± 15.894 mM; irradiation group: 69.932 ± 12.311 mM sucrose). The irradiation group also had significantly larger post-pre-differences in thresholds on day 4 when compared to the saline injected mice (Table 2).
Table 2.
Summary of alpha levels of post hoc tests of difference scores for the treatment condition by day interaction
| Day | Radiation vs. Saline | Radiation vs. Avertin | Saline vs. Avertin |
|---|---|---|---|
| 2 | P = 0.015* | P = 0.025* | P = 0.998 |
| 3 | P = 0.647 | P = 0.933 | P = 0.630 |
| 4 | P = 0.022* | P = 0.081 | P = 0.720 |
| 5 | P = 0.132 | P = 0.260 | P = 0.803 |
| 6 | P = 0.001* | P = 0.006* | P = 0.735 |
| 7 | P = 0.003* | P = 0.041* | P = 0.507 |
| 8 | P < 0.0005* | P = 0.004* | P = 0.494 |
| 9 | P = 0.120 | P = 0.283 | P = 0.731 |
| 10 | P = 0.375 | P = 0.851 | P = 0.548 |
| 11 | P < 0.0005* | P = 0.018* | P = 0.324 |
| 12 | P = 0.580 | P = 0.809 | P = 0.473 |
*Significant difference.
To determine if the mice might have altered their response patterns or strategies following their treatment, the percent of hits (identifying water when water [S+] was presented) and false alarms (responding as if water was detected when sucrose [S−] was present) were evaluated. Evaluation of pretreatment percentages did not find group differences for either measure. The percentage scores for hits were analyzed using ANOVA procedures with treatment groups as a between subject variable and days as a with-subject variable. The most notable finding was a significant treatment effect, F(2,25) = 3.345, P = 0.048. Post hoc t-tests revealed that the TBE group had significantly lower hit rates (mean P(Hit) ± SEM = 81% ± 2.5%) than either control (88% ± 2.5%) or irradiation (86% ± 2.5%) groups. The percentages for false alarms were analyzed for treatment, days, and concentration effects. This analysis found significant group differences, F(2,187) = 12.787, P < 0.0005, and post hoc testing indicated that irradiation mice made significantly more false alarms (mean ± SEM = 53% ± 1.9%) than control (44% ± 2.1%) or TBE mice (38% ± 2.4%). This ANOVA also detected a significant interaction between treatment and concentration, F(10,208) = 3.124, P < 0.001. Further evaluation revealed that the TBE group made fewer false alarms for the 100 and 175 mM sucrose concentrations than the other 2 groups (P < 0.05).
Discussion
Sucrose thresholds of irradiated mice were significantly elevated compared to either saline control or TBE control mice. Mice in the saline group had sucrose thresholds consistently around 0.5–3 mM over the course of the study. These thresholds, and the pretreatment thresholds for all of the mice, are comparable to sucrose thresholds reported for C57BL/6J mice (Delay et al. 2006; Mukherjee et al. 2013) and Sprague-Dawley rats (Sclafani and Nissenbaum 1987; Bachmanov et al. 2001; Stapleton et al. 2002). In contrast, irradiated mice had elevated thresholds immediately after irradiation treatment and beginning postirradiation day 4 their thresholds gradually increased to above 60 mM 6–8 days postirradiation, then began gradually to decrease over the rest of the experimental period.
In our previous study (Nguyen et al. 2012), we showed that a single dose of 8 Gy irradiation leads to a transient reduction of Type II TSCs, a subset of which detect sweet taste stimuli. Consequently, we hypothesized that irradiation would adversely affect sucrose detection thresholds. Whereas both control groups exhibited relatively consistent thresholds post-treatment, thresholds of irradiated mice changed in a more phasic manner with the most severe increases in thresholds on days 6–8 and 11 postirradiation, and smaller increases on days 2 and 4. The timing of some of the major disturbances matched the temporal interval proposed for replacement of Type II TSCs in mice after a single dose of 8 Gy. Specifically, in Nguyen et al. (2012), we showed that an 8 Gy dose, administered in the same manner and with the same model of irradiator as this experiment, caused a rapid interruption in progenitor proliferation (within 2 days of treatment), while the number of mature Type II TSCs in circumvallate taste buds was unchanged early on. Because it takes 5 days for progenitor proliferation to return to control levels, this results in a transient interruption of new cells. Thus, when functional TSCs die at the end of their life cycle, and are temporarily not replaced, we postulate that there are not enough mature TSCs to maintain normal taste functions until cell renewal as recommenced and new cells mature. The population of mature sucrose-detecting Type II cells that persist following irradiation, gradually decrease and are lowest at 7 days (Nguyen et al. 2012), consistent with the 6–8-day window within which sucrose thresholds were elevated in this behavior study.
Importantly, Nguyen et al. (2012) developed this cellular model by evaluating the effects of irradiation on circumvallate papillae. When considering the impact of irradiation on sweet taste in this study, it is important to note that T1R3+T1R2 expressing Type II TSCs are most dense in fungiform papillae (Hoon et al. 1999) which were also exposed to irradiation in this experiment. Work with a chemotherapy drug, cyclophosphamide, has shown that TSCs in fungiform papillae are more sensitive than cells in circumvallate taste buds to the alkylating effects of the drug (Mukherjee and Delay 2011; Mukherjee et al. 2013). It is possible that the magnitude and temporal patterns of the shifts in behavioral thresholds were enhanced by the effects of irradiation on fungiform papillae. Further investigation into the effects of irradiation on fungiform papillae is needed.
The elevations in thresholds on day 2 and 11 suggest that there may be other factors that contributed to the elevated sucrose thresholds of the irradiated mice. For example, some of the shift in thresholds, especially those observed shortly after irradiation (e.g., postirradiation day 2), may be mediated by inflammation of the lingual epithelium induced by radiotherapy. Inflammation of the taste system has been reported to occur within a 48-h window after an insult and can disrupt normal functioning of taste buds or neurites synapsing with taste buds (Wang et al. 2009). Other factors such as mucositis or xerostomia are often seen after irradiation. However, Nguyen et al. (2012) did not observe evidence of mucositis or xerostomia with the same procedure and radiation dose. Irradiation may also have injured the brain, as only the body was shielded during exposure. It has been shown that radiation can facilitate the development of a conditioned taste aversion to sweet tastes, especially if the irradiation involves the whole body (Garcia and Koelling 1966). However, this was unlikely in this study since the mice had extensive exposure to sucrose during training prior to irradiation, making the development of a conditioned aversion highly unlikely. Irradiation can also repress hippocampal neurogenesis (Brown et al. 2010), although there is little evidence that radiation treatment disrupts well-learned operant behavior in rodents. Damage to olfactory tissues may also contribute, as rodents are able to use olfactory cues to discriminate between sucrose solutions (Rhinehart-Doty et al. 1994). Despite the olfactory controls in place, odor cues might have been used in part to perform the discrimination task in this study. If so, this would be more properly assessed by olfactory threshold testing as there is evidence that olfactory epithelium is susceptible to irradiation damage at the 8 Gy dose used in this experiment (Ophir et al. 1988; Cunha et al. 2012). The spike in threshold increases on day 11 also suggests there may be other mechanisms involving cell renewal which need further exploration.
Even though the anesthesia control group (TBE injections) had thresholds comparable to saline mice prior to treatment, they exhibited a small, gradual, post-treatment increase in sucrose thresholds to 5–10 mM that remained elevated over the remainder of the study. This was an unexpected finding since a recent report indicated that a dose nearly double the dose used in this study had little effect on weight, or water or food intake after a single IP injection in C57BL/6NHsd mice (Hill et al. 2013). In addition, a pilot study comparing TBE with several other anesthetics suggested that TBE induced the least amount of cell death in the taste epithelium assayed 24 h postanesthetic (Ross B, Barlow L, unpublished data). While TBE was selected because of its apparent minimal effect on taste tissues, TBE may have side-effects that could have altered motivational states during threshold testing. For example, IP injections of TBE have been shown to cause some apoptosis in renal tissues in mice up to 6 h after injection (Thompson et al. 2002), as well as fibrous tissues in ilea and hepatic issues (Reid et al. 1999; Thompson et al. 2002). Although these side-effects were found after administration of higher doses than used in this study, it is possible that TBE caused minor injury to the kidneys which challenged renal function. In a water-deprived mouse, this condition might be expected to increase motivational levels slightly, inducing these mice to err towards more false alarms. However, this was not supported by the analysis of hit or false alarm rates. Our small sample size limits speculation but the small shift in threshold suggests further investigation is required. Nevertheless, it does not appear that TBE was responsible for the large shift in sucrose thresholds observed after irradiation.
Disturbances in taste functions of human patients undergoing radiotherapy were far more profound than the changes seen in this study (Bryant 1919; Lindemann 1949; Conger 1973; Mossman and Henkin 1978). This is likely due to differences in treatment regimens. Patients often are given a daily treatment regimen over the course of a few days up to 7 weeks, that is, fractionated radiotherapy. This continual assault on taste progenitor cells could cause a much more prolonged and impactful disturbance to taste cell renewal, and therefore to taste function than seen with a single dose of radiation. Additionally, radiotherapy and chemotherapy are often administered together. Chemotherapeutics have been shown to have destructive effects on the taste system (Wang et al. 2009; Mukherjee and Delay 2011; Mukherjee et al. 2013; Kumari et al. 2015; Castillo-Azofeifa et al. 2017) and it is plausible that combined radiation and chemotherapy has a more deleterious effect. The fractionated pattern of these dosages is also more likely to cause unpleasant side effects such as xerostomia and mucositis, worsening the disruption to normal function in the mouth (Pico et al. 1998; Wie et al. 2017). Further research into the effects of different treatment regimens could show how administration of radiotherapy could impact patient quality of life, resulting in better treatment for patients.
Funding
This work was supported by National Institutes of Health RO1DC012829 and K18DC011787 awarded to E.R.D., NIH R01 DC12383 awarded to L.A.B. and a University of Vermont Summer Undergraduate Research Fellowship awarded to B.C.J.
Acknowledgments
We wish to thank Dr Dany Gaillard for his valuable input to the manuscript.
References
- Bachmanov AA, Tordoff MG, Beauchamp GK. 2001. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 26:905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosvic GM, Slotnick BM. 1986. Absolute and intensity-difference taste thresholds in the rat: evaluation of an automated multi-channel gustometer. Physiol Behav. 38:711–717. [DOI] [PubMed] [Google Scholar]
- Brown TE, Lee BR, Ryu V, Herzog T, Czaja K, Dong Y. 2010. Reducing hippocampal cell proliferation in the adult rat does not prevent the acquisition of cocaine-induced conditioned place preference. Neurosci Lett. 481:41–46. [DOI] [PubMed] [Google Scholar]
- Bryant F. 1919. Radiotherapy. Boston Med Surg J. 181:270–276. [Google Scholar]
- Castillo-Azofeifa D, Losacco JT, Salcedo E, Golden EJ, Finger TE, Barlow LA. 2017. Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development. 144:3054–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger AD. 1973. Loss and recovery of taste acuity in patients irradiated to the oral cavity. Radiat Res. 53:338–347. [PubMed] [Google Scholar]
- Cunha C, Hort Y, Shine J, Doyle KL. 2012. Morphological and behavioural changes occur following the X-ray irradiation of the adult mouse olfactory neuroepithelium. BMC Neurosci. 13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delay ER, Hernandez NP, Bromley K, Margolskee RF. 2006. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses. 31:351–357. [DOI] [PubMed] [Google Scholar]
- Dhanani NM, Jiang Y. 2012. Anosmia and hypogeusia as a complication of general anesthesia. J Clin Anesth. 24:231–233. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Emerton S, Kolbinson DA, Le ND, Phillips N, Stevenson-Moore P, Osoba D. 1999. Quality of life and oral function following radiotherapy for head and neck cancer. Head Neck. 21:1–11. [DOI] [PubMed] [Google Scholar]
- Garcia J, Koelling RA. 1966. Relation of cue to consequence in avoidance learning. Psychon Sci. 4:123–124. [Google Scholar]
- Hill WA, Tubbs JT, Carter CL, Czarra JA, Newkirk KM, Sparer TE, Rohrbach B, Egger CM. 2013. Repeated administration of tribromoethanol in C57BL/6NHsd mice. J Am Assoc Lab Anim Sci. 52:176–179. [PMC free article] [PubMed] [Google Scholar]
- Hoon MA, Adler E, Lindemeier J, Battey JF, Ryba NJ, Zuker CS. 1999. Putative mammalian taste receptors: a class of taste-specific GPCRs with distinct topographic selectivity. Cell. 96:541–551. [DOI] [PubMed] [Google Scholar]
- Howell DC. 2016. Fundamental Statistics for the Behaviorial Sciences. Belmont (CA): Wadsworth Publishing. [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. 2006. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 103:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Ermilov AN, Allen BL, Bradley RM, Dlugosz AA, Mistretta CM. 2015. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J Neurophysiol. 113:1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. 1949. Zur frage der radiosensibilität des peripheren nervensystems. RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. 71:988–993. [Google Scholar]
- Mossman KL, Henkin RI. 1978. Radiation-induced changes in taste acuity in cancer patients. Int J Radiat Oncol Biol Phys. 4:663–670. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Delay ER. 2011. Cyclophosphamide-induced disruption of umami taste functions and taste epithelium. Neuroscience. 192:732–745. [DOI] [PubMed] [Google Scholar]
- Mukherjee N, Carroll BL, Spees JL, Delay ER. 2013. Pre-treatment with amifostine protects against cyclophosphamide-induced disruption of taste in mice. PLoS One. 8:e61607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. 2001. Mammalian sweet taste receptors. Cell. 106:381–390. [DOI] [PubMed] [Google Scholar]
- Nguyen HM, Reyland ME, Barlow LA. 2012. Mechanisms of taste bud cell loss after head and neck irradiation. J Neurosci. 32:3474–3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Clark C, Hogan BL. 2009. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 27:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir D, Guterman A, Gross-Isseroff R. 1988. Changes in smell acuity induced by radiation exposure of the olfactory mucosa. Arch Otolaryngol Head Neck Surg. 114:853–855. [DOI] [PubMed] [Google Scholar]
- Perea-Martinez I, Nagai T, Chaudhari N. 2013. Functional cell types in taste buds have distinct longevities. PLoS One. 8:e53399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico JL, Avila-Garavito A, Naccache P. 1998. Mucositis: Its Occurrence, Consequences, and Treatment in the Oncology Setting. Oncologist. 3:446–451. [PubMed] [Google Scholar]
- Reid WC, Carmichael KP, Srinivas S, Bryant JL. 1999. Pathologic changes associated with use of tribromoethanol (Avertin) in the Sprague Dawley rat. Comp Med. 49:665–667. [PubMed] [Google Scholar]
- Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. 1994. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses. 19:425–431. [DOI] [PubMed] [Google Scholar]
- Roper SD. 1989. The cell biology of vertebrate taste receptors. Annu Rev Neurosci. 12:329–353. [DOI] [PubMed] [Google Scholar]
- Rose-Ped AM, Bellm LA, Epstein JB, Trotti A, Gwede C, Fuchs HJ. 2002. Complications of radiation therapy for head and neck cancers. The patient’s perspective. Cancer Nurs. 25:461–7; quiz 468. [DOI] [PubMed] [Google Scholar]
- Sandow PL, Hejrat-Yazdi M, Heft MW. 2006. Taste loss and recovery following radiation therapy. J Dent Res. 85:608–611. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Nissenbaum JW. 1987. Taste preference thresholds for Polycose, maltose, and sucrose in rats. Neurosci Biobehav Rev. 11:181–185. [DOI] [PubMed] [Google Scholar]
- Stapleton JR, Luellig M, Roper SD, Delay ER. 2002. Discrimination between the tastes of sucrose and monosodium glutamate in rats. Chem Senses. 27:375–382. [DOI] [PubMed] [Google Scholar]
- Thompson JS, Brown SA, Khurdayan V, Zeynalzadedan A, Sullivan PG, Scheff SW. 2002. Early effects of tribromoethanol, ketamine/xylazine, pentobarbitol, and isoflurane anesthesia on hepatic and lymphoid tissue in ICR mice. Comp Med. 52:63–67. [PubMed] [Google Scholar]
- Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. 2007. Breadth of tuning and taste coding in mammalian taste buds. J Neurosci. 27: 10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbeuch A, Clapp TR, Kinnamon SC. 2008. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. 2009. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci. 1170:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner J, Kirsch C, Duong DK. 1899. A case of radiotherapy associated eosinophilic pneumonia. Am J Respir Crit Care Med 191: 2015. [Google Scholar]
- Wie SM, Wellberg E, Karam SD, Reyland ME. 2017. Tyrosine kinase inhibitors protect the salivary gland from radiation damage by inhibiting activation of protein kinase C-δ. Mol Cancer Ther. 16:1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]