Abstract
Rodents consume solutions of phosphates and pyrophosphates in preference to water. Recently, we found that the preference for trisodium pyrophosphate (Na3HP2O7) was greater in T1R3 knockout (KO) mice than wild-type (WT) controls, suggesting that T1R3 is a pyrophosphate detector. We now show that this heightened Na3HP2O7 preference of T1R3 KO mice extends to disodium phosphate (Na2HPO4), disodium and tetrasodium pyrophosphate (Na2H2PO4 and Na4H2PO4), a tripolyphosphate (Na5P3O10), a non-sodium phosphate [(NH4)2HPO4], and a non-sodium pyrophosphate (K4P2O7) but not to non-P salts with large anions (sodium gluconate, acetate, or propionate). Licking rates for Na3HP2O7 are higher in T1R2 KO mice than WT controls; Na3HP2O7 preference scores are increased even more in T1R2 KO mice and T1R2+T1R3 double KO mice than in T1R3 KO mice; preference scores for Na3HP2O7 are normal in T1R1 KO mice. These results implicate each subunit of the T1R2+T1R3 dimer in the behavioral response to P-containing taste compounds.
Keywords: gustometer, mineral taste, phosphate taste, preference tests.
Phosphorus (P) is ubiquitous in biological systems, where it exists as phosphate (PO43−), pyrophosphate (P2O74−), or tripolyphosphate (P3O105−). P is a component of DNA, RNA, ATP, phospholipids, ribonucleotides, bone, and teeth; it is eponymous for phosphorylation, and it is required for the maintenance of acid–base balance. To support these multiple vital functions, blood P concentrations are scrupulously defended, which involves both physiological counterregulatory mechanisms (reviewed in Civitelli and Ziambaras (2011) and Takeda et al. (2004)) and behavioral ones. With respect to the latter, P-deficient ungulates, hens, and rodents display a specific P appetite; that is, they seek out and select P-containing bones, foods, and taste solutions (Theiler et al. 1924; Green 1925; Richter et al. 1938; Wilens and Waller 1941; Richter and Helfrick 1943; Sutcliffe 1973; Holcombe et al. 1976; Siu et al. 1981, 1984; Denton 1984; Blair-West et al. 1992; Sweeny et al. 1998; Czarnogorski et al. 2004; Ohnishi et al. 2007 ). Animals can identify P indirectly, by flavor-nutrient learning (Villalba et al. 2008, 2006), but they also respond rapidly to P-containing tastes the first time they receive them, which suggests that the behavior involves an innate component (Green 1925; Denton 1984). This rapid recognition and ingestion of P raises the possibility that P is detected by taste, and thus, there must be at least one P taste receptor (or transduction element).
Another reason to suspect there is a P taste transducer is that oral P salts enhance the acceptance of pet food, particularly cat food (Brunner 2001; Shao and Stammer 2005; McCaughey et al. 2007; Brand et al. 2013). Boudreau identified “Xa” and “group 1” units in cat, “α” units in goat, and “amino acid” units in dog and rat geniculate ganglion that respond to tetrasodium pyrophosphate (Na4P2O7), monophosphate nucleotides, and other P salts (Boudreau and Nelson 1977; Boudreau et al. 1982; Boudreau 1987). Indeed P-containing compounds were among the most effective taste stimuli to activate these units (Boudreau and Nelson 1977). However, in later work, the geniculate ganglion response to Na4P2O7 and other P compounds was classified with sodium-responsive units in rats (Boudreau et al. 1983). Moreover, interpretation of the pet food preference behavior is not straightforward because, in addition to imparting taste, P salts alter the physical properties of foods to influence palatability; for example, they inhibit discoloration, chelate metals, stabilize emulsions, solubilize protein, and add “juiciness” (Wu et al. 1990; Agnihotri and Pal 1997; Xiong and Kupski 1999; International Food Additives Council 2013; Roldán et al. 2014).
A third line of evidence that P is detected in the oral cavity comes from studies conducted by our group (McCaughey et al. 2007; Tordoff et al. 2014a). We found that replete Sprague Dawley rats and 3 strains of mice spontaneously consumed moderate concentrations of trisodium pyrophosphate (Na3HP2O7) and other pyrophosphate solutions in preference to water. The most preferred pyrophosphates were sodium salts, but preferences for the sodium ion could not explain the results: First, the inverted U-shaped concentration-preference function observed with Na3HP2O7 persisted even when it was mixed with the sodium transduction inhibitor, amiloride. Second, the CBA/J mouse strain preferred several concentrations of Na3HP2O7 to water but did not prefer any concentration of NaCl to water. Third, the pattern of electrophysiological activity in the nucleus of the solitary tract elicited by orally applied pyrophosphates was distinct from those generated by sodium and the other 4 basic tastes. This latter finding has recently been substantiated by the observation that rats trained to make an operant response when they recognized a basic taste did not respond when they tasted Na3HP2O7 (De Ratauld et al. 2015).
We have begun studies to investigate the mechanism responsible for detecting the taste of P. Taste transduction mechanisms can be broadly dichotomized into those involving G protein-coupled receptors (GPCRs) and those involving ion channels. The GPCR-mediated tastes include sweet, umami, and bitter, and these are transduced in Type 2 taste cells; the ion channel-mediated tastes include salty and sour, and these are transduced in Type 1 and/or Type 3 taste cells. P is an anion, so it would be expected to fall with the ionic tastes. To investigate which transduction pathways are indeed involved in the detection of P, we assessed the Na3HP2O7 2-bottle preferences of mice with genetic ablation of 2 components of the GPCR transduction cascade, ITPR3 and CALHM1. Knockout of either component almost entirely eliminates sensitivity to sweet, umami, and bitter tastes whereas salty and sour tastes remain largely intact (Hisatsune et al. 2007; Taruno et al. 2013; Tordoff and Ellis 2013; Tordoff et al. 2014b). Contrary to expectation, the inverted U-shaped concentration function present in WT control mice was absent in ITPR3 KO and CALHM1 KO mice (Tordoff and Ellis 2013; unpublished results). Thus, despite being an ion, P appears to be transduced by a GPCR.
Because T1R3 detects the taste of calcium ions (Tordoff 2008; Tordoff et al. 2008, 2012), we wondered whether T1R3 might also be involved in P taste detection. We found, much to our surprise, that T1R3 KO mice had heightened preferences for Na3HP2O7 (Tordoff et al. 2014a). That is, they had higher preference scores for some concentrations of Na3HP2O7 than did WT controls. We now bolster these findings by showing that the heightened preference scores of T1R3 KO mice for Na3HP2O7 extend to several phosphates, pyrophosphates, and a tripolyphosphate. We also show that T1R2 KO mice, but not T1R1 KO mice, have enhanced preferences for Na3HP2O7, and that the double knockout (dKO) of T1R2+T1R3 is no more effective than T1R2 KO alone at enhancing P preference scores. Finally, we show that relative to WT mice, T1R2 KO mice lick more often for Na3HP2O7 in brief-access tests.
Materials and methods
Experiments followed the principles outlined in the National Research Council’s Guide for the Care and Use of Laboratory Animals, 8th edition. Protocols were approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
Mice and maintenance
All mice were maintained in a vivarium at 23 °C on a 12:12 h light:dark cycle with lights off at 7 PM. They were housed in plastic “tub” cages (26.5 cm × 17 cm × 12 cm) with stainless-steel grid lids and wood shavings scattered on the floor [details are available online (Tordoff and Bachmanov 2001)]. The mice ate pelleted AIN-76A diet and drank deionized water.
The T1R3 KO mouse line used here was originally generated by Damak et al. (2003). T1R3 WT and KO mice were obtained from a colony maintained at Monell since 2006 by a combination of heterozygote crosses and KO backcrosses to the C57BL/6J strain. T1R1 and T1R2 WT and KO mice were progeny of colonies originally generated by Iwatsuki et al. (2010) and maintained at Monell by Dr. R. Margolskee. For one experiment, we produced T1R2+T1R3 dKO mice. The dKO mice and T1R3 KO mice were offspring of (C57BL/6J × T1R3 KO) × (B6 × T1R2) parents; the T1R2 and WT mice were offspring of C57BL/6J × T1R2 KO parents. Producing the 4 pertinent genotypes from 2 sets of parents was imperfect design but necessary to generate the mice expeditiously.
All mice were bred in our facility. Male and female KO heterozygotes were housed together until the female was clearly pregnant, when the male was removed. Pregnant and lactating females were disturbed only for routine husbandry procedures. Pups were weaned at 21–23 days. During the 3rd–4th week after birth, a 1-mm tail tip snip was collected from each pup, and this was processed and analyzed by a commercial genotyping service, Transnetyx, Inc., to identify the pup’s genotype. The weanlings were housed in groups of the same sex until 7–10 days before tests began, at which time they were housed alone.
We did not phenotype heterozygote mice; they were required for breeding. We showed previously that T1R3 heterozygotes have Na3HP2O7 preference scores indistinguishable from those of T1R3 WT mice (Tordoff et al. 2014a).
Procedures
T1R3 WT and KO mice given 2-bottle choice tests with P salts and sodium salts with large anions
At the start of their 2-bottle choice tests, T1R3 WT and KO mice ranged in age from 42 to 115 days and weighed from 14.3 to 28.8 g. The knockout had no effect on body weight (mean ± standard error [SE], g [n]: WT males = 24.1 ± 0.5 [31], WT females = 19.6 ± 0.3 [51], KO males = 24.8 ± 0.3 [26], KO females = 18.9 ± 0.3 [41]).
The mice were tested in 8 cohorts, with each cohort containing 12–16 WT mice and approximately the same number of KO littermates of the same sex (Table 1). Six cohorts of T1R3 WT and KO mice received ascending concentration series such that every 48 h, the concentration of the taste compound was increased in 0.25- or 0.5-log steps, from 1 mM to a concentration where mean preference scores of both WT and KO groups fell under 20% (i.e., 100, 178, or 320 mM). Concentrations of sodium acetate and gluconate were 2, 6.4, 20, 64, 200, and 640 mM so that they were of equivalent sodium concentration to the disodium P salts. The number of cohorts (and thus mice) receiving each ascending taste compound series was determined by the availability of mice and by the general principle that more mice were needed for choice tests of the large-anion sodium salts because these tests involved failing to reject the null hypothesis that T1R3 has no effect on preference.
Table 1.
Two-bottle choice tests of T1R3 WT and KO mice: Compounds and mice tested
| Test solution | Cohort and order | Group sizes | ||
|---|---|---|---|---|
| Common name | Formula | T1R3 WT | T1R3 KO | |
| Disodium phosphate | Na2HPO4 | 6c | 3 M, 9 F | 2 M, 8 F |
| Diammonium phosphate | (NH4)2HPO4 | 1b | 12 F | 10 F |
| Disodium pyrophosphate | Na2H2P2O7 | 3c, 6e | 7 M, 13 F | 7 M, 13 F |
| Trisodium pyrophosphate | Na3HP2O7 | published | 11 M, 16 F | 11 M, 16 F |
| Tetrasodium pyrophosphate | Na4P2O7 | 6f, 7c | 7 M, 15 F | 7 M, 12 F |
| Tetrapotassium pyrophosphate | K4P2O7 | 1a | 12 F | 10 F |
| Pentasodium tripolyphosphate | Na5P3O10 | 2a, 3b | 14 M, 4 F | 12 M, 5 F |
| Sodium acetate | C2H3NaO2 | 4b, 6b, 7b | 12 M, 26 F | 9 M, 19 F |
| Sodium propionate | C3H5NaO2 | 3a, 6d | 7 M, 13 F | 7 M, 13 F |
| Sodium gluconate | C6H11NaO7 | 4a, 6a, 7a | 12 M, 26 F | 9 M, 19 F |
| Seven 32 mM salts | — | 5a, 8a | 9 M, 13 F | 8 M, 12 F |
“Cohort and order” identifies which batches of mice were tested and with which compounds. For example, the notation “3c, 6e” for Na2H2P2O7 indicates there were 2 cohorts tested with this compound; it was Cohort 3's 3rd test series and Cohort 6's 5th test series. Group sizes were the sum of M = male, F = female. Most P salts have several names in addition to the one given here. For example, disodium phosphate is also called disodium hydrogen orthophosphate, sodium hydrogen phosphate, and sodium phosphate dibasic. The salts were purchased from Sigma Aldrich except for Na3HP2O7, which was donated by the SPF-Diana Company. The results with Na3HP2O7 have been published previously (Tordoff et al. 2014a).
For each 48-h test, each mouse was presented with 2 fluid-filled graduated drinking tubes, which allowed the contents to be measured to the nearest 0.1 mL. After 24 h, the positions of the drinking tubes were switched to control for any side preferences. After 48 h, intakes were recorded and the test of the next concentration of taste solution began. During the first test in a concentration-preference series, both drinking tubes contained deionized water. In subsequent tests, one drinking tube contained water and the other contained the taste solution. Mice tested with more than one taste compound received 2–4 days with only water to drink before starting a new concentration series.
To substantiate the findings of the ascending concentration-preference experiments, 2 additional cohorts were tested with a single, 32 mM, concentration of 5 P compounds and 2 large-anion sodium salts. The mice received 48-h 2-bottle tests with a choice, first between 2 drinking tubes with water, then between water and 32 mM concentrations of the following in the order listed: Na3HP2O7, Na·propionate, Na4P2O7, K4P2O7, Na·gluconate, Na5P3O10, and Na2H2P2O7. Each 48-h choice test was followed by a day or two with a single bottle of water.
For each 48-h 2-bottle test, preference scores were derived based on taste solution intake divided by total intake (i.e., taste solution intake + water intake) expressed as a percentage. Values from the ascending concentration-preference tests were analyzed by mixed-design analysis of variance (ANOVA) with factors of group (WT or KO) and concentration. When the group × concentration interaction was significant, the statistical significance of differences between the WT and KO groups at each concentration was assessed with post hoc Least Significant Difference (LSD) tests. Values from the test involving 32 mM concentrations of several compounds were analyzed using t tests for each compound. Analyses were also conducted as follows: 1) on raw solution intakes and 2) with sex as an additional factor, but these yielded almost identical information so we do not present them here.
T1R2 WT and KO mice given two-bottle choice tests with Na3HP2O7, CaCl2, and saccharin
At the start of 2-bottle choice tests, T1R2 WT and KO mice ranged in age from 50 to 148 days and weighed from 15.8 to 30.2 g. The knockout had no effect on body weight (mean ± SE, g [n]: WT males = 25.1 ± 0.7 [18], WT females = 19.4 ± 0.3 [21], KO males = 23.7 ± 0.4 [25], KO females = 20.2 ± 0.3 [21]).
The mice were tested in 4 cohorts. Two cohorts received 3 taste compounds in the order Na3HP2O7, CaCl2, and saccharin; one was tested with the same 3 taste compounds in the order CaCl2, Na3HP2O7, and saccharin. The fourth cohort was tested with Na3HP2O7 only. Procedures were identical to those used for testing the T1R3 mice (described previously). The results of 4 mice were excluded from statistical analysis: Two WT and 1 KO mouse spilled fluid from a drinking tube, and 1 KO mouse died.
WT, T1R2 KO, T1R3 KO, and T1R2+T1R3 dKO mice given 2-bottle choice tests with Na3HP2O7
This experiment involved comparing groups of 15–19 T1R3 KO, T1R2 KO, and T1R2+T1R3 dKO mice with WT controls (age range 51–126 days). At the start of the test series, there were no differences in body weight between genotypes of the same sex: (mean ± SE, g [n]: WT males = 24.1 ± 0.5 [12], WT females = 19.7 ± 0.47 [7]; T1R2 KO males = 24.6 ± 0.4 [13], T1R2 KO females = 20.4 ± 0.2 [3]; T1R3 KO males = 23.5 ± 1.5 [7], T1R3 KO females = 18.7 ± 1.0 [8]; and T1R2+T1R3 dKO males = 23.6 ± 1.3 [7], T1R2+T1R3 dKO females = 18.9 ± 0.4 [9]).
The mice were tested in 2 cohorts, treated identically. After a 48-h choice between 2 bottles of water, the mice received 9 successive 48-h tests with a choice between water and the following Na3HP2O7 concentrations: 1.78, 3.2, 5.6, 10, 17.8, 32, 56, 100, and 178 mM.
T1R1 WT and KO mice given 2-bottle choice tests with Na3HP2O7
There were 14 WT and 17 KO mice, aged 53–124 days. The knockout had no effect on body weight (mean ± SE, g [n]: WT males = 23.8 ± 1.1 [5], WT females = 20.6 ± 1.0 [8] and KO males = 24.5 ± 0.9 [9], KO females = 21.6 ± 0.1 [8]).
The mice were tested in 2 cohorts, treated identically. They received an ascending series of Na3HP2O7 concentrations according to the procedures described for mice in the T1R2+T1R3 dKO experiment.
T1R2 WT and KO mice given brief-access gustometer tests with Na3HP2O7
Na3HP2O7 licking rates were assessed using the procedures for “hedonically positive” taste compounds, as described previously (Tordoff et al. 2014a). The experiment involved 13 T1R2 WT mice and 17 T1R2 KO mice, aged 83–141 days. The knockout had no effect on body weight (mean ± SE, g [n]: WT males = 26.4 ± 0.9 [7], WT females = 20.2 ± 0.5 [6] and KO males = 25.4 ± 0.9 [14], KO females = 20.0 ± 0.6 [3]).
The mice were trained to drink water in a gustometer (MS160; DiLog Instruments) and then received 3 test sessions. At 24 h before each session, the mice received 1 g of food and 2 mL of water. The test session consisted of 19 repeated series of 5-s exposures to 0, 17.8, 31.6, 56, and 75 mM Na3HP2O7 (with “0” being water), presented in a quasi-random order. There was no requirement to respond to a taste solution; most mice licked assiduously during the first 3–5 series of presentations and then stopped behaving, presumably because they were no longer thirsty. After the session, the mice received free access to food and water for at least 24 h.
The mean number of licks in response to each Na3HP2O7 concentration made by each mouse was obtained by averaging the results of identical exposures. These values for individual mice were then used in a mixed-design ANOVA with factors of group (WT or KO) and Na3HP2O7 concentration. Significant differences between pairs of means were assessed using post hoc LSD tests. All data from one WT and one KO mouse were excluded from analyses because they failed to lick one of the Na3HP2O7 concentrations.
Results
Preference scores of T1R3 WT and KO mice
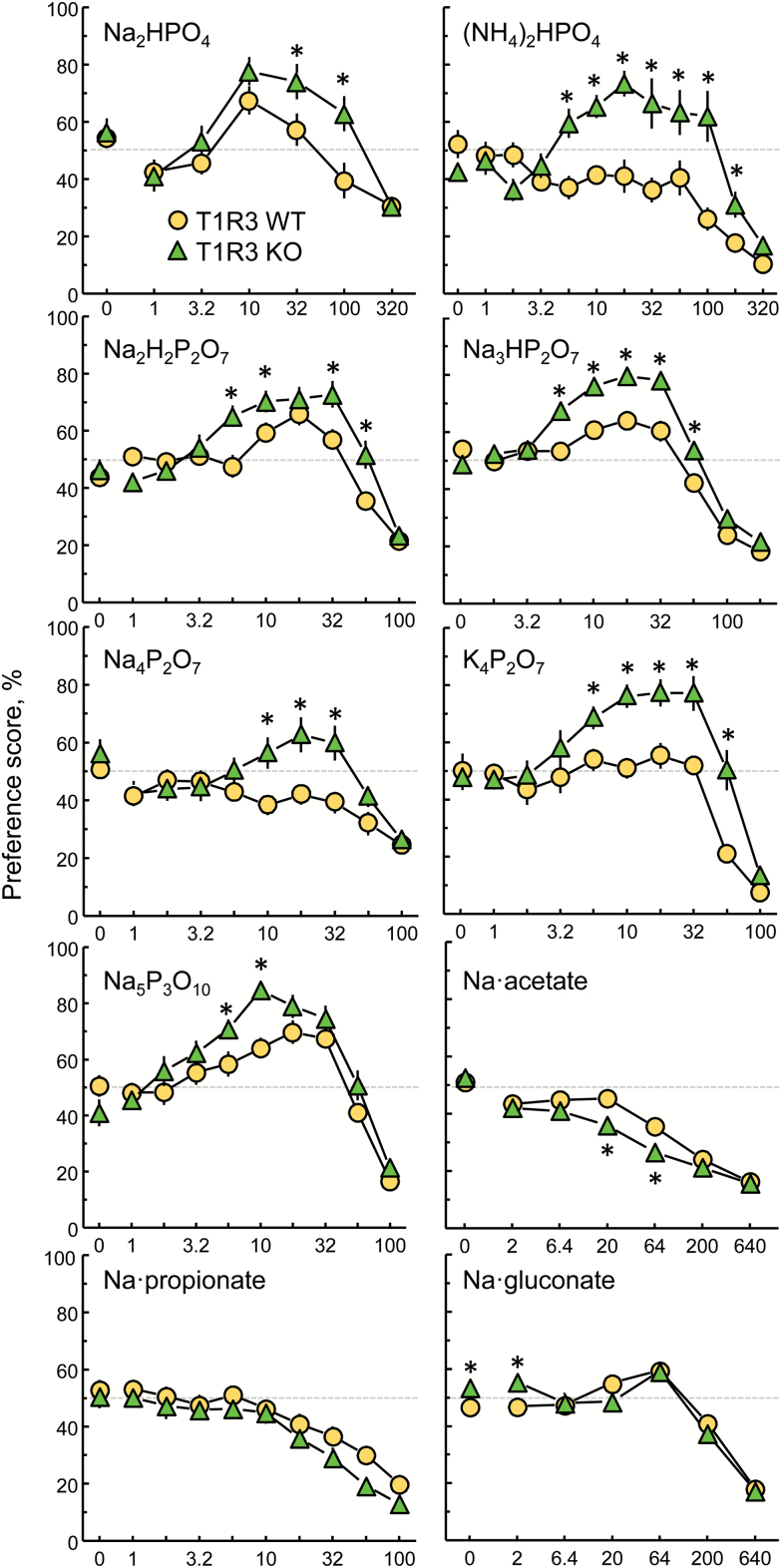
T1R3 KO mice had higher preference scores than did their WT controls for 2 or more concentrations of each of the P salts tested (Table 1 and Figure 1; water intakes, solution intakes, total fluid intakes, and preference scores of each mouse are available in an online supplement). There was a small but significant difference between WT and KO mice in response to 2 mM Na·gluconate, which was most likely an idiosyncrasy because the same difference was present in the response to water. There were significant differences between WT and KO mice in response to 20 and 64 mM sodium acetate, and a main effect of genotype on preference scores for sodium propionate but, in contrast to the results found with the P salts, acetate and propionate preference scores were significantly lower in KO than WT mice.
Figure 1.
Preferences of T1R3 WT and KO mice for 7 phosphorus salts and 3 large-anion sodium salts presented in 48-h 2-bottle tests with a choice between water and a taste solution. *P < 0.05 according to post hoc tests (Table 1 gives group sizes; Table 2 gives statistics). Symbols with vertical bars depict means and SEs. Most SEs were smaller than the symbols. The results with Na3HP2O7 were published previously (Tordoff et al. 2014a). SE, standard error.
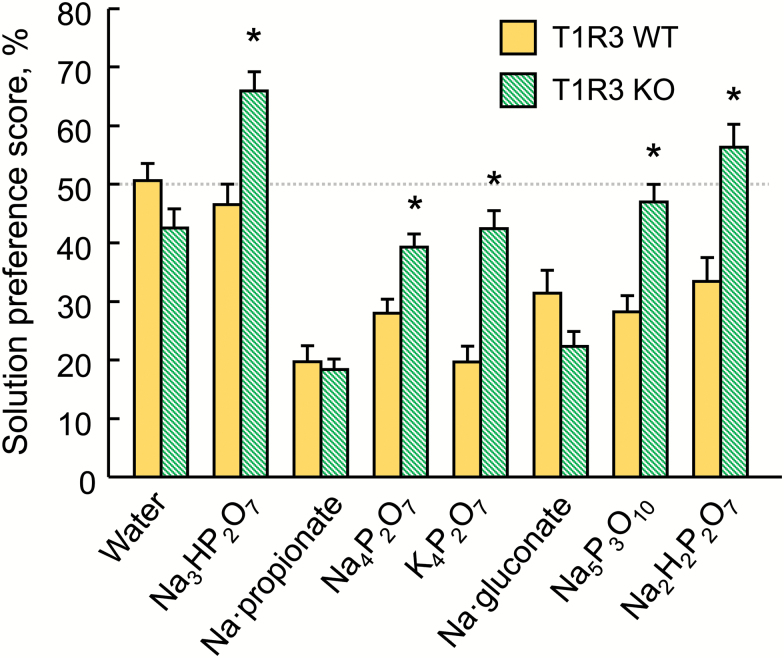
In the study comparing the response of T1R3 WT and KO mice to 32 mM concentrations of 5 P salts and 2 large-anion sodium salts, the T1R3 KO mice had significantly higher preference scores for all the P salts (all Ps < 0.002) but the 2 genotype groups did not differ in response to 32 mM sodium propionate or sodium gluconate (Figure 2).
Figure 2.
Preferences of T1R3 WT and KO mice for 32 mM concentrations of 5 phosphorus salts and 2 large-anion sodium salts presented in 48-h 2-bottle tests with a choice between water and a taste solution. Tests were conducted in the order presented. *P < 0.05 relative to WT mice. Bars depict means and SEs; n = 22 WT and 20 KO.
Table 2.
T1R3 WT and KO mice 2-bottle choice tests: Results of analyses of variance for each ascending concentration series
| Formula | Group (WT or KO) | Concentration | Group × concentration |
|---|---|---|---|
| Na2HPO4 | F(1,20) = 4.60, P = 0.0445 | F(6,120) = 20.8, P < 0.0001 | F(6,120) = 2.22, P = 0.0457 |
| (NH4)2HPO4 | F(1,19) = 16.6, P = 0.0006 | F(11,209) = 16.5, P < 0.0001 | F(11,209) = 7.02, P < 0.0001 |
| Na2H2P2O7 | F(1,42) = 3.22, P = 0.0797 | F(9,378) = 42.4, P < 0.0001 | F(9,378) = 4.52, P < 0.0001 |
| Na3HP2O7 | F(1,52) = 18.1, P < 0.0001 | F(9,468) = 121.9, P < 0.0001 | F(9,468) = 6.07, P < 0.0001 |
| Na4P2O7 | F(1,39) = 0.65, P = 0.4233 | F(9,351) = 13.0, P < 0.0001 | F(9,351) = 3.90, P < 0.0001 |
| K4P2O7 | F(1,20) = 19.7, P = 0.0002 | F(9,180) = 33.8, P < 0.0001 | F(9, 180) = 4.09, P < 0.0001 |
| Na5P3O10 | F(1,37) = 3.36, P = 0.0750 | F(8,296) = 23.9, P < 0.0001 | F(8,296) = 3.00, P = 0.0029 |
| C2H3NaO2 | F(1,66) = 4.85, P = 0.0311 | F(6,396) = 92.4, P < 0.0001 | F(6,396) = 2.46, P = 0.0240 |
| C3H5NaO2 | F(1,42) = 7.46, P = 0.0092 | F(9,378) = 31.7, P < 0.0001 | F(9,378) = 0.47, P = 0.8930 |
| C6H11NaO7 | F(1,66) = 0.21, P = 0.6523 | F(6,396) = 85.5, P < 0.0001 | F(6,396) = 3.34, P = 0.0032 |
Values for Na3HP2O7 are from Tordoff et al. (2014a).
Preference scores of T1R2 WT and KO mice
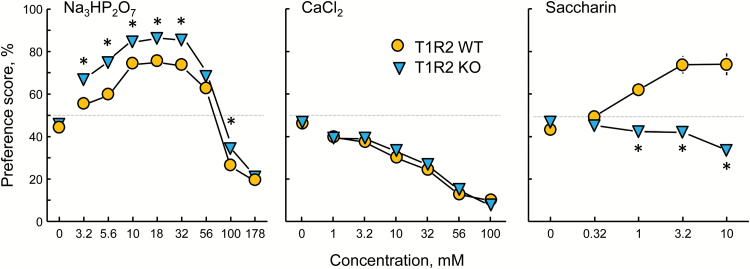
Relative to WT controls, T1R2 KO mice had significantly higher preference scores for 3.2–32 and 100 mM Na3HP2O7 (but not at 56 or 178 mM Na3HP2O7; Table 3, Figure 3). The T1R2 WT and KO groups did not differ in response to any concentration of CaCl2. The 2 groups differed significantly in their preference scores for 1, 3.2, and 10 mM saccharin. The WT mice preferred these concentrations to water but the KO mice did not prefer any concentration of saccharin.
Table 3.
T1R2 WT and KO mice 2-bottle choice tests: Results of analyses of variance for each ascending concentration series
| Formula | Group (WT or KO) | Concentration | Group × concentration |
|---|---|---|---|
| Na3HP2O7 | F(1,79) = 28.3, P < 0.0001 | F(8,632) = 209.7, P < 0.0001 | F(8,632) = 2.33, P = 0.0180 |
| CaCl2 | F(1,61) = 1.15, P = 0.2859 | F(6,366) = 72.7, P < 0.0001 | F(6,366) = 0.97, P = 0.4471 |
| Saccharin | F(1,62) = 24.2, P < 0.0001 | F(4,248) = 5.67, P < 0.0001 | F(4,248) = 18.9, P < 0.0001 |
Figure 3.
Preferences of T1R2 WT and KO mice for trisodium pyrophosphate (Na3HP2O7), calcium chloride (CaCl2), and saccharin presented in 48-h 2-bottle tests with a choice between water and a taste solution. *P < 0.05 according to post hoc tests (Table 3 gives statistics). Symbols with vertical bars depict means and SEs; n = 39 WT and 46 KO.
Preference scores of WT, T1R2 KO, T1R3 KO, and T1R2+T1R3 dKO mice
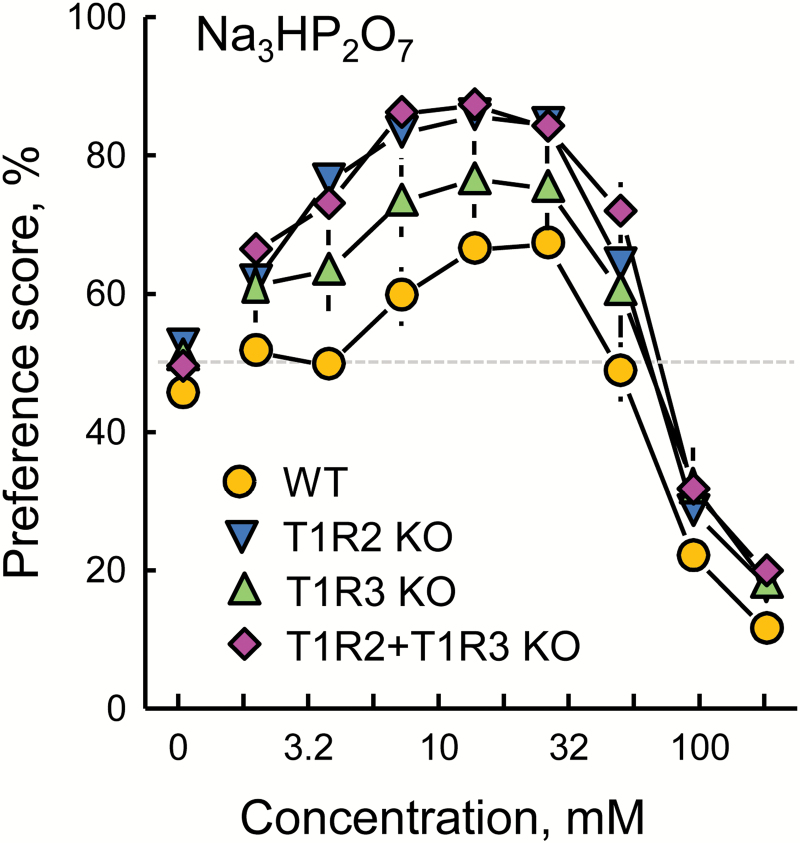
All 3 groups of KO mice (T1R2 KO, T1R3 KO, and T1R2+T1R3 dKO) had Na3HP2O7 preference scores that were significantly higher than those of the WT group. Preference scores of the T1R2 KO mice were significantly higher than those of the T1R3 KO mice. Preference scores of the dKO mice were also significantly higher than those of the T1R3 KO mice and statistically similar to those of the T1R2 KO mice; the interaction of the 2 KOs was marginally nonsignificant (Table 4, Figure 4).
Table 4.
WT, T1R2 KO, T1R3 KO, and T1R2+T1R3 double KO mice 2-bottle choice tests: Results of 3-way analysis of variance for ascending Na3HP2O7 concentration series
| Effect | F-test results |
|---|---|
| T1R2 genotype | F(1,60) = 24.1, P < 0.0001 |
| T1R3 genotype | F(1,60) = 8.39, P = 0.0053 |
| T1R2 × T1R3 | F(1,60) = 3.49, P = 0.0665 |
| Concentration | F(8,480) = 199.6, P < 0.0001 |
| T1R2 × concentration | F(8,480) = 3.79, P = 0.0003 |
| T1R3 × concentration | F(8,480) = 0.91, P = 0.5083 |
| T1R2 × T1R3 × concentration | F(8,480) = 0.40, P = 0.9208 |
Figure 4.
Preferences of WT, T1R2 KO, T1R3 KO, and T1R2+T1R3 double KO mice for trisodium pyrophosphate (Na3HP2O7) presented in 48-h 2-bottle tests with a choice between water and the taste solution. Significant effects are presented in Table 4. Symbols with vertical bars depict means and SEs; n = 15–18.
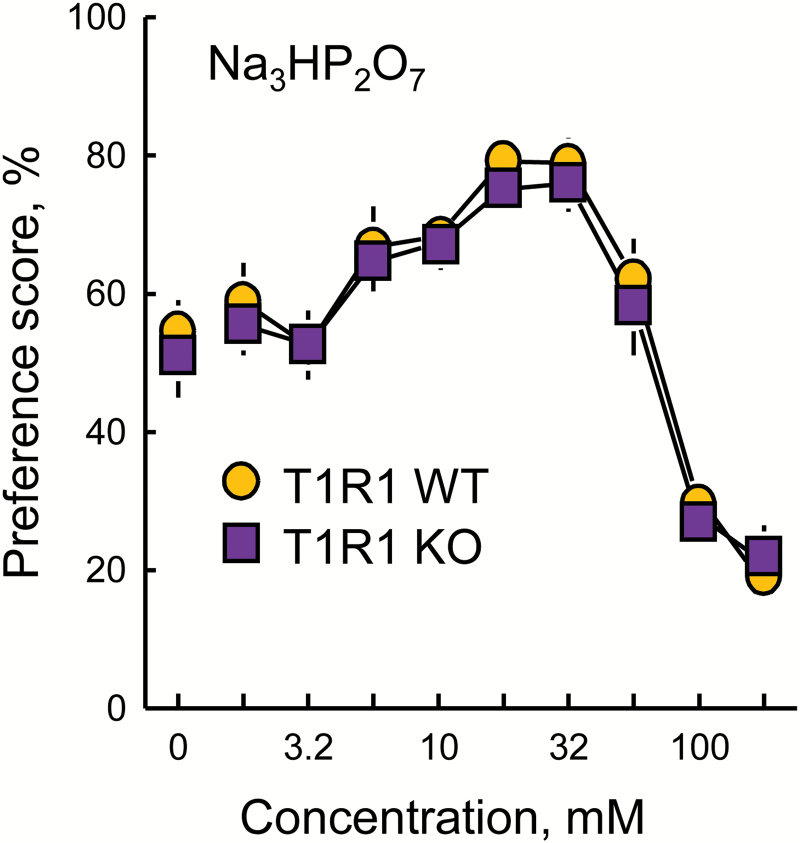
Preference scores of T1R1 WT and KO mice
T1R1 WT and KO mice had similar Na3HP2O7 preference scores [Figure 5; main effect of genotype, F(1,29) = 0.81, P = 0.3744; main effect of concentration, F(9, 261) = 66.2, P < 0.0001; genotype × concentration interaction, F(9,261) = 0.20, P = 0.9946].
Figure 5.
Preferences of T1R1 WT and KO mice for trisodium pyrophosphate (Na3HP2O7) presented in 48-h 2-bottle tests with a choice between water and the taste solution. The 2 groups did not differ significantly. Symbols with vertical bars depict means and SEs; n = 14 WT and 17 KO.
Brief-access gustometer tests of T1R2 WT and KO mice
On the last day of training, when the severely water-deprived mice were licking for water, there was no difference in the number of trials initiated or lick rates between the WT and T1R2 KO mice (means ± standard error of the mean [SEM]; WT = 27.9 ± 3 licks/5 s; KO = 30.5 ± 2.2 licks/5 s).
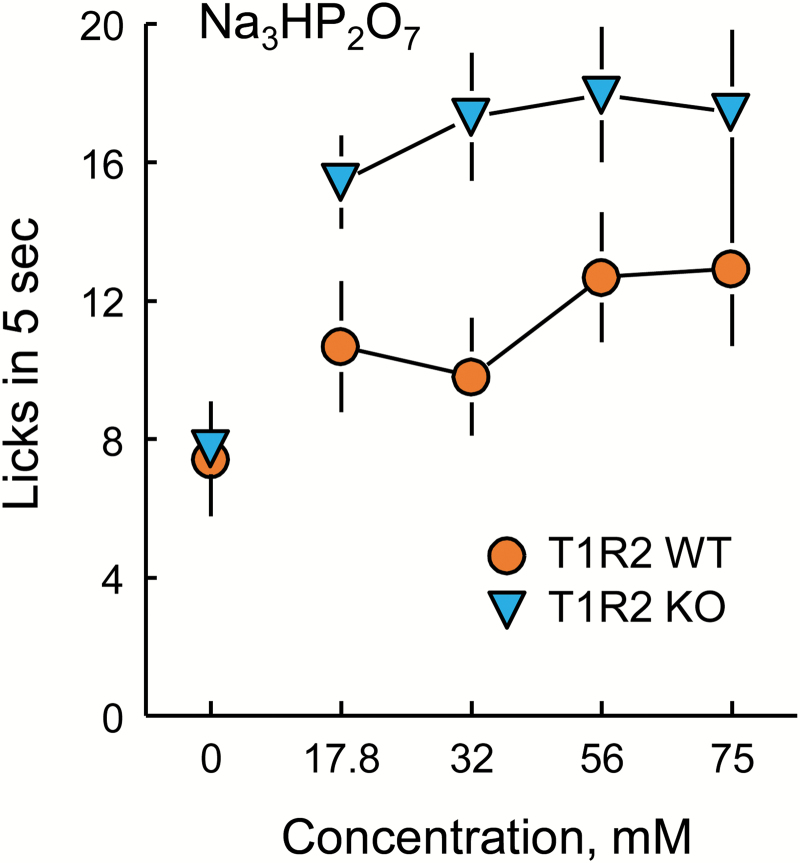
During test sessions, the mice completed with each concentration of taste solution (including water) an average 6.2 trials (range 1–26 trials). There was no effect of genotype or Na3HP2O7 concentration on the number of trials completed [main effect of genotype, F(1,25) = 0.10, P = 0.7533; main effect of concentration, F(4,100) = 1.18, P = 0.3423; genotype × concentration interaction, F(4, 100) = 0.97, P = 0.4263]. Both genotypes licked more to the 4 concentrations of Na3HP2O7 than they did to water [Figure 6; main effect of concentration, F(4,100) = 11.6, P < 0.0001]. T1R2 KO mice licked significantly more for Na3HP2O7 than did T1R2 WT mice [Figure 6; main effect of genotype, F(1,25) = 4.94, P = 0.0354]. According to the ANOVA, the effect of T1R2 KO to increase licking was not confined to a particular Na3HP2O7 concentration [i.e., the genotype × concentration interaction was not significant; F(4,100) = 1.98, P = 0.1035]. Nevertheless, post hoc LSD tests indicated the T1R2 KO mice licked more than did the WT mice for 32 and 56 mM Na3HP2O7 but not for water or the 17.8 or 75 mM concentrations.
Figure 6.
Lick rates of T1R2 WT and KO mice for trisodium pyrophosphate (Na3HP2O7). The T1R2 KO mice licked significantly more than did T1R2 WT mice for all concentrations of Na3HP2O7 combined according to ANOVA and to the 32 and 56 mM concentrations according to post hoc LSD tests. Symbols with vertical bars depict means and SEs; n = 12 WT and 16 KO.
Discussion
In previous work, we found that Na3HP2O7 preference scores and brief-access lick rates were higher in T1R3 KO than WT mice (Tordoff et al. 2014a). This was unexpected for 2 reasons. First, manipulating T1R3, the “sweet and umami taste receptor,” would not be expected to influence the taste response to a pyrophosphate because pyrophosphates are neither sweet nor umami-like ( McCaughey et al. 2007; De Ratauld et al. 2015). Second, ablating a receptor for a hedonically positive taste typically eliminates the preference but knockout of T1R3 heightened preferences for pyrophosphate. Our intention here was to investigate the boundaries of this phenomenon. One set of experiments characterized the specificity of the behavioral response, by comparing the preference scores of T1R3 WT and KO mice for a range of P and non-P salts. The other set of experiments characterized the specificity of the receptor(s) involved, by testing the behavioral responses to Na3HP2O7 of T1R1, T1R2, and T1R2+T1R3 KO mice. It is convenient to discuss each set of experiments separately:
Specificity of the behavioral response
We compared the preference scores of T1R3 WT and KO mice to 2 phosphates, 4 pyrophosphates, and a tripolyphosphate. In each case, the T1R3 KO mice had significantly higher preference scores than did the WT mice for moderate concentrations of these P salts. That this phenomenon encompassed 3 forms of phosphate and 3 cations (Na+, K+, and NH4+), with a wide pH range but did not encompass 3 non-P anions [or NaCl (McCaughey et al. 2007; Tordoff et al. 2014a)], strongly suggests that the higher preference scores of KO mice are driven by a response to the P-containing anion and not to the cation or to nonspecific effects, such as those involving anionic strength or acidity.
T1R3 WT mice did not prefer (i.e., have preference scores >50%) any concentration of (NH4)2HPO4, Na4P2O7, or K4P2O7 whereas T1R3 KO mice preferred moderate concentrations of these P salts. Indeed, WT mice avoided some concentrations of (NH4)2HPO4 and Na4P2O7 that T1R3 KO mice preferred, indicating that T1R3 influences the hedonic value of the salts.
A concern with the use of long-term 2-bottle choice tests is that preference scores can be obscured by carryover effects; that is, responses to a taste solution can be influenced by experience with taste solutions consumed earlier. It would be ideal to avoid carryover effects altogether by using naive mice for every test, but this is impractical. Here, we employed 2 strategies to ameliorate the problem: First, we tested most taste compounds either in naive mice or in 2 or more cohorts of mice with different previous experiences (Table 1). Second, we tested some mice with a short series of taste compounds at one informative concentration (32 mM; Figure 2). Despite different past experiences, the same differences between T1R3 WT and KO mice were present for P preference scores and absent for large-anion sodium preference scores. Thus, the phenotype was sufficiently robust that it overwhelmed any carryover effects, if they were present.
Our results do not allow us to distinguish whether the taste detected is for P or phosphate (PO43-). To our knowledge, there is no way to present an inert molecule containing P that is not a (mono, di, or poly) phosphate. Pyrophosphates and tripolyphosphates are simply 2 or 3 PO43- tetrahedrons that share an oxygen atom. But irrespective of the precise stimulus, the present results add to behavioral and gustatory electrophysiological studies in the rat that point to a unique P or PO43- taste that is distinct from the 5 traditional tastes (McCaughey et al. 2007; De Ratauld et al. 2015).
Umami taste can be enhanced by nucleotides, which are P compounds. It would not be surprising, then, to discover that P taste shares a common overlapping substrate with the “umami2” neural subsystem [using Boudreau’s terminology (Boudreau 1987)], although there is evidence against this: sodium phosphate does not synergize with monosodium glutamate to enhance taste in humans (Ugawa et al. 1992). Umami taste is often considered to be a signal that alerts an animal it is eating protein. We suspect that P taste is an equally good, if not better, signal for protein.
Avidity for P involves T1R2 and T1R3 but not T1R1
We confirmed our previous finding that T1R3 KO mice have heightened Na3HP2O7 preference scores [(Tordoff et al. 2014a) and Figure 4] and showed that the same is true of T1R2 KO mice and T1R2+T1R3 dKO mice but not T1R1 KO mice. In fact, T1R2 KO and T1R2+T1R3 dKO mice had significantly higher Na3HP2O7 preference scores than did T1R3 KO mice. It is noteworthy how very high were the Na3HP2O7 preference scores of the T1R2 KO mice: that is, 80–85% for 10–32 mM Na3HP2O7 (Figures 3 and 4).
Our results suggest the normal behavioral response to P is tempered by both T1R2 and T1R3 and that these receptor subunits provide a hedonically negative P taste signal; that is, they are normally responsible for the rejection of P salts. Because of its role as a sweet detector, the T1R2 + T1R3 dimer is usually considered to generate a positively hedonic signal; however, T1R3 is responsible for the negatively hedonic component of calcium taste (Tordoff et al. 2008, 2012). It is unknown whether the hedonic nature and quality of the signal depends on T1R3's dimer partner, the taste cell it occupies, or both.
The concentration-preference function for most of the P salts was inverted U shaped. For other taste compounds having this inverted U-shaped relationship between concentration and preference scores, the underlying mechanism appears to involve 2 or more receptors. For example, the response to saccharin and acesulfam-K involves the combined action of the positively hedonic sweet-detecting T1R2+T1R3 dimer and negatively hedonic bitter receptors, hT2R43, and/or hT2R44 (Kuhn et al. 2004). The response to sodium salts involves the combined action of the positively hedonic amiloride-sensitive epithelial sodium channel (Chandrashekar et al. 2010) and negatively hedonic amiloride-insensitive receptors (Oka et al. 2013; Lewandowski et al. 2016). If the same is true for P salts, then there most likely must be another receptor (or receptors) responsible for the hedonically positive P taste (i.e., attraction to P). We do not know the identity of this receptor, but it is likely a GPCR located in Type 2 taste cells because preferences for Na3HP2O7 are eliminated by ablation of components of the GPCR transduction cascade, ITPR3 or CALHM1 (Tordoff and Ellis 2013; unpublished results), and CALHM1 is confined to Type 2 taste cells (Taruno et al. 2013).
Our finding that the double knockout of T1R2 and T1R3 enhances the avidity for P argues that the effects observed with the single knockouts are due to loss of the ablated receptor subunit rather than to compensatory activity of the remaining receptor subunit. So, for example, the heightened Na3HP2O7 avidity produced by ablating T1R2 cannot be ascribed to increased activity of T1R3. We can only speculate why T1R2 KO mice have stronger preferences for Na3HP2O7 than do T1R3 KO mice. Perhaps there are P-binding pockets in both T1R2 and T1R3, and these differ in sensitivity. This would be similar to the multiple binding sites for sweeteners in the T1R2+T1R3 dimer (Nie et al. 2005; DuBois 2016). Alternately, perhaps P interacts with a binding pocket in one subunit and elimination of the other subunit prevents efficient conformation of the dimer. It will require in vitro experiments to fully understand these behavioral results.
T1Rs are located in the gastrointestinal tract, pancreas, and other organs [reviewed in Laffitte et al. (2014), Meyer-Gerspach et al. (2014) and Wauson et al. (2013)], and we used mice with global (whole-body) knockouts here, raising the possibility that postingestive actions of P could account for the effects we observed in 48-h 2 bottle choice preference scores. However, this cannot explain the increased licking rates of T1R2 KO mice or T1R3 KO mice observed in 5-s brief-access tests (Tordoff et al. 2014a; this study). The most parsimonious explanation is that oral P taste receptors are responsible for both the brief-access licking and the 2-bottle choice preference phenotypes. However, a T1R-mediated physiological action of P would, in many ways, be equally interesting because this would augment current understanding of P homeostasis [reviewed in Takeda et al. (2004) and Civitelli and Ziambaras (2011)].
T1R2 KO mice and T1R3 KO mice have the same strong avidity for pyrophosphate salts as do domestic cats (Brunner 2001; Shao and Stammer 2005; Brand et al. 2013), which lack a functional T1R2 (Li et al. 2005). Perhaps, this mutation causes the loss of a P-related negative hedonic or other inhibitory signal. Another possibility is that, in the absence of active sweet signaling pathways, neural connectivity is “rewired” such that P-sensitive receptors activate neural reward or pleasure circuitry that normally receives input from sweet-responsive taste cells.
One interpretation of the results is that P taste involves at least 2 transduction sites: At least one P-binding pocket in the T1R2+T1R3 dimer produces a hedonically negative signal, and an unknown P-sensitive GPCR produces a hedonically positive signal. We speculate that the positive signal dominates at low P concentrations, and the negative signal dominates at high P concentrations; their opposition thus accounts for the inverted U-shaped concentration-preference function observed in intact mice. In T1R2, T1R3, or T1R2+T1R3 KO mice, the hedonically negative signal is absent, leading to an increased avidity for P. But it is jeopardous to speculate about receptor mechanisms based solely on behavioral observations. We hope the current work will stimulate others to investigate P taste using molecular and electrophysiological methods that are better-suited to identify the underlying physiological mechanisms.
Supplementary material
Supplementary material are available at Chemical Senses online.
Funding
This work was supported by National Institutes of Health [R01 DK-46791 and DC-40099]. Genotyping was performed using equipment of the Monell Genotyping Core, and gustometry was performed using equipment of the Monell Phenotyping Core, which are supported by funding from National Institutes of Health [P30 DC-011735].
Supplementary Material
Acknowledgments
The authors thank Dr. R. Margolskee and Dr. K. Iwatsuki for their collaboration. Hillary Ellis and Tiffany Weiss provided superlative technical assistance. The SPF-Diana company generously provided trisodium pyrophosphate.
References
- Agnihotri MK, Pal UK. 1997. Effect of tetrasodium pyrophosphate (TSPP) on quality of chevon sausages. Ind J Anim Sci. 67:1000–1003. [Google Scholar]
- Blair-West JR, Denton DA, McKinley MJ, Radden BG, Ramshaw EH, Wark JD. 1992. Behavioral and tissue responses to severe phosphorus depletion in cattle. Am J Physiol. 263(3 Pt 2):R656–R663. [DOI] [PubMed] [Google Scholar]
- Boudreau JC. 1987. Mammalian neural taste responses to amino acids and nucleotides. In Kawamura Y, Kare MR, editor, Umami: a basic taste. New York: Marcel Dekker, p. 201–217. [Google Scholar]
- Boudreau JC, Hoang NK, Oravec J, Do LT. 1983. Rat neurophysiological taste responses to salt solutions. Chem Senses. 8:131–150. [Google Scholar]
- Boudreau JC, Nelson TE. 1977. Chemical stimulus determinants of cat geniculate ganglion chemoresponsive group I unit discharge. Chem Senses Flavour. 2:353–374. [Google Scholar]
- Boudreau JC, Oravec JJ, Hoang NK. 1982. Taste systems of goat geniculate ganglion. J Neurophysiol. 48(5):1226–1242. [DOI] [PubMed] [Google Scholar]
- Brand J, Bryant BP, Niceron C, Levesque A, Guillier I. 2013. Cats’ preference for pyrophosphates and search for a feline taste receptor using molecular biology http://www.petfoodindustry-digital.com/201211/Default/9/1#&pageSet=9&contentItem=0.
- Brunner JJ. 2001. Applying tetrasodium pyrophosphate. US Patent no: 6254920 B1. [Google Scholar]
- Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, Zuker CS. 2010. The cells and peripheral representation of sodium taste in mice. Nature. 464(7286):297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitelli R, Ziambaras K. 2011. Calcium and phosphate homeostasis: concerted interplay of new regulators. J Endocrinol Invest. 34(7 Suppl):3–7. [PubMed] [Google Scholar]
- Czarnogorski M, Woda CB, Schulkin J, Mulroney SE. 2004. Induction of a phosphate appetite in adult male and female rats. Exp Biol Med (Maywood). 229(9):914–919. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 301(5634):850–853. [DOI] [PubMed] [Google Scholar]
- De Ratauld A, Fournier M, Palmer KR, Long DJ. 2015. Taste and palatability of pyrophosphates by rats: a sensorial qualitative and quantitative approach. Association for Chemoreception Sciences Annual Meeting, Bonita Springs, FL. [Google Scholar]
- Denton D. 1984. The hunger for salt: An anthropological, physiological and medical analysis. Berlin:Springer-Verlag. [Google Scholar]
- DuBois GE. 2016. Molecular mechanism of sweetness sensation. Physiol Behav. 164(Pt B):453–463. [DOI] [PubMed] [Google Scholar]
- Green HH. 1925. Perverted appetites. Physiol Rev. 5:336–348. [Google Scholar]
- Hisatsune C, Yasumatsu K, Takahashi-Iwanaga H, Ogawa N, Kuroda Y, Yoshida R, Ninomiya Y, Mikoshiba K. 2007. Abnormal taste perception in mice lacking the type 3 inositol 1,4,5-trisphosphate receptor. J Biol Chem. 282(51):37225–37231. [DOI] [PubMed] [Google Scholar]
- Holcombe DJ, Roland DA, Sr, Harms RH. 1976. The ability of hens to regulate phosphorus intake when offered diets containing different levels of phosphorus. Poult Sci. 55(1):308–317. [DOI] [PubMed] [Google Scholar]
- International Food Additives Council 2013. Phosphates use in foods http://www.foodadditives.org/phosphates/phosphates_used_in_food.html.
- Iwatsuki K, Nomura M, Shibata A, Ichikawa R, Enciso PL, Wang L, Takayanagi R, Torii K, Uneyama H. 2010. Generation and characterization of T1R2-LacZ knock-in mouse. Biochem Biophys Res Commun. 402(3):495–499. [DOI] [PubMed] [Google Scholar]
- Kuhn C, Bufe B, Winnig M, Hofmann T, Frank O, Behrens M, Lewtschenko T, Slack JP, Ward CD, Meyerhof W. 2004. Bitter taste receptors for saccharin and acesulfame K. J Neurosci. 24(45):10260–10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffitte A, Neiers F, Briand L. 2014. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. 17(4):379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski BC, Sukumaran SK, Margolskee RF, Bachmanov AA. 2016. Amiloride-insensitive salt taste is mediated by two populations of Type III taste cells with distinct transduction mechanisms. J Neurosci. 36(6):1942–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, et al. 2005. Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS Genet. 1(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughey SA, Giza BK, Tordoff MG. 2007. Taste and acceptance of pyrophosphates by rats and mice. Am J Physiol Regul Integr Comp Physiol. 292(6):R2159–R2167. [DOI] [PubMed] [Google Scholar]
- Meyer-Gerspach AC, Wölnerhanssen B, Beglinger C. 2014. Gut sweet taste receptors and their role in metabolism. Front Horm Res. 42:123–133. [DOI] [PubMed] [Google Scholar]
- Nie Y, Vigues S, Hobbs JR, Conn GL, Munger SD. 2005. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr Biol. 15(21):1948–1952. [DOI] [PubMed] [Google Scholar]
- Ohnishi R, Segawa H, Kawakami E, Furutani J, Ito M, Tatsumi S, Kuwahata M, Miyamoto K. 2007. Control of phosphate appetite in young rats. J Med Invest. 54(3-4):366–369. [DOI] [PubMed] [Google Scholar]
- Oka Y, Butnaru M, von Buchholtz L, Ryba NJ, Zuker CS. 2013. High salt recruits aversive taste pathways. Nature. 494(7438):472–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, Helfrick S. 1943. Decreased phosphorus appetite of parathyroidectomized rats. Endocrinol. 33:349–352. [Google Scholar]
- Richter CP, Holt LE, Barelare B. 1938. Nutritional requirements for normal growth and reproduction in rats studied by the self-selection method. Am J Physiol. 122:734–744. [Google Scholar]
- Roldán M, Antequera T, Pérez-Palacios T, Ruiz J. 2014. Effect of added phosphate and type of cooking method on physico-chemical and sensory features of cooked lamb loins. Meat Sci. 97(1):69–75. [DOI] [PubMed] [Google Scholar]
- Shao CC, Stammer Y. 2005. Potassium pyrophosphate pet food palatability enhancers. Patent: US 2005/0170067 A1. [Google Scholar]
- Siu GM, Hadley M, Agwu DE, Draper HH. 1984. Self-regulation of phosphate intake in the rat: the influence of age, vitamin D and parathyroid hormone. J Nutr. 114(6):1097–1105. [DOI] [PubMed] [Google Scholar]
- Siu GM, Hadley M, Draper HH. 1981. Self-regulation of phosphate intake by growing rats. J Nutr. 111(9):1681–1685. [DOI] [PubMed] [Google Scholar]
- Sutcliffe AJ. 1973. Similarity of bones and antlers gnawed by deer to human artefacts. Nature. 246(5433):428–430. [DOI] [PubMed] [Google Scholar]
- Sweeny JM, Seibert HE, Woda C, Schulkin J, Haramati A, Mulroney SE. 1998. Evidence for induction of a phosphate appetite in juvenile rats. Am J Physiol. 275(4 Pt 2):R1358–R1365. [DOI] [PubMed] [Google Scholar]
- Takeda E, Taketani Y, Sawada N, Sato T, Yamamoto H. 2004. The regulation and function of phosphate in the human body. Biofactors. 21(1-4):345–355. [DOI] [PubMed] [Google Scholar]
- Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. 2013. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 495(7440):223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler A, Green HH, Toit PJ. 1924. Phosphorus in the livestock industry. J Dept Agricul S Africa. 8:460–504. [Google Scholar]
- Tordoff MG. 2008. Gene discovery and the genetic basis of calcium consumption. Physiol Behav. 94(5):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Alarcón LK, Valmeki S, Jiang P. 2012. T1R3: a human calcium taste receptor. Sci Rep. 2:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Aleman TR, McCaughey SA. 2014a. Heightened avidity for trisodium pyrophosphate in mice lacking Tas1r3. Chem Senses. 40(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Bachmanov AA. 2001. Monell mouse taste phenotyping project www.monell.org/MMTPP.
- Tordoff MG, Ellis HT. 2013. Taste dysfunction in BTBR mice due to a mutation of Itpr3, the inositol triphosphate receptor 3 gene. Physiol Genomics. 45(18):834–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Ellis HT, Aleman TR, Downing A, Marambaud P, Foskett JK, Dana RM, McCaughey SA. 2014b. Salty taste deficits in CALHM1 knockout mice. Chem Senses. 39(6):515–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordoff MG, Shao H, Alarcón LK, Margolskee RF, Mosinger B, Bachmanov AA, Reed DR, McCaughey S. 2008. Involvement of T1R3 in calcium-magnesium taste. Physiol Genomics. 34(3):338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugawa T, Konosu S, Kurihara K. 1992. Enhancing effects of NaCl and Na phosphate on human gustatory responses to amino acids. Chem Senses. 17:811–815. [Google Scholar]
- Villalba JJ, Provenza FD, Hall JO. 2008. Learned appetites for calcium, phosphorus, and sodium in sheep. J Anim Sci. 86(3):738–747. [DOI] [PubMed] [Google Scholar]
- Villalba JJ, Provenza FD, Hall JO, Peterson C. 2006. Phosphorus appetite in sheep: dissociating taste from postingestive effects. J Anim Sci. 84(8):2213–2223. [DOI] [PubMed] [Google Scholar]
- Wauson EM, Lorente-Rodríguez A, Cobb MH. 2013. Minireview: nutrient sensing by G protein-coupled receptors. Mol Endocrinol. 27(8):1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens SL, Waller RK. 1941. Voluntary intake of calcium and phosphorus in partially nephrectomized and parathyroidectomized rats. Endocrinol. 28:828–834. [Google Scholar]
- Wu CK, Ramsey CB, Davis GW. 1990. Effects of infused glucose, sodium and potassium chlorides and polyphosphates on palatability of hot-boned pork. J Anim Sci. 68(10):3212–3216. [DOI] [PubMed] [Google Scholar]
- Xiong YL, Kupski DR. 1999. Time-dependent marinade absorption and retention, cooking yield, and palatability of chicken filets marinated in various phosphate solutions. Poult Sci. 78(7):1053–1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.