Abstract
Cigarettes are an often-used consumer product, and flavor is an important determinant of their product appeal. Cigarettes with strong nontobacco flavors are popular among young people, and may facilitate smoking initiation. Discriminating flavors in tobacco is important for regulation purposes, for instance to set upper limits to the levels of important flavor additives. We provide a simple and fast method to determine the human odor difference threshold for flavor additives in a tobacco matrix, using a combination of chemical and sensory analysis. For an example, the human difference threshold for menthol odor, one of the most frequently used tobacco flavors, was determined. A consumer panel consisting of 20 women compared different concentrations of menthol-flavored tobacco to unflavored cigarette tobacco using the 2-alternative forced choice method. Components contributing to menthol odor were quantified using headspace GC-MS. The sensory difference threshold of menthol odor corresponded to a mixture of 43 (37–50)% menthol-flavored tobacco, containing 1.8 (1.6–2.1) mg menthol, 2.7 (2.3–3.1) µg menthone, and 1.0 (0.9–1.2) µg neomenthyl acetate per gram of tobacco. Such a method is important in the context of the European Tobacco Product Directive, and the US Food and Drug Administration Tobacco Control Act, that both prohibit cigarettes and roll-your-own tobacco with a characterizing flavor other than tobacco. Our method can also be adapted for matrices other than tobacco, such as food.
Keywords: consumer research, difference testing, headspace GC-MS, novel sensory method, odor discrimination, tobacco flavor
Introduction
The tobacco industry has become a major economic force in many industrial and developing countries (Abdallah 2004). Tobacco manufacturers stimulate tobacco use by making tobacco products as attractive as possible, for instance by using flavor additives. Tobacco products with a flavor, such as vanilla, menthol, or cherry, stimulate young and inexperienced people to start smoking (Talhout et al. 2016). To diminish tobacco product attractiveness, the new European tobacco product directive (TPD) 2014/40/EU prohibits cigarettes and roll-your-own tobacco products with a “characterizing flavor”, defined as a “clearly noticeable smell or taste other than one of tobacco, resulting from an additive or a combination of additives, including, but not limited to, fruit, spice, herb, alcohol, candy, menthol, or vanilla, which is noticeable before or during the consumption of the tobacco product” (European Union 2014).
Recently, the Health Effects Tobacco Composition (HETOC) Consortium has proposed to assess characterizing flavors by using a combination of headspace gas chromatography–mass spectrometry (GC-MS), and a trained expert panel that assesses odors by smelling tobacco samples (HETOC Consortium 2016). HETOC advised a smelling procedure for technical and ethical reasons. Smelling experiments are less time-consuming, less expensive and probably more sensitive than smoking experiments. In addition, smoking experiments relate to complex ethical issues and health hazards.
Besides odors that can be clearly discriminated, many flavors are present in tobacco at lower concentrations. Such flavors are not characterizing or clearly noticeable, but most likely also contribute to the attractiveness of smoking. For instance, already at low levels that do not impart a characterizing flavor, cocoa affects cigarette flavor and improves product acceptability (Sokol et al. 2014). We describe a simple and fast method to determine the minimal concentration of flavor components in tobacco products that is discriminated by humans. As such, our method allows to detect flavors at a concentration that is just noticeable by consumers, and can also be used as a first screening, before a more expensive expert panel will be composed for legislative purposes.
As an example, the sensory difference threshold of menthol odor, one of the most frequently used tobacco flavors, was determined using a combination of sensory and chemical analysis. Olfactory thresholds can be measured through discrimination between stimuli, for example discriminating a target from a blank (Hedner et al. 2010), and is a function of odorant concentration (Wright and Smith 2004). In our study, a consumer panel discriminated target samples with different concentrations of menthol-flavored tobacco from blank samples with tobacco of the same brand without a characterizing menthol flavor, hereafter called unflavored tobacco. To this purpose, the 2-alternative forced choice (2-AFC) method was used, (Gescheider 1997; Wysocki and Wise 2004). Forced-choice approaches are commonly used to measure sensory thresholds (Lawless and Heymann 2010; Wysocki and Wise 2004). For instance, bitterness of chocolate milk has been measured using this method (Harwood et al. 2012). Linschoten et al. (2001) considered this method to be reliable and precise, since the effect of response bias is minimized.
In the second part of this study, chemical analysis was used to establish the menthol concentration representing the sensory threshold discriminated by the panel. Headspace GC-MS was used to quantify the additives contributing to the menthol odor of tobacco. Because of the volatility of flavor and odor molecules and the complexity of natural products such as food or tobacco, GC-MS is well suited for this type of analysis. For instance, several components that contribute to the flavor of tobacco leaves have been identified with GC-MS (Hasebe and Suhara 1999). More recently, liquid GC-MS analysis showed a high degree of similarity between the flavorings found in candies and those of flavored tobacco products (Brown et al. 2014), and headspace GC-MS has been used to analyze strawberry-flavored tobacco products (Paschke et al. 2015). Headspace GC-MS is also extensively used in the food industry for flavor analysis, for instance in the analysis of beverages (Montesinos and Gallego 2014; Xiao et al. 2015), and olive oil (Cecchi and Alfei 2013). Taste and aroma in a tobacco matrix can also be assessed by GC-MS olfactometry (Frauendorfer et al. 2008). After separation by GC, the sample is split into 2 detectors, a human assessor, and an MS. For our research, we are however interested in the flavor and odor of the entire tobacco product caused by the combination of flavor compounds, not in those of the separate flavor compounds. By combining chemical and sensory analysis, the human difference threshold of odor components that have been added to tobacco products can be established. This difference threshold can be used for regulatory purposes, for example to set a maximum allowable level for flavor additives. This study has been performed in a matrix of tobacco, but our method can also be applied to other matrices, such as food.
Materials and methods
Two types of cigarette tobacco, a menthol and a unflavored variant of the same brand, were used for this study. Eleven mixtures of menthol and unflavored tobacco, taken from cigarette packs recently purchased, were prepared, varying from 0 to 100% menthol-flavored tobacco.
Sensory analysis
Twenty non-smoking female volunteers, aged between 20 and 51 years, participated in the sensory consumer panel. The panelists participated individually in 3 smelling sessions on 3 different days within 4 weeks. The participants gave informed consent. The Clinical Expertise Center (KEC, contact: mensgebonden-onderzoek@rivm.nl) at RIVM has assessed and approved the study protocol (RIVM study number GBO-318), and considered the individual burden for the subjects negligible. The study complies with the Declaration of Helsinki for Medical Research involving Human Subjects.
Smelling sessions occurred at room temperature in a large office, to make sure that the odor molecules of the previous cigarette were diffused before the next cigarette was tested. Furthermore, between each smelling sessions, the research subjects waited 1 min to allow the sensitivity of their nostrils to be recovered (Linschoten et al. 2001; Wysocki and Wise 2004). The sessions were performed using the 2-alternative forced choice (2-AFC) method. In each session, the panelists first smelled pure menthol tobacco and unflavored tobacco. This allows them to become accustomed to the odor of menthol and unflavored tobacco, and the relative intensity of menthol odor in the menthol-flavored tobacco. Subsequently, the panelists compared each of the 11 tobacco mixtures against the blank samples containing tobacco of unflavored cigarettes. The panel members were asked to indicate which of the 2 samples contained menthol odor.
The samples containing 0.7 g of each tobacco mixture in a 20-mL glass vial were closed immediately after preparation and stored in the dark at room temperature when not used. The vials of the mixtures as well as the unflavored tobacco were labeled with a random 3-digit code to avoid sequence bias. The tobacco mixtures were randomly paired with the vials containing unflavored tobacco. These 11 pairs were put next to each other in a random sequence. The research subjects were asked to randomly select 1 of the 11 pairs from that sequence in order to test them. For each research participant, a fresh sample of the 11 tobacco mixtures was prepared that was used during each of the 3 experiments. The unflavored tobacco samples were presented multiple times, and refreshed every week of the 4-week experiment to avoid loss of tobacco odor due to frequent use. All samples were produced from the same batch of menthol tobacco and unflavored tobacco respectively.
Chemical analysis
Reagents and equipment
The reference compounds (-)-menthol, menthone, menthyl acetate, and p-xylene-d10 were of analytical grade and purchased from Sigma-Aldrich (Zwijndrecht, the Netherlands). Methanol came from Biosolve BV (Valkenswaard, the Netherlands).
Headspace GC-MS analysis was performed on an Agilent 7890B gas chromatograph equipped with a 5977 single quadrupole mass spectrometer (Agilent Technologies, Amstelveen, the Netherlands) and a multi-purpose sampler (MPS-2, Gerstel, Mühlheimander Ruhr, Germany).
Sample preparation
The tobacco was pulverized by hand using a mortar and pestle immediately after opening the package. A tobacco sample was weighed and placed in a glass vial. Approximately 20 mg of tobacco was used to quantify menthol and 200 mg of tobacco was used for quantification of menthone and neomenthyl acetate. As internal standard, 50 µL of p-xylene-d10 (948 µg/µL in methanol for measurement of menthol, and 474 µg/µL for menthone and neomenthyl acetate) was then added to the sample. The vials were immediately capped and measured. Samples were measured in duplicate. Between tobacco samples, 2 air samples were run to eliminate carry over.
Headspace GC-MS parameters
The tobacco samples were incubated for 30 min in an agitator oven at a temperature of 140 °C. Subsequently, a volume of 1 mL of vapor was injected on the GC column using a 1.5-mL HS syringe (Gerstel). The syringe temperature was 130 °C.
An HP-5ms Ultra Inert capillary column (30 × 0.25 × 0.25 µm, Agilent) was used. The GC conditions were as follows: split ratio: 1:100 (menthol) or 1:20 (other components); split flow: 150 ml/min (menthol) or 30 mL/min (other components). GC oven temperature program: initial: 50 °C; gradient: 10 °C/s; end: 200 °C; hold time: 5.0 min. Total run time was 20 min. Helium was used as carrier gas at a constant flow of 1.5 mL/min. The injection temperature was 250 °C.
The MS settings were as follows: acquisition mode: full scan (m/z 40–500); transfer line: 280 °C; ionization mode: EI; ionization voltage: 70 eV; ion source: 230 °C; quadrupole: 150 °C; solvent delay: 3 min; data acquisition rate: 3.2 scans/s. Chromatograms were processed with GC-MS MassHunter Workstation software (Agilent Technologies).
Identification and quantification of components responsible for menthol odor
Peaks were identified using the Flavors and Fragrances of Natural and Synthetic Compounds, version 2 (FFNSC2) mass spectral library. Components with a peak-to-peak signal-to-noise ratio below 3 were discarded, corresponding to a “SNR Ratio” of 55 as indicated by the Automated Mass Spectral Deconvolution and Identification System (AMDIS) software. “Weighted” and “reverse” scores are AMDIS software-specific values associated with the probability (%) of correct identification. Components with a “weighted” and/or “reverse” score below 70% were excluded. Components described as menthol-like according to the Leffingwell flavor database were included (Leffingwell et al. 2012). These components (menthol, menthone, and neomenthyl acetate) were identified and quantified.
Calibration curves of menthol and menthone were generated. A calibration curve of menthyl acetate was used for quantification of neomenthyl acetate. Calibration samples were made in methanol as follows: menthol 522.5–6270.0 µg/mL; menthone 14.0–48.0 µg/mL; and menthyl acetate from 5.8 to 115.3 µg/mL. These samples and p-Xylene-d10 (948 µg/µL in methanol for measurement of menthol, 474 µg/µL for menthone and menthyl acetate) were added to tobacco of the unflavored cigarette (20 or 200 mg, respectively) in a glass headspace vial. The vial was immediately capped to avoid loss of volatile flavor components. For each component, 7 calibration levels were measured in duplicate. Calibration curves of respectively menthol (R2 = 0.994), menthone (R2 = 0.995), and menthyl acetate (R2 = 0.995) were made using GC-MS MassHunter Workstation software. Calibration curves were linear, forced through origin, with no weighted points. Specifications of the method are shown in Table 1.
Table 1.
Specifications of the analytical method used to measure (−)-menthol, menthone, and menthyl acetate
| Component | Recovery (%) | Internal standard | Internal standard (µg/µL) | CAS number | Retention time (min) | Quantifier (m/z) | Qualifier (m/z) |
|---|---|---|---|---|---|---|---|
| (−)-Menthol | 99.8 | p-xylene-d10 | 948 | 1490-04-6 | 9.44 | 95.1 | 81.1 71.1 123.2 138.1 |
| Menthone | 89.4 | p-xylene-d10 | 474 | 14073-97-3 | 9.19 | 112.1 | 139.2 154.1 97.1 69.1 |
| Menthyl acetate | 96.1 | p-xylene-d10 | 474 | 89–485 | 11.16 | 95.1 | 123.2 138.1 81.1 67.0 |
p-xylene-d10 was used as internal standard for all 3 components. The selected quantifiers were the most abundant for each component.
Statistical analysis
Data were analyzed using R statistical software. For each of the tobacco mixtures, the percentage of correctly identified samples was calculated, along with the 95% confidence interval (CI). The sensory difference threshold was determined by logistic regression on the sensory data, with boundary conditions being 50% (the percentage correct answers in case of random guessing) and 100% (the maximum percentage correct answers). The regression model was used to determine the sensory difference limit and its 95% CI at 75% correct answers, which corresponds to a majority of correct identification after correcting for random guessing (Linschoten et al. 2001; Ulrich and Miller 2004).
Results
Sensory analysis
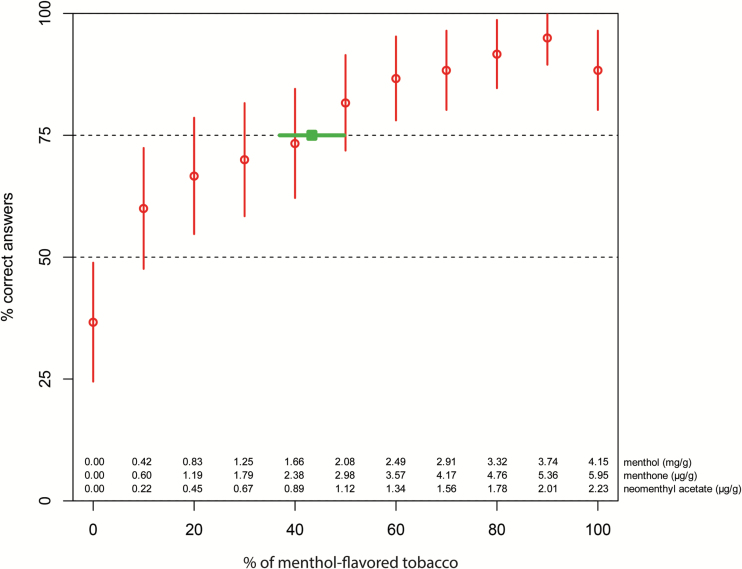
Because no significant differences between the smelling sessions were found (P = 0.5), the data from individual smelling sessions were combined. The results of the sensory analysis (n = 60) are shown in Figure 1. The consumer panel was found to discriminate menthol odor in a tobacco mix of menthol-flavored and unflavored tobacco containing at least 43% menthol-flavored tobacco with a 95% CI from 37 to 50%.
Figure 1.
Results of the sensory experiment (n = 60). The percentages of menthol-flavored tobacco in the tested tobacco mixture (x-axis) are plotted against the percentage of correct answers (y-axis). Red circles indicate the percentage of correct answers, with red bars indicating the 95% CI. Horizontal dotted lines represent 50% (random guessing) and 75% (majority correct after correcting for random guessing) correct answers, respectively. Green box and bar: sensory limit and 95% CI at which menthol odor can be determined. The calculated concentration of menthol, menthone, and neomenthylacetate per gram of tobacco is shown in the table for each mixture.
In the test with 0% menthol tobacco, 22 out of 60 panelists identified the “correct” instead of the “alternative” vial. Given that the content of these vials was the same, one might expect this percentage to be close to 50% instead of the 37% found here, but the overall deviation from 50% is not significant (P > 0.05, 2-sided binomial test). As could be expected, the percentage of correct answers increases with an increasing percentage of menthol-flavored tobacco (Figure 1). However, for the sample containing 100% menthol tobacco, the percentage of correct answers is 53/60 = 88.3%, which is lower than the value of 57/60 = 95% for the sample containing 90% menthol tobacco. Again, this is not a significant difference (P > 0.05, Fisher’s exact test).
Chemical analysis
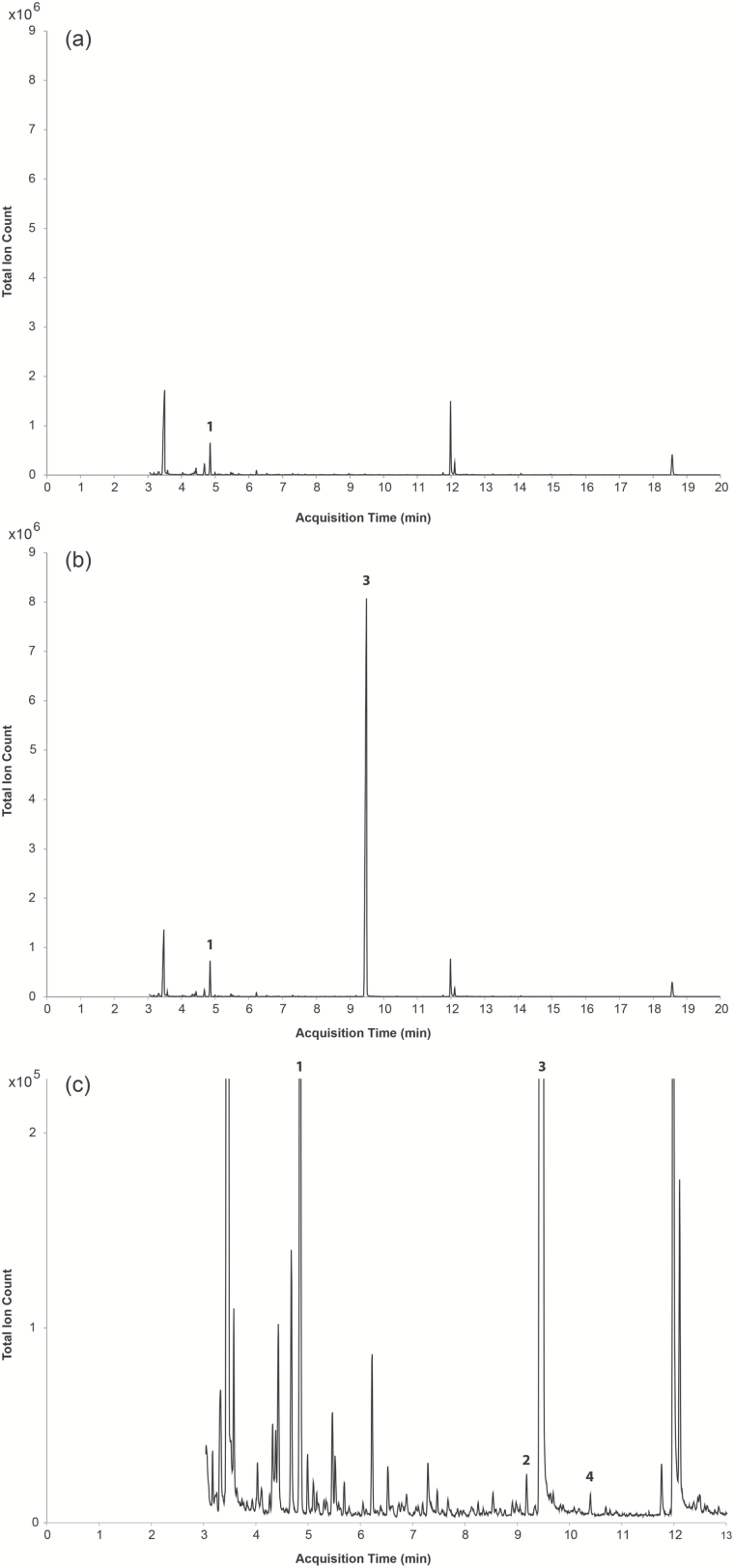
Typical chromatograms of mentholated and unflavored tobacco are shown in Figure 2. Although menthol saturates the column and detector when 200 mg of the tobacco sample is used, this was required to allow detection of menthone and neomenthyl acetate.
Figure 2.
Three chromatograms of 200 mg tobacco measured with a split ratio of 1:20. (a) the chromatogram of unflavored tobacco; (b) the chromatogram of menthol-flavored tobacco; (c) the chromatogram of menthol-flavored tobacco zoomed in to show the peaks representing menthone and neomenthyl acetate. At this concentration (200 mg), menthol saturates the column and detector. However, this relatively large quantity of tobacco is required to detect menthone and neomenthyl acetate. Peak identification: 1, p-xylene-d10 (internal standard); 2, menthone; 3, menthol; 4, neomenthyl acetate.
The concentrations of menthol, menthone, and neomenthyl acetate were determined by headspace GC–MS in 11 mixtures containing 0–100% menthol-flavored tobacco (Supplementary Figure S1). From these measurements, it can be calculated that a mixture containing 43% menthol-flavored tobacco (the human sensory difference limit) contains 1.8 mg menthol, 2.5 µg menthone and 1.0 µg neomenthyl acetate per gram of tobacco mixture.
Discussion
Design of experiment
In this study, we established a difference threshold of menthol odor in a flavored tobacco product as an example of our method that allows to detect flavors at a concentration that is just noticeable by consumers. The TPD states that a characterizing flavor “is noticeable before or during the consumption of the tobacco product” (European Union 2014). We performed smelling experiments because they are less time-consuming, less expensive, and probably more sensitive than smoking experiments. In addition, the complex ethical issues and health hazards associated with smoking experiments are avoided. However, only the odor before consumption is assessed, and not the flavor during consumption. Some flavors may result from tobacco burning (Talhout et al. 2016), although this is not expected for menthol.
Worch et al. (2010) found that consumer and expert sensory panels generally give similar results. We chose a consumer panel, because it resembles the natural situation more closely than a trained expert panel. In addition, it is less time-consuming and more cost-effective. Because olfactory function strongly decreases above the age of 55 years (Hummel et al. 2007), and woman are superior in odor identification compared to men (Larsson et al. 2004), the sensory panel of this experiment consisted of women aged between 20 and 51 years. Although the volunteers RIVM employee or interns, we see no reason to believe that our volunteers are in some way different from other consumers, even more so, because we performed odor discrimination experiments rather than testing the acceptance of the product.
We used commercially available menthol and commercially available unflavored cigarettes of the same brand to determine the sensory difference threshold of menthol odor. This study design allows a more realistic scenario compared to a design in which tobacco from natural tobacco leaves or from a commercially available product is spiked with an analytical standard of the odor additive of interest. Since most odors consist of multiple odor components, spiking requires a complex mix identical to the odor of the commercially available tobacco product of interest. This makes spiking a more complex approach, hence our approach is more realistic and convenient. Preliminary analysis with headspace GC-MS (not shown) indicated that the chemical composition of tobacco from commercially available menthol and unflavored cigarettes is the same, except for the flavor components responsible for menthol odor (menthol, menthone, and neomenthyl acetate).
Determination of sensory difference threshold
This study shows that chemical and sensory analysis can be combined to determine a human sensory difference threshold of odors in tobacco products. Components in flavored tobacco products contributing to the odor other than tobacco were identified using AMDIS and the Leffingwell Flavor Base—Tobacco version (Leffingwell et al. 2012). Components with a menthol-like description (menthol, menthone, and neomenthyl acetate) were quantified in order to determine the olfactory difference threshold of menthol odor in menthol-flavored tobacco (Supplementary Figure S1).
It is important to keep in mind that this difference threshold is specific for the tested brands. Other brands of tobacco products might consist of a different combination of components that give rise to its menthol odor.
Application of this method to more complex odors
Menthol odor of the tested brand is a relatively simple odor, consisting of 3 menthol-specific components. Other cigarettes may have more than 3 components that are responsible for their odor and flavor. For instance, it is known that strawberry, cherry, and other fruity flavors consist of multiple constituents (Brown et al. 2014; Paschke et al. 2015), which makes quantification of flavor additives in fruity-flavored tobacco products more complex.
If this method is used for analysis of other brands that do not have a unflavored form besides the flavored one, the flavored product could be diluted with a manually composed tobacco blend that is similar to the blend of the flavored product of interest. This requires that the blend of the flavored tobacco product is known, which is compulsory information to be provided by the manufacturer since the implementation of the new TPD. Alternatively, although more complex, tobacco leaves can be spiked with flavorant, using the exact flavorant mixture that is present in the flavored tobacco product.
Results compared to ingredient list of manufacturers
We found that one menthol-flavored cigarette contains 2.79 (2.71–2.84, 95% CI) mg menthol (Supplementary Figure S1, assuming 672 mg of tobacco per cigarette), which is less than the amount added by the manufacturer (3.9 mg) (RIVM 2013). The loss of menthol can be explained by partial evaporation prior to chemical analysis, for example during processing in the tobacco factory, or during storage of the tobacco products. Another explanation is that the composition of the tobacco product changed between 2013 and 2015 when the products were, respectively, registered in the ingredient database and purchased for this experiment.
Other components contributing to menthol odor
In addition to menthol, we noted the presence of menthone and neomenthyl acetate in the menthol-flavored tobacco sample (respectively, 5.95 µg and 2.23 µg per gram of tobacco). Furthermore, minor peaks of isomenthone and menthyl acetate were observed, but these components could not be reliably quantified because the signal-to-noise ratio was too low.
Conclusions and applications
Our study demonstrated how to determine a human difference threshold of a certain odor in tobacco products using sensory and chemical (headspace GC-MS) analysis. The current method can be adapted for matrices other than tobacco, such as food. We used a consumer panel, as this is fast, efficient, and resembles the natural situation more closely than a trained expert panel. For an example, we determined the difference threshold of menthol odor in commercially available menthol-flavored cigarettes, because menthol is one of the most frequently used tobacco flavors. Headspace GC-MS was used to identify and quantify the additives imparting this flavor, in this case menthol, menthone, and neomenthyl acetate. Olfactory difference thresholds of flavor additives can be used for regulation of characterizing flavors in tobacco products, for instance as a basis for dictating maximum allowed levels of specific flavor additives. Our method also allows detection of flavors at a concentration that is just noticeable by consumers. Such flavors are not characterizing or clearly noticeable, but most likely also contribute to the attractiveness of smoking, as for example shown in a study on cocoa as tobacco additive (Sokol et al. 2014). To study such effects, research with low doses of additives in both smokers and nonsmokers would therefore be interesting, for example effects on the brain reward system, or sensory acceptance studies (Talhout et al. 2016).
Our method is relevant for implementation of the European Tobacco Product Directive, and the US Food and Drug Administration Tobacco Control Act, that both prohibit cigarettes and roll-your-own tobacco with a characterizing flavor other than tobacco. Eventually this should lead to a database of difference thresholds, which may be used to establish whether a tobacco product has a characterizing flavor on the basis of analytical chemical data alone. This reduces the need for expensive human sensory panels. Still, for new flavors, and to prevent manufacturers from evading allowed levels by using several additives that individually remain below the allowable limit but together cause a strong characterizing odor, sensory analysis will always be required in flavor analysis for regulatory purposes.
Supplementary material
Supplementary data are available at Chemical Senses online
Funding
This work was supported by the Dutch Ministry of Health, Welfare and Sport (project V/050057).
Conflict of interest
Pieter Punter is the research director of OP&P Product Research BV. There are no other potential conflicts of interest.
Supplementary Material
Acknowledgements
The authors are grateful to Jeroen Pennings for his generous contribution to the statistical part of this research. The technical assistance of Ramon Ramlal concerning the usage of chemical software is gratefully acknowledged. The authors wish to thank Eugène Jansen and Peter Keizers for their critical feedback.
References
- Abdallah FM. 2004. Cigarette product development. Blending and processing know-how, sensory testing of cigarette smoke. Tobacco Reporter, Raleigh. [Google Scholar]
- Brown JE, Luo W, Isabelle LM, Pankow JF. 2014. Candy flavorings in tobacco. N Engl J Med. 370:2250–2252. [DOI] [PubMed] [Google Scholar]
- Cecchi T, Alfei B. 2013. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME-GC-MS: newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem. 141:2025–2035. [DOI] [PubMed] [Google Scholar]
- European Union. 2014. Directive 2014/40/EU of the European Parliament. Available from:http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ%3AJOL_2014_127_R_0001. [Google Scholar]
- Gescheider GA. 1997. Psychophysics: the fundamentals. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Harwood ML, Ziegler GR, Hayes JE. 2012. Rejection thresholds in chocolate milk: evidence for sementation. Food Qual Prefer. 26:128–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe H, Suhara S. 1999. The quality estimation of different tobacco types examined by headspace vapor analysis. Beiträge zur Tabakforschung Int. 18:213–222. [Google Scholar]
- Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. 2010. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 32:1062–1067. [DOI] [PubMed] [Google Scholar]
- HETOC Consortium 2016. Mapping of best practices and development of testing methods and procedures for identification of characterising flavours in tobacco products. Available from: http://eceuropaeu/health/tobacco/docs/hetoc_frep_enpdf. [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A. 2007. Normative data for the ‘‘Sniffin’ Sticks’’ including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 264:237–243. [DOI] [PubMed] [Google Scholar]
- Larsson M, Nilsson LG, Olofsson JK, Nordin S. 2004. Demographic and cognitive predictors of cued odor identification: evidence from a population-based study. Chem Senses. 29:547–554. [DOI] [PubMed] [Google Scholar]
- Lawless HT, Heymann H. 2010. Sensory evaluation of food - principles and practices. Chapter 6 - Measurement of sensory thresholds series: Food Science Text Series. Newyork (NY): Springer. [Google Scholar]
- Leffingwell & Associates. . 2012. Flavor-Base 9 - Tobacco Version. Available from: http://www.leffingwell.com/tob2001.htm. [Google Scholar]
- Linschoten MR, Harvey LO, Eller PM, Jafek BW. 2001. Fast and accurate measurement of taste and smell thresholds using a maximum-likelihood adaptive staircase procedure. Percept Psychophys. 63:1330–1347. [DOI] [PubMed] [Google Scholar]
- Montesinos I, Gallego M. 2014. How the inclusion of treated water in beverages influences the appearance of halogenated volatile organic compounds. J Agric Food Chem. 62:10240–10247. [DOI] [PubMed] [Google Scholar]
- Paschke M, Hutzler C, Henkler F, Luch A. 2015. Toward the stereochemical identification of prohibited characterizing flavors in tobacco products: the case of strawberry flavor. Arch Toxicol. 89:1241–1255. [DOI] [PubMed] [Google Scholar]
- RIVM 2013. Toevoegingen tabaksproducten - Producten verkrijgbaar in Nederland. Available from: http://rivm.nl/toevoegingentabaksproducten/products.html. [Google Scholar]
- Sokol NA, Kennedy RD, Connolly GN. 2014. The role of cocoa as a cigarette additive: opportunities for product regulation. Nicotine Tob Res. 16:984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhout R, van de Nobelen S, Kienhuis AS. 2016. An inventory of methods suitable to assess additive-induced characterising flavours of tobacco products. Drug Alcohol Depend. 161:9–14. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Miller J. 2004. Treshold estimation in two-alternative forced-choice (2AFC) tasks: the Spearman-Kärber method. Percept Psychophys. 66:517–533. [DOI] [PubMed] [Google Scholar]
- Worch T, Lê S, Punter P. 2010. How reliable are the consumers? Comparison of sensory profiles from consumers and experts. Food Qual Prefer. 21:309–318. [Google Scholar]
- Wright GA, Smith BH. 2004. Different thresholds for detection and discrimination of odors in the honey bee (Apis mellifera). Chem Senses. 29:127–135. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Wise P. 2004. Handbook of flavor characterization: sensory analysis, chemistry, and physiology - chapter v measuring olfaction and chemesthesis. New York, NY: Marcel Dekker, Inc. [Google Scholar]
- Xiao Z, Zhou X, Niu Y, Yu D, Zhu J, Zhu G. 2015. Optimization and application of headspace-solid-phase micro-extraction coupled with gas chromatography–mass spectrometry for the determination of volatile compounds in cherry wines. J Chromatogr B. 978–979:122–130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.