Abstract
Interactions with the environment depend not only on sensory perception of external stimuli but also on processes of neuromodulation regulated by the internal state of an organism. These processes allow regulation of stimulus detection to match the demands of an organism influenced by its general brain state (satiety, wakefulness/sleep state, attentiveness, arousal, learning etc.). The sense of smell is initiated by sensory neurons located in the nasal cavity that recognize environmental odorants and project axons into the olfactory bulb (OB), where they form synapses with several types of neurons. Modulations of early synaptic circuits are particularly important since these can affect all subsequent processing steps. While the precise mechanisms have not been fully elucidated, work from many labs has demonstrated that the activity of neurons in the OB and cortex can be modulated by different factors inducing specific changes to olfactory information processing. The symposium “Neuromodulation in Chemosensory Pathways” at the International Symposium on Olfaction and Taste (ISOT 2016) highlighted some of the most recent advances in state-dependent network modulations of the mouse olfactory system including modulation mediated by specific neurotransmitters and neuroendocrine molecules, involving pharmacological, electrophysiological, learning, and behavioral approaches.
Keywords: hormonal status, learning, mouse olfactory system, network modulations, state-dependent, surprise and expectation, top–down inputs
Introduction
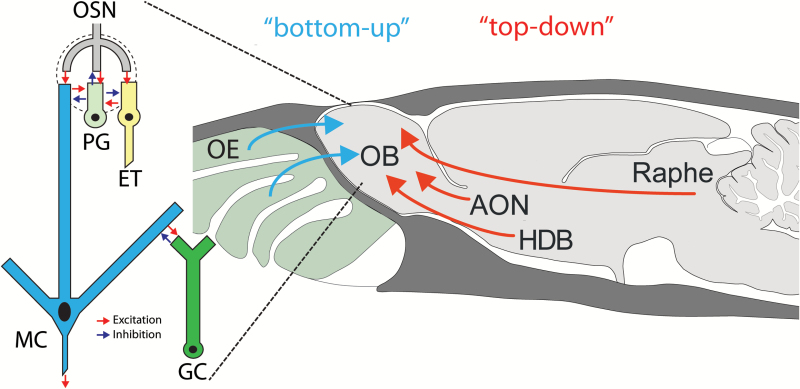
Sensory systems have evolved to transmit information about the external environment from the peripheral detection organ to the central nervous system. However, this process is not just a one-way transfer of information. Along the way are numerous sites for modulation of the sensory information, from the initial detection to behavior. In the olfactory system, olfactory sensory neurons (OSNs) in the nasal cavity detect volatile chemicals in the environment and transmit that information to the olfactory bulb (OB) (Figure 1). In recent years, mechanisms have been identified that modulate the detection and perception of this information, even at the periphery (Jiang et al. 2015). Modulation can occur at the level of OSN axons and at local interneurons and output neurons of the OB. Understanding all the different mechanisms requires the elucidation of the sources and types of top–down pathways that are present. To address this, Markus Rothermel presented data on some of the pathways involved.
Figure 1.
Diagram of bottom-up and top-down inputs into the olfactory bulb. Olfactory sensory neurons (OSNs) located in the olfactory epithelium project axons into the olfactory bulb (OB), transmitting sensory information to this brain region. OSN axons synapse with both projection neurons (mitral cells, MC) and interneurons (periglomerular, PG and external tufted, ET). Mitral cells also make connections with granule cells (GCs) and PGs. Several regions of the brain, including the Raphe, anterior olfactory nucleus (AON) and horizontal limb of the diagonal band of Broca (HDB) also project back to the OB (top–down) to modulate the perception of odors. Courtesy of A.C. Puche, modified from (Aungst et al. 2003) and “The Rat Nervous System” 2nd Edition.
Cortical and neuromodulatory inputs to the mouse OB
The OB receives centrifugal input from two major types of modulatory systems whose role in shaping early olfactory processing remains unclear: classical neuromodulatory inputs and cortical inputs. The functional investigation of these systems in vivo is complicated by the fact that most of these centers are comprised of heterogeneous cell populations and typically project to multiple brain areas that themselves are interconnected. The ability to drive optogenetic tools in restricted neuronal subpopulations and their axonal processes (Atasoy et al. 2008; Betley and Sternson 2011; Rothermel et al. 2013; Wachowiak et al. 2013) has enabled versatile new approaches to specifically investigate the contribution of selectively-targeted modulatory systems to sensory information processing.
To examine how neuromodulatory projections to the OB shape output neuron activity, optogenetic activation of the axons of cholinergic neurons projecting from the horizontal limb of the diagonal band of Broca (HDB) to the OB in ChAT (choline acetyltransferase)-Cre animals was used (Rothermel et al. 2014) (Figure 1). Cholinergic projections regulate OB output by increasing the spiking frequency of mitral/tufted cells, the principal OB output neurons. This modulation was rapid and transient. Cholinergic enhancement of mitral/tufted cell odorant responses was robust and occurred independent of the strength or even polarity of the odorant-evoked response, indicating that cholinergic modulation adds an excitatory bias to mitral/tufted cell responses as opposed to increasing response gain or sharpening response spectra. These findings contrast with a recent study that reported robust suppression of mitral/tufted cells spontaneous activity and preferential suppression of weak excitatory responses by optogenetic stimulation of cholinergic somata in the basal forebrain of anesthetized transgenic mice expressing channelrhodopsin-2 (ChR2) under control of the ChAT promoter (Ma and Luo 2012). Replication of the experimental setup in this publication, using the same mouse line, method of anesthesia and stimulation protocol, was not able to clarify all discrepancies however, it was clearly shown that axonal versus somatic stimulation (i.e. local vs. network) can affect modulation outcome.
To investigate another source of neuromodulatory input to the OB, activation of serotonergic centrifugal projections originating in the raphe nucleus in Slc6a4 (solute carrier family 6, serotonin transporter)-cre animals was used (Brunert et al. 2016) (Figure 1). Using extracellular recordings, activating serotonergic projections had a bidirectional effect on mitral/tufted cell firing under baseline conditions while modestly enhancing odor responses. These results are in general agreement with a recent publication that used an imaging approach and observed that mitral cell activity increases as well as decreases in response to raphe stimulation (Kapoor et al. 2016).
Projections to the OB from the anterior olfactory nucleus (AON) provide a major source of cortical feedback to the OB (Rothermel and Wachowiak 2014) (Figure 1). Selective expression of GCaMP in AON projection neurons revealed that odorants evoked large signals in the axon terminals in the OB that were transient and coupled to odorant inhalation both in the anesthetized and awake mouse. These data suggest that feedback from AON to the OB is rapid and robust across different brain states. In comparison to other work (Boyd et al. 2015; Otazu et al. 2015), showing cortical feedback projections to the OB originating in the piriform cortex have no spatial (odor-specific) organization, AON feedback projections produced odorant-specific patterns. The strength of AON feedback signals increased during wakefulness, suggesting a state-dependent modulation of cortical feedback. Finally, AON feedback projections were also activated when stimulating other neuromodulatory centers—for example, the HDB. These results point to the AON as a multifunctional cortical area that provides ongoing feedback to the OB and also serves as a descending relay for other neuromodulatory systems.
The work presented by Rothermel detailed the different pathways that provide top–down input to the OB. These pathways can be used by multiple, different behavior states, and by a large number of neurotransmitters and hormones. Following this talk, Debi Fadool presented work identifying the roles that specific neurohormones can play to regulate neuronal function and the pathways to which they converge.
Neuromodulation of OB activity driven by neuroendocrine hormones and diet-induced obesity
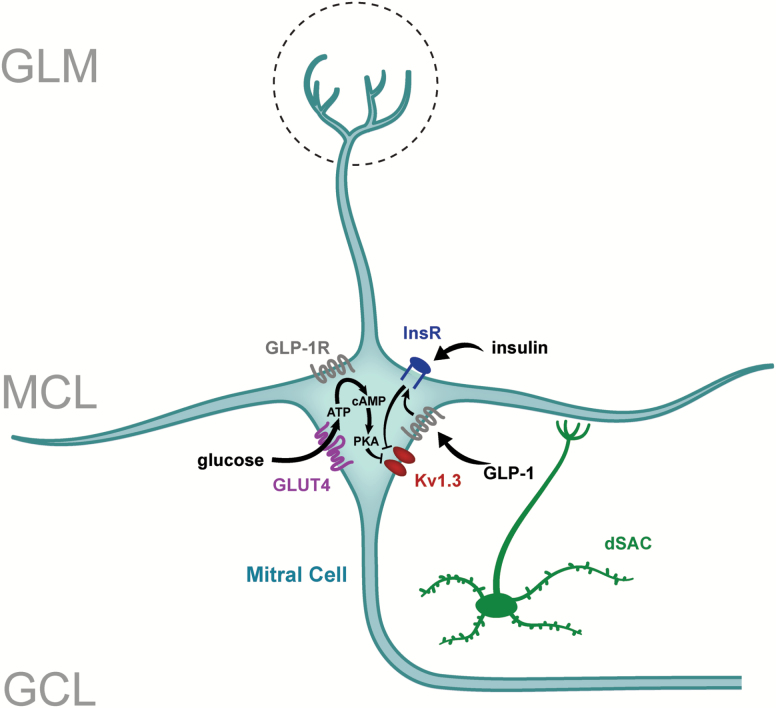
In mammals, olfactory perception has an intricate link with feeding behavior, reward and appetite. It has long been known that odors are a determinant for food choice or rejection, but also that feeding state, by controlling internal energy homeostasis, can regulate the perception of odors at both central and peripheral levels [for review see (Palouzier-Paulignan et al. 2012)]. In this scope, Fadool and colleagues investigated if an unbalanced energy homeostasis could disrupt olfactory anatomy and function. These studies found that mice challenged with fatty diets exhibit a loss of OSNs, a reduction in glomerular projections, an associated reduction in electro-olfactogram amplitude, and olfactory dysfunction in odor discrimination and odor reversal learning assessed by olfactometry (Tucker et al. 2012; Thiebaud et al. 2014). They currently hypothesize that the olfactory system is designed to encode external and internal chemical information, the latter being energy important molecules that modulate mitral cell firing frequency to give information about the state of nutrient availability. Over the years, the Fadool laboratory has found electrophysiological evidence for the modulation of the primary output neurons of the OB, mitral cells, by several nutrients and hormones, such as insulin (Fadool et al. 2000; Fadool et al. 2011), glucose (Tucker et al. 2010; Tucker et al. 2013), or more recently glucagon-like peptide-1 (GLP-1) (Thiebaud et al. 2016). All of these neurohormones or metabolic factors are found to modulate firing frequency of mitral cells by targeting the voltage-dependent potassium channel, Kv1.3, whereby channel inhibition leads to an increase in firing frequency (Figure 2). The consumption of food, changes in energy availability, or circulating/released changes in these metabolic factors could trigger differential phosphorylation or other post-translational modification of Kv1.3 to alter its biophysical properties leading to modulation in mitral cell excitability. Most recently they have revealed parallel lines of evidence for the expression of the GLP-1 receptor (GLP-1R) on mitral cells using immunocytochemistry, RT-PCR, peptide binding assays, and tracking of fluorescently-conjugated Exendin-4. These approaches have allowed the discovery that insulin and GLP-1 receptors are co-localized in mitral cells and thereby have the capacity to coordinate excitability. In current-clamp recordings of ex vivo OB slices, GLP-1 and its synthetic agonist, Exendin-4, increased firing frequency of mitral cells in a dose-dependent manner that is dependent upon Kv1.3 ion channel protein (Thiebaud et al. 2016). Spike analysis revealed that increased excitability was attributed to a decrease in the action potential pause duration (interburst interval) rather than interspike interval. Those results were also confirmed by using voltage ramps and ion substitution to support a decrease in potassium conductance following GLP-1 application, which was not present in transgenic mice deficient for Kv1.3. Interestingly, a transgenic mouse line with the preproglucagon promoter (PPG) upstream of a YFP reporter (Reimann et al. 2008) revealed a unique class of deep short axon cells (dSACs) that may be the source of GLP-1 peptide in the OB. In order to further characterize the dSACs, a Cre/lox recombination technology was used to selectively express ChR2 to achieve light activation in PPG-positive neurons. Current-clamp recordings showed that optogenetic activation of PPG-neurons resulted in a biphasic inhibition-excitation control of action potential firing in mitral cells, revealing a novel microcircuit involving the deep short axon cells, granule cells, and mitral cells in the OB. This unique modulation of mitral cells by PPG-neurons suggests a fine tuning of the olfactory output. Further investigation will be needed to determine the reciprocal molecular interaction between glucose sensing, insulin, and GLP-1 pathways in the OB, as well as their combined action on the output of olfactory processing. The importance of centrifugal projections to the OB, in particular coming from the hypothalamic areas, remains to be systemically studied to determine the origin of the signals linking the regulation of olfaction with feeding states.
Figure 2.
Model of neuromodulatory signaling in the olfactory bulb relying upon metabolic cues. Glucose, insulin, and glucagon-like peptide (GLP-1) levels are sensed in mitral cells of the OB through phosphorylation and post-translational changes of a potassium ion channel, Kv1.3. dSAC, deep short axon cells; GLUT4, glucose transporter 4; GLP-1R, glucagon-like peptide receptor 1; GCL, granule cell layer; GLM, glomerular cell layer; MCL, mitral cell layer; PKA, protein kinase A; InsR, insulin receptor kinase.
While hunger and satiety are easily identifiable physiological processes incorporating learning and expectation into neuromodulation are less well defined. Animals come in contact with numerous odors in their daily lives. How does the nervous system know to give more attention to some odors over others? Experience is one way in which this can occur, modulating the system to prime it for detection of specific odors.
Surprise and expectation modulate early olfactory processing in mice
In his talk, John McGann presented a range of data supporting the claim that neural processing of olfactory information incorporates learned information about the world as early as the axon terminals of the OSNs potentially through synapses with periglomular (PG) cells (Figure 1). He framed expectations as the use of pre-existing information, generally learned from previous experience, to anticipate or interpret incoming sensory input. Prior information about the olfactory world is potentially available directly to the olfactory system through long-term experience-dependent plasticity, but information about how odors relate to non-olfactory stimuli (e.g. cross-modal cueing) or potential outcomes (e.g. odors that predict aversive or appetitive stimuli) must involve projections from non-olfactory brain regions. In the olfactory system, critical forms of these expectations include (i) which odorants are common in the environment, (ii) which odorants predict ecologically-important events, and (iii) when can a given odor be expected based on other stimuli in the environment. The talk applied this conceptual framework to explore how the population-level neural representations of odorants in the OB could exhibit each form of expectation.
Optical neurophysiological methods were used to observe the odorant-evoked neural activity of the OSN presynaptic terminals, including presynaptic calcium signaling in dye-loaded mice (Wachowiak and Cohen 2001) and exocytosis in OMP-spH mice (Bozza et al. 2004), which constitutes the primary olfactory input to the brain. Population-level activity of GAD65-expressing periglomerular interneurons (PG cells, Figure 1) was also observed in GAD2-GCaMP mice (Wachowiak et al. 2013) in order to visualize the representation of odorants in the activity of some of the first neurons in the olfactory system to integrate “bottom-up” peripheral input with “top-down” signaling from other brain regions (Figure 1).
To address the first form of expectation, the incorporation of odorant frequency information, data were presented showing that artificially adding an odorant to a mouse’s environment selectively sparsened the neural representation of that odorant (and closely related odorants), measured at the synaptic output of the OSNs in OMP-spH mice (Kass et al. 2013b). This neural plasticity is correlated with changes in OSN neurotransmitter release dynamics during odorant presentation and a modest increase in olfactory discrimination acuity (Kass et al. 2016). This result doesn’t require “top-down” information because olfactory information is directly available to the OSNs, and in fact, the locus of the plasticity must be within the OSNs themselves because odor-selectiveness of the effect requires olfactory marker protein (Kass et al. 2013b). This result was obtained using a strong exposure paradigm. In this paradigm, the mouse lives in an exposure chamber where an odorant is pumped into the chamber for 4 out of every 8 hours. This paradigm is unlike the briefer odor exposures employed by Takaki Komiyama in his experiments (Kato et al. 2012). In addition, the McGann Lab’s study used both male and female mice, which recent data indicates a new appreciation for the exhibition of differences in baseline OSN selectivity (Kass et al. 2017).
To address the second form of plasticity, previously published data were shown illustrating that discriminative odor-cued fear conditioning, where the mouse learns that one odorant predicts an impending electric shock while another odorant does not, evokes odor-specific enhancement of the OSN synaptic output evoked by the shock-predictive odorant (Kass et al. 2013a). Unpublished data were then presented extending these findings to PG cells, including exploring how long the effect of fear learning persists. Here the McGann lab considered the neural plasticity induced in single-odorant conditioning paradigms where the mouse’s learned fear generalizes across olfactory stimuli.
The work presented by McGann demonstrates that learning and experience can influence the odotopic map representation in the OB in both the OSN terminal and the PG cells. These results raise interesting questions on the impact of neuromodulation through learning on the mitral cells themselves, the output neurons to higher regions of the brain. In the last talk of the session, Takaki Komiyama presented work from his lab investing how learning affects odor representation of mitral cells.
Balancing the robustness and efficiency of olfactory representations during learning
Odor representations in the OB are profoundly dynamic and experience-dependent. What are the principles governing the types of changes in odor representations? Information theory articulates two factors affecting information coding, namely robustness and efficiency (Shannon 1948). A reliable code must be robust against noise. A common way to combat noise is to increase redundancy, which enhances the robustness of the code. However, increasing redundancy sacrifices the efficiency, decreasing the capacity of distinct information that a system can encode. Then, how is the balance between robustness and efficiency achieved? It has been proposed that neural circuits should adapt these factors to achieve optimality with learning (Tkacik et al. 2010). However, experimental support for this notion has been scarce.
Recent applications of two-photon calcium imaging in the OB have allowed longitudinal recording of odor responses of neural ensembles in awake mice (Kato et al. 2012; 2013). This was recently combined with a discrimination learning paradigm developed for head-fixed mice (Komiyama et al. 2010). By monitoring the responses of mitral cells (Figure 1) over a week-long learning paradigm, it was found that the similarity of experienced odorants was an important determinant of changes in mitral cell odor representations. Namely, when mice were trained to discriminate between a very dissimilar pair of odorants, mitral cell representations of these two odorants were initially very distinct but gradually became closer. This was as if the system prioritized the efficiency of the code while sacrificing the robustness. In contrast, when mice learned to discriminate between a highly similar pair of odorants, initially overlapping representations became progressively more distinct, increasing the robustness. Intriguingly, these bidirectional changes resulted in a degree of separation of representations of odorant pairs that was nearly independent of the chemical difference between the odorants. It is therefore tempting to speculate that the learning paradigm led the system to approach an optimal balance between robustness and efficiency of the code.
Furthermore, this study found that qualitatively similar, bidirectional changes were observed when mice experienced the same odorant pairs passively without task engagement (Chu et al. 2016). Thus, changes to balance the robustness and efficiency of odor representations do not require active task learning. Rather, changes appear to affect all familiarized odorants. A possible explanation for these results is that the OB attempts to achieve an optimal separation between representations of familiar odorants based on the statistics of experienced odorants.
In summary, the ISOT 2016 symposium “Neuromodulation in Chemosensory Pathways” shed light on some of the recent findings in state-dependent network modulations in the mouse olfactory system. Top-down inputs, hormonal status, as well as the animals’ diet are clearly potent modulators of OB circuit activity. However, part of the modulatory effects observed in learning, surprise and expectation paradigms seem to be mediated by OSNs or the local OB circuitry. Therefore, our symposium nicely illustrates the complexity of modulations in chemosensory pathways, which is taking place on multiple levels and involving many different systems. More studies are needed to fully elucidate the underlying mechanisms and combinations of modulatory pathways that together shape our sensory perception.
Funding
This work was supported by the NIH grants DC013080 (D.A.F); DC013090, MH101293 (J.P.M); DC014690, DC012641, NS091010A, NS094342, and EY025349 (T.K). N.T. was supported by awards from the NIH (DC013988) and the American Heart Association (AHA; 14POST20380615). Work from M.R was supported by the Deutsche Forschungsgemeinschaft (RO4046/2-1, Emmy Noether Program) and by NIH grant DC012718 (to Matt Wachowiak). Work from T.K. was also supported by the Human Frontier Science Program, Japan Science and Technology Agency (PRESTO), New York Stem Cell Foundation, David & Lucile Packard Foundation, Pew Charitable Trusts, and McKnight Foundatiom. T.K. is a NYSCF-Robertson Investigator.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
We thank Drs. Looger, Akerboom, and Kim and the Genetically Encoded Calcium Indicator (GECI) Project at Janelia Farm Research Campus in collaboration with Penn Vector Core for providing with GCaMP-expressing viruses. We would like to thank Debra Ann Fadool for participating in the symposium and editing of the manuscript. We would like to thank Charles Badland and Adam C. Puche for graphic assistance with the figures.
References
- Atasoy D, Aponte Y, Su HH, Sternson SM. 2008. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 28(28):7025–7030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. 2003. Centre-surround inhibition among olfactory bulb glomeruli. Nature. 426(6967):623–629. [DOI] [PubMed] [Google Scholar]
- Betley JN, Sternson SM. 2011. Adeno-associated viral vectors for mapping, monitoring, and manipulating neural circuits. Hum Gene Ther. 22(6):669–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AM, Kato HK, Komiyama T, Isaacson JS. 2015. Broadcasting of cortical activity to the olfactory bulb. Cell Rep. 10(7):1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. 2004. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 42(1):9–21. [DOI] [PubMed] [Google Scholar]
- Brunert D, Tsuno Y, Rothermel M, Shipley MT, Wachowiak M. 2016. Cell-type-specific modulation of sensory responses in olfactory bulb circuits by serotonergic projections from the raphe nuclei. J Neurosci. 36(25):6820–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu MW, Li WL, Komiyama T. 2016. Balancing the robustness and efficiency of odor representations during learning. Neuron. 92(1):174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Pedarzani P. 2011. Mitral cells of the olfactory bulb perform metabolic sensing and are disrupted by obesity at the level of the Kv1.3 ion channel. PLoS One. 6(9):e24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Phillips JJ, Simmen JA. 2000. Brain insulin receptor causes activity-dependent current suppression in the olfactory bulb through multiple phosphorylation of Kv1.3. J Neurophysiol. 83(4):2332–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Li YR, Tian H, Ma M, Matsunami H. 2015. Muscarinic acetylcholine receptor M3 modulates odorant receptor activity via inhibition of β-arrestin-2 recruitment. Nat Commun. 6:6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor V, Provost AC, Agarwal P, Murthy VN. 2016. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels. Nat Neurosci. 19(2):271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Czarnecki LA, McGann JP. 2017. Sexually-dimorphic neurophysiology in the olfactory input to the brain in adult mice. Sci Rep. [Google Scholar]
- Kass MD, Rosenthal MC, Pottackal J, McGann JP. 2013a. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 342(6164):1389–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Guang SA, Moberly AH, McGann JP. 2016. Changes in olfactory sensory neuron physiology and olfactory perceptual learning after odorant exposure in adult mice. Chem Senses. 41(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Moberly AH, Rosenthal MC, Guang SA, McGann JP. 2013b. Odor-specific, olfactory marker protein-mediated sparsening of primary olfactory input to the brain after odor exposure. J Neurosci. 33(15):6594–6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Chu MW, Isaacson JS, Komiyama T. 2012. Dynamic sensory representations in the olfactory bulb: modulation by wakefulness and experience. Neuron. 76(5):962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato HK, Gillet SN, Peters AJ, Isaacson JS, Komiyama T. 2013. Parvalbumin-expressing interneurons linearly control olfactory bulb output. Neuron. 80(5):1218–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O’Connor DH, Zhang YX, Huber D, Hooks BM, Gabitto M, Svoboda K. 2010. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 464(7292):1182–1186. [DOI] [PubMed] [Google Scholar]
- Ma M, Luo M. 2012. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci. 32(30):10105–10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otazu GH, Chae H, Davis MB, Albeanu DF. 2015. Cortical feedback decorrelates olfactory bulb output in awake mice. Neuron. 86(6):1461–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palouzier-Paulignan B, Lacroix MC, Aimé P, Baly C, Caillol M, Congar P, Julliard AK, Tucker K, Fadool DA. 2012. Olfaction under metabolic influences. Chem Senses. 37(9):769–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. 2008. Glucose sensing in L cells: a primary cell study. Cell Metab. 8(6):532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Wachowiak M. 2014. Functional imaging of cortical feedback projections to the olfactory bulb. Front Neural Circuits. 8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Brunert D, Zabawa C, Díaz-Quesada M, Wachowiak M. 2013. Transgene expression in target-defined neuron populations mediated by retrograde infection with adeno-associated viral vectors. J Neurosci. 33(38):15195–15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermel M, Carey RM, Puche A, Shipley MT, Wachowiak M. 2014. Cholinergic inputs from Basal forebrain add an excitatory bias to odor coding in the olfactory bulb. J Neurosci. 34(13):4654–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon CE. 1948. A mathematical theory of communication. Bell Syst Tech 27:379–423. [Google Scholar]
- Thiebaud N, Llewellyn-Smith IJ, Gribble F, Reimann F, Trapp S, Fadool DA. 2016. The incretin hormone glucagon-like peptide 1 increases mitral cell excitability by decreasing conductance of a voltage-dependent potassium channel. J Physiol. 594(10):2607–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaud N, Johnson MC, Butler JL, Bell GA, Ferguson KL, Fadool AR, Fadool JC, Gale AM, Gale DS, Fadool DA. 2014. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J Neurosci. 34(20):6970–6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacik G, Prentice JS, Balasubramanian V, Schneidman E. 2010. Optimal population coding by noisy spiking neurons. Proc Natl Acad Sci USA. 107(32):14419–14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K, Cho S, Thiebaud N, Henderson MX, Fadool DA. 2013. Glucose sensitivity of mouse olfactory bulb neurons is conveyed by a voltage-gated potassium channel. J Physiol. 591(10):2541–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K, Cavallin MA, Jean-Baptiste P, Biju KC, Overton JM, Pedarzani P, Fadool DA. 2010. The Olfactory Bulb: A Metabolic Sensor of Brain Insulin and Glucose Concentrations via a Voltage-Gated Potassium Channel. Results Probl Cell Differ. 52:147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KR, Godbey SJ, Thiebaud N, Fadool DA. 2012. Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity. Physiol Behav. 107(3):424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. 2001. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron. 32(4):723–735. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Economo MN, Díaz-Quesada M, Brunert D, Wesson DW, White JA, Rothermel M. 2013. Optical dissection of odor information processing in vivo using GCaMPs expressed in specified cell types of the olfactory bulb. J Neurosci. 33(12):5285–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]