Abstract
Olfactory sensitivity has traditionally been viewed as a trait that varies according to individual differences but is not expected to change with one’s momentary state. Recent research has begun to challenge this position and time of day has been shown to alter detection levels. Links between obesity and the timing of food intake further raise the issue of whether odor detection may vary as a function of circadian processes. To investigate this question, 37 (21 male) adolescents (M age = 13.7 years) took part in a 28-h forced desynchrony (FD) protocol with 17.5 h awake and 10.5 h of sleep, for 7 FD cycles. Odor threshold was measured using Sniffin’ Sticks 6 times for each FD cycle (total threshold tests = 42). Circadian phase was determined by intrinsic period derived from dim light melatonin onsets. Odor threshold showed a significant effect of circadian phase, with lowest threshold occurring on average slightly after the onset of melatonin production, or about 1.5○ (approximately 21:08 h). Considerable individual variability was observed, however, peak olfactory acuity never occurred between 80.5○ and 197.5○ (~02:22–10:10 h). These data are the first to show that odor threshold is differentially and consistently influenced by circadian timing, and is not a stable trait. Potential biological relevance for connections between circadian phase and olfactory sensitivity are discussed.
Keywords: adolescents, food intake, forced desynchrony, individual differences, odor threshold, trait-state
Introduction
In the absence of deliberate efforts to alter our sensory systems, such as extensive training, the capacity of our senses is relatively stable. Acuity varies within and between individuals along major trait dimensions such as genetics, age, sex, and health but is not expected to change with one’s momentary state (McKee and Westheimer 1978; Deveau et al. 2014). Recent evidence in the chemical senses, however, has begun to challenge this principle. For example, olfactory sensitivity was shown to decrease following a negative mood induction (Pollatos et al. 2007). In other research, sourness perception decreased when participants were in a negative mood, and sweet taste sensitivity increased in a positive mood (Noel and Dando 2015; Platte et al. 2013). Thus, transient changes in mood can influence smell and taste perception.
Circadian rhythms impose continuous transient states, as they underlie the approximately 24-h cycle of human physiology through which sleep, alertness, mood, hormone release, and many other processes vary with one’s internal clock. The term “circadian phase” refers to the instantaneous state of a circadian oscillation (e.g., the onset of melatonin release) and represents a marker for the time of the internal circadian clock. The primary circadian clock in mammals is located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus. Recent evidence indicates that neural structures outside of the SCN also have independent circadian pacemakers—including the olfactory bulb (Guilding and Piggins 2007). Therefore, olfactory perception may be especially well suited for examination as a function of circadian phase.
Limited prior research has examined the possibility that olfactory sensitivity is affected by time of day. In the first studies to address this issue, Goetzl and colleagues (Goetzl and Stone 1947; Goetzl et al. 1950) reported that olfactory acuity varied diurnally in accordance with food intake. Among subjects who ate lunch, acuity was found to decrease shortly after lunch, and then increase to prelunch levels in the later afternoon. Among participants who skipped lunch, however, diurnal variability was not observed. Gilbert and Rosenwasser (1987) examined variability in nasal patency (the rate of airflow in each nostril) every 10 min over the course of 8 h from 09:00 to 17:00. Nasal patency can be considered related to olfactory sensitivity in that greater airflow implies a higher volume of odorous molecules available to be sensed. In their study, however, no consistent pattern in nasal patency was seen among subjects or across time. More recently, Nordin and colleagues (2003) performed a neurological test of olfactory processing as a function of time of day by examining event related potentials (ERPs) in response to hydrogen sulfide in 5 healthy men at 04:00, 08:00, 12:00, 16:00, 20:00, and 24:00. Here, a diurnal pattern was observed, with ERP amplitudes largest at 16:00 and 20:00 and smallest at 04:00.
Of the olfactory research to date, the study by Nordin et al. (2003) most closely targets an assessment of circadian factors, in that recordings were made across 24 h. On the other hand, no physiological measurements of participants’ internal circadian phase were taken, and various extraneous factors were unaccounted for, including prior sleep/wake history. Most important, odor detection thresholds were not evaluated, and participants’ subjective ratings of odor intensity did not show any significant differences at the various test times. We performed the present study to investigate directly how olfactory sensitivity varies as a function of measured circadian phase.
To test the influence of circadian phase, we used a forced desynchrony (FD) protocol (Dijk and Czeisler 1994), with which the contributions of circadian timing can be separated from the influence of time of day. The method uses a non-24-h schedule to “force” the components of circadian physiology apart from the number of hours awake. In the present study, we used a validated protocol that extended the day length to 28 h (Wu et al. 2015) and odor detection was repeatedly tested at various circadian phases while controlling for the length of time awake. As this research was entirely novel, specific hypotheses were not made and our study was exploratory in nature.
Methods
Participants
Thirty-seven (21 male) adolescents (mean age: 13.7 years; range 12.4–15.9 years) completed the study. The age range selected in this study was based on a parent study on circadian timing and food intake in adolescents. Participants were selected based on the following criteria: no history of sleep, medical, or psychological disorders; no first-degree relative with a psychopathology or genetically transmitted neurological disorder; regular sleep schedules; English language proficiency; no evidence of learning disabilities or a physical handicap that would interfere with testing; no travel beyond 2 time zones in the 2 months before in-laboratory assessments; and no evidence of impaired sense of taste or smell. Current use of psychoactive substances or other drugs that might affect the sleep/wake cycle, sleepiness/alertness, or circadian timing (including contraceptive pills) were exclusionary and confirmed with urine toxicology screening. All participants were in normal ranges for the Child Behavior Checklist (Achenbach and Rescorla 2001a), Youth Self Report (Achenbach and Rescorla 2001b), and Center for Epidemiological Studies Depression Scale (Radloff 1977). The Smith (1989) Questionnaire was used to assess Morningness-Eveningness in the current study and 12% of participants scored as “morning type,” 86% as “intermediate type,” and 2% as “evening type.” This study was approved by the Institutional Review Board for Human Subjects of Lifespan Hospitals. Participants were treated in accordance with the Declaration of Helsinki for Medical Research involving Human Subjects and were compensated for their time. Parental consent and participant assent was obtained.
Procedures
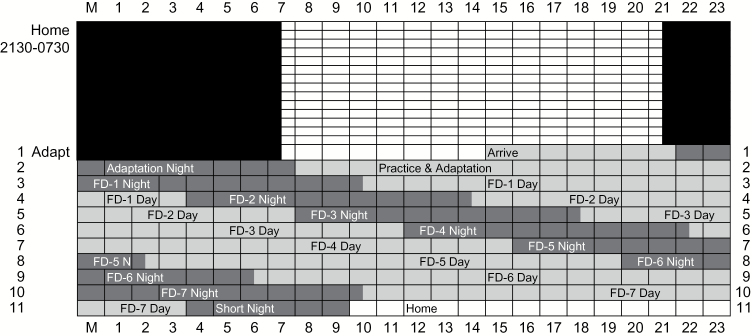
All participants slept on a fixed 10-h (21:30–07:30) stabilization schedule at home for a minimum of 14 nights wearing eye-shades before coming to the laboratory (“at-home” protocol) (see Figure 1). Adherence to this schedule was confirmed by actigraph monitoring (Acebo and LeBourgeois 2006), sleep diary, and evening and morning phone calls to the laboratory’s time-stamped answering machine. After the 2-week at-home stabilization period, participants arrived at the laboratory at approximately 15:00 to begin the in-laboratory study. For the in-laboratory study, participants remained in the laboratory for 10 consecutive earth nights and the intervening 9 days. The study began with a 10-h (22:00–08:00) “adaptation night” to screen for sleep-disordered breathing and periodic limb movements (none were detected) and to allow participants to adapt to the laboratory setting. The adaptation night was followed by an “adaptation day” during which participants learned and practiced the olfactory tasks.
Figure 1.
Schematic of study protocol. Study day is on the y axis and clock time on the x axis. Black cells represent scheduled sleep during the at-home portion of the study (21:30–07:30). Dark gray cells represent scheduled sleep during in-lab protocol and light gray scheduled wake. Of note, sleep is scheduled for 4 h later each day, as part of the FD protocol.
The 28-h FD schedule began at bedtime on the adaptation day and continued for 7 cycles (see Figure 1), in which 17.5 h were scheduled for wake and 10.5 h were scheduled for sleep. Participants were never informed of the clock time of day to minimize expectancies based on knowledge of time. To avoid suppressing melatonin production—our measure of circadian phase—the light level in the laboratory was completely dark (0 lux) during the scheduled hours of sleep and very dim (less than 20 lux) during the waking periods. By completing 7 FD “days,” participants were scheduled for both sleep and wake across the full range of circadian phases. Odor detection threshold was measured at consistent times after scheduled waking on each FD, thus holding time-awake constant so that the independent effect of circadian phase on odor detection could be examined.
Olfactory testing
Olfactory measures
Psychophysical testing of olfactory function was performed using “Sniffin’ Sticks,” a widely used and validated test battery that assesses odor discrimination, odor identification, and odor threshold (“Sniffin’ Sticks” Burghart GmbH, Wedel, Germany; Hummel et al. 1997; Kobal et al. 2000; Hummel et al. 2007; Tekeli et al. 2013). Sniffin’ Sticks comprise felt tip pen-like odor-dispensing devices (hereafter referred to as “pen/pens”) in which the tampon of the pen is filled with the test odorant diluted in the odorless solvent, propylene glycol. The discrimination test involves 16 triplets of pens, 2 of which contain the same odor (e.g., anethole, smells like camphor) and one that contains a different odor (e.g., isoamylacetate, smells like banana); for each triplet, the participant is asked to indicate the pen that smells different. The identification test includes 16 pens, each containing a familiar odor (e.g., lemon, peppermint, cinnamon). For each pen, a 4-alternative forced-choice procedure is used where the participant is asked to identify the respective odor from a list of 4 descriptors. Threshold testing was performed using 16 concentrations of the target odor phenylethyl alcohol (PEA, smells like rose). Starting with the lowest concentration of PEA (0.00000028%), participants were presented with 3 pens in random order, one containing diluted PEA and 2 containing propylene glycol (blank controls). Following a standard psychophysical staircase procedure, if the odorized pen was not correctly identified, progressively higher concentration pen sets were presented until correct detection was achieved. When an odorized pen was correctly identified, the same triplet was presented again for confirmation. Two successive correct identifications of the pen containing the odor or one incorrect identification triggered a reversal of the staircase to the next higher or the next lower dilution step, respectively.
For all olfactory testing, the pen cap was removed by the experimenter who then waved the pen beneath the participant’s nostrils 3 times. Research technicians wore gloves while administering the olfactory tests. The total time the participant was exposed to each pen was 3–5 s. For odor threshold testing, the interval between presentations of individual pens in a triplet was 3–5 s and presentation of each triplet to the participant occurred roughly every 30 s.
Initial olfactory assessments
Approximately 2 weeks before the in-laboratory study, participants came to the laboratory for an orientation session where the experiment was explained and consent forms were signed. At this session, participants were also presented with the highest concentration of PEA (4%) from the Sniffin’ Sticks threshold test so that an evaluation of basic olfactory function could be established, as has been done in prior research (Tekeli et al. 2013). All participants were able to perceive the odor at this concentration. The next olfactory tests were performed during the “adaptation day” in the laboratory. On this day, the Sniffin’ Sticks tests were explained and demonstrated, and participants performed the odor discrimination and identification tests following standard procedures. This was the only occasion when odor discrimination and identification was assessed. Scores for olfactory performance are shown in Table 1. Sniffin’ Sticks odor threshold tests were also administered that day and practiced 3 times over the course of the afternoon. For the first threshold test, the standard procedure of starting with the lowest concentration of the 16 pens and then presenting triplets in increasing concentration until the target odor pen was correctly identified was followed; for all subsequent threshold testing trials throughout the study, the threshold test began with a concentration that was 3 steps lower than the final reversal point of the previous threshold test. Scores from the threshold tests obtained on the “adaptation-practice” day were not included in the analyses of interest, but were used to assess baseline detection levels (see Table 1).
Table 1.
Scores on Sniffin’ sticks tests performed on the adaptation day
| Threshold | Discrimination | Identification | TDI | |
|---|---|---|---|---|
| All Participants (N = 37) | ||||
| Mean (SD) | 9.86 (2.53) | 11.51 (2.14) | 12.08 (1.69) | 33.45 (4.52) |
| Minimum | 4.5 | 7 | 7 | 23.50 |
| Maximum | 14.5 | 16 | 15 | 40.50 |
SD, standard deviation; TDI, sum of the threshold, discrimination and identification tests.
Odor threshold testing during FD protocol
Smell sensitivity to PEA was measured 6 times each FD cycle for a total of 42 threshold tests per participant. For each FD cycle, testing began approximately 55 min after waking and was repeated at 3-h intervals. Standard threshold testing with Sniffin’ Sticks calls for the participant to wear a blindfold. Pilot testing of this procedure, however, indicated that adolescents were prone to fall asleep when wearing the blindfold if testing occurred at adverse circadian phases. We therefore modified the procedure such that a partition was used to hide the Sniffin’ Sticks, and participants were asked to close their eyes when the pens were presented. To allow for repeated trials with adolescent participants in this rigorous paradigm, we also changed the number of reversals to 4 instead of the standard method of 7 reversals. Odor threshold was therefore calculated as the average of 4 reversal scores.
Circadian phase determination
Dim Light Melatonin Onset (DLMO) is the gold standard for determining circadian phase in humans (Carskadon et al. 1997). Saliva samples (2 mL) were collected using salivettes (Sarstedt Inc.) during FD waking episodes at 20–45 min intervals for determination of melatonin levels. Samples were centrifuged and frozen (−20 °C) within 4 h of collection and subsequently analyzed by radioimmunoassay (Solid Phase using Alpco melatonin kits, Bühlmann Laboratories) with sensitivity of 0.9 pg/mL, intra-assay coefficient of variation 7.9%, and interassay coefficient of variance 11.7%. Dim light melatonin onset (DLMO) phase (i.e., circadian phase) was measured for each participant by linear interpolation between rising values crossing a threshold value of 4 pg/mL (the standard threshold to determine melatonin onset in this age group (Carskadon et al. 1997). This measure was calculated for each day when the rising threshold occurred during waking hours. The intrinsic circadian period for each participant was subsequently estimated using all DLMO phase determinations, with period computed by linear regression. Each odor threshold measure was then assigned a circadian phase (0–360°, where 0° = estimated DLMO based on fitting an individual’s period to the data set; the initial baseline DLMO in the sample was a mean clock time of ~20:59 [95% confidence interval = 20:42; 21:16]).
Analytical approach
To isolate the within-participant variability across circadian time and time awake, smell thresholds were z-scored within each participant. We used a mixed effects model to account for the nesting of assessments within participants. A multilevel cosinor model was applied (Mikulich et al. 2003), and linearizing cosinor transformations were used to account for the cyclical pattern of circadian data. Specifically, the mixed-effects model included the following fixed effects: intercept, sine and cosine transformations, and time awake (centered at 8 h awake). Sine and cosine transformations were included as random effects. The model was estimated using restricted maximum likelihood. Mesor (mid-value of a circadian cycle), acrophase (phase of circadian peak), and amplitude (height of the circadian cycle; see Figure 2) were then calculated from model parameters through nonlinear transformations described by Refinetti et al. (2007) and standard errors were calculated using the delta method (Mikulich et al. 2003). Model parameters for each participant were estimated using the Best Linear Unbiased Predictions (BLUPs) generated by the mixed effects model. Analyses were performed using the following R packages: nlme v3.1–128, msm v1.6.4.
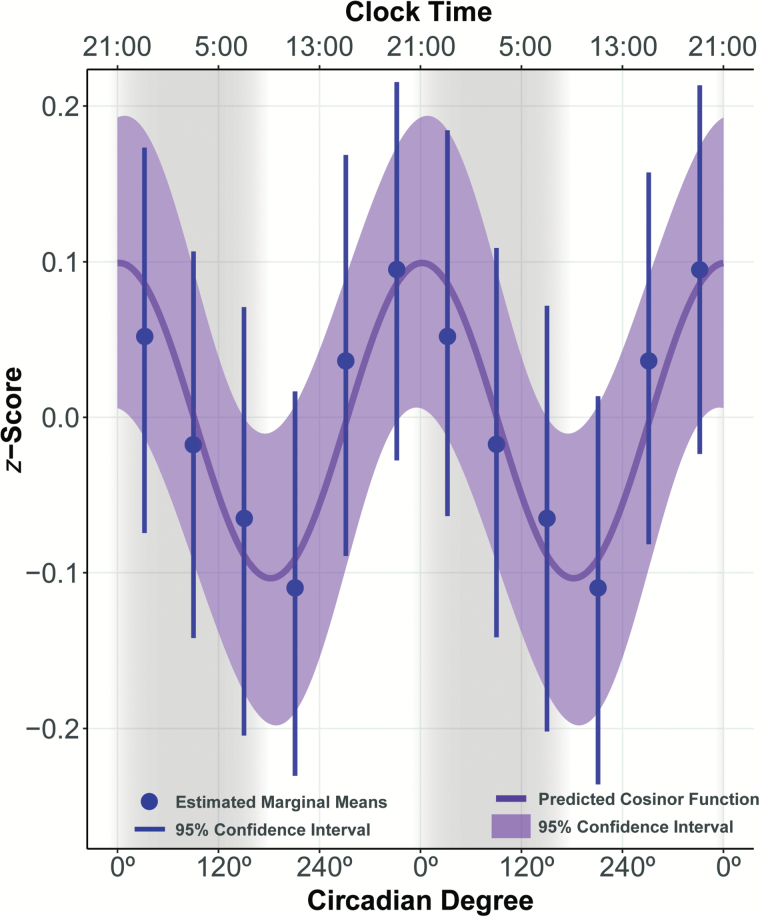
Figure 2.
Predicted circadian function for olfactory sensitivity. Sample average circadian function with 95% confidence interval estimated using multilevel cosinor model. Twenty-four-hour clock time was pegged to the average initial dim light melatonin onset phase (20:59). Shaded bands represent biological night. Mesor, mid-value of a circadian cycle; Acrophase, phase of circadian peak; Amplitude, height of the circadian cycle (distance from Mesor to Acrophase). Higher amplitude indicates greater olfactory sensitivity.
Results
Initial odor testing
Descriptive Statistics for the scores obtained on the Sniffin’ Sticks tests performed on the adaptation day (discrimination, identification, threshold) are shown in Table 1. Values obtained were similar to those reported by Hummel et al. (2007) for this age group, and thereby confirm that our participants had a normal sense of smell.
Circadian influence on odor threshold
Results from the multilevel cosinor analysis indicated that, on average, a significant circadian influence was observed on participants’ odor thresholds (amplitude = 0.10 [95% confidence interval 0.01; 0.18] z-score units), with peak odor sensitivity occurring slightly after onset of melatonin production (acrophase = 1.93 [I = 309.82; 54.77] circadian degrees) which corresponds to about 21:08 h, referencing average time of baseline DLMO phase (see Figure 2). As expected, given that the data were z-scored, the mesor was essentially zero (0.002 [I = −0.050; 0.046] z-score units). Odor sensitivity decreased by 0.02 (−0.032; −0.012) z-score units per hour awake. To validate the cosinor model, individuals’ data were binned into 60° circadian intervals and analyzed using a linear mixed model, with circadian bin and time awake as fixed effects and the intercept as a random effect. The estimated marginal means for each bin are shown in Figure 3 and show general agreement with the cosinor model.
Figure 3.
Fit of Cosinor model. The cosinor model predicted function was compared to data estimated using 60° circadian bins. Marginal Mean Estimates and 95% confidence interval for each circadian bin were estimated using a linear mixed effect model. Higher amplitude indicates greater olfactory sensitivity.
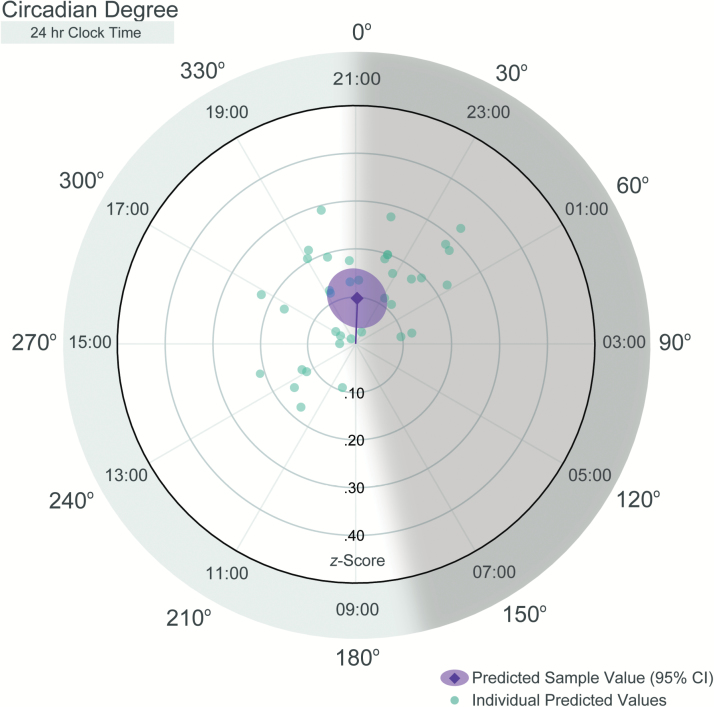
Importantly, the data also showed substantial interindividual variability among participants for both the strength and timing of the circadian influence on odor sensitivity (see Figures 3 and 4). Participants’ amplitudes (i.e., strength of the circadian signal) ranged from 0.02 to 0.33 z-score units, with some individuals showing little variation in odor sensitivity across the circadian cycle (i.e., amplitude < 0.10), while others showed a robust circadian signal (i.e., amplitude > 0.20). The timing of peak odor sensitivity also varied across participants, with individual peaks (acrophases) evenly distributed across about two-thirds of the circadian day (running clockwise from 197.52° [about 10:10] to 80.45° [about 02:22]). However, no peaks were ever observed between 197.52° and 80.45°.
Figure 4.
Radial plot of predicted circadian functions for each participant. Radial plot depicts Amplitude (distance from center) and Acrophase (clockwise angle from 0°). Sample average and 95% confidence interval are equivalent to those depicted in Figure 2. Individual Predicted Values were estimated using the best linear unbiased predictor for each participant estimated from the multilevel cosinor model. Shaded area represents the biological night.
Discussion
Our results revealed that olfactory sensitivity varies with circadian phase. Specifically, we observed peak olfactory sensitivity (lowest odor threshold) in what would be the early biological night that is approximately 21:00, of a 24-h light–dark cycle. By contrast, no participants ever evinced peak sensitivity during the circadian phase range that would map on to clock times of approximately 02:22–10:10. These data demonstrate for the first time that olfactory sensitivity is not a stable trait; rather, it is modulated by circadian phase. Although Nordin et al. (2003) did not assess odor thresholds in direct relation to circadian phase, our data are somewhat in line with their time of day findings that showed peak amplitude of ERPs to an olfactory stimulus at 16:00 and 20:00 and a minimum at 04:00. Our results also showed that olfactory sensitivity is related to time awake; however, the impact of sleep quantity and sleep stages on the quality of wakefulness coupled with our relatively small sample size preclude assigning too much significance to the time-awake data. As our sample size increases with future data collection, we plan to provide further examination of these features of the data, and we will also be able to identify interactions between circadian phase and time awake.
When interpreting those findings, it is important to bear in mind that our participants were given an entrainment schedule with sleep and darkness occurring from 21:30 to 07:30. Hence, the peak circadian phases of olfactory sensitivity correspond to afternoon through about the middle of darkness; peaks were never observed during the second half of their entrained night or morning. This sleep schedule is unlikely to be typical for many, especially adolescents, in an artificial light and screen-filled world; however, it may have relevance for some naturalistic conditions. For example, the finding that olfactory sensitivity tended to be best in the early night may have evolutionary significance for the detection of predators. After the evening meal, which anthropological records indicate has been the main meal of the day (Walker et al. 2003), carnivorous animals may be especially drawn to human groups. The ability to detect the presence of predators through odor cues when visual cues are less effective due to dusk and darkness would be adaptive. Relatedly, heightened olfactory acuity for the main meal of the day may have aided with satiation—greater olfactory sensitivity increases flavor perception and satiation (Ruijschop et al. 2010)—which would be beneficial when limited resources were available. Another evolutionary explanation for olfactory acuity in early night may be related to the selection of reproductive mates. Many species mate at the end of the activity cycle (Goldman 1999; Fergus et al. 2012), and olfactory assessments of potential mates for health and immunological viability has been shown an important variable mediating reproduction in both rodents and humans (Yamazaki et al. 1976; Wedekind et al. 1995). These hypotheses are speculative.
The finding that olfactory sensitivity was never at its best late in the nocturnal phase has important implications for olfaction as a sentinel system. We have previously shown that during slow wave and REM sleep, people are unresponsive to external olfactory cues (Carskadon and Herz 2004). Our current findings further underscore that odors cannot be relied upon as danger warnings during the early morning hours when awake or asleep.
Consistent with Goetzl and colleagues (Goetzl and Stone 1947; Goetzl et al. 1950), our results indicated that olfactory sensitivity decreased across the waking day. Goetzl’s group found that meal consumption influenced olfactory sensitivity over a day’s meals. Alternatively, rising sleep pressure could be a factor influencing olfactory sensitivity. Unfortunately, our protocol does not allow for the separation of time awake from order of meal consumption; thus, it is unclear whether this decrease is related to increasing sleep pressure or to the cumulative effect of meal consumption. Moreover, our sample size is currently insufficient to examine the interplay between time awake and circadian timing, an interplay that may have important implications for populations with misalignment between their sleep-wake and circadian cycles, such as shift workers.
The interplay we show among bio-regulatory systems and olfactory sensitivity has important methodological implications for olfactory research and clinical applications. Assessing odor detection at different times within the same participants, for example, may be confounded by the influence of bio-regulatory systems such as circadian timing and sleep pressure. Further, the timing of chemical assessments may need to be examined in terms of these findings. Additional systems affecting olfaction may include sleep architecture, caloric intake/satiety, and the effects of light; for example, the present study was conducted under dim light and it unknown if this may have affected olfactory responses. Our results suggest that olfactory sensitivity is influenced by the circadian system and time-awake, but more work is needed to understand how these systems work together to influence olfactory sensitivity.
Beyond the intraindividual differences in olfactory sensitivity due to bio-regulatory systems, we note interindividual differences in how the systems operate. Potential factors that may contribute to individual variability include sex, circadian phase preference, chronotype, weight, and pubertal development. For example, olfactory threshold has been shown to vary with BMI, with most evidence suggesting olfactory threshold increases with increasing BMI (Skrandies and Zschieschang 2015), although opposite trends have also been reported (Obrebowski et al. 2000; Stafford and Whittle 2015). Additionally considerable evidence shows that females outperform men on tests of olfactory function (e.g., Doty and Cameron, 2009), and sex may influence circadian mechanisms (Cain et al. 2010; Duffy et al. 2011; Van Reen et al. 2013). Additional work with larger subject samples is needed to begin to understand individual variability in how the regulatory systems influence olfactory sensitivity. A constraint of the present research was that our participants were healthy adolescents, aged 12–15 years, and findings may not generalize to younger or older cohorts, particularly as adolescence is a period when circadian processes shift towards later phase (Crowley et al. 2007). Additionally, we only tested odor thresholds to PEA, and while this is a standard test of general olfactory function, it is not known whether the same pattern of results would be obtained with biologically relevant odors (e.g., food, animal and human body odors), which would represent more ecologically meaningful responses. Finally, the FD protocol focuses on separating circadian timing and time awake, and does not facilitate the separation of circadian processes from other potentially important systems such as sleep architecture. Despite these constraints our results indicate that the circadian system does influence olfactory sensitivity, and support the need for more research to understand this connection.
Funding
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (R01 DK101046-0) and further supported by the Periodic Breathing Foundation and Craig Cogut.
Acknowledgments
We wish to thank Daniel Baum and Thomas Hummel for valuable assistance with Sniffin’ Sticks preparation and administration.
References
- Acebo C, LeBourgeois MK. 2006. Actigraphy. Respir Care Clin N Am. 12:23–30, viii. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. 2001a. Manual for the ASEBA Child Behavior Checklist for Ages 6–18. Burlington (VT): Research Center for Children, Youth, and Families, University of Vermont. [Google Scholar]
- Achenbach TM, Rescorla LA. 2001b. Manual for the ASEBA Youth Self-Report for Ages 11-18. Burlington (VT): Research Center for Children, Youth, and Families, University of Vermont. [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, Schoen MW, Czeisler CA, Duffy JF. 2010. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 25:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. 1997. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 12:278–289. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Herz RS. 2004. Minimal olfactory perception during sleep: why odor alarms will not work for humans. Sleep. 27:402–405. [PubMed] [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. 1993. Association between puberty and delayed phase preference. Sleep. 16:258–262. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Acebo C, Carskadon MA. 2007. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 8:602–612. [DOI] [PubMed] [Google Scholar]
- Deveau J, Ozer DJ, Seitz AR. 2014. Improved vision and on-field performance in baseball through perceptual learning. Curr Biol. 24:R146–R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. 1994. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 166:63–68. [DOI] [PubMed] [Google Scholar]
- Doty RL, Cameron EL. 2009. Sex differences and reproductive hormone influences on human odor perception. Physiol Behav. 97:213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJ, Münch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP Jr, Czeisler CA. 2011. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci USA. 108(Suppl 3):15602–15608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergus DJ, Bell RC, Shaw KL. 2012. Rhythmic male reproductive behavior controls timing of courtship and mating in Laupala cerasina. Behav Ecol Sociobiol. 66:1333–1340. [Google Scholar]
- Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee YC, Ordovás JM, Scheer FA. 2013. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 37:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AN, Rosenwasser AM. 1987. Biological rhythmicity of nasal airway patency: a re-examination of the ‘nasal cycle’. Acta Otolaryngol. 104:180–186. [DOI] [PubMed] [Google Scholar]
- Goetzl FR, Abel MS, Ahokas AJ. 1950. Occurrence in normal individuals of diurnal variations in olfactory acuity. J Appl Physiol. 2:553–562. [DOI] [PubMed] [Google Scholar]
- Goetzl FR, Stone F. 1947. Diurnal variations in acuity of olfaction and food intake. Gastroenterology. 9:444–453. [PubMed] [Google Scholar]
- Goldman BD. 1999. The circadian timing system and reproduction in mammals. Steroids. 64:679–685. [DOI] [PubMed] [Google Scholar]
- Guilding C, Piggins HD. 2007. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain?Eur J Neurosci. 25:3195–3216. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 22:39–52. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A. 2007. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 264:237–243. [DOI] [PubMed] [Google Scholar]
- Kobal G, Klimek L, Wolfensberger M, Gudziol H, Temmel A, Owen CM, Seeber H, Pauli E, Hummel T. 2000. Multicenter investigation of 1,036 subjects using a standardized method for the assessment of olfactory function combining tests of odor identification, odor discrimination, and olfactory thresholds. Eur Arch Otorhinolaryngol. 257:205–211. [DOI] [PubMed] [Google Scholar]
- McKee SP, Westheimer G. 1978. Improvement in vernier acuity with practice. Percept Psychophys. 24:258–262. [DOI] [PubMed] [Google Scholar]
- Mikulich SK, Zerbe GO, Jones RH, Crowley TJ. 2003. Comparing linear and nonlinear mixed model approaches to cosinor analysis. Stat Med. 22:3195–3211. [DOI] [PubMed] [Google Scholar]
- Noel C, Dando R. 2015. The effect of emotional state on taste perception. Appetite. 95:89–95. [DOI] [PubMed] [Google Scholar]
- Nordin S, Lötsch J, Murphy C, Hummel T, Kobal G. 2003. Circadian rhythm and desensitization in chemosensory event-related potentials in response to odorous and painful stimuli. Psychophysiology. 40:612–619. [DOI] [PubMed] [Google Scholar]
- Obrebowski A, Obrebowska-Karsznia Z, Gawliński M. 2000. Smell and taste in children with simple obesity. Int J Pediatr Otorhinolaryngol. 55:191–196. [DOI] [PubMed] [Google Scholar]
- Platte P, Herbert C, Pauli P, Breslin PA. 2013. Oral perceptions of fat and taste stimuli are modulated by affect and mood induction. PLoS One. 8:e65006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Kopietz R, Linn J, Albrecht J, Sakar V, Anzinger A, Schandry R, Wiesmann M. 2007. Emotional stimulation alters olfactory sensitivity and odor judgment. Chem Senses. 32:583–589. [DOI] [PubMed] [Google Scholar]
- Radloff LS. 1977. The CES-D scale a self-report depression scale for research in the general population. Appl Psych Meas. 1:385–401. [Google Scholar]
- Refinetti R, Lissen GC, Halberg F. 2007. Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res. 38:275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruijschop RM, Boelrijk AE, Burgering MJ, de Graaf C, Westerterp-Plantenga MS. 2010. Acute effects of complexity in aroma composition on satiation and food intake. Chem Senses. 35:91–100. [DOI] [PubMed] [Google Scholar]
- Skrandies W, Zschieschang R. 2015. Olfactory and gustatory functions and its relation to body weight. Physiol Behav. 142:1–4. [DOI] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. 1989. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 74:728–738. [DOI] [PubMed] [Google Scholar]
- Stafford LD, Whittle A. 2015. Obese individuals have higher preference and sensitivity to odor of chocolate. Chem Senses. 40:279–284. [DOI] [PubMed] [Google Scholar]
- Tekeli H, Altundağ A, Salihoğlu M, Cayönü M, Kendirli MT. 2013. The applicability of the “Sniffin’ Sticks” olfactory test in a Turkish population. Med Sci Monit. 19:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reen E, Sharkey KM, Roane BM, Barker D, Seifer R, Raffray T, Bond TL, Carskadon MA. 2013. Sex of college students moderates associations among bedtime, time in bed, and circadian phase angle. J Biol Rhythms. 28:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AR, Walker BF, Adam F. 2003. Nutrition, diet, physical activity, smoking, and longevity: from primitive hunter-gatherer to present passive consumer–how far can we go?Nutrition. 19:169–173. [DOI] [PubMed] [Google Scholar]
- Wedekind C, Seebeck T, Bettens F, Paepke AJ. 1995. MHC-dependent mate preference in humans. Proc Roy Soc London (B). 260:245–249. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Acebo C, Seifer R, Carskadon MA. 2015. Sleepiness and cognitive performance among younger and older adolescents across a 28-hour forced desynchrony protocol. Sleep. 38:1965–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Boyse EA, Miké V, Thaler HT, Mathieson BJ, Abbott J, Boyse J, Zayas ZA, Thomas L. 1976. Control of mating preferences in mice by genes in the major histocompatibility complex. J Exp Med. 144:1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]