Abstract
The intestine is continuously exposed to commensal microorganisms, food and environmental agents, and also serves as a major portal of entry for many pathogens. A critical defense mechanism against microbial invasion in the intestine is the single layer of epithelial cells that separates the gut lumen from the underlying tissues. The barrier function of the intestinal epithelium is supported by cells and soluble factors of the intestinal immune system. Chiefly among them are intestinal intraepithelial lymphocytes (iIELs), which are embedded in the intestinal epithelium, and represent one of the single largest populations of lymphocytes in the body. Compared with lymphocytes in other parts of the body, iIELs exhibit unique phenotypic, developmental and functional properties that reflect their key roles in maintaining the intestinal epithelial barrier. Here, we review the biology of iIELs in supporting normal health and how their dysregulation can contribute to disease.
Introduction
Our bodies are continuously exposed to microbial organisms present in the environment, including commensal microbiota as well as many infectious agents. These encounters with microbial organisms primarily occur at external or internal body surfaces, including the skin and the mucosal membranes of the gastrointestinal, respiratory and genitourinary tracts. To deal with this onslaught of microbes, the immune system at mucosal surfaces has evolved specialized features to balance immune responsiveness against invaders with tolerance against commensal microorganisms and other environmental agents, such as food particles (1). These dynamic interactions between the host and microorganisms are particularly apparent in the intestine, which contains more than 1,000 distinct bacterial species and numerous viruses, fungi, and protozoa (2). It has been suggested that many features of the adaptive immune system, such as organized lymphoid structures, may have evolved initially in the gut of vertebrates in response to interactions with microbiota (3). Particularly intriguing is that the thymus, which is critical for the maturation of the majority of T lymphocytes, is derived from the embryonic intestine in vertebrates (4). The gut has been implicated in the development of a subset of γδT cells (5, 6), suggesting similarities in the functions and evolutionary origin of the thymus and gut-associated lymphoid tissues. Based on these considerations it has been postulated that the thymus may have evolved from gut-associated lymphoid tissues in the gills of early vertebrates (7).
The intestinal immune system consists of multiple levels to defend against microbial invaders (8). First is immunity provided by the gut microbiota, which compete with pathogens for nutrients and ecological niches, produce bacteriocins and proteinaceous toxins that can inhibit the growth of related bacterial species, and play a critical role in shaping host immunity (9). The second level of defense is provided by the single layer of intestinal epithelial cells (IECs) that separates the luminal contents from the gut tissue (10). IECs are securely joined together via distinct types of cell junctions, generating a barrier against harmful insults such as pathogenic microbes. Moreover, the epithelium contains specialized IECs such as mucus-producing goblet cells, anti-microbial – producing Paneth cells, and microfold (M) cells that sample antigens from the lumen for delivery to underlying lymphoid structures. The epithelium is protected by the mucus layer, which prevents microorganisms and toxic substances from reaching the surface of the epithelium (11). IECs can also respond to microbial products to produce a variety of mediators, including proinflammatory cytokines (e.g., IL-1β and IL-18) and factors that promote cell survival and repair (e.g., EGFR ligands), barrier function (e.g., mucins and anti-microbial products), and immunoregulatory responses (e.g., IL-25, TGF-β, TSLP and retinoic acid). Furthermore, IECs actively transport IgA antibodies, secreted by plasma cells in the mucosa, into the lumen. The third level of immune defense consists of innate and adaptive immunity. Dendritic cells (DCs) and macrophages are found throughout the intestinal lamina propria, the layer of connective tissue underlying the intestinal epithelium. Interestingly, mononuclear phagocytes found underneath the epithelium can sample the luminal contents by extending dendrites through the epithelial layer into the lumen (12, 13). The gut mucosa contains organized lymphoid structures such as the mesenteric lymph nodes and the Peyer’s patches, and diffuse lymphoid follicles (14). Many innate and adaptive lymphocytes are also scattered throughout the lamina propria and other parts of the intestinal mucosa. Additionally, abundant lymphocytes, called intestinal intraepithelial lymphocytes (iIELs), are interspersed between IECs at the basolateral side of the epithelium (Figure 1) (15–18). The epithelium of the small intestine contains approximately 1 iIEL for every 10 IECs (19–21), making it one of the largest immune compartments of the body. While iIELs serve critical roles in supporting the barrier function of the intestinal epithelium, they sometimes also contribute to gastrointestinal inflammation and disease. Here, we review the unique features, developmental requirements and functions of iIELs.
FIGURE 1.
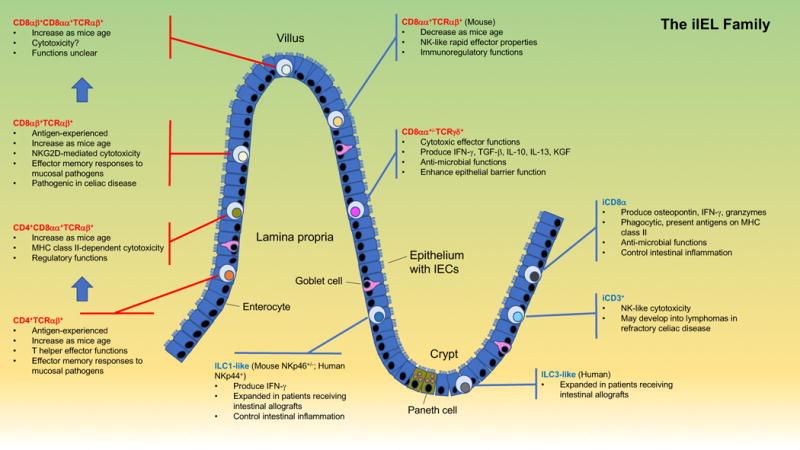
Subsets and salient features of iIELs. iIELs include TCR+ (red font) and TCR− (blue font) populations, with subsets that are derived from conventional, antigen-experienced T cells (called induced iIELs, red brackets) and subsets that home to the intestinal epithelium immediately after their generation (called natural iIELs, blue brackets). Upon entry into the epithelium induced TCR+ iIELs often initiate expression of CD8αα (indicated by blue arrows). The TCR− iIEL population includes subsets resembling ILCs found in barrier tissues outside the intestinal epithelium (i.e., ILC1- and ILC3-like iIELs), cells expressing iCD3 chains (iCD3+TCR− iIELs), and cells co-expressing iCD3 and surface CD8αα (iCD8α cells). A few differences between mouse and human iIEL subsets are highlighted. Key features of distinct iIEL subsets, mostly derived from studies with mice, are listed. Localization of iIEL subsets within the figure does not represent their normal distribution among villi and crypts.
General properties of iIELs
Recruitment of iIELs to the intestinal epithelium is mediated in part by the chemokine CCL25, which is produced by IECs and is recognized by the chemokine receptor CCR9 expressed by all iIELs (22, 23). Entry and retention of iIELs into the epithelium is further facilitated by interaction of E-cadherin on enterocytes with the integrin αEβ7 (β7 is also called CD103) on iIELs (24, 25). Unlike lymphocytes in many other tissues, iIELs do not recirculate (26–28). Most iIELs exhibit an effector or memory phenotype, which endows these cells with the capacity to rapidly respond to activating signals (29–31). Many of them also express surface receptors, including both activating and inhibitory receptors, that are characteristically expressed by natural killer (NK) cells (15, 17, 32). iIELs predominantly consist of T lineage cells, and compared with most other tissues, are enriched for T cells expressing γδT cell receptors (TCRs) (33). While TCRγδ+ iIELs exhibit a strongly restricted TCR repertoire since birth, TCRαβ+ iIELs exhibit a polyclonal TCR repertoire in newborn mice and humans (34). However, in adults, the repertoire of TCRαβ+ iIELs becomes largely oligoclonal, in a manner that is driven by microbial colonization (34–37), suggesting that these cells recognize intestinal commensal antigens under physiological conditions. Another intriguing feature of iIELs is that many of them express a homodimer of CD8α (CD8αα), either in the presence, but more commonly in the absence of CD8αβ expression (15, 17). While CD8αβ functions as a T cell co-receptor to enhance interactions between the TCR and MHC class I-peptide complexes, the function of CD8αα on TCR+ iIELs appears to be distinct (38, 39). CD8αβ and CD8αα exhibit similar affinity for multiple classical MHC class I molecules, yet CD8αα but not CD8αβ binds with high affinity to the thymus leukemia (TL) antigen (40, 41), a nonclassical MHC class I protein (42). TL, which is selectively expressed in the intestinal epithelium (43, 44), is not known to bind antigen of any kind (45), and therefore does not function as a traditional antigen-presenting molecule. Instead, TL engagement redirects CD8αα and its associated lck tyrosine kinase away from the TCR, leading to negative signaling in iIELs (46–48). In this manner, interactions between CD8αα and TL may assist in dampening uncontrolled immune responses in the intestinal epithelium (38, 39). Of note, CD8αα is also expressed by a subset of TCR− iIELs (49).
iIEL subsets
Approximately 90% of all iIELs are TCR+ (Figure 1). These cells can be further divided into induced and natural TCR+ iIELs (also called conventional or type a, and unconventional or type b iIELs, respectively) (15, 17). Induced TCR+ iIELs are derived from conventional, antigen-specific T cells that were activated in the periphery and subsequently entered the epithelium. This group of iIELs includes both CD4+ and CD8αβ+ subsets. Natural TCR+ iIELs include TCRαβ+ and TCRγδ+ subsets, which immediately enter the iIEL compartment following their generation. The prevalence of distinct TCR+ iIEL subsets differs substantially between mice and humans (15, 32). The subset distribution of TCR+ iIELs in mice consists of 10–15% CD4+TCRαβ+ and 20–30% CD8αβ+TCRαβ+ induced iIELs, and 20–50% CD8αβ−CD8αα+TCRαβ+ and 40–70% CD4−CD8αβ−CD8αα+/−TCRγδ+ natural iIELs (50, 51). Humans contain 10–15% CD4+TCRαβ+ and 70–80% CD8αβ+TCRαβ+ induced iIELs, and <1% CD8αβ−CD8αα+TCRαβ+ and 5–20% CD4−CD8αβ−CD8αα+/−TCRγδ+ natural iIELs (52, 53).
Although TCR− iIELs were described two decades ago (31, 54, 55), these cells have been characterized only in recent years (Figure 1) (17). TCR− iIELs include subsets resembling innate lymphoid cells (ILCs) found outside the intestinal epithelium. Cells resembling peripheral ILC1 cells have been identified in both mice and humans (56–59). Mice contain subsets of ILC1-like iIELs with or without expression of the natural cytotoxicity receptor (NCR) NKp46 (NCR1/CD335) (56, 59), and humans contain ILC1-like iIELs expressing NKp44 (NCR2/CD336) (56–58). A subset of iIELs expressing NKp44 and resembling peripheral ILC3 cells has also been identified in humans (59). Another subset of TCR− iIELs, present in both mice and humans, expresses intracellular CD3 (iCD3) chains, and is called iCD3+TCR− iIELs (60). One subset of TCR− iIELs, identified in both mice and humans, expresses iCD3 chains together with surface CD8αα, and is referred to as innate CD8α+ (iCD8α) cells (49, 60, 61).
iIEL development and maintenance
Induced TCR+ iIELs
These are derived from conventional antigen-experienced T cells that enter the intestinal epithelium (Figure 2). Their ontogeny follows the conventional intrathymic development pathway, which will not be discussed here.
FIGURE 2.
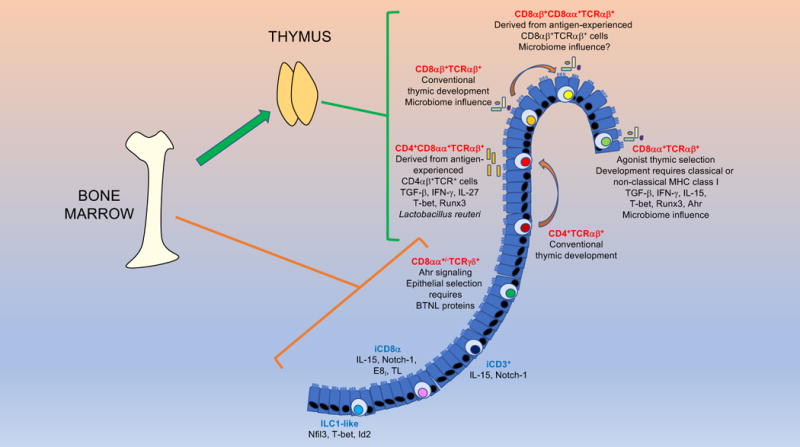
Development and homeostasis of iIEL subsets. Except for TCRγδ+ iIELs, all TCR+ (red font) iIEL develop in the thymus. All TCR− (blue font) iIELs develop extrathymically. Induced TCR+ iIEL follow a conventional thymic development and selection pathway, whereas natural CD8αα+TCRαβ + iIELs undergo agonist selection. iIELs require a variety of transcription factors for their development and function. Many TCR+ iIELs initiate CD8αα expression upon entry into the epithelium. The development, maintenance and homeostasis of iIELs requires a variety of factors, as indicated.
A fraction of CD4+ and CD8αβ+ T cells that migrate into the intestinal epithelium upregulate expression of CD8αα (15, 39). Recent studies have provided insight into the acquisition of CD8αα by CD4+ iIELs. Traditionally, it was thought that the CD4+ and CD8+ T lineages were fixed, without converting from one to the other. However, some CD4+ T cells that migrate into the intestine gain features of the CD8+ T cell lineage, such as cytotoxicity, thus becoming CD4+CD8αα+TCRαβ+ iIELs (62). This transition is governed primarily by the interplay between two transcription factors: ThPOK (encoded by Zbtb7b), which promotes the CD4+ T cell lineage while suppressing the CD8+ T cell lineage, and Runx3, which acts inversely to ThPOK (63–65). For peripheral CD4+ T cells to gain expression of CD8αα, they must downregulate ThPOK and increase expression of Runx3 (and other factors, such as T-bet) (62, 66, 67). The microenvironment of the intestinal epithelium provides signals in the form of TGF-β, retinoic acid, IFN-γ and IL-27 to induce CD8αα expression in CD4+ T cells entering the epithelium (66–69).
Other signals involved in the selection of CD4+CD8αα+TCRαβ+ iIELs are provided by the microbiome. CD4+CD8αα+TCRαβ+ iIELs are reduced in number in germ-free mice as compared with mice maintained under specific pathogen-free conditions (34, 70). In particular, Lactobacillus reuteri has been shown to promote the development of these cells, as mice deficient in this microorganism have significantly reduced numbers of CD4+CD8αα+TCRαβ+ iIELs (71). Mechanistic studies showed that L. reuteri generates indol derivatives of tryptophan that activate the aryl hydrocarbon receptor (AhR) in CD4+ T cells, allowing these cells to differentiate into CD4+CD8αα+TCRαβ+ iIELs.
Natural TCR+ iIELs
CD8αα+TCRαβ+ iIELs are of particular interest because they are exclusively found in the iIEL compartment. Although their antigen-specificity remains unclear, reactivity of these cells is restricted by classical or non-classical MHC class I molecules (72–76). Since their discovery, the origin and development of CD8αα+TCRαβ+ iIEL has been debated (77, 78). This controversy stems from the observation that athymic nude mice harbor considerable numbers of these cells (79, 80). Cryptopatches, small aggregates of lymphoid cells in the lamina propria, were suggested as the relevant site for their extrathymic development (26, 81, 82). However, athymic mice transplanted with a normal thymus reconstitute CD8αα+TCRαβ + cells in the iIEL compartment (83, 84), and neither iIELs nor cryptopatches express RAG proteins (85), providing strong evidence for thymus-dependent development (Figure 2). This conclusion was supported by fate mapping studies (86) and by analyzing the iIEL compartment of mice expressing TCR transgenes (87–89). The precursors for CD8αα+TCRαβ+ iIELs are comprised of CD4−CD8−TCRβ+ thymocytes (87, 89, 90). These precursors undergo agonist positive selection (91), which is responsible for the tendency of mature CD8αα+TCRαβ+ iIELs to exhibit self-reactivity (92). CD8αα+TCRαβ+ iIEL precursors include two subpopulations: PD-1+T-bet− and PD-1−T-bet+ cells (90). PD-1+T-bet− precursors are restricted by classical MHC class I molecules and are enriched in self-reactive thymocytes, whereas the PD-1−T-bet+ population includes cells restricted by non-classical MHC class I molecules (90). Thymic emigrants derived from CD4−CD8−TCRβ+ precursors migrate into the intestinal epithelium where they receive the appropriate cues for their final differentiation, such as expression of CD8αα (Figure 2) (87, 89, 90). This final differentiation step involves TGF-β, as revealed by defective CD8αα+TCRαβ+ iIEL development in TGF-β- and TGF-β signaling-deficient mice, and enrichment of these cells in TGF-β transgenic mice (68).
The maintenance of CD8αα+TCRαβ+ iIEL is influenced by the intestinal microbiota, as shown by their reduced prevalence in germ-free animals (34, 70). Mice deficient in the NOD2 pattern recognition receptor also harbor reduced numbers of CD8αα+TCRαβ+ iIEL (93). Mechanistic studies showed that microbiota activate NOD2 in DCs and IECs, triggering IL-15 secretion, which promotes the survival and maintenance of CD8αα+TCRαβ+ iIELs. IEC-derived IL-15 induces T-bet expression in CD8αα+TCRαβ+ iIELs, which is critical for expression of CD8αα (94).
Although it is now generally accepted that the vast majority of natural TCRαβ+ iIELs are selected in the thymus, accumulating evidence indicates that natural TCRγδ+ iIELs predominantly develop extrathymically (Figure 2) (5, 6, 26, 33, 95). While a contribution for the thymus in generating TCRγδ+ iIEL progenitors cannot be excluded, a critical role for cryptopatches has been proposed (82, 96). Consistent with their lack of MHC-restriction, the development of murine TCRγδ+ iIEL is largely unaffected by MHC antigens that shape αβ T cell repertoires (97).
The size of the TCRγδ+ subset of natural iIELs in mice does not appear to be influenced by the intestinal microbiota (6, 98, 99), but these cells require AhR signaling for their maintenance in the epithelium (100). TCRγδ+ iIELs have a highly restricted TCR repertoire, with a dominant Vγ7 subset in mice and a dominant Vγ4 subset in humans. How these TCRγδ+ subsets are selected and retained within the intestinal epithelium has remained unclear until recently. Two studies showed that G protein-coupled receptors (GPRs) expressed by TCRγδ+ iIELs play a critical role in their recruitment and migration into the intestinal epithelium (101, 102). The orphan receptor GPR18 augmented accumulation of TCRγδ+ cells in the epithelium (101), whereas GPR55, which mediates migration inhibition in response to lysophosphatidylinositol, counteracted this accumulation (102), suggesting tight control over the entry of these cells into the iIEL compartment. Another recent study reported that IECs selectively express certain members of a family of receptors, called butyrophilin-like (BTNL) proteins, that are structurally related to CD80 co-stimulatory and PD-L1 inhibitory molecules (6). These investigators showed that BTNL1 and BTNL6 heterodimers expressed by murine IECs are critically important for the selection and function of Vγ7+ iIELs in mice, and that human gut epithelial cells expressing BTNL3 and BTNL8 heterodimers induce responses of human colonic Vγ4+ iIELs. Surprisingly, these effects of BTNL products on TCRγδ+ iIEL selection and function are TCR-dependent, suggesting TCR-specificity for individual (or pairs of) BTNL family members (6, 103). Additionally, intestinal inflammation and colon cancer alters expression of BTN and BTNL genes (104), which might contribute to some of the alterations in TCRγδ+ iIEL numbers and activity observed in these conditions. These findings also raise the possibility that unique BTNL products expressed in different epithelia may play a role in the selection and function of specific γδT cell subsets in these tissues. The latter hypothesis is supported by studies showing that the murine BTNL protein SKINT1 is selectively expressed by thymic epithelial cells and keratinocytes, and mediates selection of Vγ5+ dendritic epidermal T cells in the skin of mice (33, 103, 105, 106). Whether selection of specific γδT cell subsets in the epithelium of the respiratory and reproductive systems similarly involves expression of tissue-specific BTNL proteins remains to be determined.
TCR− iIELs
Consistent with their lack of antigen-specific receptors, TCR− iIELs develop extrathymically (Figure 2) (17). The development of murine NKp46+ ILC1-like iIELs is dependent on the transcription factors Nfil3 and Id2 (56), which are also required for the development of all peripheral ILC subsets. The development of these cells also requires T-bet expression (56), as would be expected from its role in ILC1 development. Development of murine NKp46− ILC1-like iIELs similarly depends on T-bet expression (59). Both iCD3+TCR− iIELs and iCD8α cells differentiate in the absence of Id2 and require Notch1 signals for their development (49, 60).
The NKp46− subset, but not the NKp46+ subset, of murine ILC1-like cells requires IL-15 for its survival in the intestinal epithelium (56, 59). Similarly, both iCD3+TCR- iIELs and iCD8α cells require IL-15 signaling for their homeostasis or survival (49, 60). iCD8α cells also require interactions between CD8αα and TL for selection or survival in the epithelium, as shown in mice lacking TL expression or defective for the CD8α enhancer E8I (49).
iIEL functions
Induced TCR+ iIELs
Induced iIELs are comprised of conventional, MHC-restricted CD4+ and CD8αβ+ T cells that, after activation in the mesenteric lymph nodes or the lamina propria, migrate into the intestinal epithelium. Once in the epithelium, these cells remain as sentinels to protect the mucosal barrier, either as bona fide effector cells or tissue-resident memory T cells (Table 1) (29). Many of these cells also induce expression of CD8αα upon entry into the epithelium, which not only increases their activation threshold, but may also modulate their functional properties (38, 39).
Table 1.
Examples illustrating the diverse immune functions of iIELs in disease.
| iIEL subset | Infection | Inflammation/colitis | Celiac disease | References |
|---|---|---|---|---|
| CD4+TCRαβ+ | Effector memory Th1 and Th17 responses against mucosal pathogens |
Pathogenic | Unknown | (29) |
| CD4+CD8αα+TCRαβ+ | Unknown | Possibly regulatory functions mediated by IL-10 | Unknown | (107–110) |
| CD8αβ+TCRαβ+ | Effector memory responses against mucosal pathogens, such as vesicular stomatitis virus and L. monocytogenes | Unknown | Lyse IECs via NKG2D-MICA interactions | (29, 32, 111, 115) |
| CD8αβ+CD8αα+TCRαβ+ | Unknown | Unknown | Unknown | |
| CD8αα+TCRαβ+ | Unknown | Possibly regulatory functions mediated by TGF-β3, Lag-3, IL-10 | Unknown | (116–118) |
| CD8αα+/−TCRγδ+ | Anti-microbial activity against pathogens such as S. typhimurium, T. gondii, N. brasiliensis; RegIIIγ-mediated | Pro-inflammatory in early stages of murine colitis; promote healing and protect epithelial integrity in late stages of inflammation and colitis | Expansion during disease but function is not well understood | (15, 32, 118, 122–125, 128–136, 139, 140) |
| ILC1-like | Unknown | Cause IFN-γ-mediated pathology in anti-CD40 antibody-induced colitis | Unknown | (56, 58) |
| iCD8α | Promote bacterial clearance of organisms such as C. rodentium; phagocytic; capable of MHC class II-restricted antigen presentation | Decreased numbers in necrotizing enterocolitis; promote intestinal inflammation via granzymes | Unknown | (17, 49) |
| iCD3+ | Unknown | Unknown | May develop into lymphomas in some patients with refractory celiac disease | (60, 141) |
The frequencies of CD4+CD8αα+TCRαβ+ iIELs are reduced in individuals with chronic intestinal inflammation (107, 108), suggesting an anti-inflammatory role for these cells. This possibility was tested in an animal model following adoptive transfer of in vitro polarized Th2 cells into RAG-deficient mice (109). The transferred cells were able to enter the intestinal epithelium of the recipient animals and also acquired CD8αα expression. Secondary transfer of these CD4+CD8αα+TCRαβ+ iIELs protected recipient RAG-deficient mice against Th1 cell-driven inflammation in a manner that required IL-10. These findings suggested that CD4+ T cells activated and polarized anywhere outside the epithelium may yield CD4+CD8αα+TCRαβ+ iIELs. A recent study provided further support for these findings by showing that regulatory Foxp3+ T cells from the lamina propria can migrate into the intestinal epithelium, where they gain CD8αα expression but lose Foxp3 expression, yet retain regulatory properties and can suppress inflammation (Table 1) (110). The regulatory properties of these iIELs complemented the immunosuppressive functions of CD4+Foxp3+ T cells resident to the lamina propria.
Similar to other tissue-resident CD8+ T cells, CD8αβ+TCRαβ+ iIELs represent effector or memory cells activated in immune organs associated with the intestines (Table 1) (111). For example, in mice infected with vesicular stomatitis virus or Listeria monocytogenes, CD8αβ+TCRαβ+ T cells migrate to the iIEL compartment as early as 5 days post infection, and are retained there over 250 days (111). Additional studies have provided evidence that CD8αβ+TCRαβ+ iIELs expressing CD8αα are enriched for high-affinity TCRs (112), suggesting that TL on IECs preferentially retains such high-affinity memory T cells in the epithelium. Once in the iIEL compartment, CD8αβ+TCRαβ+ iIELs do not require antigen to persist in the epithelium, which suggests that CD8αβ+TCRαβ+ iIELs exhibit effector-like qualities (111, 113). Despite this similarity, the phenotype of antigen-experienced iIELs is distinct from CD8+ T cells in peripheral immune organs such as the spleen and lymph nodes (114). For example, unlike conventional CD8+ T cells, CD8αβ+TCRαβ+ iIELs constitutively express granzyme B, CD69, CD103 and β7 integrin, and produce lower amounts of TNF-α and IFN-γ. In humans with celiac disease, CD8αβ+TCRαβ+ iIELs contribute to disease pathogenesis (Table 1) (32, 115). Gluten-derived products can induce IL-15 production by IECs, which in turn augments expression of activating NK cell receptors such as NKG2D on CD8αβ+TCRαβ+ iIELs. Engagement of NKG2D on the iIELs with its ligand MICA (MHC class I polypeptide-related sequence A), an MHC class I-related protein expressed by stressed IECs, subsequently leads to IEC lysis. However, there is currently no evidence that the TCRs expressed by these iIELs recognize specific antigens derived from the IECs.
Natural TCR+ iIELs
The role of CD8αα+TCRαβ+ iIELs in the intestinal epithelium remains incompletely understood. However, these cells possess a transcriptional profile consistent with regulatory potential (116). For example, CD8αα+TCRαβ+ iIELs express NK cell receptors and their signaling components, such as Ly49 family members, DNAX activating protein of 12KD (DAP-12), 2B4, CD94 and others. These cells are also enriched in immunomodulatory factors, including TGF-β3, lymphocyte activating 3 (LAG-3, which is involved in immunosuppression by regulatory T cells), and fibrinogen-like protein 2 (Fg1–2, which suppresses DC maturation) (116). Consistent with this transcriptional profile, CD8αα+TCRαβ+ iIEL protect mice against colitis induced following adoptive transfer of naïve CD4+ T cells into immunodeficient animals, in a manner that requires IL-10 production (Table 1) (117, 118).
The TCRγδ+ subset of iIELs can produce a variety of pro-inflammatory cytokines (IFN-γ and TNF-α), anti-inflammatory cytokines (TGF-β and IL-10), factors associated with wound healing (TGF-β, prothymosin β4, and keratinocyte growth factor [KGF]), antimicrobial proteins (RegIIIγ), pro-fibrotic factors (IL-13), and granzymes (15). A number of recent studies have shown that TCRγδ+ iIELs are highly motile and rapidly migrate within the space between the epithelial layer and the basement membrane (119–121), while contacting enterocytes via homotypic interactions mediated by the tight-junction protein occludin (119). This motility of TCRγδ+ iIELs is driven by commensal bacteria, resulting in an efficient immune surveillance program. Consistent with these findings, it has been shown that TCRγδ+ iIELs play a critical role in mucosal immune responses against gut microbiota, in a manner that involves MyD88-mediated signaling in IECs and production of the antibacterial lectin RegIIIγ (122, 123). Following infection with microbial pathogens, TCRγδ+ iIELs quickly change their motility and pattern of movement within the epithelium. Interestingly, this response is associated with a metabolic switch toward glycolysis, indicating pathogen-induced alterations in energy utilization pathways (121). Such crosstalk between TCRγδ+ iIELs and IECs is critical for the antimicrobial properties of these cells (Table 1), which were previously implicated in limiting bacterial invasion of Salmonella typhimurium (123) and Toxoplasma gondii infection (124), and to clear Nippostrongylus brasiliensis parasites (125). In addition to promoting pathogen clearance, TCRγδ+ iIELs can also limit tissue damage after infection, as seen during infection with Listeria monocytogenes (126) and Eimeria vermiformis (127). The latter properties of TCRγδ+ iIELs are likely mediated by their capacity to produce factors such as TGF-β, prothymosin β4 and KGF that promote healing and fortify the mucosal barrier (Table 1).
In patients with inflammatory bowel disease, disease severity correlates with increased numbers of TCRγδ+ cells in the intestinal mucosa (128, 129). In several models of colitis, TCRγδ+ T cells promote inflammation (118, 130–133). However, in later stages of colitis, these cells can protect the epithelium against inflammation-induced damage (118, 134–136). Consistent with this role in strengthening the epithelial barrier, TCRγδ+ iIELs have been implicated in promoting the induction and maintenance of oral tolerance in mice (137, 138). The iIEL lymphocytosis observed in patients with celiac disease, which is due to loss of tolerance against gluten, includes a significant expansion of TCRγδ+ iIELs (32, 139, 140). However, whether TCRγδ+ iIELs contribute to disease pathogenesis or are involved in the healing process following tissue damage remains unclear.
TCR− iIELs
Both NKp46+ and NKp46− ILC1-like iIELs in mice can produce IFN-γ in response to cytokine stimulation (56, 59). The NKp46+ subset has been shown to cause pathology in a model of innate cell-mediated colitis induced by anti-CD40 antibodies (Table 1) (56). In humans, NKp44+ ILC1-like iIELs exhibit a memory-activated phenotype and produce IFN-γ in response to IL-12 and IL-15 stimulation (56). Further, these cells are expanded in patients with Crohn’s disease (56) and in patients who received intestinal allografts (58). Human ILC3-like iIELs were shown to produce IL-22 and, like ILC1-like cells, were expanded in patients who received intestinal allografts (58).
iCD3+TCR− iIELs produce granzyme B in response to IL-15 stimulation (60). In some patients with a refractory form of celiac disease, iCD3+TCR− iIELs undergo massive expansion in response to IEC-derived IL-15 production and develop NK-like cytotoxicity against IECs (60, 141). Eventually, some of these cells may develop into clonal lymphomas (Table 1).
iCD8α cells produce a variety of innate cytokines such as MCP-1, IFN-γ and osteopontin, and these cells exhibit cytotoxic and phagocytic properties (49). They also express MHC class II proteins and are capable of presenting antigens to MHC class II-restricted T cells. In mice, these cells provide protection against infection with Citrobacter rodentium (49), and exacerbate colitis induced by anti-CD40 antibodies (17) (Table 1). In humans, the numbers of these cells are decreased in newborns with necrotizing enterocolitis (49).
Crosstalk between iIEL subsets
Due to their close proximity, it is likely that distinct iIEL subsets are engaged in extensive interactions with each other. Yet, few examples of such crosstalk are available. A human study has provided evidence that TCRγδ+ iIELs control the number and activation of CD8αβ+TCRαβ + iIELs, in part by reducing expression of the activating NK cell receptor NKG2D on these cells, in a process that may be relevant to the pathogenesis of celiac disease (142). Additionally, the finding that iCD8α cells have a functional MHC class II antigen presentation pathway (49) suggests that these cells might be able to present antigens to CD4+TCRαβ +TCRαβ+ iIELs, a possibility that remains to be explored. Clearly, crosstalk between iIEL subsets is an important area for future research.
Crosstalk between iIELs and other immune cells
Many studies have shown that iIELs interact with immune cells outside the intestinal epithelium, especially the lamina propria. For example, lamina propria DCs constitutively produce retinoid acid and TGF-β, which induce gut-homing and differentiation of induced iIELs (143–146). DCs also are important for presenting antigens to induced TCRαβ+ iIELs (147, 148). In human celiac disease, anti-gluten CD4+ T cells in the lamina propria contribute to activation of induced CD8αβ+TCRαβ+ iIELs (149). In vitro co-culture studies have shown that TCRαβ+ iIELs from mice primed with antigen by the oral route can function as helper cells to antigen-pulsed B cells to induce IgG and IgA antibodies (150), suggesting that these cells can influence antibody responses. In colitis models, IL-10-producing natural CD8αα+TCRαβ+ iIELs were able to prevent inflammation induced by CD4+CD45RBhi T cells (109, 117). Finally, the finding that TCRγδ+ iIELs can promote oral tolerance (137, 138) suggests that these cells can influence CD4+ T cell responses in the lamina propria.
Outstanding Questions
Although recent studies have provided new insight into the development, homeostasis and functions of distinct iIEL subsets, a variety of outstanding questions remain. First, it is likely that additional subsets of TCR− iIEL are yet to be discovered. Whether all subsets of iIELs are conserved between mice and humans also requires further investigation. Second, the precise developmental relationships between the different subsets of iIELs and with other lymphoid cells residing outside the intestinal epithelium remain to be elucidated. Third, a key question regarding the population of natural TCRγδ+ iIELs is the tissue location and pathway for their development, which appears to be largely thymus-independent. Fourth, the factors that are responsible for the induction and maintenance of CD8αα expression by the majority of iIELs remain incompletely defined. The functional implications of this expression also require further attention. Fifth, while it is clear that the intestinal microbiota greatly impacts iIEL numbers and functions, a better understanding of the effects of individual microbial species on iIEL biology could be employed to devise methods to enhance or suppress iIEL functions. Finally, the precise interactions of distinct iIEL subsets with IECs, with each other, and with immune cells outside the intestinal epithelium remain incompletely understood. Answers to these questions should inform efforts to manipulate iIELs for the development of vaccines and immunotherapies.
Conclusions
The intestinal epithelium contains a wide variety of lymphoid cells with diverse developmental requirements and effector functions. The majority of iIELs are TCR+, and may enter the epithelium after antigen encounter elsewhere (for induced TCR+ iIELs), or immediately after their generation (for natural TCR+ IELs). In recent years, several subsets of TCR− iIELs have been identified, some of which resemble ILCs found in many other mucosal tissues, and cells expressing intracellular CD3 chains. The latter population includes a subset of iIELs that co-express iCD3 chains and surface CD8α homodimers (iCD8α cells). While their main function is to promote the integrity of the epithelial barrier, iIELs can also occasionally contribute to inflammation and disease. Further studies in this field should be instrumental in the development of iIEL-based vaccines and immunotherapies for infectious and inflammatory diseases.
Acknowledgments
Work by the authors was supported by grants from the National Institutes of Health (DK104817 to L.V.K. and AI115419 and DK111617 to D.O.-V.), the Department of Defense (W81XWH-15-1-0543 to L.V.K.) and the Crohn’s and Colitis Foundation (326979 to L.V.K.).
Abbreviations
- AhR
aryl hydrocarbon receptor
- BTNL
butyrophilin-like
- iCD3
intracellular CD3
- iCD8α
innate CD8αα+
- IEC
intestinal epithelial cell
- iIEL
intestinal intraepithelial lymphocyte
- ILC
innate lymphoid cell
- NCR
natural cytotoxicity receptor
- TL
thymus leukemia
References
- 1.Smith PD, MacDonald TT, Blumberg RS. Principles of Mucosal Immunology. CRC Press; 2012. [Google Scholar]
- 2.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 3.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon J, Manley NR. Mechanisms of thymus organogenesis and morphogenesis. Development. 2011;138:3865–3878. doi: 10.1242/dev.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poussier P, Julius M. Thymus independent T cell development and selection in the intestinal epithelium. Annu Rev Immunol. 1994;12:521–553. doi: 10.1146/annurev.iy.12.040194.002513. [DOI] [PubMed] [Google Scholar]
- 6.Di Marco Barros R, Roberts NA, Dart RJ, Vantourout P, Jandke A, Nussbaumer O, Deban L, Cipolat S, Hart R, Iannitto ML, Laing A, Spencer-Dene B, East P, Gibbons D, Irving PM, Pereira P, Steinhoff U, Hayday A. Epithelia Use Butyrophilin-like Molecules to Shape Organ-Specific gammadelta T Cell Compartments. Cell. 2016;167:203–218 e217. doi: 10.1016/j.cell.2016.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsunaga T, Rahman A. In search of the origin of the thymus: the thymus and GALT may be evolutionarily related. Scand J Immunol. 2001;53:1–6. doi: 10.1046/j.1365-3083.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid Y. The mucosal immune system. In: Paul WE, editor. Fundamental Immunology. 7. Lippincott Williams and Wilkins; 2012. pp. 833–849. [Google Scholar]
- 9.Kamada N, Chen GY, Inohara N, Nunez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013;14:685–690. doi: 10.1038/ni.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 11.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3:e982426. doi: 10.4161/21688370.2014.982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 13.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu Rev Immunol. 2004;22:217–246. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- 15.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheridan BS, Lefrancois L. Intraepithelial lymphocytes: to serve and protect. Curr Gastroenterol Rep. 2010;12:513–521. doi: 10.1007/s11894-010-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar AA, Delgado AG, Piazuelo MB, Van Kaer L, Olivares-Villagomez D. Innate CD8alphaalpha+ lymphocytes enhance anti-CD40 antibody-mediated colitis in mice. Immun Inflamm Dis. 2017;5:109–123. doi: 10.1002/iid3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 19.Beagley KW, Fujihashi K, Lagoo AS, Lagoo-Deenadaylan S, Black CA, Murray AM, Sharmanov AT, Yamamoto M, McGhee JR, Elson CO, et al. Differences in intraepithelial lymphocyte T cell subsets isolated from murine small versus large intestine. J Immunol. 1995;154:5611–5619. [PubMed] [Google Scholar]
- 20.Mysorekar IU, Lorenz RG, Gordon JI. A gnotobiotic transgenic mouse model for studying interactions between small intestinal enterocytes and intraepithelial lymphocytes. J Biol Chem. 2002;277:37811–37819. doi: 10.1074/jbc.M205300200. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson A. Intraepithelial lymphocytes of the small intestine. Gut. 1977;18:921–937. doi: 10.1136/gut.18.11.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la Rosa M, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, Agace WW. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. J Clin Invest. 2002;110:1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schon MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, Marsal J, Donohue JP, Her H, Beier DR, Olson S, Lefrancois L, Brenner MB, Grusby MJ, Parker CM. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 25.Kim SK, Schluns KS, Lefrancois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- 26.Poussier P, Edouard P, Lee C, Binnie M, Julius M. Thymus-independent development and negative selection of T cells expressing T cell receptor alpha/beta in the intestinal epithelium: evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J Exp Med. 1992;176:187–199. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki S, Sugahara S, Shimizu T, Tada T, Minagawa M, Maruyama S, Watanabe H, Saito H, Ishikawa H, Hatakeyama K, Abo T. Low level of mixing of partner cells seen in extrathymic T cells in the liver and intestine of parabiotic mice: its biological implication. Eur J Immunol. 1998;28:3719–3729. doi: 10.1002/(SICI)1521-4141(199811)28:11<3719::AID-IMMU3719>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 28.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montufar-Solis D, Garza T, Klein JR. T-cell activation in the intestinal mucosa. Immunol Rev. 2007;215:189–201. doi: 10.1111/j.1600-065X.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebert EC. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology. 1998;115:1439–1445. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 31.Guy-Grand D, Cuenod-Jabri B, Malassis-Seris M, Selz F, Vassalli P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T cell intraepithelial lineages. Eur J Immunol. 1996;26:2248–2256. doi: 10.1002/eji.1830260942. [DOI] [PubMed] [Google Scholar]
- 32.Abadie V, Discepolo V, Jabri B. Intraepithelial lymphocytes in celiac disease immunopathology. Seminars in immunopathology. 2012;34:551–566. doi: 10.1007/s00281-012-0316-x. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen MM, Witherden DA, Havran WL. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733–745. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probert CS, Saubermann LJ, Balk S, Blumberg RS. Repertoire of the alpha beta T-cell receptor in the intestine. Immunol Rev. 2007;215:215–225. doi: 10.1111/j.1600-065X.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- 35.Blumberg RS, Yockey CE, Gross GG, Ebert EC, Balk SP. Human intestinal intraepithelial lymphocytes are derived from a limited number of T cell clones that utilize multiple V beta T cell receptor genes. J Immunol. 1993;150:5144–5153. [PubMed] [Google Scholar]
- 36.Van Kerckhove C, Russell GJ, Deusch K, Reich K, Bhan AK, DerSimonian H, Brenner MB. Oligoclonality of human intestinal intraepithelial T cells. J Exp Med. 1992;175:57–63. doi: 10.1084/jem.175.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jabri B, Selby JM, Negulescu H, Lee L, Roberts AI, Beavis A, Lopez-Botet M, Ebert EC, Winchester RJ. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 2002;17:487–499. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 38.Cheroutre H, Lambolez F. Doubting the TCR Coreceptor Function of CD8alphaalpha. Immunity. 2008;28:149–159. doi: 10.1016/j.immuni.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Olivares-Villagomez D, Van Kaer L. TL and CD8alphaalpha: Enigmatic partners in mucosal immunity. Immunol Lett. 2010;134:1–6. doi: 10.1016/j.imlet.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leishman AJ, Naidenko OV, Attinger A, Koning F, Lena CJ, Xiong Y, Chang HC, Reinherz E, Kronenberg M, Cheroutre H. T cell responses modulated through interaction between CD8alphaalpha and the nonclassical MHC class I molecule, TL. Science. 2001;294:1936–1939. doi: 10.1126/science.1063564. [DOI] [PubMed] [Google Scholar]
- 41.Tsujimura K, Obata Y, Matsudaira Y, Ozeki S, Yoshikawa K, Saga S, Takahashi T. The binding of thymus leukemia (TL) antigen tetramers to normal intestinal intraepithelial lymphocytes and thymocytes. J Immunol. 2001;167:759–764. doi: 10.4049/jimmunol.167.2.759. [DOI] [PubMed] [Google Scholar]
- 42.Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–471. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- 43.Wu M, van Kaer L, Itohara S, Tonegawa S. Highly restricted expression of the thymus leukemia antigens on intestinal epithelial cells. J Exp Med. 1991;174:213–218. doi: 10.1084/jem.174.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hershberg R, Eghtesady P, Sydora B, Brorson K, Cheroutre H, Modlin R, Kronenberg M. Expression of the thymus leukemia antigen in mouse intestinal epithelium. Proc Natl Acad Sci U S A. 1990;87:9727–9731. doi: 10.1073/pnas.87.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Xiong Y, Naidenko OV, Liu JH, Zhang R, Joachimiak A, Kronenberg M, Cheroutre H, Reinherz EL, Wang JH. The crystal structure of a TL/CD8alphaalpha complex at 2.1 A resolution: implications for modulation of T cell activation and memory. Immunity. 2003;18:205–215. doi: 10.1016/s1074-7613(03)00027-x. [DOI] [PubMed] [Google Scholar]
- 46.van Oers NS, Teh SJ, Garvin AM, Forbush KA, Perlmutter RM, Teh HS. CD8 inhibits signal transduction through the T cell receptor in CD4-CD8- thymocytes from T cell receptor transgenic mice reconstituted with a transgenic CD8 alpha molecule. J Immunol. 1993;151:777–790. [PubMed] [Google Scholar]
- 47.Madakamutil LT, Christen U, Lena CJ, Wang-Zhu Y, Attinger A, Sundarrajan M, Ellmeier W, von Herrath MG, Jensen P, Littman DR, Cheroutre H. CD8alphaalpha-mediated survival and differentiation of CD8 memory T cell precursors. Science. 2004;304:590–593. doi: 10.1126/science.1092316. [DOI] [PubMed] [Google Scholar]
- 48.Levelt CN, de Jong YP, Mizoguchi E, O’Farrelly C, Bhan AK, Tonegawa S, Terhorst C, Simpson SJ. High- and low-affinity single-peptide/MHC ligands have distinct effects on the development of mucosal CD8alphaalpha and CD8alphabeta T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:5628–5633. doi: 10.1073/pnas.96.10.5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Kaer L, Algood HM, Singh K, Parekh VV, Greer MJ, Piazuelo MB, Weitkamp JH, Matta P, Chaturvedi R, Wilson KT, Olivares-Villagomez D. CD8alphaalpha(+) Innate-Type Lymphocytes in the Intestinal Epithelium Mediate Mucosal Immunity. Immunity. 2014;41:451–464. doi: 10.1016/j.immuni.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guy-Grand D, Cerf-Bensussan N, Malissen B, Malassis-Seris M, Briottet C, Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991;173:471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guy-Grand D, Rocha B, Mintz P, Malassis-Seris M, Selz F, Malissen B, Vassalli P. Different use of T cell receptor transducing modules in two populations of gut intraepithelial lymphocytes are related to distinct pathways of T cell differentiation. J Exp Med. 1994;180:673–679. doi: 10.1084/jem.180.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundqvist C, Baranov V, Hammarstrom S, Athlin L, Hammarstrom ML. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7:1473–1487. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- 53.Jarry A, Cerf-Bensussan N, Brousse N, Selz F, Guy-Grand D. Subsets of CD3+ (T cell receptor alpha/beta or gamma/delta) and CD3- lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur J Immunol. 1990;20:1097–1103. doi: 10.1002/eji.1830200523. [DOI] [PubMed] [Google Scholar]
- 54.Eiras P, Leon F, Camarero C, Lombardia M, Roldan E, Bootello A, Roy G. Intestinal intraepithelial lymphocytes contain a CD3- CD7+ subset expressing natural killer markers and a singular pattern of adhesion molecules. Scand J Immunol. 2000;52:1–6. doi: 10.1046/j.1365-3083.2000.00761.x. [DOI] [PubMed] [Google Scholar]
- 55.Leon F, Roldan E, Sanchez L, Camarero C, Bootello A, Roy G. Human small-intestinal epithelium contains functional natural killer lymphocytes. Gastroenterology. 2003;125:345–356. doi: 10.1016/s0016-5085(03)00886-2. [DOI] [PubMed] [Google Scholar]
- 56.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, Cella M, Colonna M. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38:769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simoni Y, Fehlings M, Kloverpris HN, McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang CL, Kam MH, Dennis K, Lim TK, Fui AC, Hoong CW, Chan JK, Curotto de Lafaille M, Narayanan S, Baig S, Shabeer M, Toh SE, Tan HK, Anicete R, Tan EH, Takano A, Klenerman P, Leslie A, Tan DS, Tan IB, Ginhoux F, Newell EW. Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency. Immunity. 2017;46:148–161. doi: 10.1016/j.immuni.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Talayero P, Mancebo E, Calvo-Pulido J, Rodriguez-Munoz S, Bernardo I, Laguna-Goya R, Cano-Romero FL, Garcia-Sesma A, Loinaz C, Jimenez C, Justo I, Paz-Artal E. Innate Lymphoid Cells Groups 1 and 3 in the Epithelial Compartment of Functional Human Intestinal Allografts. Am J Transplant. 2016;16:72–82. doi: 10.1111/ajt.13435. [DOI] [PubMed] [Google Scholar]
- 59.Van Acker A, Gronke K, Biswas A, Martens L, Saeys Y, Filtjens J, Taveirne S, Van Ammel E, Kerre T, Matthys P, Taghon T, Vandekerckhove B, Plum J, Dunay IR, Diefenbach A, Leclercq G. A Murine Intestinal Intraepithelial NKp46-Negative Innate Lymphoid Cell Population Characterized by Group 1 Properties. Cell Rep. 2017;19:1431–1443. doi: 10.1016/j.celrep.2017.04.068. [DOI] [PubMed] [Google Scholar]
- 60.Ettersperger J, Montcuquet N, Malamut G, Guegan N, Lopez-Lastra S, Gayraud S, Reimann C, Vidal E, Cagnard N, Villarese P, Andre-Schmutz I, Gomes Domingues R, Godinho-Silva C, Veiga-Fernandes H, Lhermitte L, Asnafi V, Macintyre E, Cellier C, Beldjord K, Di Santo JP, Cerf-Bensussan N, Meresse B. Interleukin-15-Dependent T-Cell-like Innate Intraepithelial Lymphocytes Develop in the Intestine and Transform into Lymphomas in Celiac Disease. Immunity. 2016;45:610–625. doi: 10.1016/j.immuni.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 61.Olivares-Villagomez D, Van Kaer L. iCD8alpha cells: living at the edge of the intestinal immune system. Oncotarget. 2015;6:19964–19965. doi: 10.18632/oncotarget.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mucida D, Husain MM, Muroi S, van Wijk F, Shinnakasu R, Naoe Y, Reis BS, Huang Y, Lambolez F, Docherty M, Attinger A, Shui JW, Kim G, Lena CJ, Sakaguchi S, Miyamoto C, Wang P, Atarashi K, Park Y, Nakayama T, Honda K, Ellmeier W, Kronenberg M, Taniuchi I, Cheroutre H. Transcriptional reprogramming of mature CD4(+) helper T cells generates distinct MHC class II-restricted cytotoxic T lymphocytes. Nat Immunol. 2013;14:281–289. doi: 10.1038/ni.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He X, He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 64.Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 65.Woolf E, Xiao C, Fainaru O, Lotem J, Rosen D, Negreanu V, Bernstein Y, Goldenberg D, Brenner O, Berke G, Levanon D, Groner Y. Runx3 and Runx1 are required for CD8 T cell development during thymopoiesis. Proc Natl Acad Sci U S A. 2003;100:7731–7736. doi: 10.1073/pnas.1232420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reis BS, Hoytema van Konijnenburg DP, Grivennikov SI, Mucida D. Transcription factor T-bet regulates intraepithelial lymphocyte functional maturation. Immunity. 2014;41:244–256. doi: 10.1016/j.immuni.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reis BS, Rogoz A, Costa-Pinto FA, Taniuchi I, Mucida D. Mutual expression of the transcription factors Runx3 and ThPOK regulates intestinal CD4(+) T cell immunity. Nat Immunol. 2013;14:271–280. doi: 10.1038/ni.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Konkel JE, Maruyama T, Carpenter AC, Xiong Y, Zamarron BF, Hall BE, Kulkarni AB, Zhang P, Bosselut R, Chen W. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nat Immunol. 2011;12:312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Kaer L, Rabacal WA, Scott Algood HM, Parekh VV, Olivares-Villagomez D. In vitro induction of regulatory CD4+CD8alpha+ T cells by TGF-beta, IL-7 and IFN-gamma. PloS one. 2013;8:e67821. doi: 10.1371/journal.pone.0067821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mota-Santos T, Masmoudi H, Voegtle D, Freitas A, Coutinho A, Cazenave PA. Divergency in the specificity of the induction and maintenance of neonatal suppression. Eur J Immunol. 1990;20:1717–1721. doi: 10.1002/eji.1830200814. [DOI] [PubMed] [Google Scholar]
- 71.Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, Cella M, Gordon JI, Hsieh CS, Colonna M. Lactobacillus reuteri induces gut intraepithelial CD4+CD8alphaalpha+ T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olivares-Villagomez D, Mendez-Fernandez YV, Parekh VV, Lalani S, Vincent TL, Cheroutre H, Van Kaer L. Thymus leukemia antigen controls intraepithelial lymphocyte function and inflammatory bowel disease. Proc Natl Acad Sci U S A. 2008;105:17931–17936. doi: 10.1073/pnas.0808242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gapin L, Cheroutre H, Kronenberg M. Cutting edge: TCR alpha beta+ CD8 alpha alpha+ T cells are found in intestinal intraepithelial lymphocytes of mice that lack classical MHC class I molecules. J Immunol. 1999;163:4100–4104. [PubMed] [Google Scholar]
- 74.Park SH, Guy-Grand D, Lemonnier FA, Wang CR, Bendelac A, Jabri B. Selection and expansion of CD8alpha/alpha(1) T cell receptor alpha/beta(1) intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J Exp Med. 1999;190:885–890. doi: 10.1084/jem.190.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Das G, Gould DS, Augustine MM, Fragoso G, Sciutto E, Stroynowski I, Van Kaer L, Schust DJ, Ploegh H, Janeway CA., Jr Qa-2-dependent selection of CD8alpha/alpha T cell receptor alpha/beta(+) cells in murine intestinal intraepithelial lymphocytes. J Exp Med. 2000;192:1521–1528. doi: 10.1084/jem.192.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayans S, Stepniak D, Palida S, Larange A, Dreux J, Arlian B, Shinnakasu R, Kronenberg M, Cheroutre H, Lambolez F. alphabetaT cell receptors expressed by CD4(-)CD8alphabeta(-) intraepithelial T cells drive their fate into a unique lineage with unusual MHC reactivities. Immunity. 2014;41:207–218. doi: 10.1016/j.immuni.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lefrancois L, Puddington L. Extrathymic intestinal T-cell development: virtual reality? Immunol Today. 1995;16:16–21. doi: 10.1016/0167-5699(95)80065-4. [DOI] [PubMed] [Google Scholar]
- 78.Rocha B. The extrathymic T-cell differentiation in the murine gut. Immunol Rev. 2007;215:166–177. doi: 10.1111/j.1600-065X.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 79.Klein JR. Ontogeny of the Thy-1-, Lyt-2+ murine intestinal intraepithelial lymphocyte. Characterization of a unique population of thymus-independent cytotoxic effector cells in the intestinal mucosa. J Exp Med. 1986;164:309–314. doi: 10.1084/jem.164.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Geus B, Van den Enden M, Coolen C, Nagelkerken L, Van der Heijden P, Rozing J. Phenotype of intraepithelial lymphocytes in euthymic and athymic mice: implications for differentiation of cells bearing a CD3-associated gamma delta T cell receptor. Eur J Immunol. 1990;20:291–298. doi: 10.1002/eji.1830200210. [DOI] [PubMed] [Google Scholar]
- 81.Guy-Grand D, Vassalli P, Eberl G, Pereira P, Burlen-Defranoux O, Lemaitre F, Di Santo JP, Freitas AA, Cumano A, Bandeira A. Origin, trafficking, and intraepithelial fate of gut-tropic T cells. J Exp Med. 2013;210:1839–1854. doi: 10.1084/jem.20122588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saito H, Kanamori Y, Takemori T, Nariuchi H, Kubota E, Takahashi-Iwanaga H, Iwanaga T, Ishikawa H. Generation of intestinal T cells from progenitors residing in gut cryptopatches. Science. 1998;280:275–278. doi: 10.1126/science.280.5361.275. [DOI] [PubMed] [Google Scholar]
- 83.Lin T, Matsuzaki G, Kenai H, Nakamura T, Nomoto K. Thymus influences the development of extrathymically derived intestinal intraepithelial lymphocytes. Eur J Immunol. 1993;23:1968–1974. doi: 10.1002/eji.1830230836. [DOI] [PubMed] [Google Scholar]
- 84.Lin T, Matsuzaki G, Kenai H, Nomoto K. Progenies of fetal thymocytes are the major source of CD4-CD8+ alpha alpha intestinal intraepithelial lymphocytes early in ontogeny. Eur J Immunol. 1994;24:1785–1791. doi: 10.1002/eji.1830240810. [DOI] [PubMed] [Google Scholar]
- 85.Guy-Grand D, Azogui O, Celli S, Darche S, Nussenzweig MC, Kourilsky P, Vassalli P. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med. 2003;197:333–341. doi: 10.1084/jem.20021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 87.Gangadharan D, Lambolez F, Attinger A, Wang-Zhu Y, Sullivan BA, Cheroutre H. Identification of pre- and postselection TCRalphabeta+ intraepithelial lymphocyte precursors in the thymus. Immunity. 2006;25:631–641. doi: 10.1016/j.immuni.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 88.Baldwin TA, Sandau MM, Jameson SC, Hogquist KA. The timing of TCR alpha expression critically influences T cell development and selection. J Exp Med. 2005;202:111–121. doi: 10.1084/jem.20050359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McDonald BD, Bunker JJ, Ishizuka IE, Jabri B, Bendelac A. Elevated T cell receptor signaling identifies a thymic precursor to the TCRalphabeta(+)CD4(−)CD8beta(−) intraepithelial lymphocyte lineage. Immunity. 2014;41:219–229. doi: 10.1016/j.immuni.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA. CD8alphaalpha intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol. 2017;18:771–779. doi: 10.1038/ni.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8alphaalpha(+) T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 92.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jiang W, Wang X, Zeng B, Liu L, Tardivel A, Wei H, Han J, MacDonald HR, Tschopp J, Tian Z, Zhou R. Recognition of gut microbiota by NOD2 is essential for the homeostasis of intestinal intraepithelial lymphocytes. J Exp Med. 2013;210:2465–2476. doi: 10.1084/jem.20122490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Klose CS, Blatz K, d’Hargues Y, Hernandez PP, Kofoed-Nielsen M, Ripka JF, Ebert K, Arnold SJ, Diefenbach A, Palmer E, Tanriver Y. The transcription factor T-bet is induced by IL-15 and thymic agonist selection and controls CD8alphaalpha(+) intraepithelial lymphocyte development. Immunity. 2014;41:230–243. doi: 10.1016/j.immuni.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 95.Lefrancois L. Extrathymic differentiation of intraepithelial lymphocytes: generation of a separate and unequal T-cell repertoire? Immunol Today. 1991;12:436–438. doi: 10.1016/0167-5699(91)90015-L. [DOI] [PubMed] [Google Scholar]
- 96.Podd BS, Thoits J, Whitley N, Cheng HY, Kudla KL, Taniguchi H, Halkias J, Goth K, Camerini V. T cells in cryptopatch aggregates share TCR gamma variable region junctional sequences with gamma delta T cells in the small intestinal epithelium of mice. J Immunol. 2006;176:6532–6542. doi: 10.4049/jimmunol.176.11.6532. [DOI] [PubMed] [Google Scholar]
- 97.Hayday A, Vantourout P. A long-playing CD about the gammadelta TCR repertoire. Immunity. 2013;39:994–996. doi: 10.1016/j.immuni.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 98.Bandeira A, Itohara S, Bonneville M, Burlen-Defranoux O, Mota-Santos T, Coutinho A, Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci U S A. 1991;88:43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawaguchi M, Nanno M, Umesaki Y, Matsumoto S, Okada Y, Cai Z, Shimamura T, Matsuoka Y, Ohwaki M, Ishikawa H. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc Natl Acad Sci U S A. 1993;90:8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147:629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Sumida H, Cyster JG. GPR18 is required for a normal CD8alphaalpha intestinal intraepithelial lymphocyte compartment. J Exp Med. 2014;211:2351–2359. doi: 10.1084/jem.20140646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sumida H, Lu E, Chen H, Yang Q, Mackie K, Cyster JG. GPR55 regulates intraepithelial lymphocyte migration dynamics and susceptibility to intestinal damage. Sci Immunol. 2017;2 doi: 10.1126/sciimmunol.aao1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vantourout P, Laing A, Woodward MJ, Zlatareva I, Apolonia L, Jones AW, Snijders AP, Malim MH, Hayday AC. Hteromeric interactions regulate butyrophilin (BTN) and BTN-like molecules governing gd T cell biology. Proc Natl Acad Sci U S A. 2018 doi: 10.1073/pnas.1701237115. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lebrero-Fernandez C, Wenzel UA, Akeus P, Wang Y, Strid H, Simren M, Gustavsson B, Borjesson LG, Cardell SL, Ohman L, Quiding-Jarbrink M, Bas-Forsberg A. Altered expression of Butyrophilin (BTN) and BTN-like (BTNL) genes in intestinal inflammation and colon cancer. Immun Inflamm Dis. 2016;4:191–200. doi: 10.1002/iid3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boyden LM, Lewis JM, Barbee SD, Bas A, Girardi M, Hayday AC, Tigelaar RE, Lifton RP. Skint1, the prototype of a newly identified immunoglobulin superfamily gene cluster, positively selects epidermal gammadelta T cells. Nat Genet. 2008;40:656–662. doi: 10.1038/ng.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Barbee SD, Woodward MJ, Turchinovich G, Mention JJ, Lewis JM, Boyden LM, Lifton RP, Tigelaar R, Hayday AC. Skint-1 is a highly specific, unique selecting component for epidermal T cells. Proc Natl Acad Sci U S A. 2011;108:3330–3335. doi: 10.1073/pnas.1010890108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carton J, Byrne B, Madrigal-Estebas L, O’Donoghue DP, O’Farrelly C. CD4+CD8+ human small intestinal T cells are decreased in coeliac patients, with CD8 expression downregulated on intra-epithelial T cells in the active disease. Eur J Gastroenterol Hepatol. 2004;16:961–968. doi: 10.1097/00042737-200410000-00003. [DOI] [PubMed] [Google Scholar]
- 108.Senju M, Wu KC, Mahida YR, Jewell DP. Coexpression of CD4 and CD8 on peripheral blood T cells and lamina propria T cells in inflammatory bowel disease by two colour immunofluorescence and flow cytometric analysis. Gut. 1991;32:918–922. doi: 10.1136/gut.32.8.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Das G, Augustine MM, Das J, Bottomly K, Ray P, Ray A. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A. 2003;100:5324–5329. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sujino T, London M, Hoytema van Konijnenburg DP, Rendon T, Buch T, Silva HM, Lafaille JJ, Reis BS, Mucida D. Tissue adaptation of regulatory and intraepithelial CD4(+) T cells controls gut inflammation. Science. 2016;352:1581–1586. doi: 10.1126/science.aaf3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 112.Huang Y, Park Y, Wang-Zhu Y, Larange A, Arens R, Bernardo I, Olivares-Villagomez D, Herndler-Brandstetter D, Abraham N, Grubeck-Loebenstein B, Schoenberger SP, Van Kaer L, Kronenberg M, Teitell MA, Cheroutre H. Mucosal memory CD8(+) T cells are selected in the periphery by an MHC class I molecule. Nat Immunol. 2011;12:1086–1095. doi: 10.1038/ni.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Masopust D, Jiang J, Shen H, Lefrancois L. Direct analysis of the dynamics of the intestinal mucosa CD8 T cell response to systemic virus infection. J Immunol. 2001;166:2348–2356. doi: 10.4049/jimmunol.166.4.2348. [DOI] [PubMed] [Google Scholar]
- 114.Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- 115.Jabri B, Sollid LM. T Cells in Celiac Disease. J Immunol. 2017;198:3005–3014. doi: 10.4049/jimmunol.1601693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Denning TL, Granger SW, Mucida D, Graddy R, Leclercq G, Zhang W, Honey K, Rasmussen JP, Cheroutre H, Rudensky AY, Kronenberg M. Mouse TCRalphabeta+CD8alphaalpha intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–4239. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- 117.Poussier P, Ning T, Banerjee D, Julius M. A unique subset of self-specific intraintestinal T cells maintains gut integrity. J Exp Med. 2002;195:1491–1497. doi: 10.1084/jem.20011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hu MD, Edelblum KL. Sentinels at the frontline: the role of intraepithelial lymphocytes in inflammatory bowel disease. Curr Pharmacol Rep. 2017;3:321–334. doi: 10.1007/s40495-017-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Edelblum KL, Shen L, Weber CR, Marchiando AM, Clay BS, Wang Y, Prinz I, Malissen B, Sperling AI, Turner JR. Dynamic migration of gammadelta intraepithelial lymphocytes requires occludin. Proc Natl Acad Sci U S A. 2012;109:7097–7102. doi: 10.1073/pnas.1112519109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Edelblum KL, Singh G, Odenwald MA, Lingaraju A, El Bissati K, McLeod R, Sperling AI, Turner JR. gammadelta Intraepithelial Lymphocyte Migration Limits Transepithelial Pathogen Invasion and Systemic Disease in Mice. Gastroenterology. 2015;148:1417–1426. doi: 10.1053/j.gastro.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell. 2017;171:783–794 e713. doi: 10.1016/j.cell.2017.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ismail AS, Behrendt CL, Hooper LV. Reciprocal interactions between commensal bacteria and gamma delta intraepithelial lymphocytes during mucosal injury. J Immunol. 2009;182:3047–3054. doi: 10.4049/jimmunol.0802705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ismail AS, Severson KM, Vaishnava S, Behrendt CL, Yu X, Benjamin JL, Ruhn KA, Hou B, DeFranco AL, Yarovinsky F, Hooper LV. Gammadelta intraepithelial lymphocytes are essential mediators of host-microbial homeostasis at the intestinal mucosal surface. Proc Natl Acad Sci U S A. 2011;108:8743–8748. doi: 10.1073/pnas.1019574108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dalton JE, Cruickshank SM, Egan CE, Mears R, Newton DJ, Andrew EM, Lawrence B, Howell G, Else KJ, Gubbels MJ, Striepen B, Smith JE, White SJ, Carding SR. Intraepithelial gammadelta+ lymphocytes maintain the integrity of intestinal epithelial tight junctions in response to infection. Gastroenterology. 2006;131:818–829. doi: 10.1053/j.gastro.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 125.Inagaki-Ohara K, Sakamoto Y, Dohi T, Smith AL. gammadelta T cells play a protective role during infection with Nippostrongylus brasiliensis by promoting goblet cell function in the small intestine. Immunology. 2011;134:448–458. doi: 10.1111/j.1365-2567.2011.03503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mombaerts P, Mizoguchi E, Grusby MJ, Glimcher LH, Bhan AK, Tonegawa S. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:274–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]
- 127.Roberts SJ, Smith AL, West AB, Wen L, Findly RC, Owen MJ, Hayday AC. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kanazawa H, Ishiguro Y, Munakata A, Morita T. Multiple accumulation of Vdelta2+ gammadelta T-cell clonotypes in intestinal mucosa from patients with Crohn’s disease. Dig Dis Sci. 2001;46:410–416. doi: 10.1023/a:1005669319556. [DOI] [PubMed] [Google Scholar]
- 129.Yeung MM, Melgar S, Baranov V, Oberg A, Danielsson A, Hammarstrom S, Hammarstrom ML. Characterisation of mucosal lymphoid aggregates in ulcerative colitis: immune cell phenotype and TcR-gammadelta expression. Gut. 2000;47:215–227. doi: 10.1136/gut.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Simpson SJ, Hollander GA, Mizoguchi E, Allen D, Bhan AK, Wang B, Terhorst C. Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur J Immunol. 1997;27:17–25. doi: 10.1002/eji.1830270104. [DOI] [PubMed] [Google Scholar]
- 131.Kawaguchi-Miyashita M, Shimada S, Kurosu H, Kato-Nagaoka N, Matsuoka Y, Ohwaki M, Ishikawa H, Nanno M. An accessory role of TCRgammadelta (+) cells in the exacerbation of inflammatory bowel disease in TCRalpha mutant mice. Eur J Immunol. 2001;31:980–988. doi: 10.1002/1521-4141(200104)31:4<980::aid-immu980>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 132.Mizoguchi A, Mizoguchi E, de Jong YP, Takedatsu H, Preffer FI, Terhorst C, Bhan AK. Role of the CD5 molecule on TCR gammadelta T cell-mediated immune functions: development of germinal centers and chronic intestinal inflammation. Int Immunol. 2003;15:97–108. doi: 10.1093/intimm/dxg006. [DOI] [PubMed] [Google Scholar]
- 133.Park SG, Mathur R, Long M, Hosh N, Hao L, Hayden MS, Ghosh S. T regulatory cells maintain intestinal homeostasis by suppressing gammadelta T cells. Immunity. 2010;33:791–803. doi: 10.1016/j.immuni.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuhl AA, Pawlowski NN, Grollich K, Loddenkemper C, Zeitz M, Hoffmann JC. Aggravation of intestinal inflammation by depletion/deficiency of gammadelta T cells in different types of IBD animal models. J Leukoc Biol. 2007;81:168–175. doi: 10.1189/jlb.1105696. [DOI] [PubMed] [Google Scholar]
- 135.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsuchiya T, Fukuda S, Hamada H, Nakamura A, Kohama Y, Ishikawa H, Tsujikawa K, Yamamoto H. Role of gamma delta T cells in the inflammatory response of experimental colitis mice. J Immunol. 2003;171:5507–5513. doi: 10.4049/jimmunol.171.10.5507. [DOI] [PubMed] [Google Scholar]
- 137.Mengel J, Cardillo F, Aroeira LS, Williams O, Russo M, Vaz NM. Anti-gamma delta T cell antibody blocks the induction and maintenance of oral tolerance to ovalbumin in mice. Immunol Lett. 1995;48:97–102. doi: 10.1016/0165-2478(95)02451-4. [DOI] [PubMed] [Google Scholar]
- 138.Fujihashi K, Dohi T, Kweon MN, McGhee JR, Koga T, Cooper MD, Tonegawa S, Kiyono H. gammadelta T cells regulate mucosally induced tolerance in a dose-dependent fashion. Int Immunol. 1999;11:1907–1916. doi: 10.1093/intimm/11.12.1907. [DOI] [PubMed] [Google Scholar]
- 139.Saborido R, Martinon N, Regueiro A, Crujeiras V, Eiras P, Leis R. Intraepithelial lymphocyte immunophenotype: a useful tool in the diagnosis of celiac disease. J Physiol Biochem. 2017;11:EC17–EC21. doi: 10.1007/s13105-017-0586-9. [DOI] [PubMed] [Google Scholar]
- 140.Sollid LM. The roles of MHC class II genes and post-translational modification in celiac disease. Immunogenetics. 2017;69:605–616. doi: 10.1007/s00251-017-0985-7. [DOI] [PubMed] [Google Scholar]
- 141.Malamut G, Meresse B, Cellier C, Cerf-Bensussan N. Refractory celiac disease: from bench to bedside. Seminars in immunopathology. 2012;34:601–613. doi: 10.1007/s00281-012-0322-z. [DOI] [PubMed] [Google Scholar]
- 142.Bhagat G, Naiyer AJ, Shah JG, Harper J, Jabri B, Wang TC, Green PH, Manavalan JS. Small intestinal CD8+TCRgammadelta+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J Clin Invest. 2008;118:281–293. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 144.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 145.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 147.Moretto MM, Weiss LM, Combe CL, Khan IA. IFN-gamma-producing dendritic cells are important for priming of gut intraepithelial lymphocyte response against intracellular parasitic infection. J Immunol. 2007;179:2485–2492. doi: 10.4049/jimmunol.179.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Moretto MM, Harrow DI, Hawley TS, Khan IA. Interleukin-12-producing CD103+ CD11b- CD8+ dendritic cells are responsible for eliciting gut intraepithelial lymphocyte response against Encephalitozoon cuniculi. Infect Immun. 2015;83:4719–4730. doi: 10.1128/IAI.00820-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Setty M, Discepolo V, Abadie V, Kamhawi S, Mayassi T, Kent A, Ciszewski C, Maglio M, Kistner E, Bhagat G, Semrad C, Kupfer SS, Green PH, Guandalini S, Troncone R, Murray JA, Turner JR, Jabri B. Distinct and Synergistic Contributions of Epithelial Stress and Adaptive Immunity to Functions of Intraepithelial Killer Cells and Active Celiac Disease. Gastroenterology. 2015;149:681–691 e610. doi: 10.1053/j.gastro.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fujihashi K, Taguchi T, Aicher WK, McGhee JR, Bluestone JA, Eldridge JH, Kiyono H. Immunoregulatory functions for murine intraepithelial lymphocytes: gamma/delta T cell receptor-positive (TCR+) T cells abrogate oral tolerance, while alpha/beta TCR+ T cells provide B cell help. J Exp Med. 1992;175:695–707. doi: 10.1084/jem.175.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]


