Abstract
Aberrant activation of the Wnt/β-catenin pathway contributes to the development of diabetic nephropathy (DN); however, treatment with Tripterygium wilfordii (TW) may be beneficial for patients with DN. The aim of the present study was to evaluate the effect of TW on Wnt/β-catenin expression in the kidneys of diabetic rats. Male Sprague-Dawley rats were randomly injected with vehicle (control) or streptozotocin to induce diabetes. Diabetic rats were then randomly treated with vehicle (sodium carboxymethyl cellulose; SCC), TW combined with SCC (8 or 16 mg/kg) or irbesartan (50 mg/kg) daily for 8 weeks. Metabolic parameter levels and renal pathological changes were examined. mRNA and protein expression of Wnt-1, glycogen synthase kinase (GSK)-3β, β-catenin, nuclear factor (NF)-κB and transforming growth factor (TGF)-β1 in the kidneys of rats from all groups were measured. Compared with the DM group, metabolic parameters and morphological parameters, apart from blood glucose levels, were significantly improved in TW-treated rats (all P<0.01). Furthermore, levels of Wnt-1, β-catenin, NF-κB-p65 and TGF-β1 mRNA and protein were significantly reduced in the kidneys of TW-treated rats compared with DM rats, whereas levels of GSK-3β mRNA and protein did not differ significantly between any of the groups; however, the expression of P-GSK-3β protein was significantly decreased in the kidneys of TW-treated rats compared with the DM group. The protective effects of TW tended to be dose-dependent and were an improvement compared with irbesartan treatment in diabetic rats. Therefore, the results of the present study indicated that treatment with TW mitigated hyperglycemia-induced upregulated Wnt-1 and β-catenin expression in kidney tissues and ameliorated diabetes-induced kidney injury in rats.
Keywords: diabetic nephropathy, Tripterygium wilfordii, Wnt-1, β-catenin, glycogen synthase kinase 3β, nuclear factor-κB, transforming growth factor-β1
Introduction
Diabetic nephropathy (DN) is the main cause of end stage renal disease (ESRD), accounting for ~44% of all ESRD cases, and seriously affects patients' quality of life (1). Pathologically, early DN is characterized by glomerular hyperfiltration, glomerular and renal hypertrophy, increased urinary albumin excretion, and increased basement membrane thickening and mesangial expansion with the accumulation of extracellular matrix and podocyte injury (2). Currently, the pathogenesis of DN is attributed to abnormal cell signal transduction, an immune response, and metabolic disorders, renin-angiotensin system (RAS) and other associated disorders. Previous studies have demonstrated that Wnt/β-catenin signaling is able to downregulate nephrin expression in podocytes and impair the glomerular filtration barrier, resulting in proteinuria; however, it has been demonstrated that inhibiting this pathway reduces podocyte damage (3,4). Furthermore, aberrant activation of Wnt/β-catenin signaling may induce extracellular matrix accumulation and glomerulosclerosis by raising TGF-β1 expression, thus affecting the development of DN (5). Certain therapeutic strategies are able to effectively inhibit the progression of DN and recent studies have confirmed that treatment with angiotensin II receptor blockers reduces proteinuria and delays DN progression (6,7). However, the identification of novel therapeutic reagents, particularly those able to potently inhibit Wnt/β-catenin signaling, may provide an improved treatment option.
Tripterygium wilfordii (TW) is a type of traditional Chinese medicine that has been used to treat a variety of primary and secondary glomerular diseases due to its effectiveness in attenuating proteinuria (8). Previous studies have demonstrated that TW has potent anti-inflammatory and regulatory properties, as well as the ability to promote mesangial cell apoptosis and protect podocytes from inflammatory injury (9,10). TW has been extensively used in China for the treatment of autoimmune diseases, including rheumatoid arthritis, systemic lupus erythematosus (SLE), nephrotic syndrome (11), and has been used to treat patients with DN (12); however, it has not yet been determined whether TW is able to modulate Wnt/β-catenin signaling in kidney tissue. Furthermore, the association between the downregulation of Wnt/β-catenin signaling and improvement in kidney function in individuals with DN is currently unclear.
In the present study, a rat model of DN was induced by streptozotocin. Diabetic rats were then randomly treated with vehicle (sodium carboxymethyl cellulose; SCC), TW combined with SCC (8 or 16 mg/kg) or irbesartan (50 mg/kg) daily for 8 weeks. Metabolic parameter levels and renal pathological changes were examined. mRNA and protein expression levels of Wnt-1, glycogen synthase kinase (GSK)-3β, β-catenin, nuclear factor (NF)-κB and transforming growth factor (TGF)-β1 in the kidneys of rats from all groups were measured. The aim of the present study was to observe the association between renal injury and Wnt/β-catenin expression by establishing a DN rat model, and investigate the possible mechanisms of repairing renal injury in DN with TW.
Materials and methods
Animals
A total of 60 male Sprague-Dawley (SD) rats (8 weeks old, weighing 180–200 g) were obtained from the Laboratory Animal Center of the Bengbu Medical College (license no. SYXK, Anhui 2012–002; Bengbu, China). SD rats were housed in a specific pathogen free facility at 24±1°C and 60% humidity, and exposed to a 12-h dark-light cycle. During the experiment, the rats accessed food and water freely, and were not administered with insulin. The present study was approved by the Animal Ethics Committee of Bengbu Medical College (Bengbu, China).
Diabetes induction and treatment
SD rats were randomly divided into two groups: A control group (n=10) and a diabetes mellitus (DM) group (n=50). All rats were fasted overnight. The control group was injected intraperitoneally (i.p.) with vehicle (0.1 mol/l citrate buffer, pH 4.5) and the DM group received an i.p. injection of 55 mg/kg streptozotocin (STZ; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to induce diabetes. After 3 days, the blood glucose (BG) levels of rats were measured using a glucose analyzer (One Touch SureStep; Johnson & Johnson, New Brunswick, NJ, USA) and individual rats with three consecutive BG readings >16.7 mmol/l were considered diabetic (n=48). The BG of two mice were <16.7 mmol/l, therefore they were not considered diabetic and were excluded from the rest of the study. In the DM group (n=12), diabetic rats were randomly fed with 0.5% sodium carboxymethyl cellulose (CMC-Na, 5 ml/kg) once daily by gavage. Rats in the Control group (n=10) received the same amount of CMC-Na (5 ml/kg) once daily by gavage. Diabetic rats in the TW8 groups (n=12) received 8 mg/kg and TW16 groups (n=12) received 16 mg/kg TW (Shanghai Fudan Fuhua Pharmaceutical Co., Ltd., Shanghai, China) combined with 5 ml/kg CMC-Na by once daily by gavage. Diabetic rats in the Irbesartan group received 50 mg/kg irbesartan [Sanofi-Aventis Minsheng (Hangzhou) Co., Ltd., Hangzhou, China] combined with 5 ml/kg CMC-Na once daily by gavage. All rats received treatment for 8 consecutive weeks. The body weights of individual rats were measured weekly to allow adjustment of dosages. During the experiment, rats were fed a standard diet, had free access to food and drinking water and did not receive insulin.
Blood sample and tissue collection
One day prior to the end of the experiment, 24-h urine samples were collected from all rats. The concentration of urinary protein in the 24-h urine samples (24 h U-PRO; normal reference range, 0–30 mg daily) was determined using urine protein determination kit (cat. no. 59400370314; Shaoxing Medical Biotech, Inc., Zhejiang, China), according to manufacturer's protocol. At the end of the experiment, the body weights of rats from each group were measured. Individual rats were injected i.p. with 50 mg/kg pentobarbital (Sigma-Aldrich; Merck KGaA) to induce anesthesia and their blood samples were collected. BG (normal reference range, 4.3–7.3 mmol/l), blood urea nitrogen (BUN; normal reference range, 4.3–9.8 mmol/l) and serum creatinine (Scr; normal reference range, 35–63 µmol/l) levels were analyzed using an OLYMPUS automatic biochemical analyzer (Olympus Corporation, Tokyo, Japan). The rats were sacrificed and the kidneys were perfused in vivo via the right carotid artery with 100 ml of normal saline at 4°C, while the renal vein was punctured to permit the perfusate to drain, then the kidneys were removed immediately. The left kidneys of individual rats were weighed to calculate the ratio of kidney weight/body weight (KW/BW). Kidney tissues (8-mm thick) were fixed in 10% formalin solution at room temperature for 48 h and paraffin-embedded. Kidney sections were cut (3-µm thick) and subjected to histological and immunohistochemistry examinations. Remaining kidney tissues were cut into small pieces (1-mm thick) and immersed in 4°C ethyl alcohol for 4 h prior to transmission electron microscopy. The right kidneys were stored at −70°C until they were assayed.
Renal pathology and immunohistochemistry examination
The 3-µm thick formalin-fixed kidney sections were stained with Periodic Acid-Schiff (PAS) or Masson's reagent. The digital images of glomeruli and interstitial areas of individual kidney samples were captured under a light microscope (magnification, ×400). The mean glomerular area (AG) and area of extracellular matrix (AECM) in six glomeruli of individual kidney samples was calculated using Image-Pro Plus 6.0 analysis software (Media Cybernetics, Inc., Rockville, MD, USA). The mean glomerular volume (VG) of individual kidney samples was calculated using the formula VG=β/K[AG] 3/2 [as described previously (13)], where β=1.38 is the size distribution coefficient and K=1.1 is the shape coefficient for glomeruli idealized as a sphere. The mean Masson-positive stained areas in six glomeruli and interstitial areas of individual kidney samples were analyzed.
The impact of treatment on Wnt-1 and β-catenin expression in the kidney tissues of rats from the different groups was determined using immunohistochemistry. Kidney sections (3-µm thick) were rehydrated, treated with 3% H2O2 for 10 min at room temperature and subjected to antigen retrieval. The sections were blocked with 5% fat-free dry milk for 60 min at room temperature and probed with rabbit anti-rat Wnt-1 polyclonal antibodies (1:200; cat. no. sc-514531; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and mouse anti-rat β-catenin monoclonal antibodies (1:200; cat. no. sc-221398A; Santa Cruz Biotechnology, Inc.) overnight at 4°C. Following washing, bound antibodies were detected with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibodies (1:1,000) and visualized with 3,3′-Diaminobenzidine, the antibodies and reagent were supplied by the Ultra-Sensitive Streptavidin-Peroxidase (mouse) IHC kit (cat. no. 9701; Fuzhou Maixin Biotech. Co., Ltd., Fuzhou, China) according to the manufacturer's protocol. The immunostained sections were viewed under a light microscope. A total of 10 glomerular and interstitial regions were randomly selected and imaged (magnification, ×400). The immunostaining intensity (optical density) of six glomerular and interstitial areas in individual kidney samples was analyzed by Image-Pro Plus 6.0 analysis software (Media Cybernetics, Inc.) in a blinded manner.
Electronic microscopy
Tissue specimens were cut into small pieces, washed in pH 7.6 phosphate buffer and fixed with 2.5% glutaraldehyde for 4 h at 4°C. The fixed tissues were dehydrated with graded concentrations of acetone, embedded in Araldite and cut into ultrathin sections (50 nm). Following staining with uranyl acetate and lead citrate, the kidney sections were examined under a Hitachi Hu-12A transmission electron microscope (Hitachi, Ltd., Tokyo, Japan; magnification, ×7,000).
Isolation of total mRNA and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from individual right kidney samples using TRIzol reagent. Following the determination of RNA concentration and purity by an ultraviolet spectrophotometer (NanoDrop™ 2000; Thermo Fisher Scientific Inc., Waltham, MA, USA), RNA samples (5 µg/sample) were reverse transcribed into cDNA using a PrimeScript™ RT reagent kit (cat. no. RR047A; Takara Biotechnology Co., Ltd., Dalian, China). qPCR was performed using the SYBR-Green kit (cat. no. RR820A; Takara Biotechnology Co., Ltd.) and specific primers (Sangon Biotech Co., Ltd., Shanghai, China) in an Applied Biosystems StepOne Real-Time PCR machine (Applied Biosystems; Thermo Fisher Scientific Inc.). The sequences of primers and product lengths are presented in Table I. qPCR was performed using SYBR® Premix Ex Taq™ II in a 20 µl reaction volume. Each reaction was comprised of 2 µl of the cDNA solution, 10 µl of SYBR Premix Ex Taq (2X), 1.6 µl of primers, 0.4 µl of ROX Reference Dye and 6 µl of nuclease-free water. The thermocycling protocol was as follows: 95°C for 30 sec for initial denaturation, 40 cycles of 95°C for 5 sec and 60°C for 30 sec for PCR, and 95°C for 15 sec, 60°C for 30 sec and 95°C for 15 sec for separation. β-actin was used as a reference gene and primer sequences are provided in Table I. The relative gene expression levels of Wnt-1, glycogen synthase kinase (GSK)-3β, β-catenin, nuclear factor (NF)-κB and transforming growth factor (TGF)-β1 to control β-actin mRNA transcripts were determined using the 2−ΔΔCq method (14).
Table I.
Primer sequences and lengths for reverse transcription-quantitative polymerase chain reaction.
| Primer | Sequence (5′-3′) | Product length (bps) |
|---|---|---|
| Wnt-1 | Forward: GGTGGGGCATCGTGAACATAG | 296 |
| Reverse: GGAGGTGATTGCGAAGATAAACG | ||
| GSK-3β | Forward: ATGCCTGTCTCCTCTAACGC | 321 |
| Reverse: GGTCTTGGTGGCGGGTTT | ||
| β-catenin | Forward: GCTGACCAAACTGCTAAATGACGA | 192 |
| Reverse: TGTAGGGTCCCAGCGGTACAA | ||
| NF-κB | Forward: CTGGAAGCACGAATGACAGA | 297 |
| Reverse: TTTCAAGTTGGATGCATTGG | ||
| TGF-β1 | Forward: CCAACTA TTGCTTCAGCTCCA | 154 |
| Reverse: GTGTCCAGGCTCCAAATGT | ||
| β-actin | Forward: CACCCGCGAGTACAACCTTC | 207 |
| Reverse: CCCATACCCACCATCACACC |
GSK, glycogen synthase kinase; NF, nuclear factor; TGF, transforming growth factor.
Western blot analysis
Frozen kidney tissue samples were homogenized in radioimmunoprecipitation assay lysis solution (cat. no. P0013B; Beyotime Institute of Biotechnology, Jiangsu, China) and centrifuged (12,000 × g) for 10 min at 4°C. Following quantification of protein concentrations using a BCA protein assay kit (cat. no. P0012; Beyotime Institute of Biotechnology), tissue lysates (30 µg/lane) were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were blocked with 5% fat-free dry milk for 1 h at room temperature and incubated with primary antibodies directed against Wnt-1 (cat. no. sc-514531), GSK-3β (cat. no. sc-81462), p-GSK-3β (cat. no. sc-81495), β-catenin (cat. no. sc-221398A), NF-κB-p65 (cat. no. sc-71675) and TGF-β1 (cat. no. sc-130348; all 1:500) or anti-β-actin (cat. no. sc-69879; 1:800; all Santa Cruz Biotechnology, Inc.) overnight at 4°C. Following washing, membranes were incubated with HRP-conjugated goat anti-mouse IgG (1:2,000; cat. no. BA1051; Wuhan Boster Biological Technology, Ltd., Wuhan, China) for 1 h at room temperature. Membranes were incubated with the secondary antibodies alone as a negative control. Bound antibodies were detected by enhanced chemiluminescence reagents. The relative levels of target protein expression to control were determined by densitometric scanning using an AlphaEase FC 4.0 image analysis system (Alpha Innotech Corporation, San Leandro, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation. The difference among groups was analyzed by repeated one-way analysis of variance followed by least significant difference and the data between two groups were analyzed by t-test with SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA). The potential correlation between variants was analyzed by the Pearson correlation test. P<0.05 was considered to indicate a statistically significant difference.
Results
Treatment with TW significantly mitigates hyperglycemia-related metabolic parameter values in rats
To determine the effect of TW on hyperglycemia-related kidney injury, SD rats were injected with STZ to induce hyperglycemia and treated by gavage with vehicle, different doses (8 or 16 mg/kg) of TW, or irbesartan (50 mg/kg) daily for 8 weeks. During the observation period, two rats from each of the DM, TW8, and Irbesartan groups and one from the TW16 group succumbed from gavage-related causes and were therefore excluded from the statistical analyses. Laboratory examinations indicated that rats in the DM group had significantly higher levels of BG, Scr, BUN and 24-h urinary protein (24-h U-PRO) than rats in the control group (all P<0.01), indicating that they developed hyperglycemia and renal functional impairment (Table II). Although treatment with either TW (8 or 16 mg/kg) or irbesartan did not significantly alter the levels of BG, treatment with TW (8 or 16 mg/kg) or irbesartan did significantly reduce the levels of Scr, BUN and 24-h U-PRO in rats compared with the DM group (P<0.01; Table II). These results indicate that the therapeutic effect of TW may be dose-dependent. In the present rat model, although treatment with irbestartan elicited a similar biochemical response on rats as treatment with TW, TW treatment appeared to have an improved effect on reducing hyperglycemia-related renal functional impairment. The present data suggest that treatment with TW or irbesartan significantly mitigated hyperglycemia-related renal functional impairment in rats.
Table II.
Metabolic parameters in rats from all five groups.
| Group | Dose (mg/kg) | N | BG (mmol/l) | Scr (µmol/l) | BUN (mmol/l) | 24 h U-PRO (mg/24 h) |
|---|---|---|---|---|---|---|
| Control | – | 10 | 5.23±0.45 | 54.62±9.23 | 5.98±0.82 | 9.37±1.66 |
| DM | – | 10 | 27.40±3.37a | 99.46±7.67a | 12.62±2.83a | 76.73±10.27a |
| TW8 | 8 | 10 | 26.31±3.12a | 72.39±7.52a,b | 8.48±1.2a,b | 42.55±4.86a,b |
| TW16 | 16 | 11 | 26.01±3.15a | 66.26±7.46a,b | 7.87±1.06b,c | 36.13±6.07a,b |
| Irbesartan | 50 | 10 | 27.42±3.07a | 74.81±7.23a,b | 9.10±1.91a,b | 43.41±6.35a,b |
Data are expressed as the mean ± standard deviation of each group.
P<0.01 vs. Control group
P<0.01 vs. DM group
P<0.05 vs. Control group. BG, blood glucose; Scr, serum creatinine; BUN, blood urea nitrogen; 24-h U-PRO, 24-h urinary protein; DM, diabetes mellitus; TW8, 8 mg/kg Tripterygium wilfordii; TW16, 16 mg/kg Tripterygium wilfordii.
Treatment with TW reduces hyperglycemia-induced kidney injury in diabetic rats
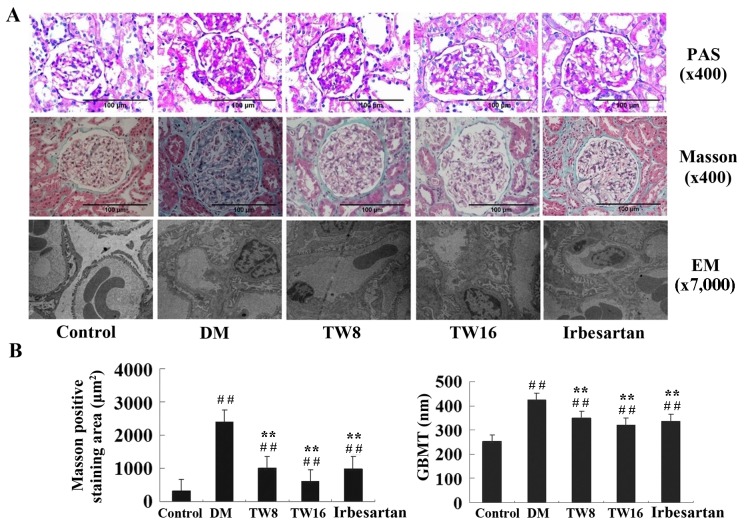
The impact of TW treatment on pathological changes in the kidney tissues of rats was investigated. The KW/BW ratio of rats in the DM group was significantly increased compared with the healthy rats in the Control group (P<0.01; Table III). Treatment with irbesartan or 8 mg/kg TW significantly reduced the ratio of KW/BW in diabetic rats compared with the DM group (P<0.05). Rats treated with 16 mg/kg TW exhibited a significantly reduced ratio of KW/BW compared with rats in the DM group (P<0.01), which was notably decreased compared with rats treated with 8 mg/kg TW (Table III). PAS staining indicated no abnormalities in the kidney sections of rats in the Control group. Conversely, glomerular and renal hypertrophy, thickened basement membranes and mesangial expansion with the accumulation of extracellular matrix was detected in the kidney sections of rats in the DM group. However, reduced pathological changes were observed in the kidney sections of rats in the TW8, TW16 and Irbesartan groups, particularly in the TW16 group (Fig. 1). Similarly, potent Masson staining was detected in the kidney sections of rats in the DM group; however, significantly reduced Masson staining intensity was observed in rats from the TW8, TW16 and Irbesartan groups compared with the DM group (P<0.01; Fig. 1B). Notably, limited Masson staining was observed in the kidney sections of rats in the TW16 group. The results indicated that treatment with TW reduced hyperglycemia-induced kidney fibrosis in rats (Fig. 1). In addition, hypertrophy and fusion of podocytes, and thickened basement membranes were detected in the glomeruli of rats in the DM group using electron microscopy. However, less severe pathological changes were observed in the kidney sections of rats in the TW and Irbesartan groups (Fig. 1). Quantitative analysis of the glomerular size revealed that although the AG and AECM in rats from the TW8, TW16 and Irbesartan groups were increased significantly compared with the Control group, these values were significantly decreased compared with the DM group (P<0.01; Table III). Notably, the lowest values of AG and AECM were observed in the TW16 group. A similar pattern was observed regarding the values of VG in rats from all five groups (Table III). The present results indicated that treatment with TW effectively reduced hyperglycemia-induced kidney injury in diabetic rats.
Table III.
Morphological parameters in glomerular and KW/BW in rats from all five groups.
| Group | Dose (mg/kg) | N | KW/BW (g/100 g) | AG (×103 µm2) | VG (×105 µm3) | AECM (×102 µm2) | AECM/AG (%) |
|---|---|---|---|---|---|---|---|
| Control | – | 10 | 0.34±0.03 | 5.45±0.29 | 5.03±0.41 | 8.20±0.40 | 15.09±1.01 |
| DM | – | 10 | 0.63±0.10a | 8.03±0.64a | 9.02±1.07a | 22.54±1.33a | 28.23±2.74a |
| TW8 | 8 | 10 | 0.54±0.07a,b | 7.15±0.51a,c | 7.57±0.82a,c | 16.38±0.64a,c | 23.02±1.85a,c |
| TW16 | 16 | 11 | 0.46±0.06a,c | 6.49±0.42a,c | 6.54±0.62a,c | 12.98±0.53a,c | 20.11±1.85a,c |
| Irbesartan | 50 | 10 | 0.55±0.07a,b | 7.35±0.47a,c | 7.89±0.75a,c | 15.47±0.66a,c | 21.13±1.51a,c |
Data are expressed as the mean ± standard deviation of each group.
P<0.01 vs. Control group
P<0.05
P<0.01 vs. DM group. KW/BW, kidney weight/body weight; AG, mean glomerular area; AECM, area of extracellular matrix; VG, mean glomerular volume; DM, diabetes mellitus; TW8, 8 mg/kg Tripterygium wilfordii; TW16, 16 mg/kg Tripterygium wilfordii.
Figure 1.
Histological examination of the kidney sections. (A) Kidney tissue sections from rats in the Control (n=10), DM (n=10), TW8 (n=10), TW16 (n=11) and Irbesartan (n=10) groups were stained with PAS or Masson's reagent. Ultrathin kidney 50-nm-thick sections were examined under an electronic microscope. (B) Data are expressed as the mean ± standard deviation. ##P<0.01 vs. Control group; **P<0.01 vs. DM group. PAS, Periodic Acid-Schiff; EM, electronic microscopy; GBMT, glomerular basement membrane thickness; DM, diabetes mellitus; TW8, 8 mg/kg Tripterygium wilfordii; TW16, 16 mg/kg Tripterygium wilfordii.
Treatment with TW impairs hyperglycemia-induced upregulated Wnt-1, p-GSK-3β, β-catenin, NF-κB and TGF-β1 expression in the kidneys of diabetic rats
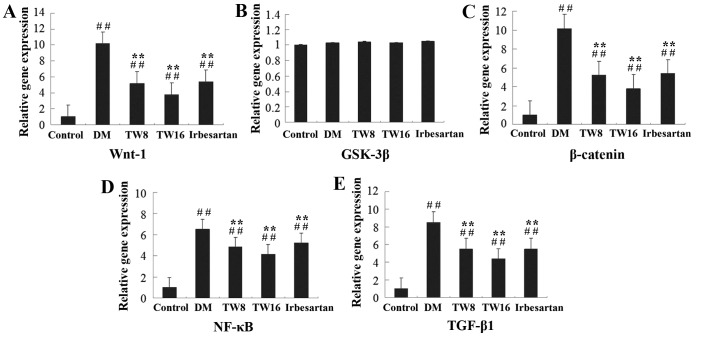
To understand the molecular mechanisms underlying the action of TW, the impact of TW treatment on Wnt-1, GSK-3β, β-catenin, NF-κB and TGF-β1 expression in the kidney tissues of rats was assessed. Compared with the DM group, the relative expression of Wnt-1, β-catenin, NF-κB and TGF-β1 mRNA transcripts in the kidneys of rats in the TW8, TW16 and Irbesartan groups were significantly reduced (P<0.01), particularly in the TW16 group, although levels of gene expression in all treatment groups remained significantly higher compared with the Control group (all P<0.01; Fig. 2). No significant differences in the expression of GSK-3β mRNA were observed in any of the groups (Fig. 2). Treatment with 16 mg/kg TW provided an increased inhibitory effect compared with 8 mg/kg TW or 50 mg/kg irbesartan in the present study (Fig. 2).
Figure 2.
Relative gene expression of (A) Wnt-1, (B) GSK-3β, (C) β-catenin, (D) NF-κB and (E) TGF-β1 in the kidney tissues of rats from the 5 groups. The relative gene expression levels of Wnt-1, GSK-3β, β-catenin, NF-κB and TGF-β1 mRNA transcripts to control β-actin in individual rats in the Control (n=10), DM (n=10), TW8 (n=10), TW16 (n=11) and Irbesartan (n=10) groups were determined by reverse transcription-quantitative polymerase chain reaction. Data are expressed as the mean ± standard deviation. ##P<0.01 vs. Control group; **P<0.01 vs. DM group. GSK, glycogen synthase kinase; NF, nuclear factor; TGF, transforming growth factor; DM, diabetes mellitus; TW8, 8 mg/kg Tripterygium wilfordii; TW16, 16 mg/kg Tripterygium wilfordii.
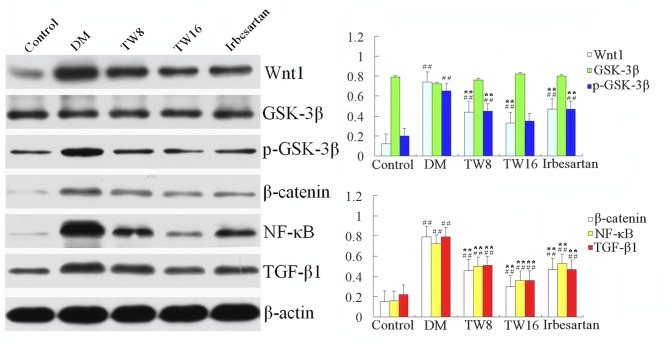
A similar pattern was detected in the levels of Wnt-1, GSK-3β, p-GSK-3β, β-catenin, NF-κB-p65 and TGF-β1 protein expression in the kidneys of rats in all groups (Fig. 3), further supporting that treatment with TW mitigated hyperglycemia-induced Wnt-1, p-GSK-3β, β-catenin, NF-κB p65 and TGF-β1 overactivation in the kidney of rats. Again, no significant differences in the protein expression of GSK-3β were detected in any of the groups (Fig. 3).
Figure 3.
Expression of Wnt-1, GSK-3β, p-GSK-3β, β-catenin, NF-κB and TGF-β1 protein relative to control β-actin in the kidney tissues of individual rats from the Control (n=10), DM (n=10), TW8 (n=10), TW16 (n=11) and Irbesartan (n=10) groups were determined by western blotting. Data are representative images of each group of rats. ##P<0.01 vs. Control group; **P<0.01 vs. DM group. GSK, glycogen synthase kinase; p-, phosphorylated; NF, nuclear factor; TGF, transforming growth factor; DM, diabetes mellitus; TW8, 8 mg/kg Tripterygium wilfordii; TW16, 16 mg/kg Tripterygium wilfordii.
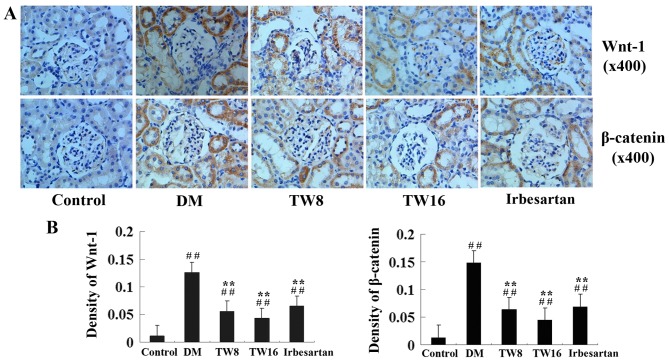
Furthermore, immunohistochemistry analysis revealed that significantly increased anti-Wnt-1 and anti-β-catenin staining occurred in the glomeruli and tubulointerstitial areas in the kidneys of rats in the DM group compared with the Control group (P<0.01; Fig. 4). However, compared with the DM group, the kidney sections of rats in the TW8, TW16 and Irbesartan groups exhibited significantly reduced intensities of anti-Wnt-1 and anti-β-catenin staining (Fig. 4). Quantitative analysis indicated that treatment with TW significantly mitigated the hyperglycemia-induced Wnt and β-catenin over-expression in the kidney of diabetic rats (P<0.01; Fig. 4). Therefore, these data demonstrate that treatment with TW impairs hyperglycemia-upregulated Wnt-1 and β-catenin expression in the kidney of diabetic rats.
Figure 4.
Immunohistochemical analysis of Wnt-1 and β-catenin expression in the kidney tissues of rats from the Control (n=10), DM (n=10), TW8 (n=10), TW16 (n=11) and Irbesartan (n=10) groups. The expression of Wnt-1 and β-catenin in the kidney tissues of individual rats were determined by (A) immunohistochemistry and (B) quantitatively analyzed. Data are representative images of the kidney tissues of each group of rats (magnification, ×400). ##P<0.01 vs. Control group; **P<0.01 vs. DM group. GSK, glycogen synthase kinase; NF, nuclear factor; TGF, transforming growth factor; DM, diabetes mellitus; TW8, 8 mg/kg Tripterygium wilfordii; TW16, 16 mg/kg Tripterygium wilfordii.
Correlation analysis
Correlation analysis of data from all five groups indicated a significant positive linear correlation between Wnt-1 mRNA and protein expression, and β-catenin mRNA and protein expression (P<0.01). Notably, the levels of Wnt-1 and β-catenin expression were significantly positively correlated with the ratios of KW/BW and the levels of 24U-PRO, AG, VG, AECM/AG and GBMT in all five groups of rats (P<0.01; Table IV).
Table IV.
Correlation analysis.
| Index | Wnt-1 protein | β-catenin protein |
|---|---|---|
| 24-h U-PRO | r=0.953, P<0.001 | r=0.919, P<0.001 |
| KW/BW | r=0.592, P=0.006 | r=0.580, P=0.007 |
| AG | r=0.882, P<0.001 | r=0.824, P<0.001 |
| VG | r=0.882, P<0.001 | r=0.825, P<0.001 |
| AECM/AG | r=0.857, P<0.001 | r=0.889, P<0.001 |
| GBMT | r=0.932, P<0.001 | r=0.945, P<0.001 |
| Wnt-1 mRNA | r=0.972, P<0.001 | – |
| β-catenin mRNA | – | r=0.943, P<0.001 |
24-h U-PRO, 24-h urine protein; KW/BW, kidney weight/body weight; AG, mean glomerular area; AECM, area of extracellular matrix; VG, mean glomerular volume; GBMT, glomerular basement membrane thickness.
Discussion
Previous studies have demonstrated that treatment with TW benefits patients with DN (12). However, the mechanisms underlying the action of TW have not been clarified. The Wnt/β-catenin signaling pathway is crucial for the pathogenesis of DN, particularly in the early process of DN (15); therefore, the current study investigated the effect of treatment with TW on Wnt/β-catenin expression in the kidney tissues of rats in the early stage of DN. The present results indicated that treatment with TW did not significantly alter BG levels; however, TW treatment did significantly reduce the levels of BUN, Scr and 24-h U-PRO, particularly when a higher dose of TW (16 mg/kg) was used in rats. Administration of 16 mg/kg TW provoked an improved therapeutic effect compared with the positive control, irbesartan, in the present experimental system. Treatment with TW also significantly reduced the ratios of KW/BW and the values of AG, VG, AECM/AG and GBMT compared with those in the DM group. In addition, treatment with TW dramatically reduced hyperglycemia-induced fibrosis and kidney injury and the relative levels of Wnt-1 and β-catenin expression in the kidney tissues of rats. Furthermore, Wnt-1 and β-catenin expression was positively correlated with the ratios of KW/BW, as well as levels of 24-h U-PRO, AG, VG, AECM/AG and GBMT in rats from all five groups. The present data supports the notion that Wnt/β-catenin signaling is crucial in the pathogenesis of DN.
Various factors may contribute to the early development of DN, including aberrant action of cell signaling, abnormal immune and inflammatory responses, and increased levels of the cytokines, TGF-β1 and NF-κB (16,17). In the present study glomerular and renal hypertrophy, thickened basement membranes and mesangial expansion with the accumulation of extracellular matrix in the kidney sections of rats in the DM group were observed. Furthermore, potent Masson staining was identified in the kidney sections of rats in the DM group and increased basement membrane thickness and podocyte hypertrophy and fusion were detected in the glomeruli of the kidney sections of rats in the DM group. Moreover, the values of AG, VG, AECM/AG and GBMT were significantly increased in rats from the DM group. Notably, upregulated Wnt-1, p-GSK-3β, β-catenin, NF-κB and TGF-β1 expression levels were detected in the kidney tissues of rats in the DM group. These data indicate that during the early process of DM, aberrant activation of Wnt/β-catenin signaling contributes to NF-κB and TGF-β1 over-expression, as well as hyperglycemia-induced functional impairment and structural damage in the kidneys of rats. The present findings were in agreement with those from a previous study, which suggested that activation of Wnt/β-catenin signaling was associated with hyperglycemia-induced podocyte apoptosis and glomerular injury (18). In addition, the present results extended on previous findings demonstrating that the aberrant activation of the Wnt/β-catenin signaling contributes to the development of multiple chronic kidney disease (19). Therefore, the Wnt/β-catenin signaling pathway may be a potential target for the development of novel therapies for DN.
TW is a type of traditional Chinese medicine that has potent anti-inflammatory activity and provides protection from hyperglycemia-induced kidney injury. It is associated with the downregulation of NF-κB, osteopontin and TGF-β1 expression (20,21). The present study indicated that treatment with TW significantly reduced the ratios of KW/BW and the levels of 24-h U-PRO, indicating a reduction of kidney injury in rats. The present findings are consistent with those from a previous study, which used a DN model (10). The present results indicated that TW is able to protect hyperglycemia-induced kidney injury in animals and notably, that treatment with a higher dose of TW (16 mg/kg) provided an improved protective effect compared with irbesartan treatment. This is similar to the results of a previous study (22). Furthermore, the present study indicated that treatment with TW significantly attenuated hyperglycemia-upregulated Wnt-1 and β-catenin expression in the kidney tissues of rats. Given that Wnt/β-catenin signaling positively regulates pro-inflammatory responses, it is possible that TW may inhibit activation of the Wnt/β-catenin signaling pathway and in turn, mitigate hyperglycemia-induced inflammation, such as NF-κB and TGF-β1, to protect the kidney from hyperglycemia-induced injury (20). Notably, the current study observed that treatment with irbesartan alone significantly mitigated hyperglycemia-induced upregulated Wnt-1 and β-catenin expression in the kidney tissues of diabetic rats. These findings may explain why the combination of TW and irbesartan synergistically protect against hyperglycemia-induced podocyte injury in patients with DN (23).
In conclusion, the present study demonstrated that treatment with TW mitigated hyperglycemia-induced functional impairment and structural injury of the kidney in a rat model of DM. Furthermore, treatment with TW significantly mitigated hyperglycemia-induced upregulated Wnt-1 and β-catenin expression in the kidney of diabetic rats, suggesting that the aberrant activation of Wnt/β-catenin signaling may contribute to the early development of DN. The present findings may provide new insights into the pathogenesis of DN and molecular mechanisms underlying the action of TW in treatment of DN.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Program of Natural Science Foundation of Higher Education of Anhui Province (grant nos. KJ2013A191 and KJ2015B008by) and Natural Science Foundation of Bengbu Medical College (grant no. BYKY1457).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors contributed significantly to the conception and implementation of the study. BC initiated the study, was responsible for the experiment implementation, statistical analysis and wrote the first draft and subsequent drafts. WC initiated the study, was responsible for the experiment implementation, and critically revised the first and subsequent drafts for important intellectual content. PY was responsible for the detection of biochemical indexes. YZ and LL assisted animal feeding, gavage and collection of samples. All authors read and approved the final draft.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Doi T, Mima A, Matsubara T, Tominaga T, Arai H, Abe H. The current clinical problems for early phase of diabetic nephropathy and approach for pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S21–S24. doi: 10.1016/j.diabres.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Zhu B, Wang Y, Jardine M, Jun M, Lv JC, Cass A, Liyanage T, Chen HY, Wang YJ, Perkovic V. Tripterygium preparations for the treatment of CKD: A systematic review and meta-analysis. Am J Kidney Dis. 2013;62:515–530. doi: 10.1053/j.ajkd.2013.02.374. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Dai C, Li Y, Liu Y. Canonical Wnt/β-catenin signaling mediates transforming growth factor-β1-driven podocyte injury and proteinuria. Kidney Int. 2011;80:1159–1169. doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai C, Stolz DB, Kiss LP, Monga SP, Holzman LB, Liu Y. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL. Sustained Wnt/β-catenin signaling rescues high glucose induction of transforming growth factor-β1-mediated renal fibrosis. Am J Med Sci. 2012;344:374–382. doi: 10.1097/MAJ.0b013e31824369c5. [DOI] [PubMed] [Google Scholar]
- 6.Zain M, Awan FR. Renin angiotensin aldosterone system (RAAS): Its biology and drug targets for treating diabetic nephropathy. Pak J Pharm Sci. 2014;27:1379–1391. [PubMed] [Google Scholar]
- 7.Ren F, Tang L, Cai Y, Yuan X, Huang W, Luo L, Zhou J, Zheng Y. Meta-analysis: The efficacy and safety of combined treatment with ARB and ACEI on diabetic nephropathy. Ren Fail. 2015;37:548–561. doi: 10.3109/0886022X.2015.1012995. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Ren K, Liang C, Yuan L, Qi X, Dong J, Shen J, Lin S. Renoprotective effect of total glucosides of paeony (TGP) and its mechanism in experimental diabetes. J Pharmacol Sci. 2009;109:78–87. doi: 10.1254/jphs.08112FP. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Shi S, Zhang R, Shen W, Tu J, Ding Z, Fan Y. In vitro immunosuppressive and cytotoxic activities of Tripterygium wilfordii extract. Drug Chem Toxicol. 2015;38:145–151. doi: 10.3109/01480545.2014.919583. [DOI] [PubMed] [Google Scholar]
- 10.Hao L, Pan MS, Zheng Y, Wang RF. Effect of Cordyceps sinensis Tripterygium wilfordii polyglycosidium on podocytes in rats with diabetic nephropathy. Exp Ther Med. 2014;7:1465–1470. doi: 10.3892/etm.2014.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu S, Li X, Li H, Liang Q, Chen J, Chen J. Comparison of Tripterygium wilfordii multiglycosides and tacrolimus in the treatment of idiopathic membranous nephropathy: A prospective cohort study. BMC Nephrol. 2015;16:200. doi: 10.1186/s12882-015-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ge Y, Xie H, Li S, Jin B, Hou J, Zhang H, Shi M, Liu Z. Treatment of diabetic nephropathy with Tripterygium wilfordii Hook F extract: A prospective, randomized, controlled clinical trial. J Transl Med. 2013;11:134. doi: 10.1186/1479-5876-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ota T, Takamura T, Ando H, Nohara E, Yamashita H, Kobayashi K. Preventive effect of cerivastatin on diabetic nephropathy through suppression of glomerular macrophage recruitment in a rat model. Diabetologia. 2003;46:843–851. doi: 10.1007/s00125-003-1099-3. [DOI] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.He W, Kang YS, Dai C, Liu Y. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández Fernández B, Elewa U, Sánchez-Niño MD, Rojas-Rivera JE, Martin-Cleary C, Eqido J, Ortiz A. 2012 update on diabetic kidney disease: The expanding spectrum, novel pathogenic insights and recent clinical trials. Minerva Med. 2012;103:219–234. [PubMed] [Google Scholar]
- 17.Zhang W, Zhao L, Su SQ, Xu XX, Wu YG. Total glucosides of paeony attenuate renal tubulointerstitial injury in STZ-induced diabetic rats: Role of Toll-like receptor 2. J Pharmacol Sci. 2014;125:59–67. doi: 10.1254/jphs.13173FP. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Xu J, Xu P, Liu S, Yang Z. Wnt/β-catenin signalling pathway mediates high glucose induced cell injury through activation of TRPC6 in podocytes. Cell Prolif. 2013;46:76–85. doi: 10.1111/cpr.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma R, Liu L, Liu X, Wang Y, Jiang W, Xu L. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp Ther Med. 2013;6:649–656. doi: 10.3892/etm.2013.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma ZJ, Zhang XN, Li L, Yang W, Wang SS, Guo X, Sun P, Chen LM. Tripterygium glycosides tablet ameliorates renal tubulointerstitial fibrosis via the toll-like receptor 4/nuclear factor kappa B signaling pathway in high-fat diet fed and streptozotocin-induced diabetic rats. J Diabetes Res. 2015;2015:390428. doi: 10.1155/2015/390428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao Q, Shen W, Qin W, Zheng C, Zhang M, Zeng C, Wang S, Wang J, Zhu X, Liu Z. Treatment of db/db diabetic mice with triptolide: a novel therapy for diabetic nephropathy. Nephrol Dial Transplant. 2010;25:3539–3547. doi: 10.1093/ndt/gfq245. [DOI] [PubMed] [Google Scholar]
- 23.Ma RX, Zhao N, Zhang W. The effects and mechanism of Tripterygium wilfordii Hook F combination with irbesartan on urinary podocyte excretion in diabetic nephropathy patients. Zhonghua Nei Ke Za Zhi. 2013;2:469–473. (In Chinese) [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.