Abstract
Gold nanoparticles, because of their high radiation absorption coefficient and efficient generation of secondary photoelectrons, have been predicted to enhance therapeutic efficacy in radiation therapy. However, high dose for effective treatment limits their use. We have synthesized multifunctional gold nanoclusters (GNCs) that can be used for imaging and radiation therapy. The designed GNCs have been characterized for their physicochemical properties, biocompatibility, and their radiation dose enhancement potential on PC3 cell lines.
Keywords: gold nanoclusters, radiation therapy
Introduction
Noble metal (gold, silver, and platinum) nanoclusters (NCs) comprising of few atoms are emerging as good alternatives to quantum dots which are intrinsically toxic for bioapplications. These nanoclusters exhibit strong fluorescence which is tunable in the range of visible to near-infrared (NIR). Gold nanoclusters (GNCs) have gained tremendous attention in the field of theranostics owing to their excellent optical fluorescence, high quantum yield, easy conjugation with biological molecules, photostability, and biocompatibility.
Radiotherapy is still considered as a major modality in cancer therapy. From a therapeutic perspective, despite significant advances in technology, radiation therapy is unable to achieve local control of the primary tumor and normal tissue toxicity. The interaction of X-rays with high atomic number materials is expected to enhance the radiation doses in biological tissues through low-energy electrons produced as a result of photoelectric absorption.1 The gold nanoparticles owing to their high Z value have been investigated as potential radiosensitizers in radiation therapy. However, enhancing tumor radiation dose with high atomic number (high-Z) requires large amounts of the material to be delivered to the tumor site, which poses a constraint on their practical use.
GNCs, which are clusters of 20–25 gold atoms embedded in a protein scaffold, have been shown to enhance the radiation therapy as well as have efficient clearance from the body.2 In the present study, we have synthesized gold nanoclusters using a wet chemical route for their application in radiation therapy for cancer. We investigated their biocompatibility and radiation dose enhancement potential on PC3 prostate cancer cell lines. The initial results are encouraging.
Materials and methods
All the reagents used were purchased from Sigma Aldrich (St Louis, MO, USA). The PC3 cell lines were procured from the American Type Culture Collection (Manassas, VA, USA).
Synthesis
GNCs have been synthesized using an earlier reported method.3 Five milliliters of 10 mM HAuCl4 in deionized water was dispensed in a 25 mL conical flask and 5 mL of 0.5 mM aqueous solution of bovine serum albumin (BSA) in water was added with vigorous stirring. Further, 0.5 mL of 0.5 M aqueous sodium hydroxide solution was added to the stirring solution, and pH of the solution was maintained at about 10–12. The whole reaction mixture was kept in a water bath and incubated for 12 h at the temperature of 37°C. After the completion of the reaction, transparent orange brown coloration was observed, indicating the formation of GNCs. The as-synthesized GNCs were filtered using 0.22 µm syringe filter and lyophilized.
Results and discussion
The synthesized GNCs were characterized using Fourier transform infrared (FTIR) spectroscopy, UV-Visible spectroscopy, and photoluminesence. The size of GNCs was measured by transmission electron microscopy (TEM). GNCs were found to emit red color under illumination by ultraviolet light lamp of 365 nm.
The FTIR spectra (data not shown) of GNCs exhibit the characteristic vibration peaks of protein BSA. The characteristic amide-I and amide-II bands of BSA related to secondary structure of protein are observed at 1,665 cm−1 and 1,529 cm−1. Peaks at 3,276 cm−1 and at 2,958 cm−1 correspond to primary amines and C-H vibration, respectively.4 The GNCs exhibited the UV-vis absorption spectrum (data not shown) with a maximum absorption at 280 and 520 nm. The absorption peaks at 280 nm are ascribed to the π→π* transition of the aromatic amino acid residues of BSA, and feeble absorption at 520 nm could be attributed to the formation of a few gold nanoparticles. TEM image depicts formation of GNCs less than 5 nm (Figure 1).
Figure 1.
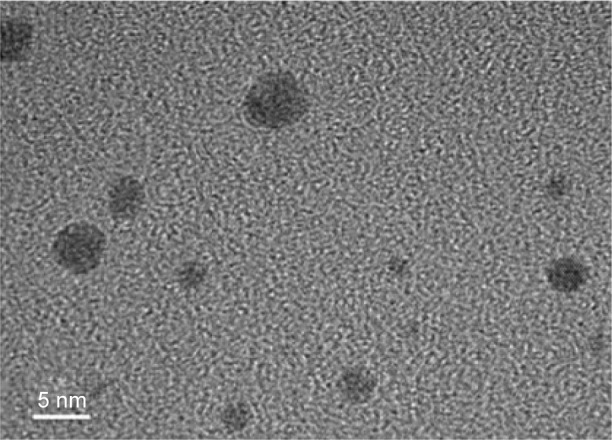
TEM image of GNCs.
Abbreviations: GNC, gold nanocluster; TEM, transmission electron microscopy.
Photoluminescence measurements illustrate that GNCs exhibited fluorescence peaks between 650 and 665 nm resulting from the gold atoms contained in GNCs core, upon excitation at wavelengths between 350 and 490 nm. The Gaussian behavior of fluorescence emission peaks is attributed to the presence of 18, 22, 23, and 25 atom gold clusters.4 Photo-luminescence emission at around 656 nm with excitation of 390 nm indicates that our sample is dominated by the presence of an 18 gold cluster. This emission was considered to arise from intraband transitions of free electrons of the GNCs (Figure 2).
Figure 2.
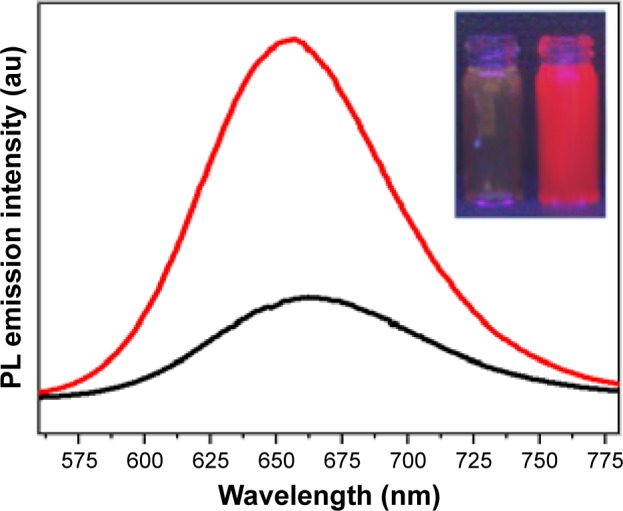
Photoluminescence of GNCs and fluorescence of GNCs under UV Illumination (inset).
Notes: Black line: 350 nm excitation wavelength; red line: 490 nm excitation wavelength.
Abbreviations: GNC, gold nanocluster; PL, photoluminescence; UV, ultraviolet.
The cell viability was assessed using 3-(4,5-dimethy-lthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to check the biocompatibility of synthesized GNCs. For this, PC3 prostate cancer cells were dispensed in 96-well plates and allowed to adhere for 24 h. After adherence, different concentrations of the GNCs were then added to each well. The effect of the concentration of GNCs was assessed using cell viability assay on PC-3 prostate cancer cell lines. The percentage of cell growth inhibition was assayed by the number of surviving cells after 24 h incubation. It was observed that there was no significant cell death, and a 97% cell viability was recorded even at the higher concentration (0.4 mg/mL) of GNCs (Figure 3).
Figure 3.
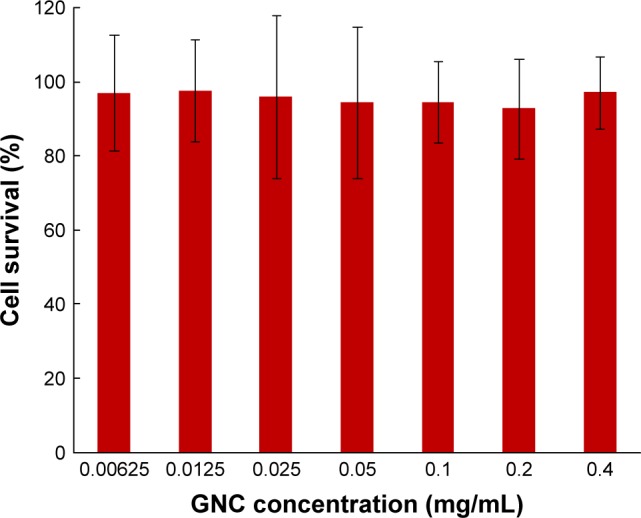
Cell viability assay.
Abbreviation: GNC, gold nanocluster.
Radiation dose enhancement
The clonogenic assay is a more stringent assay for cell viability studies upon irradiation with X-rays. In vitro radiation dose enhancement studies with GNCs were carried out on PC3 cell lines using clonogenic cell survival assay. The colony formation of PC3 cells treated with GNCs at 2Gy X-ray radiation was assessed for 2 weeks post-irradiation. Figure 4 shows the cell survival fraction for X-ray irradiated cells without or with GNCs. A 45% cell death is observed at very low concentration (0.4 mg/mL) of GNCs, which is comparable to a dose of 2 mg/mL of gold nanoparticles for a similar effect.1 Results suggest that GNCs are a potential candidate for enhancing the therapeutic radiation effect.
Figure 4.
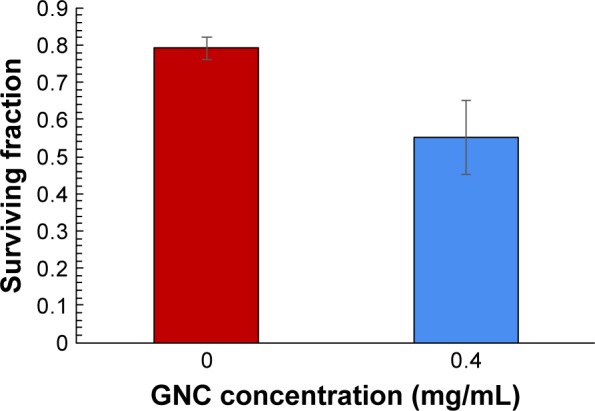
Clonogenic assay on PC3 cells without and with GNCs using 2Gy of X-ray dose.
Abbreviation: GNC, gold nanocluster.
Conclusion
We have successfully synthesized highly luminescent gold nanoclusters of around 2–4 nm in size. The observation of 97% in cell viability assays clearly indicates the nontoxic nature of these GNCs and their potential use in bioapplications such as imaging and drug delivery. Further, the possibility of using these GNCs as radiosensitizers may bring a paradigm shift in cancer radiation therapy.
Acknowledgments
Funding from IUSSTF funded joint center on nanomedicines for head and neck cancer (GAP 130932) and NSF-IGERT are gratefully acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kumar R, Korideck H, Ngwa W, Berbeco RI, Makrigiorgos GM, Sridhar S. Third generation gold nanoplatform optimized for radiation therapy. Transl Cancer Res. 2013;2(4):228–239. doi: 10.3978/j.issn.2218-676X.2013.07.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorsey JF, Sun L, Joh DY, et al. Gold nanoparticles in radiation research: potential applications for imaging and radiosensitization. Transl Cancer Res. 2013;4:280–291. doi: 10.3978/j.issn.2218-676X.2013.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie J, Zheng Y, Ying JY. Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc. 2009;131(3):888–889. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]
- 4.Guevel XL, Hotzer B, Jung G, Schneider M. NIR-emitting fluorescent gold nanoclusters doped in silica nanoparticles. J Mater Chem. 2011;21:2974. [Google Scholar]


