Abstract
Titanium dioxide has been proven for toxicity by in vitro and in vivo approaches, however, further studies are needed in nano-toxicological research using in silico analysis. In this study, Autodock 4.0.5 was used in an attempt to evaluate the interaction of titanium dioxide with proteins. Different cellular proteins were sorted to study the interaction, binding sites, and active sites as a pocket. These pockets have been determined using CastP – an online server. The analysis for the docked structures was performed with regard to the most efficient binding with amino acids. This study is the first of its kind to report on the in silico docking interaction of titanium dioxide nanoparticles without any surface modification. The higher negative binding energy shows strong binding of titanium dioxide with proteins. A strong interaction with different cellular proteins was observed, and more specifically, titanium dioxide nanoparticles showed frequent interaction with proline, lysine, as well as leusine.
Keywords: docking, titanium dioxide nanoparticle, interaction, CASTp
Introduction
Titanium dioxide has been widely used in consumer products, mainly food and cosmetics industry. Although bulk titanium dioxide is considered as safe, there are reports wherein these particles are converted into nano form in the processing steps. Titanium dioxide nanoparticles (TNPs) are now detected in the consumer products without any information about their shape, size or intention. The deliberate use of bulk titanium dioxide has exposed TNPs also to human body and environment. This exposure is undetected at various stages – synthesis (laboratory), manufacture (industry), use (consumer products, devices, medicines, etc) and through environmental exposure (through disposal). A lot of studies are in process about the genotoxic and carcinogenic potential of TNP. However, the classical toxicology methods are not applicable to TNPs as the shape and size greatly depends upon the synthesis method and the unique physicochemical properties of the TNPs. Thus, there is a need to develop an in silico approach that can validate the data observed from the in vitro and in vivo experiments.1,2 Docking provides a quick and convenient method to predict the interaction of cellular proteins with nanoparticles to predict toxicity. In this work, we have docked the TNP with several cellular proteins and tried to predict the TNP–protein interaction.
Materials and methods
Protein data bank file of proteins and TNP
The protein data bank file of TNP was downloaded from the source file of University of Sydney website3 and for proteins Research Collaboratory For Structural Bioinformatics protein data bank.4 This has been summarized in Table 1.
Table 1.
Docking results of TNP–proteins interaction
| PDB ID | Binding energy | Kl | Intermolecular energy | Internal energy | Torsional energy | Unbound extended energy | Ref RMS | |
|---|---|---|---|---|---|---|---|---|
| ICAM-1 | IP53 | −11.63 | 2.97 nM | −12.73 | −2.88 | 1.1 | −2.88 | 171.59 |
| CCL-20 | IM8A | −8.25 | 901.44 nM | −9.34 | −2.75 | 1.1 | −2.75 | 39.68 |
| COX-2 | N/A (Bounded PDB available) | |||||||
| IL-8 | 1IL8 | −4.04 | 1.09 mM | −5.14 | −2.66 | 1.1 | −2.66 | 11.57 |
| NF-kB | 1SVC | −8.29 | 841.35 nM | −9.39 | −2.61 | 1.1 | −2.61 | 57.33 |
| P-38 | 3W8Q | −11.73 | 2.52 nM | −12.83 | −2.49 | 1.1 | −2.49 | 35.13 |
| PIGF | 1FZV | −9.26 | 163.05 nM | −10.36 | −2.77 | 1.1 | −2.77 | 41.37 |
| CXCL-1 | 1MSG | 1.67 | U/A | 0.57 | −2.96 | 1.1 | −2.95 | 13.62 |
| CXCL-3 | N/A | |||||||
| CXCL-5 | 2MGS | 576.34 | U/A | 575.34 | 42.18 | 1.1 | 42.18 | 8.93 |
| CD 35 | 1GKG | 5420 | U/A | 5420 | 84.59 | 1.1 | 85.59 | 16.8 |
| CD 66b | N/A | |||||||
| MMP-9 | 1L6J | −9.01 | 247.86 nM | −10.11 | −2.72 | 1.1 | −2.72 | 63.94 |
Abbreviations: CCL-20, chemokine ligand 20; CD, cluster of differentiation; COX-2, cyclooxygenase; IL-8, interleukin 8; CXCL, C–X–C motif chemokine ligand; ICAM-1, intercellular adhesion molecule 1; MMP-9, matrix metallopeptidase 9; N/A, not applicable; NF-kB, nuclear factor kappa B; PIGF, placental growth factor; TNP, titanium dioxide nanoparticles; PDB, protein data bank; U/A, unassigned.
Dimensions of TNP
The dimensions of the lattice were derived from PyMol, and the size of the nanoparticle was identified.5
Determination of binding site
Computed atlas of surface topography of proteins (CASTp)6 is used to visualize the annotated functional residues. The active sites of proteins have been predicted using CASTp. The best binding pockets with high area and volume were predicted by CASTp as active sites for each protein.
Docking studies of TNP with proteins
AutoDock 4.0 docking program was applied as discussed in our earlier published work.7
Results and discussion
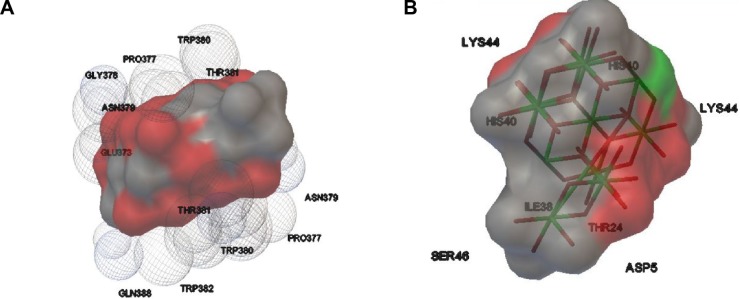
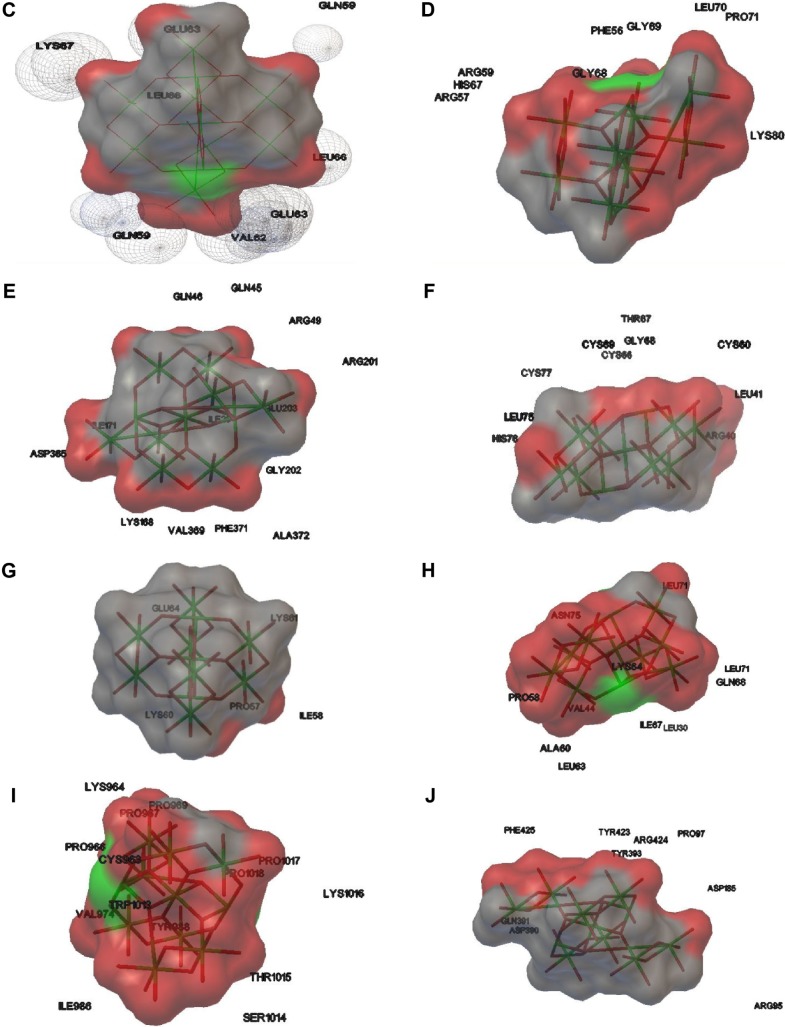
The TNP size was determined as 1.09 nm using PyMol software. The efficient formation of protein–nanoparticle complexes was identified after observing the negative binding energy and intermolecular energy of TNP–protein complexes. The higher binding energy and intermolecular energy are −11.63 and −12.73, respectively – for ICAM (inter-cellular adhesion molecule) titanium dioxide nanoparticle shows stable docked complex. Figure 1 depicts the hydrogen bond formed after docking. On the basis of the binding energy and intermolecular energy, it can be stated that ICAM and P-38 has stable binding with TNP, whereas no such complex formation takes place with CXCL-1 (C–X–C motif chemokine ligand), CXCL-5 and cluster of differentiation 35. The binding and intermolecular energies along with torsion energy and the interacting amino acids have been tabulated (Table 1). Binding energy ranges from −11.63 to −4.04 for other proteins.
Figure 1.
Interaction of TNP with (A) ICAM protein; (B) CCL-20 protein; (C) IL-8 protein; (D) NF-kB protein; (E) P-38 protein; (F) PIGF protein; (G) CXCL-1 protein; (H) CXCL-5 protein; (I) CD-35 protein; (J) MMP-9 protein.
Abbreviations: CCL-20, chemokine ligand 20; CD-35, cluster of differentiation 35; CXCL-1, C–X–C motif chemokine ligand 1; ICAM, intercellular adhesion molecule 1; IL-8, interleukin 8; MMP-9, matrix metallopeptidase 9; NF-kB, nuclear factor kappa B; PIGF, placental growth factor; TNP, titanium dioxide nanoparticle.
Analyzing the docking results, it can be concluded that TNP has frequent binding with positively charged R-group and nonpolar aliphatic R-groups amino acid containing amino acids of the proteins. More frequently, it binds with lysine and proline, and more interestingly, it does not bind with methionine. This suggests that hydrogen bond formation is prevented by the sulfur containing heavy chain R-group. Along with positively charged R-group and nonpolar aliphatic R-group amino acids, it also binds with aromatic R-group-, polar uncharged R-groups- and negatively charged R-group-containing amino acids, but the frequency of binding is very reduced in number as shown in Table 2. Least frequent binding has been observed with tyrosine, alanine, asparagine, serine and phenylalanine (Table 2). TNP has least affinity with aromatic R-group-containing amino acids, which may be attributed to the fact that TNP does not form a stable hydrogen bond because of the resonance energy stabilization of the amino acid.
Table 2.
Amino acid binding in the docked structure with different proteins
| GLY | PRO | TRP | THR | ASN | GLU | GLN | LYS | SER | ASP | HIS | ILE | LEU | VAL | ARG | PHE | ALA | CYS | TYR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICAM-1 | + | ++ | +++ | ++ | ++ | + | + | ||||||||||||
| CCL-20 | + | ++ | + | + | ++ | + | |||||||||||||
| IL-8 | ++ | ++ | + | ++ | + | ||||||||||||||
| NF-kB | ++ | ++ | + | + | + | ++ | + | ||||||||||||
| P-38 | + | + | ++ | + | + | ++ | + | ++ | + | + | |||||||||
| PIGF | + | + | + | ++ | + | ++++ | |||||||||||||
| CXCL-1 | + | + | + | + | |||||||||||||||
| CXCL-5 | + | + | + | + | + | ++++ | + | + | |||||||||||
| CD 35 | +++++ | + | + | ++ | + | + | + | + | + | ||||||||||
| MMP-9 | + | + | ++ | ++ | + | ++ | |||||||||||||
| Total frequency | 5 | 12 | 4 | 5 | 3 | 5 | 7 | 9 | 2 | 4 | 4 | 6 | 9 | 4 | 7 | 3 | 2 | 5 | 3 |
Note: +, number of times TNP interacts with the respective amino acid.
Abbreviations: CCL-20, chemokine ligand 20; CD, cluster of differentiation 35; CXCL, C–X–C motif chemokine ligand; ICAM-1, intercellular adhesion molecule 1; IL-8, interleukin 8; MMP-9, matrix metallopeptidase 9; NF-kB, nuclear factor kappa B; PIGF, placental growth factor; TNP, titanium dioxide nanoparticle.
Acknowledgments
Authors acknowledge Department of Biotechnology (DBT, India) for the funded project – BT/PR10414/PFN/20/961/2014. Authors have presented this abstract in T-Nano 2014 at Ahmedabad University, Ahmedabad, Gujarat, India.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Shukla RK, Kumar A, Vallabani NV, Pandey AK, Dhawan A. Titanium dioxide nanoparticle-induced oxidative stress triggers DNA damage and hepatic injury in mice. Nanomedicine. 2014;9(9):1423–1434. doi: 10.2217/nnm.13.100. [DOI] [PubMed] [Google Scholar]
- 2.Ranjan S, Dasgupta N, Chakraborty AR, et al. Nanoscience and nanotechnologies in food industries: opportunities and research trends. J Nanopart Res. 2014;16(6):2464. [Google Scholar]
- 3.University of Sydney TiO2.pdb 19-May-2007. 2014. [Accessed April 10, 2014]. Available from: http://firstyear.chem.usyd.edu.au/jmol/
- 4.Research Collaboratory For Structural Bioinformatics Protein Data Bank. 2014. [Accessed April 25, 2014]. Available from: http://www.rcsb.org/pdb/home/home.do. [DOI] [PMC free article] [PubMed]
- 5.TPMGS . ThePyMOL Molecular Graphics System, (Version 1.5.0.4) Schrödinger, LLC; 2014. [Google Scholar]
- 6.Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucl Acids Res. 2006;34:W116–W118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranjan S, Dasgupta N, Chinnappan S, Ramalingam C, Kumar A. A novel approach to evaluate titanium dioxide nanoparticle–protein interaction through docking: an insight into mechanism of action. Proc Natl Acad Sci India Sect B Biol Sci. 2015 In press. [Google Scholar]