Abstract
Background
The effectiveness of using point-of-care (POC) urine culture in primary care on appropriate antibiotic use is unknown.
Aim
To assess whether use of the Flexicult™ SSI-Urinary Kit, which quantifies bacterial growth and determines antibiotic susceptibility at the point of care, achieves antibiotic use that is more often concordant with laboratory culture results, when compared with standard care.
Design and setting
Individually randomised trial of females with uncomplicated urinary tract infection (UTI) in primary care research networks (PCRNs) in England, the Netherlands, Spain, and Wales.
Method
Multilevel regression compared outcomes between the two groups while controlling for clustering.
Results
In total, 329 participants were randomised to POC testing (POCT) and 325 to standard care, and 324 and 319 analysed. Fewer females randomised to the POCT arm than those who received standard care were prescribed antibiotics at the initial consultation (267/324 [82.4%] versus 282/319 [88.4%], odds ratio [OR] 0.56, 95% confidence interval [CI] = 0.35 to 0.88). Clinicians indicated the POCT result changed their management for 190/301 (63.1%). Despite this, there was no statistically significant difference between study arms in antibiotic use that was concordant with laboratory culture results (primary outcome) at day 3 (39.3% POCT versus 44.1% standard care, OR 0.84, 95% CI = 0.58 to 1.20), and there was no evidence of any differences in recovery, patient enablement, UTI recurrences, re-consultation, antibiotic resistance, and hospitalisations at follow-up. POCT culture was not cost-effective.
Conclusion
Point-of-care urine culture was not effective when used mainly to adjust immediate antibiotic prescriptions. Further research should evaluate use of the test to guide initiation of ‘delayed antibiotics’.
Keywords: antimicrobial drug resistance, bacterial infections, cost–benefit analysis, drug resistance, point-of-care testing, urinary tract infections
INTRODUCTION
Point-of-care testing (POCT) for infections is being promoted to reduce antimicrobial resistance and improve patient outcomes.1 POCT is frequently subject to evaluations of analytic performance, but is often introduced into practice without rigorous trials evaluating clinical and cost-effectiveness.2–5
Approximately 10% of adult females experience a urinary tract infection (UTI) in any given year, and around half experience a UTI at some point in their lives.6 Around 15–20% of the antibiotics prescribed in primary care are prescribed for UTI,7–9 but up to 60% of females with uncomplicated UTI who are treated with antibiotics do not have a positive urine culture.10–13 In addition, although antibiotics shorten the duration of symptoms on average, not all individuals benefit from antibiotic treatment;14,15 furthermore, some females with a positive urine laboratory culture are not prescribed antibiotics.10 Better-targeted antibiotic prescribing may reduce unnecessary risk of side effects and subsequent infections that are antibiotic resistant, and, in so doing, may reduce symptom duration and the burden on health services in the future.16–18
Current strategies to predict microbiologically confirmed UTI in adult females need to be improved so that:
more females who will benefit from antibiotic treatment are prescribed antibiotics;
antibiotic treatment is better targeted to the sensitivity of the infecting organisms; and
antibiotics are prescribed less often for females who will not benefit from them.
Better-targeted antibiotic use is important for antibiotic stewardship, as antibiotic use drives resistance. There is, therefore, an urgent need to support clinicians in the community in deciding whether to prescribe antibiotics and in selecting the most appropriate antibiotic when indicated.
POCT urine culture has been proposed as a solution in primary care because results can be available within 24 hours.19,20 The approach is already widely used in Denmark, but has never been evaluated in a rigorous randomised controlled trial to determine whether it benefits patients. Therefore, the authors aimed to determine the clinical effects and costs of POCT urine culture for symptoms of uncomplicated UTI on the overall appropriateness of antibiotic prescribing compared with current best practice.
How this fits in
Although the analytic performance of point-of-care (POC) urine culture tests has been investigated, its effect on antibiotic use that is congruent with laboratory culture results has not been evaluated in a randomised controlled trial. POC urine culture, used mainly for adjusting empirical antibiotic prescribing decisions for uncomplicated urinary tract infection in primary care, did not lead to an increase in concordant antibiotic prescribing or improve patient outcomes, and was not cost-effective.
METHOD
The POCT for UTI in primary care trial was a pragmatic, parallel, two-arm, individually randomised, open, test-treatment4 controlled trial. It aimed to assess the costs and effects of an optimised POCT-guided diagnostic and treatment strategy for symptoms of uncomplicated UTI in adult females on the overall appropriateness of antibiotic use when compared with practice based on best available local guidelines (standard care). This multinational trial was implemented in primary care research networks (PCRNs) in four countries — in England, the Netherlands, Spain, and Wales — which were selected based on past research experience and variation in resistance rates and usual management of UTI.
The full POETIC protocol has been published elsewhere.21 A brief summary of trial procedures is presented below.
Participants
Network coordinators invited clinicians (GPs or nurse prescribers) in four PCRNs to take part. Participating clinicians identified eligible patients during routine general practice consultations. Females (aged ≥18 years) were eligible if they were presenting in primary care with at least one symptom of dysuria, urgency, or frequency, and had a clinical diagnosis of uncomplicated UTI. Females were excluded if they:
had suspected pyelonephritis;
were on long-term antibiotic treatment;
had received antibiotics for UTI in the preceding 4 weeks; or
had significant genitourinary tract abnormalities or terminal illness.
Patients were randomised before any dipstick testing and management decisions were made. There was no minimum recruitment target for clinicians.
Randomisation
Remote, online randomisation was stratified by practice, and used minimisation (with a random element) to balance for the number of key symptoms (dysuria, frequency, and/or urgency) at presentation. Practice stratification was kept confidential to help with allocation concealment.
Clinical examination
Using a 7-point scale — from 0 (not affected) to 6 (as bad as it could be) — clinicians recorded the presence and severity of baseline clinical features, including:
daytime frequency (of urination);
urgency;
burning or pain when passing urine (dysuria);
night-time frequency;
feeling generally unwell;
abdominal pain;
smelly urine;
restricted activities;
pain in the side (costovertebral angle tenderness);
fever; and
blood in urine.
The scale was similar to previously used instruments22 and was part of the symptom diary that participants were asked to complete each day. Clinicians also recorded the diagnostic tests (that is, urine dipstick testing) carried out, antibiotics prescribed, and planned follow-up.
Sample collection
Participants were asked to provide a urine and optional stool sample on the day of recruitment. Urine samples were collected using the Peezy midstream urine collection kit. For participants randomised to the intervention, urine samples were split: a portion was earmarked for the intervention test and the rest was sent for culture, in containers with boric acid, to local network laboratories using routine clinical sample transport arrangements (Spain and the Netherlands) or to a central laboratory by post (England and Wales). Participants were asked to return stool samples by post to their designated local laboratory within 24 hours of collection and to provide further urine and stool samples at day 14. Stool samples were obtained to estimate the effect on resistance in faecal flora. Participants were asked to provide a stool sample at baseline and at 2 weeks.
Trial intervention
Clinicians were asked to use the Flexicult™ POCT urine culture to guide the management of participants randomised to the intervention, but it was for them to decide or negotiate with the patient on how best to use the test. For example, they could avoid empirical prescribing and use the test to:
determine whether, and what antibiotic class, to prescribe the following day;
prescribe empirically and use the test to aid in a next-day review of the initial prescribing decision; or
provide a delayed antibiotics prescription and use the test to guide use of the delayed prescription.
Management decisions were recorded after reading the test in the intervention arm.
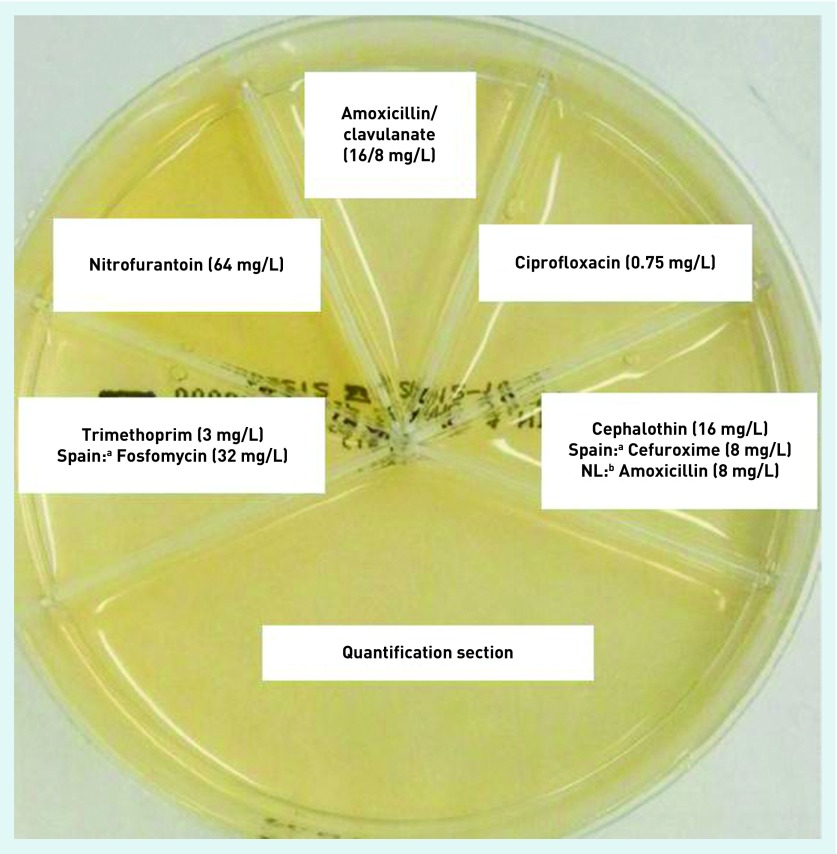
Flexicult POCT urine culture involves fresh urine being poured onto a chromogenic agar plate, which is then incubated at 35–37°C overnight in a small desktop incubator in the practice; the results are reviewed 18–24 hours later. The chromogenic agar plate is sub-divided into six segments: the largest allows for the identification of species (by colour of colonies) and bacterial growth; the other five contain agar impregnated with antibiotics and are used to assess antibiotic susceptibility (Figure 1). Clinicians were provided with face-to-face training, a country-specific Flexicult brochure, and a poster to aid interpretation of the results. Further training resources were available online at www.POETIC-study.co.uk.
Figure 1.
The UK Flexicult SSI-Urinary Kit.
aIn Spain, fosfomycin was used instead of trimethoprim, and cefuroxime instead of cephalothin.
bIn the Netherlands, amoxicillin was used instead of cephalothin.
When reading the Flexicult urine culture test, clinicians recorded:
bacterial growth, based on the number of colonies;
bacterial identification, based on the colour of colonies; and
antibiotic resistance of the pure or predominant organisms, based on the presence of bacterial growth in the antibiotic sections.
Antibiotic susceptibility was only recorded if growth was ≥103 colony-forming units per millilitre (CFU/mL) in the large plate section.
Standard care
Patients randomised to the standard-care arm received care informed by national guidelines; clinicians received a summary of relevant national treatment guidelines.
Participant follow-up
All participants were asked to complete a 2-week daily symptom diary, which covered medical history and included the Patient Enablement Instrument.23 On each of the 14 days, participants were asked to rate the presence and severity of symptoms (using the same scale as at baseline) and record any antibiotic use. On day 14, they were asked to record all resource use associated with UTI and time off work. Non-responders were reminded by telephone and given the opportunity to complete minimum data set questions, which consisted of a question about the day on which the woman felt she had recovered and about antibiotic use.
At 3 months, the primary care medical records were examined for re-consultations with primary and secondary care, recurrences of UTI, and further antibiotic use.
Microbiological procedures
Local laboratories were provided with a POETIC microbiology manual and standard operating procedures.21
UTI definition
The definition of a UTI used by the laboratory serving the participating clinicians was used to compare results of the point-of-care (POC) Flexicult test, because the POCT aimed to give the same information as the laboratory-based test, only quicker. In Wales, England, and Spain, the definition of a UTI on laboratory culture was ≥105 CFU/mL of a pure/predominant recognised uropathogen (where predominant was defined as a ≥103 CFU/mL difference between the first- and second-highest bacterial growths); in the Netherlands, the definition was 104 CFU/mL growth of a pure/predominant recognised uropathogen.
Statistical considerations
Sample size calculation
A sample of 460 patients (230 for each arm) for final analysis was required (significance level α 0.05, statistical power [1–β] 0.90) to allow for an increase in concordant antibiotic usage from 55% in the standard-care arm to 70% in the intervention arm. This was inflated to 614 to allow for a 25% loss to follow-up. The analysis took clustering by practice into account.
Primary outcome
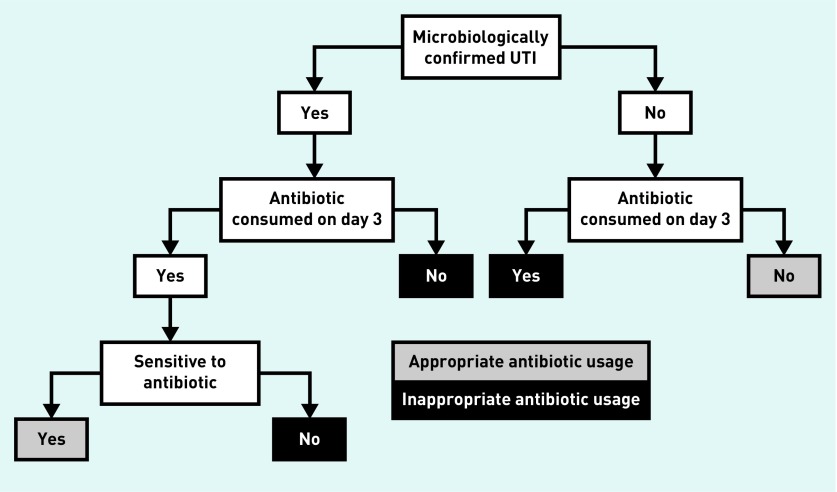
The primary outcome was concordant antibiotic use, defined as:
consumption of an antibiotic on day 3 (or day 1 or day 2 for fosfomycin), for which a pathogen considered to be causing a UTI isolated in a laboratory was sensitive in vitro; or
no antibiotic use by females who did not have a UTI on laboratory culture (Figure 2).
Figure 2.
Decision tree to ascertain whether antibiotic prescribing is concordant or discordant.
UTI = urinary tract infection.
Sensitivity analyses were undertaken using the primary outcome calculated using the Flexicult plate results and initial antibiotic prescribing.
Secondary outcomes
Secondary outcomes, also defined at the outset in the protocol, comprised:
initial antibiotic prescription (that is, on day 1) and at any point during the 2-week follow-up period;
dose and duration of antibiotic;
antibiotic consumption;
adherence to national prescribing guidelines;
antibiotic resistance in urine and stool samples at 2 weeks;
patient enablement;23
re-consultation;
recurrence of UTI;
hospitalisation;
direct/indirect costs (within a 3-month period); and
cost-effectiveness.
Statistical analysis
Analyses of primary and secondary outcomes were based on a modified intention-to-treat (ITT) population. A sensitivity analysis was conducted using multiple imputations to account for missing outcome data (supplementary material available from the authors on request). Multilevel regression models were used to account for clustering of patients within practices. Country (England, the Netherlands, Spain, and Wales), number of key symptoms (one, two, or three), and stratifying and balancing variables were included as covariates in all models.
Logistic, linear, or futility (survival) analyses were undertaken as appropriate. Urinary symptom burden was calculated using the area under the curve (AUC) of the total symptom score for urgency, daytime frequency, and night-time frequency on each day. Due to its distribution, the AUC symptom burden was natural logged, and patient enablement was dichotomised for analysis.
Health economic evaluation
The authors aimed to assess mean total cost (including the cost of the POCT) per unit increase in concordant antibiotic prescribing.21 ITT analysis was used to determine the difference in resource use and difference in effectiveness between the intervention and control groups. Where cost data were skewed, 10 000 replications and bias-corrected, non-parametric, bootstrap methods were used to determine 95% CIs; analysis accounted for cluster effect.25 A cost-effectiveness acceptability curve was used to show the probability of the intervention having an incremental cost-effectiveness ratio below a range of acceptability thresholds.26
RESULTS
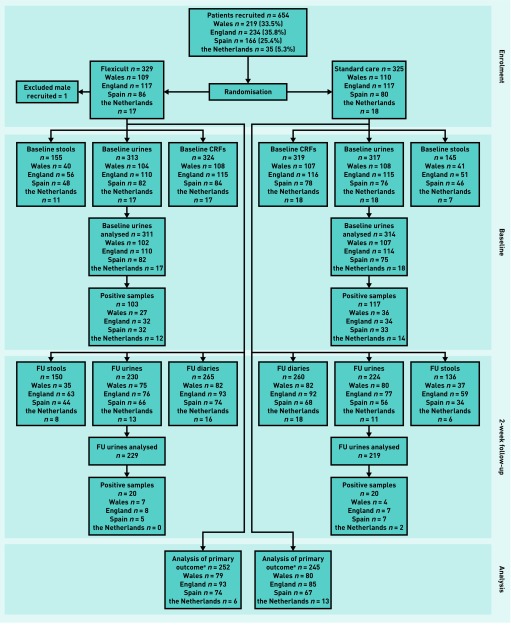
Participants were recruited between July 2013 and August 2014, with 329 randomised to the intervention (Flexicult) arm and 325 to standard care. Baseline data were available for 324 in the intervention arm and 319 in the standard-care arm. The authors ascertained initial antibiotic prescribing for 100% of participants, obtained primary outcome data for 252 (76.6%) and 245 (75.4%) respectively (Figure 3), and also had 3-month follow-up data for 98.8%.
Figure 3.
CONSORT flow diagram. aData for derivation of primary outcome analysis required each participant to have 2-week diary and urinalysis data available. CRF = case report form. FU = follow-up.
There were no important measured differences between those with and without primary outcome data (further information available from the authors on request) and the two arms were well balanced in terms of baseline variables. Frequency, urgency, and dysuria were the symptoms most often reported by patients as being a problem; those reported as being a problem the least number of times were fever, blood in the urine and feeling generally unwell (Table 1). Approximately one-third (220/612, 35.9%) of responders had a microbiologically confirmed UTI (Table 1).
Table 1.
Participant baseline data on presentation by management allocation, intervention (n = 324) versus standard care (n = 319)
| Flexicult | Standard care | Overall | ||
|---|---|---|---|---|
|
| ||||
| na | Mean (SD)b | Mean (SD)b | Mean (SD)b,c | |
| Age, years | 643 | 47.6 (22.5) | 47.6 (22.4) | 47.6 (27.6) |
|
| ||||
| Temperature, °C | 613 | 36.6 (0.95) | 36.6 (0.85) | 36.6 (1.18) |
|
| ||||
| n(%) | n(%) | n(%) | ||
|
| ||||
| Symptoms reported by patient as a problemd | ||||
| Daytime frequency (of urination) | 643 | 294 (90.7) | 295 (92.5) | 589 (91.6) |
| Urgency | 643 | 288 (88.9) | 286 (89.7) | 574 (89.3) |
| Burning or pain (dysuria) | 642 | 268 (83.0) | 252 (79.0) | 520 (81.0) |
| Night-time frequency | 643 | 261 (80.6) | 260 (81.5) | 521 (81.0) |
| Feeling generally unwell | 637 | 231 (72.0) | 220 (69.6) | 451 (70.8) |
| Abdominal pain | 641 | 209 (64.7) | 203 (63.8) | 412 (64.3) |
| Smelly urine | 642 | 177 (54.6) | 190 (59.7) | 367 (57.2) |
| Restricted activities | 643 | 171 (52.8) | 163 (51.1) | 334 (51.9) |
| Pain in the side | 642 | 155 (48.0) | 142 (44.5) | 297 (46.3) |
| Fever | 638 | 92 (28.8) | 93 (29.2) | 185 (29.0) |
| Blood in urine | 641 | 88 (27.2) | 72 (22.6) | 160 (25.0) |
|
| ||||
| Number of symptoms (dysuria, frequency, urgency)e | 643 | |||
| 1 | 34 (10.5) | 29 (9.1) | 63 (9.8) | |
| 2 | 96 (29.6) | 97 (30.4) | 193 (30.0) | |
| 3 | 194 (59.9) | 193 (60.5) | 387 (60.2) | |
|
| ||||
| Microbiologically confirmed UTI | 612 | 103 (33.4) | 117 (38.5) | 220 (35.9) |
|
| ||||
| UTIs with causative organism resistant to any first-line antibiotic (nitrofurantoin, trimethoprim, or fosfomycin) | 220 | 16 (15.5) | 24 (20.5) | 40 (18.2) |
|
| ||||
| UTI in the previous 12 months | 431 | |||
| 0 | 67 (31.3) | 70 (32.3) | 137 (31.8) | |
| 1–2 | 88 (41.1) | 85 (39.2) | 173 (40.1) | |
| ≥3 | 52 (24.3) | 58 (26.7) | 110 (25.5) | |
| Don’t know | 7 (3.3) | 4 (1.8) | 11 (2.6) | |
These represent the overall N.
Inflated for clustering by practice.
The overall SDs for age and temperature are substantially higher than those of either arm due to the nature of the inflation calculation; given that this is an individually randomised trial, the cluster size is, essentially, doubled for the overall calculation.
Symptoms reported as a problem include all categories from ‘Very little problem’ (score of 1) to ‘As bad as it could be’ (score of 6).
Balancing variable in randomisation. SD = standard deviation. UTI = urinary tract infection.
More participants in the standard-care arm reported concordant antibiotic use at day 3 than in the intervention arm (108/245 [44.1%] versus 99/252 [39.3%]), although this was not statistically significant (OR 0.84, 95% confidence interval [CI] = 0.58 to 1.20) (Table 2). The main driver of discordant usage was use of antibiotics with no laboratory microbiological confirmation of UTI (110/245 [44.9%] patients in the standard-care arm and 136/252 [54.0%] patients in the intervention arm) (data not shown). Only 10/245 (4.1%) and 5/252 (2.0%) in the standard-care and intervention arms respectively received an antibiotic to which the infecting organism was resistant (Table 2). Sensitivity analyses, including full ITT analyses using multiple imputations, did not alter the conclusions (Table 2, additional information available from the authors on request).
Table 2.
Comparison of intervention versus standard care for primary and secondary outcomesa
| Category | Flexicult n (%) | Standard care n (%) | Odds ratio | 95% CI | ICC | ||
|---|---|---|---|---|---|---|---|
| Concordant antibiotic usage | Yes | Primary outcome | |||||
|
| |||||||
| UTI + antibiotic + sensitive | 58 (23.0) | 67 (27.3) | |||||
| No UTI + no antibiotic | 41 (16.3) | 41 (16.7) | |||||
| Total | 99 (39.3) | 108 (44.1) | |||||
|
|
|||||||
| No | UTI + antibiotic + resistant | 5 (2.0) | 10 (4.1) | 0.84 | 0.58 to 1.20 | 0.02 | |
| UTI + No antibiotic | 12 (4.8) | 17 (6.9) | |||||
| No UTI + antibiotic | 136 (54.0) | 110 (44.9) | |||||
| Total | 153 (60.7) | 137 (55.9) | |||||
|
| |||||||
| Concordant antibiotic usage sensitivity: Flexicult b | Secondary analyses of primary outcome | ||||||
|
| |||||||
| 99 (39.4) | 108 (44.1) | 0.84 | 0.58 to 1.20 | n/a | |||
|
| |||||||
| Concordant antibiotic prescribing sensitivity: initial consultationc | 98 (33.1) | 114 (38.5) | 0.79 | 0.57 to 1.11 | 0.01 | ||
|
| |||||||
| Antibiotic prescribing at initial consultation | Secondary outcomes | ||||||
|
| |||||||
| 267 (82.4) | 282 (88.4) | 0.56 | 0.35 to 0.88 | 0.46 | |||
|
| |||||||
| Prescribed to guidelines at initial consultationd | 156 (58.9) | 166 (59.5) | 0.99 | 0.67 to 1.45 | 0.67 | ||
|
| |||||||
| Drug type and duratione | UTI-specific and 1–3 days | 182 (69.2) | 185 (67.8) | (ref) | n/a | ||
| UTI-specific and >3 days | 50 (19.0) | 57 (20.9) | 1.15 | 0.71 to 1.87 | |||
| Broad spectrum and 1–3 days | 0 (0.0) | 0 (0.0) | (empty) | ||||
| Broad spectrum and >3 days | 31 (11.8) | 31 (11.4) | 1.00 | 0.58 to 1.75 | |||
|
| |||||||
| Patient enablement (dichotomised) | 171 (70.1) | 177 (69.7) | 0.99 | 0.66 to 1.48 | n/a | ||
|
| |||||||
| Antibiotic consumed (day 3) | 217 (79.2) | 200 (76.6) | 1.24 | 0.81 to 1.89 | 0.24 | ||
|
| |||||||
| Antibiotic consumed (during 2 weeks) | 234 (85.1) | 217 (81.6) | 1.38 | 0.87 to 2.19 | 0.33 | ||
|
| |||||||
| New antibiotic prescribed (within 2 weeks) | 33 (10.3) | 30 (9.7) | 1.11 | 0.65 to 1.89 | n/a | ||
|
| |||||||
| Re-consultation (within 2 weeks) | 41 (12.9) | 41 (13.2) | 0.99 | 0.62 to 1.60 | n/a | ||
|
| |||||||
| Hospital stay (within 2 weeks) | 3 (0.9) | 4 (1.3) | Numbers too small for analysis | ||||
|
| |||||||
| Microbiologically confirmed UTI (at 2 weeks) | 20 (8.7) | 20 (9.2) | 0.94 | 0.49 to 1.81 | n/a | ||
|
| |||||||
| Stool sample resistance (at 2 weeks) | Ciprofloxacin | 33 (22.4) | 34 (24.5) | 0.96 | 0.54 to 1.71 | 0.396 | |
| Extended-spectrum beta-lactamases | 12 (8.2) | 8 (5.8) | 1.35 | 0.53 to 3.46 | n/a | ||
| Gentamicin | 16 (10.9) | 9 (6.5) | 1.75 | 0.74 to 4.42 | n/a | ||
| Carbapenem | 0 (0.0) | 1 (0.7) | Numbers too small for analysis | ||||
|
| |||||||
| Recurrence (within 3 months) | 54 (17.0) | 69 (22.3) | 0.72 | 0.48 to 1.07 | n/a | ||
|
| |||||||
| Duration of all symptoms | Median (IQR) | Median (IQR) | HR | 95% CI | ICC | ||
|
| |||||||
| 8.0 (5.0–14.0) | 8.0 (5.0–14.0) | 1.02 | 0.83 to 1.25 | n/a | |||
|
| |||||||
| Duration of moderately bad symptoms | 4.0 (2.0–6.0) | 4.0 (2.0–6.0) | 0.98 | 0.82 to 1.17 | n/a | ||
|
| |||||||
| Overall urinary symptom burden (AUC over 2 weeks)f | Mean (SD) | Mean (SD) | MD | 95% CI | ICC | ||
|
| |||||||
| 39.5 (36.56) | 38.2 (34.56) | 0.99 | 0.84 to 1.19 | n/a | |||
For these analyses, the denominator depends on the amount of relevant data available, which is not always 324 and 319 for Flexicult (intervention) and Standard Care respectively. As stated in the results section, the denominators for this analysis are 252 and 245 for Flexicult (intervention) and Standard Care respectively.
UTI and antibiotic resistance defined by the clinician’s reading of the Flexicult plate.
Antibiotic prescribed at initial consultation or, if available, the antibiotic prescribed after reading the Flexicult plate.
Prescribed to guidelines (England and Wales: trimethoprim, 3 days; nitrofurantoin, 3 days; Spain: fosfomycin, 1 day; nitrofurantoin, 7 days; the Netherlands: nitrofurantoin, 5 days; fosfomycin, 1 day; trimethoprim, 3 days) based on prescription made at the initial consultation. This excludes those who did not receive a prescription and those for which the prescribed drug is unknown.
As a multinomial model, relative risk ratios, rather than odds ratios, are given.
Effect/95% CI are back-transformed from a natural log transformation. AUC = area under the curve. ICC = intracluster correlation coefficient. HR = hazard ratio. IQR = interquartile range. n/a = not applicable. MD = mean difference. SD = standard deviation. UTI = urinary tract infection.
Secondary outcomes (Table 2) include fewer females who were prescribed antibiotics at initial consultation in the intervention arm than in the standard-care arm (267/324 [82.4%] versus 282/319 [88.4%], OR 0.56, 95% CI = 0.35 to 0.88), but no differences in: antibiotic prescribing at any point; antibiotic resistance and consumption; adherence to national prescribing guidelines; patient enablement; symptom severity or duration; recurrence; re-consultation; hospitalisation; or resistance in urine and stool samples at 2 weeks.
Clinicians reported using the Flexicult POC test for 313/324 (96.6%) of participants randomised to the intervention (data not shown). They indicated that they had contacted 176/303 (58.1%) females in response to the test result (data not shown) and that it had changed management for 190/301 (63.1%): 14 (7.4%) of those with a change of management were advised not to start taking an antibiotic; 10 (5.3%) were advised to stop taking an antibiotic they had already started; 29 (15.3%) to start taking an antibiotic; 63 (33.2%) to keep taking an antibiotic that was prescribed at the baseline visit; and 74 (38.9%) were prescribed a new antibiotic (Table 3). There were two reports of Flexicult use in the standard-care arm; these participants were not excluded from all analyses.
Table 3.
Clinician advice about antibiotic treatment in response to Flexicult result
| Did the Flexicult result indicate that a UTI was present? | |||||
|---|---|---|---|---|---|
|
| |||||
| No | Yes | Missing | Total | ||
|
| |||||
| n (%) | n (%) | n (%) | n (%) | ||
| ‘Was patient’s management changed in response to the test result?’ | No | 78 (38.8) | 31 (33.0) | 2 (33.3) | 111 (36.9) |
| Yes | 123 (61.2) | 63 (67.0) | 4 (66.7) | 190 (63.1) | |
| Total | 201 (100.0) | 94 (100.0) | 6 (100.0) | 301 (100.0) | |
|
| |||||
| Change of management | No antibiotic needed/don’t start antibiotic | 14 (11.4) | 0 (0.0) | 0 (0.0) | 14 (7.4) |
| Stop taking antibiotic | 10 (8.1) | 0 (0.0) | 0 (0.0) | 10 (5.3) | |
| Start taking antibiotic | 16 (13.0) | 11 (17.5) | 2 (50.0) | 29 (15.3) | |
| Continue with antibiotic | 35 (28.5) | 27 (42.9) | 1 (25.0) | 63 (33.2) | |
| New antibiotic prescribed | 48 (39.0) | 25 (39.7) | 1 (25.0) | 74 (38.9) | |
| Total | 123 (100.0) | 63 (100.0) | 4 (100.0) | 190 (100.0) | |
It took an average of 9 minutes to prepare the Flexicult test, 6 minutes to obtain and record the results, and 7 minutes to discuss the results with the patient. The total cost per person of the intervention, including the cost of the POCT, was £48 (England and Wales), €56 (the Netherlands), and €32 (Spain); the delivery costs contributed to nearly 90% of the total cost. At day 3, the average cost of antibiotic prescribing was similar between the two groups. There were no differences in any other healthcare costs by day 3, patient-borne costs at 14 days, or healthcare resource use at 3 months.
A cost-effectiveness ratio showed that the intervention is never cost saving; in addition, it is cost-effective only in limited cases and against a high willingness to pay for it. However, the cost of antibiotic resistance is not included in estimates.27
DISCUSSION
Summary
In this clinical and cost-effectiveness randomised controlled trial of POCT urine culture for uncomplicated UTI in females in primary care, it was found that marginally fewer antibiotics were prescribed at the initial consultation for patients in the POCT arm. Clinicians indicated that they had contacted nearly 60% of patients in response to the test, that it had changed their management in nearly two-thirds of cases, and they had prescribed a new antibiotic in just over one-third of those for whom they had a test result. However, they generally prescribed antibiotics empirically at the initial consultation without waiting for the test result, and seldom withdrew antibiotic treatment that had already been started when the test indicated no UTI.
Despite the test changing clinicians’ advice and decisions about antibiotic prescribing for UTI, the authors found no evidence of any difference in the primary outcome of patient-reported antibiotic use that was concordant with laboratory (as opposed to the POCT) culture results. Patient-reported recovery was not changed by test use, despite the additional costs of the test.
The main driver of discordant antibiotic use for females was antibiotic consumption without a microbiologically confirmed UTI, rather than use of antibiotics to which the UTI pathogen was not susceptible.
Strengths and limitations
Clinicians could decide or discuss with the patient about how best to use the test, for example, to aid in a next-day review of an initial antibiotic prescribing decision; to guide starting a delayed antibiotic prescription; or to determine whether, and what antibiotic class, to prescribe the following day. Flexicult is a POC test, in that it is performed where the patient is receiving care and outside of the clinical laboratory setting. However, even though the results are available within 24 hours — which is generally far quicker than laboratory culture results — it is not a rapid test. As such, it can be used in a number of ways; approximately 30% of the test results led to treatment changes in this study.
This large, pragmatic trial involved multiple sites in four countries, recruited to target. It achieved high ascertainment rates, so external validity should be high. In addition, there were no meaningful differences in baseline characteristics between those for whom the authors did, and did not, ascertain the primary outcome. Clinicians were not able to reliably keep logs of eligible patients who were not recruited, so estimating the proportion of potentially eligible participants who were invited to participate is not possible. Not all clinicians in participating practices were part of the study and many who were did not work full time and were not available to recruit on each day they worked.
Patient-reported antibiotic consumption was used in order to take into account: adherence; prescribing from other sources, such as use of leftover antibiotics; and prescribing by out-of-hours and other emergency care clinicians. However, information was retrieved on further antibiotic prescriptions and additional consultations in primary care over 3 months after the initial consultation for almost all patients. The authors found little evidence of contamination — namely, use of POCT in the standard-care arm — and clinicians reported using the POC test in 96.6% of those randomised to the intervention.
This was an open trial of the change of a POC test on clinicians’ prescribing behaviour and, ultimately, on patients’ adherence to those prescribing decisions. Open studies capture the changing of clinicians’ and patients’ expectations of interventions on behaviour, which is important to ascertain an accurate estimate of healthcare costs. Delivering the intervention took a median of 17 minutes (results were discussed with patients in about two-thirds of cases); this constitutes a substantial opportunity cost of alternative use of health professionals’ time,28 but may reduce once greater familiarity is established. POCT urine culture was, therefore, not cost-effective in primary care when used mainly to guide changes to initial antibiotic prescribing decisions; this was largely because antibiotic prescribing decisions were not delayed until results were known, and antibiotics were not always stopped when there was no evidence of a UTI on POCT.
Comparison with existing literature
Although all participants were presenting with symptoms of uncomplicated UTI, only 35.9% had a UTI confirmed on culture. This is similar to the 25–50% culture positivity rates found in observational studies.10–13 Possible explanations include UTIs caused by pathogens that are not identifiable on routine urine culture, problems with sample transportation, or contamination masking true UTIs. Any of these possibilities may have affected results according to the authors’ primary outcome. Although routine laboratory urine culture provides the diagnostic information that clinicians would usually obtain if they chose to submit a sample, it is not a perfect reference standard, as routine culture may produce inconsistent results and vary between laboratories.29
In a separate analysis, the authors examined clinicians’ interpretations of the Flexicult urine culture results in this study, and found they overestimated the proportion that was positive for UTI when compared with the results of laboratory culture of urine from the same patient.30 Observing some growth on a culture plate from fresh urine from a patient who is symptomatic may have made clinicians reluctant to stop antibiotics, despite the level of growth on the POCT culture not technically meeting the threshold for a UTI. It is also not clear whether routine laboratory culture with associated delays in transport and processing, or more rapid POC culture, give a more valid answer.
The authors have previously examined the analytic performance of the Flexicult test using routinely submitted urine samples,31 and the analytic performance of other POCT culture approaches have also been assessed.19,32–34 However, it was not possible to identify previous evaluations of intervention implementation to improve the management of UTI in terms of inappropriate antibiotic use, costs, resistance, and health outcomes. Holm et al have also found that POC culture was better at ruling in, rather than ruling out, a UTI diagnosis based on laboratory culture.34
Implications for research and practice
In test–treatment trials,4 patient outcomes are only likely to be affected if patients receive a valid test and appropriate diagnosis, management decisions are made, and treatment is implemented and adhered to. In interpreting the plates, clinicians may have assumed that low-threshold bacterial growth in females with symptoms suggestive of UTI justified antibiotic treatment and, therefore, refrained from adjusting it. Had clinicians prescribed or adjusted their initial prescriptions according to POCT urine culture results, and patients had changed their antibiotic consumption behaviour accordingly, the intervention may have been effective. This underlines the importance of paying attention to targeted behaviour-change strategies in conjunction with the introduction of new tests.
The Flexicult POC test was not clinically or cost-effective when used mainly for adjusting antibiotic prescribing decisions for UTI after the test result became available. Given the low levels of antibiotic resistance identified in these systematically sampled patients with uncomplicated UTI, along with the challenges associated with stopping short courses of antibiotics when the patient is in the community, future research should focus on assessing the effect of culture-based POC tests (such as Flexicult) in the context of advising patients to delay prescribing until the results of the test are known. Symptomatic treatments offered at the point of consultation may improve the acceptability and uptake of this approach.
Few POC tests have been subjected to rigorous, pragmatic clinical trials of cost-effectiveness using a range of outcomes, including patient-orientated measures.2 This study underlines the importance of conducting a rigorous, pragmatic trial of cost-effectiveness (and not, simply, of analytic performance) and evidence-based identification of the place of such tests in clinical pathways, before new diagnostic technologies are adapted into usual care.
Acknowledgments
The authors would like to thank: Statens Serum Institute and team for providing the Flexicult plates and delivering training to the research team and participating sites; Freda McKenzie for being the patient/public representative on the trial management group; trial steering committee members, Alastair Hay, Andrew Lovering, and Toby Provost; the local primary care research networks; comprehensive local research networks; the Coordinating Research Centre of the National Institute for Social Care and Health Research; the clinicians who implemented the study in their practices; the patients who participated; Charles Cowtan for assessing the psychometrics of the symptom scores prior to analysis; and Elinor Coulman for coordinating the close-down of the trial. In addition to the authors, the POETIC team comprises: Rhys Thomas, Anna Moragas, and José M Molero.
Funding
The research leading to these results has received funding from the European Community’s Seventh Framework Programme FP7/2007-2013 under grant agreement number 282512.
Ethical approval
The trial was approved in the UK by the Research Ethics Committee for Wales (now known as Wales REC3) (reference: 12/WA/0394), Jordi Gol i Gurina Ethics Committee in Barcelona (reference: AC13/01), and the Medical Research Ethics Committee of UMC Utrecht (reference: 13/304) in the Netherlands.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.O’Neill J. Rapid diagnostics: stopping unnecessary use of antibiotics — the review on antimicrobial resistance. 2015. https://amr-review.org/sites/default/files/Paper-Rapid-Diagnostics-Stopping-Unnecessary-Prescription-Low-Res.pdf (accessed 20 Feb 2018)
- 2.St John A, Price CP. Economic evidence and point-of-care testing. Clin Biochem Rev. 2013;34(2):61–74. [PMC free article] [PubMed] [Google Scholar]
- 3.Horvath AR, Lord SJ, St John A, et al. From biomarkers to medical tests: the changing landscape of test evaluation. Clin Chim Acta. 2014;427:49–57. doi: 10.1016/j.cca.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Ferrante di Ruffano L, Hyde CJ, McCaffery KJ, et al. Assessing the value of diagnostic tests: a framework for designing and evaluating trials. BMJ. 2012;344:e686. doi: 10.1136/bmj.e686. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox M. Assuring the quality of diagnostic tests: how do we know that they do what we think they do? BMJ. 2013;346:f836. doi: 10.1136/bmj.f836. [DOI] [PubMed] [Google Scholar]
- 6.Butler CC, Hawking MK, Quigley A, McNulty CA. Incidence, severity, help seeking, and management of uncomplicated urinary tract infection: a population-based survey. Br J Gen Pract. 2015. DOI: https://doi.org/10.3399/bjgp15X686965. [DOI] [PMC free article] [PubMed]
- 7.Salvatore S, Salvatore S, Cattoni E, et al. Urinary tract infections in women. Eur J Obstet Gynecol Reprod Biol. 2011;156(2):131–136. doi: 10.1016/j.ejogrb.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366(11):1028–1037. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 9.Ong DS, Kuyvenhoven MM, van Dijk L, Verheij TJ. Antibiotics for respiratory, ear and urinary tract disorders and consistency among GPs. J Antimicrob Chemother. 2008;62(3):587–592. doi: 10.1093/jac/dkn230. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien K, Hillier S, Simpson S, et al. An observational study of empirical antibiotics for adult women with uncomplicated UTI in general practice. J Antimicrob Chemother. 2007;59(6):1200–1203. doi: 10.1093/jac/dkm108. [DOI] [PubMed] [Google Scholar]
- 11.Nazareth I, King M. Decision making by general practitioners in diagnosis and management of lower urinary tract symptoms in women. BMJ. 1993;306(6885):1103–1106. doi: 10.1136/bmj.306.6885.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahey T, Webb E, Montgomery AA, Heyderman RS. Clinical management of urinary tract infection in women: a prospective cohort study. Fam Pract. 2003;20(1):1–6. doi: 10.1093/fampra/20.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Little P, Turner S, Rumsby K, et al. Validating the prediction of lower urinary tract infection in primary care: sensitivity and specificity of urinary dipsticks and clinical scores in women. Br J Gen Pract. 2010. DOI: https://doi.org/10.3399/bjgp10X514747. [DOI] [PMC free article] [PubMed]
- 14.Christiaens TC, De Meyere M, Verschraegen G, et al. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. Br J Gen Pract. 2002;52(482):729–734. [PMC free article] [PubMed] [Google Scholar]
- 15.Monsen TJ, Holm SE, Ferry BM, Ferry SA. Mecillinam resistance and outcome of pivmecillinam treatment in uncomplicated lower urinary tract infection in women. APMIS. 2014;122(4):317–323. doi: 10.1111/apm.12147. [DOI] [PubMed] [Google Scholar]
- 16.Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 17.Alam MF, Cohen D, Butler C, et al. The additional costs of antibiotics and re-consultations for antibiotic-resistant Escherichia coli urinary tract infections managed in general practice. Int J Antimicrob Agents. 2009;33(3):255–257. doi: 10.1016/j.ijantimicag.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007;57(543):785–792. [PMC free article] [PubMed] [Google Scholar]
- 19.Blom M, Sorensen TL, Espersen F, Frimodt-Moller N. Validation of FLEXICULT SSI-Urinary Kit for use in the primary health care setting. Scand J Infect Dis. 2002;34(6):430–435. doi: 10.1080/00365540110080601. [DOI] [PubMed] [Google Scholar]
- 20.Bongard E, Frimodt-Moller N, Gal M, et al. Analytic laboratory performance of a point of care urine culture kit for diagnosis and antibiotic susceptibility testing. Eur J Clin Microbiol Infect Dis. 2015;34(10):2111–2119. doi: 10.1007/s10096-015-2460-4. [DOI] [PubMed] [Google Scholar]
- 21.Bates J, Thomas-Jones E, Pickles T, et al. Point of care testing for urinary tract infection in primary care (POETIC): protocol for a randomised controlled trial of the clinical and cost effectiveness of FLEXICULTTM informed management of uncomplicated UTI in primary care. BMC Fam Pract. 2014;15:187. doi: 10.1186/s12875-014-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little P, Merriman R, Turner S, et al. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care: observational study. BMJ. 2010;340:b5633. doi: 10.1136/bmj.b5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howie JG, Heaney DJ, Maxwell M, Walker JJ. A comparison of a Patient Enablement Instrument (PEI) against two established satisfaction scales as an outcome measure of primary care consultations. Fam Pract. 1998;15(2):165–171. doi: 10.1093/fampra/15.2.165. [DOI] [PubMed] [Google Scholar]
- 24.Butler CC, Hood K, Verheij T, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes M, Ng ES, Grieve R, et al. Developing appropriate methods for cost-effectiveness analysis of cluster randomized trials. Med Decis Making. 2012;32(2):350–361. doi: 10.1177/0272989X11418372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves — facts, fallacies and frequently asked questions. Health Econ. 2004;13(5):405–415. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- 27.Oppong R, Smith RD, Little P, et al. Cost effectiveness of amoxicillin for lower respiratory tract infections in primary care: an economic evaluation accounting for the cost of antimicrobial resistance. Br J Gen Pract. 2016. DOI: https://doi.org/10.3399/bjgp16X686533. [DOI] [PMC free article] [PubMed]
- 28.Morris S, Devlin N, Parkin D, Spencer A. Economic analysis in healthcare. Chichester: John Wiley & Sons; 2012. [Google Scholar]
- 29.Reitsma JB, Rutjes AW, Khan KS, et al. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol. 2009;62(8):797–806. doi: 10.1016/j.jclinepi.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Hullegie S, Wootton M, Verheij TJM, et al. Clinicians’ interpretations of point of care urine culture versus laboratory culture results: analysis from the four-country POETIC trial of diagnosis of uncomplicated urinary tract infection in primary care. Fam Pract. 2017;34(4):392–399. doi: 10.1093/fampra/cmx009. [DOI] [PubMed] [Google Scholar]
- 31.Bongard E, Frimodt-Moller N, Gal M, et al. Analytic laboratory performance of a point of care urine culture kit for diagnosis and antibiotic susceptibility testing. Eur J Clin Microbiol Infect Dis. 2015;34(10):2111–2119. doi: 10.1007/s10096-015-2460-4. [DOI] [PubMed] [Google Scholar]
- 32.Yagupsky P, Rider M, Peled N. Clinical evaluation of a novel chromogenic agar dipslide for diagnosis of urinary tract infections. Eur J Clin Microbiol Infect Dis. 2000;19(9):694–698. doi: 10.1007/s100960000345. [DOI] [PubMed] [Google Scholar]
- 33.Ferry S, Burman LG, Holm SE. Uricult and Sensicult dipslides for diagnosis of bacteriuria and prediction of drug resistance in primary health care. Scand J Prim Health Care. 1989;7(2):123–128. doi: 10.3109/02813438909088659. [DOI] [PubMed] [Google Scholar]
- 34.Holm A, Cordoba G, Sorensen TM, et al. Clinical accuracy of point-of-care urine culture in general practice. Scand J Prim Health Care. 2017;35(2):170–177. doi: 10.1080/02813432.2017.1333304. [DOI] [PMC free article] [PubMed] [Google Scholar]