Abstract
It is proposed that environmental exposures in early life influence immune programming. Specifically, socioeconomic disadvantage is thought to program an immune phenotype that is prone to inflammation and associated with increased risk for inflammatory disease later in life. Existing literature shows an inverse association of early childhood socioeconomic status (SES) with adult levels of systemic inflammation. Here, we extend that literature to examine whether early childhood SES also relates to the magnitude of inflammatory response to acute psychological stress in adulthood. Healthy volunteers (N=110; 40-58 years; 59% female; 90% white) performed a laboratory stress protocol, with blood samples drawn at the end of a 30-min baseline, a 5-min speech task, and a 30-min recovery to assess interleukin (IL)-6 stress responses. An early childhood SES index was derived from reports of parental home and vehicle ownership, and number of bedrooms per child in the home across ages 1-2, 3-4, and 5-6. Regressions adjusted for current age, sex, race, and BMI showed that lower SES at age 1-2 was associated with larger IL-6 stress responses in adulthood (ΔR2 = .05, β = −.24, p = .03). This association was independent of adult SES and task-evoked affective responses. No association was found between SES at ages 3-4 or 5-6 and IL-6 responses. These results provide initial evidence for a link between disadvantage in the first 2 years of life and heightened inflammatory response to stress in adulthood; this link may contribute to the increased disease risk that accompanies being raised in disadvantaged socioeconomic circumstances.
1.0 Introduction
Physical health tracks a socioeconomic gradient that is influenced by environmental conditions in childhood. Indeed, considerable evidence shows that disadvantaged childhood socioeconomic status (SES) is associated with shorter life expectancy and poorer physical health across the lifespan (Dong et al., 2004; Galobardes et al., 2008, 2004; Shonkoff et al., 2009; Taylor et al., 2011). This is a major concern in the United States, where 23% of children under the age of six currently live in poverty (Jiang et al., 2017). However, the pathways that link environmental conditions in childhood to adult health are complex and not fully understood.
Recent attention has focused on the possibility that early life exposures program the biology of the developing organism in ways that are health protective in the short term, but may contribute to increased morbidity and mortality later in life (Miller and Chen, 2013; Shonkoff et al., 2009). In this regard, converging evidence suggests that poorer socioeconomic circumstances in early childhood can program the immune cells that produce markers of systemic inflammation (Miller et al., 2011; Taylor, 2010). This programming is proposed to result in a proinflammatory immune phenotype, characterized by immune cells that 1) respond to potential threats with greater production of inflammatory mediators and 2) are less sensitive to inhibitory signals (Miller et al., 2011, 2009; Miller and Chen, 2007). The predisposition to mount larger inflammatory responses may offer greater protection against acute infections and promote recovery from injury in the short term, but increase vulnerability to chronic inflammatory conditions (Danese et al., 2008; Miller et al., 2011).
Consistent with theories of biological programming, initial human studies support an association of poorer childhood socioeconomic conditions with increased systemic inflammation in adulthood. Findings show that lower childhood SES is associated with heightened basal levels of inflammatory mediators in adulthood (Fagundes et al., 2013; Pollitt et al., 2007). In particular, we have shown that basal circulating levels of interleukin (IL)-6 are inversely associated with indicators of parental SES during the first two years of life, but not later in childhood (Carroll et al., 2011a). These associations were independent of adult SES, suggesting that SES in early childhood makes a unique contribution to adult inflammation. Supporting the possibility that this environmental impact may begin at a very early age, recent work indicates that maternal socioeconomic disadvantage is associated with higher salivary levels of the inflammatory marker C-reactive protein (CRP) in infants (David et al., 2017). Taken together, existing evidence suggests that socioeconomic disadvantage in the earliest years of life may affect biological stress response systems and inflammatory processes.
Early socioeconomic exposures may contribute to elevated systemic inflammation through programming of stress regulatory systems. Rodent models support this hypothesis, showing that stress exposure in infancy has enduring effects on physiological stress responses later in life (Denenberg, 1964; Levine, 1957; Meaney et al., 1996). In humans, socioeconomic disadvantage in early life may program increased vigilance and mistrust of others, leading to exaggerated responses to potential threats (Chen et al., 2004; Chen and Matthews, 1999; Miller et al., 2011; Nusslock and Miller, 2016). For example, individuals reporting low childhood SES show greater amygdala responses to threatening faces (Gianaros et al., 2008). Additionally, childhood SES is associated with behavioral and physiological responses to threat. For instance, children raised in low SES environments are more likely to perceive threat in ambiguous situations and show heightened cardiovascular responses to laboratory stressors (Chen et al., 2004). This heightened threat sensitivity is proposed to contribute to the regulation of proinflammatory cytokine production by immune cells via physiological stress pathways, including the autonomic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis (Eisenberger and Cole, 2012; Nusslock and Miller, 2016). In summary, this evidence suggests that socioeconomic disadvantage in childhood may trigger alterations in stress regulatory systems, leading to enduring changes in stress responses into adulthood. To date, however, no studies have examined whether individuals exposed to low SES in early childhood show greater inflammatory responses to acute psychological stress in adulthood.
It is well established that circulating levels of inflammatory cytokines (e.g., IL-6) increase after exposure to acute psychological stress in the laboratory (Marsland et al., 2017; Steptoe et al., 2007). Notably, there are marked individual differences in the magnitude of these inflammatory responses, with some individuals exhibiting large increases and others showing little or no response (Cohen and Hamrick, 2003; Schneiderman et al., 2008). Individual differences in immune responses to acute stress are reproducible across time and type of challenge (Marsland et al., 2002, 1995), supporting the possibility that they reflect immune phenotypes that may contribute to future disease susceptibility.
Several factors have been found to predict the magnitude of inflammatory stress responses. In general, behavioral and psychosocial health risk factors are associated with larger increases in circulating IL-6; these risk factors include smoking, lower physical fitness, poorer sleep quality, lower self-compassion, and loneliness (Marsland et al., 2017). There is also some evidence that lower adult SES is associated with larger and more prolonged increases in circulating IL-6 from baseline to 45-120min after acute laboratory stress (Brydon et al., 2004; Derry et al., 2013). In addition, adults who endorse a history of maltreatment in childhood show larger IL-6 responses to acute stress (Carpenter et al., 2010).While childhood maltreatment is not synonymous with low childhood SES, early life stress is a common feature of both environmental exposures. Taken together, these findings raise the possibility that socioeconomic conditions in childhood may play a role in programming inflammatory stress responses.
Accordingly, the present study examined whether childhood SES is associated with individual differences in the magnitude of inflammatory stress responses in adulthood. Based on current theories of immune programming (Miller et al., 2011), as well as human evidence and animal models indicating a critical period for environmental sensitivity in early life (Carroll et al., 2011a; David et al., 2017; Denenberg, 1964; Levine, 1957; Meaney et al., 1996), we hypothesized that lower childhood SES in the earliest years of life would relate to larger IL-6 responses to an acute laboratory stressor among midlife adults. In addition, given that prior findings from the same parent study found negative affective responses to be associated with larger IL-6 stress responses (Carroll et al., 2011b), we also explored whether associations of childhood SES and IL-6 stress responses were independent of negative affective responses to stress.
2.0 Methods
2.1 Participants
Participants were community volunteers involved in the Vaccination Immunity Project (VIP), a study assessing behavioral, psychosocial, and physiological factors associated with antibody response to hepatitis B vaccination. Subjects were recruited by mass mail solicitation in Allegheny County, Pennsylvania. Exclusion criteria for the VIP study included: history of autoimmune disease, chronic infections, hormone disorders, asthma, severe allergies, cardiovascular disease, bleeding problems, chronic kidney or liver conditions, cancer, Type I or II diabetes, clinical depression, or psychotic disorders. Smokers and those using medications affecting the immune, endocrine, or nervous systems were also excluded. Data collection took place over multiple laboratory sessions and informed consent procedures were carried out following guidelines of the University of Pittsburgh Institutional Review Board.
The original VIP sample included 153 participants between the ages of 40-60 (58.2% female, 88.2% White). Data from the original VIP sample have previously been reported by (Carroll et al., 2011a, 2011b). The analytic sample for the present analysis consisted of 110 individuals (40-58 years, 59.1% female, 90.0% White) who completed a laboratory stress session that included both pre- and post-stressor measures of serum IL-6. Serum samples were not included at the beginning of the study and thus were not available for a portion of participants (N=29). An additional 14 participants did not have serum IL-6 due to issues with the placement of the intravenous catheter. Initial descriptive analyses did not indicate outliers on any key variables in this analytic sample.
2.2 Procedures
Data used for the present study were primarily collected during two laboratory sessions that started between 7:00 and 9:00 AM, lasted approximately 1.5 hours, and were scheduled 1 month apart. Both sessions took place at least 4 weeks before the initial hepatitis B vaccination was administered. Before both sessions, participants were asked to abstain from alcohol (48h), strenuous physical activity and non-prescription medications (24h), and caffeine, beverages, and food (12h). On arrival, participants completed questionnaires, a medical history interview, and measurements of height and weight. To collect blood samples, an intravenous catheter was inserted into the antecubital vein of one arm. Participants then completed a 30-min resting baseline period, after which the baseline blood sample was collected and participants completed a measure assessing their affective state during the baseline period. Participants then performed a speech task in which they defended themselves against an alleged accusation of shoplifting or a traffic violation (Marsland et al., 2002, 1995). The speech task included a 2-min preparation period and a videotaped 3-min speech delivery period. Immediately following the speech task, a second blood sample was drawn and affective state was reassessed. Finally, participants rested for a 30-min period, after which a final blood sample was taken and affective state during this period was assessed. Participants were not debriefed about laboratory stress procedures after the first visit.
Of the 110 participants, 92 participants completed the second laboratory stress session. Experimental procedures at the two testing sessions were nearly identical, with two notable exceptions. First, participants were told that their “performance on the first speech task was slightly below average when compared to other participants’ speeches” and that they should “try to be more persuasive when delivering this speech”. Second, the two transgression scenarios (shoplifting or traffic violation) were counterbalanced across sessions.
In addition to the laboratory visits, participants completed the SC Childhood Interview 4 weeks after completing the second laboratory session prior to receiving the first Hepatitis B vaccination.
2.3 Measures
2.3.1 Serum IL-6
IL-6 was selected as a key marker of inflammatory stress response for several reasons. Two meta-analytic reviews have indicated that circulating IL-6 shows a robust increase after exposure to acute laboratory stress, while other commonly studied inflammatory mediators either do not show this response or have not been as extensively studied (Marsland et al., 2017; Steptoe et al., 2007). IL-6 is also of primary interest, given its established relationship with cardiovascular disease risk (Danesh et al., 2008). Prior to batch analysis, serum was stored at −80°C. Serum IL-6 levels were determined using high sensitivity ELISA kits (R&D Systems, Minneapolis, MN). Samples were run in duplicate. The average inter- and intra-assay coefficients of variation were 7% and 5%, respectively.
As this study examined IL-6 stress responses on multiple occasions, these data provide the unique opportunity to characterize more stable individual differences in IL-6 stress reactivity. Aggregating stress responses across multiple testing occasions reduces measurement error and increases reliability, ultimately improving researchers’ ability to characterize individual differences (Kamarck et al., 2000, 1992). Moreover, prior work in the laboratory stress reactivity field indicates that aggregation of responses to stressors across multiple testing occasions increases the generalizability of laboratory stress responses to the responses an individual shows outside of the laboratory (Kamarck et al., 2000). As such, aggregating IL-6 stress responses across the two occasions of testing in the present study increases our ability to characterize stable individual differences in IL-6 stress responses.
2.3.2 Childhood SES Index
Participants completed the SC Childhood Interview (Cohen, 2010). This is a retrospective assessment of multiple indicators of childhood SES. For each year from ages 1-18, participants reported on parental home ownership, parental vehicle ownership, number of bedrooms in the home, and number of adults and children living in the home. These indicators of childhood SES were employed because (1) they are easier to recall than parental income/education and (2) they are sensitive to change over the childhood years (Cohen et al., 2004). Using these socioeconomic factors, a childhood SES index was calculated for ages 1-6 using the same method as Carroll et al. (2011a). Briefly, a composite score was calculated across 2-year intervals (years 1-2, 3-4, and 5-6) by summing scores across three indicators: home ownership (0= no, 1= yes), vehicle ownership (0= 0-1 vehicles, 1= 2+ vehicles), and number of bedrooms per child in the house (0= <1, 1= 1+). For each year, a participant’s score could range from 0-3. Thus, for each 2-year interval, participants had childhood SES index scores ranging from 0-6. During the interview, participants were given the option to say that they could not recall the given measure for that year. The percentage of participants selecting this option ranged from 0-10% for parental home ownership, 0-11.8% for parental vehicle ownership, and 0-15% for number of bedrooms in the home. The most difficult items to recall were number of bedrooms in the home and parental vehicle ownership at age 1. This missing data resulted in variations in sample size for each 2-year interval (Table 1).
Table 1.
Descriptive statistics
| Mean or % | SD | N | |
|---|---|---|---|
| Sex (% female) | 59% | 110 | |
| Race (% white) | 90% | 110 | |
| Age | 50.4 | 5.3 | 110 |
| BMI kg/m2 | 26.2 | 3.7 | 110 |
| BDI score | 2.5 | 3.2 | 110 |
| Adult SES index | 2.1 | 0.8 | 102 |
| Adult educational attainment (years) | 14.9 | 1.4 | 104 |
| Adult annual family income | $72,064 | $40,027 | 93 |
| Childhood SES index (age 1-2) | 2.49 | 1.7 | 85 |
| Childhood SES index (age 3-4) | 2.49 | 1.7 | 87 |
| Childhood SES index (age 5-6) | 2.85 | 1.7 | 94 |
| Baseline IL-6 | 1.3 | 0.7 | 110 |
Note: BMI = body mass index; BDI = Beck Depression Inventory; SES = socioeconomic status; IL-6 = interleukin-6
2.3.3 Adult SES
An adult SES index was computed using current self-reported SES variables selected to parallel the childhood SES index. Specifically, the adult SES index was the summed score of number of vehicles owned by the participant’s household (0 = 0-1, 1 = 2+), the number of bedrooms per occupant in the home (0 = <1, 1= 1+), and reported home ownership (0 = no, 1 = yes). Thus, adult SES index scores ranged from 0-3. Bedrooms per occupant in the home was used in place of bedrooms per child, as not all adult participants had children living in the home.
Education and family income were also used as indices of adult SES. Participants reported cumulative years of schooling and current annual household income on a bracketed scale with ranges of <$5,000; $5,000-11,999; $12,000-15,999; $16,000-24,999; $25,000-34,999; $35,000-49,000; $50,000-74,999; $75,000-99,999; >$100,000. To determine occupant adjusted family income, the midpoint of the income bracket was weighted by the square root of the number of people living in the home. This value was normalized with a cube root transformation (Schwartz, 1985). Of note, six participants did not report education and 20 did not report family income. Primary analyses were conducted with and without these participants and results remained the same.
2.3.4 Task-Evoked Affective Responses
Participants reported state affect during the stress protocol using a mood adjective rating scale derived from the Profile of Mood States (POMS) (McNair et al., 1971). Given previous evidence from this sample that acute IL-6 stress responses associate with changes in state anger and anxiety in response to the task (Carroll et al., 2011b), we examined whether any associations of childhood SES with IL-6 reactivity were independent of these affective responses. Consistent with the previous study, affect was assessed using items derived from Usala and Hertzog’s (1989) factor analysis of the POMS. Participants rated anxiety (e.g., nervous, uneasy, tense, on edge) and anger (e.g., resentful, angry, hostile) items on a scale of 0 (not at all) to 4 (extremely).
2.3.5 Additional Covariates
Participants self-reported age, sex, and race on a standard demographics questionnaire. Sex was coded as 0 = Female, 1 = Male. Race was dichotomized as White and nonwhite and coded as 0 = White, 1 = nonwhite. Body mass index (BMI) was determined using participants’ height and weight measurements and calculated as weight(kg)/height(m2). For participants who completed the second laboratory visit, BMI was averaged across the two visits. Based on previous evidence that depressive symptoms are associated with larger stress-induced increases in IL-6 (Pace et al., 2006), scores on the 13-item Beck Depression Inventory (BDI) were also included in initial analyses. Depressive symptomatology was quite low in this sample: a large number of participants (32.7%) had a score of 0 on the BDI and only 8 of the 110 participants scored above the clinical cutoff for depression. Although the BDI was included as a continuous variable in the present analyses, the skewed distribution should be kept under consideration.
2.4 Data Analysis
Preliminary analyses were conducted prior to aggregating IL-6 responses across visits. Given previous evidence that IL-6 levels show a delayed increase after stressor exposure (Steptoe et al., 2007), we used IL-6 levels at 30-min post-stressor to calculate change scores; we refer to these change scores as IL-6 stress responses. IL-6 levels at each time point and raw change scores for each visit are shown in Table 2. Prior work in this field has shown that IL-6 stress responses do not show habituation across periods of testing (von Kanel et al., 2006) but may show sensitization upon repeated stress exposure (McInnis et al., 2014; Rohleder et al., 2014). Before aggregating IL-6 responses across visits, we assessed our data for possible habituation or sensitization. Across subjects, a paired t-test indicated that IL-6 stress responses were not significantly different from Visit 1 to Visit 2 (t = .92, p = .36) and stress responses showed a modest correlation (r = .23, p = .04). We also assessed possible habituation and sensitization at the individual level, examining differences in change scores from Visit 1 to Visit 2. In this assessment, the vast majority of participants (70.6%) displayed IL-6 stress responses at Visit 2 that were within one standard deviation of their responses at Visit 1. Of the remaining participants, 12.9% showed a decrease of more than one standard deviation and 16.5% showed an increase of more than one standard deviation. Given that the large majority of participants in the present sample did not show habituation or sensitization of the IL-6 response and the theoretical rationale for increased reliability of stress responses aggregated across multiple occasions, we elected to aggregate IL-6 responses across the two visits. Specifically, IL-6 values for the 92 participants who completed both stress sessions were averaged across the two occasions of testing; for the remaining 18 participants, results of the single stress session were employed.
Table 2.
Mean IL-6 values at individual study visits and averaged across visits
| Baseline | Task | Recovery | Raw Change | |
|---|---|---|---|---|
| Visit 1 | 1.26 (.80) | 1.26 (.84) | 1.29 (.80) | .03 (.32) |
| Visit 2 | 1.28 (.76) | 1.25 (.71) | 1.39 (.74) | .08 (.41) |
| Average Across Visits | 1.25 (.66) | 1.24 (.66) | 1.32 (.66) | .05 (.28) |
Notes: Values in parentheses are standard deviations. Task values are IL-6 levels assessed immediately after the stressor task. Recovery values are IL-6 levels assessed 30-minutes after the stressor task. Raw change values were computed by subtracting Baseline values from Recovery values and averaging across participants. IL-6 was measured as pg/ml. IL-6 = interleukin-6
Pearson correlations were performed to assess bivariate associations between demographic variables (age, sex, race), BMI, depressive symptoms, IL-6 change scores, baseline IL-6, childhood SES, and adult SES. Next, a repeated measures analysis of variance (ANOVA) was carried out to confirm expected main effects of the speech task on IL-6, followed by planned contrasts. The distribution of IL-6 was non-normal and was thus log-transformed prior to the ANOVA. Greenhouse-Geisser corrections were used when the assumption of sphericity was violated. For the main analyses, we calculated baseline-adjusted IL-6 change scores. There was a negative association of baseline IL-6 with the magnitude of IL-6 stress responses. To control for baseline variation, baseline-adjusted IL-6 change scores were calculated by regressing post-task levels of IL-6 onto baseline levels and saving the residuals.
To assess primary hypotheses, we conducted a series of hierarchical linear regressions, examining the association between childhood SES index at each of the 2-year intervals (1-2, 3-4, and 5-6) and IL-6 stress responses. We ran separate regressions for each childhood SES interval. For these regressions, we entered age, sex, race, and BMI in the first step and childhood SES index for each 2-year interval in the second step of a model predicting IL-6 stress responses. Additional regressions were conducted to determine whether significant associations of childhood SES index with IL-6 stress responses were independent of adult SES. Here, demographics and BMI were entered in the first step, adult SES variables in the second step, and childhood SES index in the third step of the model. Two additional regressions were conducted to assess whether significant associations of childhood SES index with IL-6 stress responses were independent of task-evoked anger and anxiety. For this purpose, residualized affective change scores were created for both anxiety and anger by regressing immediate post-task affective ratings on baseline affective ratings. Demographics and BMI were entered in the first step, residualized anger or anxiety change scores in the second step of separate models, and childhood SES index in the third step of each model.
3.0 Results
3.1 Demographics and Correlations
Descriptive statistics are displayed in Table 1. Across subjects, childhood SES index did not change from age 1-2 to age 3-4, though there was a slight increase by age 5-6. Although there was no mean change in childhood SES across age periods, there was variation at the individual level: 21 participants showed at least a one point change in the childhood SES index from age 1-2 to 3-4 while 41 participants changed from 1-2 to 5-6. A one-point change in this measure would represent, for example, the difference between renting and owning a home.
There were a number of significant correlations between study variables. Childhood SES index scores were inter-correlated: childhood SES at age 1-2 was correlated with childhood SES at ages 3-4 (r = .91, p <.001) and 5-6 (r = .66, p <.001); childhood SES at ages 3-4 and 5-6 were also correlated (r =.81, p <.001). Lower childhood SES index score at age 1-2 was correlated with higher adult IL-6 stress responses (r = −.25, p < .009). IL-6 stress responses were not correlated with demographic characteristics, BMI, or childhood SES index at ages 3-4 or 5-6 (p’s >.10). Notably, IL-6 stress responses were not correlated with any of the adult SES variables (p’s > .10). Higher baseline IL-6 was correlated with higher BMI (r = .36, p < .001) and lower adult family income (r = −.22, p = .03), though not with childhood SES measures (p’s > .05). There were a number of significant associations between covariates: less educational attainment was correlated with higher BMI (r = −.29, p = .002); nonwhite race was correlated with lower scores on the adult SES index (r = −.25, p = .01); current family income was positively correlated with scores on the adult SES index (r = .36, p = .001) and educational attainment (r = .461, p < .001). All other correlations were non-significant. Of note, BDI scores were uncorrelated with childhood SES index scores and IL-6 stress responses (p’s > .10), and were not included in regression analyses.
3.2 Effects of Stressor Task on IL-6
Mean levels of IL-6 changed significantly over the course of the experiment (F [2, 109] = 7.93, p < .001; see Figure 1 and Table 2). Across subjects, IL-6 levels did not increase from baseline to immediately post-task (F [1, 109] = .608, p = .44). As expected, IL-6 increased significantly from baseline to 30 min post-task (F [1, 109] = 7.76, p < .001). There was substantial variation in magnitude of IL-6 change from baseline to 30-min post-stressor, with some participants showing large increases and others showing no change or even a slight decrease (mean change = 0.07 pg/ml (SD = .29), range = −0.57 to 1.11 pg/ml).
Figure. 1.
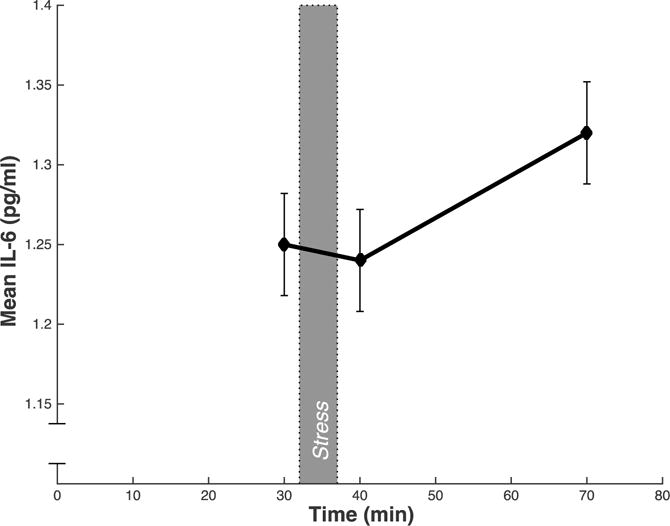
Mean circulating serum IL-6 (pg/mL) across all subjects at each time point. Blood samples were taken after a baseline period (30 min), immediately after the stressor (40 min), and after the 30 min post-task recovery period (70 min). Error bars indicate standard error of the mean of IL-6.
3.3 Childhood SES and IL-6 Response
In support of our main hypothesis, lower early childhood SES index (age 1-2) was significantly associated with larger IL-6 stress responses (ΔR2 = .05, ΔF [1, 79] =4.73, p = .03) (Table 3; Figures 2 and 3). This association was significant after adjusting for age, sex, race, and BMI. We did not find a significant association between IL-6 stress responses and childhood SES index at ages 3-4 or 5-6, suggesting that childhood SES at age 1-2 may have a unique relationship with IL-6 stress responses in later life. To ensure that differences in the relationship between childhood SES index and IL-6 response did not vary as a function of the changing sample size, the regression models for childhood SES index at age 3-4 and 5-6 were rerun with only the 85 participants included in the regression model for childhood SES index at age 1-2. The pattern of results remained the same.
Table 3.
Regressions predicting IL-6 responses with childhood SES at age 1-2, 3-4, and 5-6
| Model 1 (N=85)
|
||||
|---|---|---|---|---|
| B | SE | β | p | |
| Step 1 | ||||
| Age | .01 | .01 | .05 | .63 |
| Sex | −.08 | .07 | −.14 | .23 |
| Race | .10 | .10 | .10 | .36 |
| BMI | .01 | .01 | .14 | .22 |
| Step 2 | ||||
| Childhood SES index (age 1-2) | −.04 | .02 | −.24 | .03 |
|
| ||||
| Model 2 (N=87)
|
||||
| Step 1 | ||||
| Age | .01 | .01 | .12 | .29 |
| Sex | −.09 | .07 | −.16 | .18 |
| Race | .04 | .12 | .04 | .71 |
| BMI | .02 | .01 | .20 | .08 |
| Step 2 | ||||
| Childhood SES index (age 3-4) | −.02 | .02 | −.13 | .25 |
|
| ||||
| Model 3 (N=94)
|
||||
| Step 1 | ||||
| Age | .01 | .01 | .03 | .78 |
| Sex | −.09 | .07 | −.15 | .17 |
| Race | .09 | .11 | .09 | .39 |
| BMI | .01 | .01 | .11 | .31 |
| Step 2 | ||||
| Childhood SES index (age 5-6) | .01 | .02 | .04 | .74 |
Note: IL-6 = interleukin-6; BMI = body mass index; SES = socioeconomic status. Sex was coded as 0 = Female, 1 = Male. Race was coded as 0 = White, 1 = nonwhite.
Figure 2.
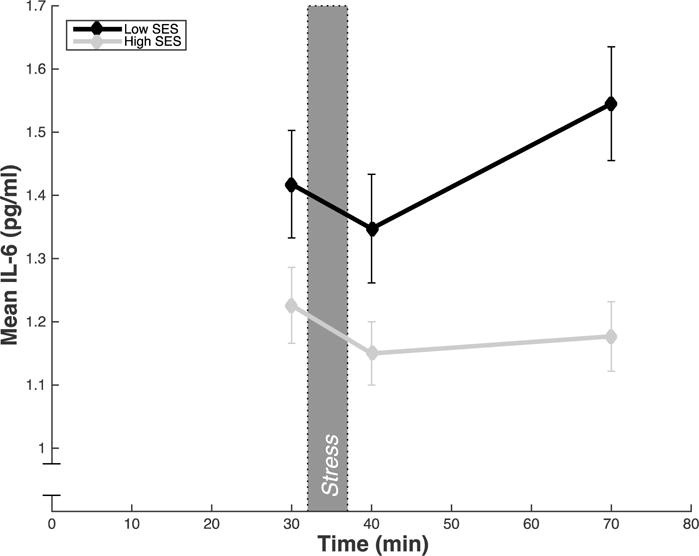
Mean circulating serum IL-6 split by low and high childhood SES at age 1-2; childhood SES index was dichotomized into high (+1 SD) and low (−1 SD) for illustrative purposes. Blood samples were taken after a baseline period (30 min), immediately after the stressor (40 min), and after the 30 min post-task recovery period (70 min). Error bars represent standard error of the mean of IL-6.
Figure 3.
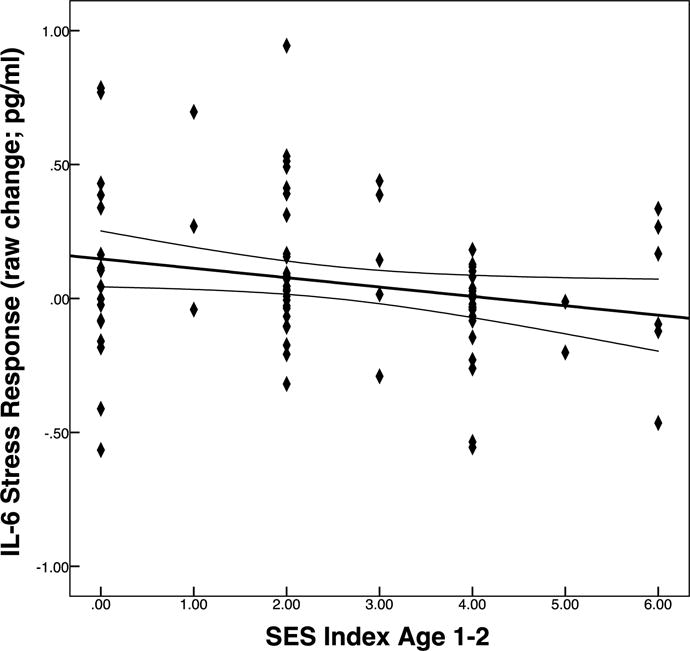
Association of childhood SES index at age 1-2 with change in IL-6 from baseline to 30 min post-task with 95% mean confidence interval.
Adult SES did not account for the association between childhood SES index at age 1-2 and IL-6 stress responses. In models that adjusted for the three adult SES measures, there was still an independent association between childhood SES index at age 1-2 and IL-6 response (ΔR2 = .07, ΔF[1, 75] = 4.83, p = .03) (Table 4). The second step of the model with the adult SES variables did not account for significant variance in IL-6 response. Thus, childhood SES at age 1-2 was significantly associated with IL-6 stress responses above and beyond the effects of age, sex, race, BMI, and indices of adult SES.
Table 4.
Regressions predicting IL-6 responses with childhood SES index at age 1-2 adjusting for adult SES and task-evoked affective responses.
| N = 83
|
||||
|---|---|---|---|---|
| B | SE | β | p | |
| Step 1 | ||||
| Age | .01 | .01 | .03 | .83 |
| Sex | −.08 | .07 | −.10 | .23 |
| Race | .15 | .11 | .16 | .17 |
| BMI | .01 | .01 | .14 | .27 |
| Step 2 | ||||
| Adult SES index | .01 | .05 | −.04 | .78 |
| Adult education | .04 | .03 | .17 | .23 |
| Adjusted family income | −.01 | .01 | −.08 | .58 |
| Step 3 | ||||
| Childhood SES index (age 1-2) | −.04 | .02 | −.26 | .03 |
|
| ||||
| N = 85
|
||||
| Step 1 | ||||
| Age | .01 | .01 | .05 | .63 |
| Sex | −.08 | .07 | −.14 | .23 |
| Race | .09 | .10 | .10 | .36 |
| BMI | .01 | .01 | .14 | .22 |
| Step 2 | ||||
| Task-evoked anger change | .04 | .01 | .29 | .007 |
| Step 3 | ||||
| Childhood SES index (age 1-2) | −.03 | .02 | −.22 | .04 |
|
| ||||
| N = 85
|
||||
| Step 1 | ||||
| Age | .01 | .01 | .05 | .63 |
| Sex | −.08 | .07 | −.14 | .23 |
| Race | .09 | .10 | .10 | .36 |
| BMI | .01 | .01 | .14 | .22 |
| Step 2 | ||||
| Task-evoked anxiety change | .01 | .01 | .16 | .092 |
| Step 3 | ||||
| Childhood SES index (age 1-2) | −.04 | .02 | −.26 | .02 |
Note: IL-6 = interleukin-6; BMI = body mass index; SES = socioeconomic status. Sex was coded as 0 = Female, 1 = Male. Race was coded as 0 = White, 1 = nonwhite.
3.3.1 Affective Changes
The association between childhood SES index at age 1-2 and IL-6 stress response was also independent of task-evoked negative affective responses. Specifically, childhood SES index at age 1-2 and IL-6 responses remained significantly associated after adjusting for both task-evoked anger (ΔR2 = .04, ΔF [1, 78] = 4.18, p = .04) and anxiety (ΔR2 = .06, ΔF[1, 78] = 5.57, p = .02) (Table 4). Consistent with our previous findings (Carroll et al., 2011b), there was an association between task-evoked anger and IL-6 stress response (ΔR2 = .09, ΔF [7, 78] = 8.38, p = .005). However, there was no significant association of task-evoked anxiety with IL-6 stress response (ΔR2 = .03, ΔF [1, 78] = 2.08, p = .15). As a final exploratory test, task-evoked anger and anxiety change were entered into the model simultaneously; the association between childhood SES index at age 1-2 and IL-6 stress response remained significant (ΔR2 = .04, ΔF [1, 77] = 4.05, p = .04).
4.0 Discussion
In the current study, we show for the first time that socioeconomic exposures in the earliest years of life relate to IL-6 responses to acute stress in adulthood. Specifically, individuals who report lower early childhood SES show larger stress-related increases in circulating IL-6 than those who recall more privileged early circumstances. This association was independent of several potential covariates, including demographic factors, BMI, and adult SES.
These findings add to a growing literature examining adverse factors that are associated with inflammatory stress responses. Prior work has shown that larger IL-6 responses are associated with state anger and anxiety (Carroll et al., 2011b), depressive symptoms (Pace et al., 2006), and childhood trauma (Carpenter et al., 2010). Indeed, in the current sample, we have previously shown a positive association between negative affective responses to the task and magnitude of IL-6 responses (Carroll et al., 2011b). The present analysis adds to our prior work, showing that early childhood SES associates with IL-6 stress responses independently of concomitant affective responses. Depressive symptoms in the present sample were not related to SES in childhood or adulthood, nor were they related to IL-6 stress responses. Although this null association is inconsistent with prior studies, it is likely due to the low prevalence of depressive symptomatology in the present sample. Childhood trauma was not assessed in the original data collection with this sample, so we were unable to test whether trauma was associated with IL-6 stress response. Although childhood SES and trauma are distinct constructs, our results are consistent with the broader hypothesis that early life stress contributes to inflammatory stress responses in adulthood. In sum, the present work makes a novel contribution to the existing literature and provides a foundation for future work examining potential behavioral and biological pathways that link early life SES with acute inflammatory responses to stress in adulthood.
4.1 Behavioral and Biological Pathways
One behavioral factor that may contribute to observed associations of early life SES with adult inflammatory stress responses is heightened vigilance for threat. Others have shown that individuals who report lower childhood SES perceive greater threat on exposure to ambiguous situations (Chen et al., 2004) and show heightened central and cardiovascular stress responses when exposed to threatening stimuli (Chen et al., 2004; Gianaros et al., 2008). We did not specifically measure perceived threat in the current study, but task-evoked anxiety changes were not significantly associated with our measures of early life SES. Thus, to the extent that our anxiety measures represent threat response to the stressor task, we did not find evidence for threat perception as a mediator. Notably, the anxiety measure used here differs from threat perception measures, which typically ask participants to rate how threatened they feel. Future work should more directly assess whether individual differences in behavioral or neural threat responses mediate the relationship between early life SES and IL-6 stress responses.
There are several biological pathways through which early childhood SES may exert an enduring effect on acute inflammatory stress responses. The HPA axis is an important modulator of the inflammatory response, with glucocorticoids acting to down-regulate the production of inflammatory mediators by immune cells (McKay and Cidlowski, 1999; Sapolsky et al., 2000). Dysregulation of the HPA axis has been linked with early life stress. For instance, rodent models show that exposure to adversity during a critical period in early life results in an adult phenotype that shows hyperactive HPA responses to stress (Fish et al., 2004; Plotsky and Meaney, 1993). In human studies, recent work indicates that exposure to stressful environments before 2 years of age is associated with HPA hyperactivity (McLaughlin et al., 2015). However, other findings are mixed, with early life adversity associating with both hyperactivity and blunted activity of the HPA axis (Fries et al., 2008; Gunnar et al., 2009; MacMillan et al., 2009). These inconsistencies may be due to the range of environmental circumstances (e.g., abuse, neglect, or poverty) or variability in the timing of exposures across studies. Notably, glucocorticoids do not always down-regulate production of inflammatory mediators. Glucocorticoid sensitivity varies considerably across individuals and early life adversity may decrease the sensitivity of immune cells to the anti-inflammatory effects of glucocorticoids (Miller et al., 2011). Low early life SES has been associated with decreased glucocorticoid sensitivity, possibly contributing to increased inflammatory response to acute stress (Miller et al., 2009). In sum, animal models support an early sensitive period during which exposure to adversity imparts enduring effects on HPA responses to stress. Although evidence for this pathway is less consistent in humans, early life socioeconomic disadvantage may influence HPA activity and the sensitivity of immune cells to the anti-inflammatory effects of glucocorticoids, ultimately contributing to increased inflammatory responses to stress.
The sympathetic nervous system (SNS) may also play a role in the modulation of inflammatory responses to stress. Rodent models show that experimental administration of epinephrine produces a dose-dependent increase in IL-6; moreover, beta-adrenergic antagonists inhibit this effect (DeRijk et al., 1994). In humans, studies show a similar positive relationship between catecholamine and inflammatory responses to acute mental stress (Kop et al., 2008; Papanicolaou et al., 1996). This relationship was eliminated with the administration of glucocorticoids (Kop et al., 2008). Together, these findings suggest that activation of the SNS and the HPA axis are likely synergistically involved in modulating inflammatory responses to acute stressors. Further research examining how these two pathways control the magnitude of inflammatory responses to stress is warranted.
It is important to note that the source of stress-related increases in circulating IL-6 remains unclear. Although immune cells are the main source discussed in the present study, muscle cells and adipose tissue also produce IL-6. Indeed, adipose tissue is a major source of circulating IL-6, with adipocytes producing 10-35% of circulating levels (Mohamed-Ali et al., 1997). Higher concentrations of IL-6 are observed among obese individuals and in vivo animal studies show larger IL-6 responses to β-agonist infusion among obese animals (Mohamed-Ali et al., 2001). Moreover, greater BMI and body fat are associated with larger IL-6 responses to repeated acute stress (McInnis et al., 2014). However, in the current study, task-related increases in IL-6 were independent of BMI, suggesting that adipocytes did not significantly contribute to observed increases in circulating levels of IL-6.
4.2 Implications
Regardless of the source of acute increases in circulating IL-6, the implications of inflammatory stress responses for health should be considered. It is proposed that immune responses to acute stress are adaptive and prepare the organism to handle acute immune challenges, such as infection or injury. While such responses may be of survival benefit in response to acute threats, there may be a long-term health cost for individuals who are predisposed to mount larger responses (Danese et al., 2008; Miller et al., 2011). One possibility is that larger inflammatory responses to acute stress contribute to higher basal levels of circulating inflammatory mediators. Indeed, our recent work indicates that larger IL-6 responses to acute stress are associated with elevated CRP among men (Lockwood et al., 2016). As such, larger IL-6 stress responses may lead to greater inflammatory disease risk via heightened basal levels of systemic inflammation. In support of this hypothesis, larger acute inflammatory stress responses relate to greater carotid artery stiffness, a preclinical marker of cardiovascular health (Ellins et al., 2008). Furthermore, prospective evidence links greater acute IL-6 stress responses to higher ambulatory blood pressure 3 years later (Brydon and Steptoe, 2005). Thus, to the extent that larger stress-induced inflammatory responses reflect a proinflammatory phenotype, they may identify individuals at increased risk for inflammatory disease in later life.
It is also worth noting that the SES index created for the present study differs from more commonly used SES indices, such as education or income, and may more accurately capture wealth than other measures. Indeed, the difference between this aggregate wealth measure and the more standard measures of SES is reflected in the moderate correlation between the adult SES index and adult family income, as well as the nonsignificant correlation between adult SES index and adult education. The current findings raise the possibility that family wealth or material assets have unique implications for a child’s stress response system development and future health. Future work should examine this question by assessing whether IL-6 stress responses in adulthood are best predicted by parental wealth, education, or income.
4.3 Limitations and Strengths
The present study has several limitations. First, the strength of the association reported here is modest and should be interpreted as such. In addition, the early life SES measures used here are retrospective in nature. The shortcomings of the childhood SES index are well discussed in previously published work (Carroll et al., 2011a) and include concerns regarding recall bias. We hoped to limit this bias by using childhood measures that are easily recalled (parental home and vehicle ownership, number of bedrooms in the home). However, a number of participants were excluded from analysis due to inability to recall this information at ages less than 6 years (see Table 1). This raises the possibility that participants’ impressions of early life SES reflect subjective assessments of their family’s socioeconomic status in early childhood. As such, our findings are in line with a growing body of research suggesting that subjective measures of early life SES are predictive of physiological outcomes (Derry et al., 2013; Gianaros et al., 2008; John-Henderson et al., 2016). In addition, our cross-sectional design prevents causal conclusions about the relationship between early life SES and IL-6 stress responses. Future prospective work linking early life SES with IL-6 stress responses in adulthood would provide more information regarding the time course of these changes and resolve recall bias issues. Finally, we only assessed changes in IL-6 up to 30 minutes post-stressor; as other studies show that IL-6 levels continue to rise up to 2-hours after stressor exposure (Marsland et al., 2017; Steptoe et al., 2007), future work should consider whether early life SES predicts more prolonged stressor-related increases in IL-6.
It is also important to note the limitations of the present sample. Participants were selected to be generally healthy, so findings may not generalize to a broader population. Replicating these results with a larger, more representative sample would further support our findings. Additionally, it should be noted that we previously found an association between low SES in the first 2 years of life and higher basal levels of circulating IL-6 in a subsample of participants drawn from the same study (Carroll et al., 2011a). At the time of that report, only basal levels of IL-6 at Visit 1 were complete. Interestingly, this association was not retained in the current analysis, which averaged pre-task levels of circulating IL-6 across the two laboratory visits. There was also no significant association of childhood SES at age 1-2 with IL-6 when we examined pre-task levels at only Visit 2. The reason for inconsistent findings across the two study visits is unclear, but does not appear to reflect differences in statistical power, as the total sample size for each analysis is comparable. One possibility is that the prior subsample differed from the present subsample, in that the current analysis included only those participants who provided both pre- and post-task blood samples.
Despite these limitations, this study had several notable strengths. First, the year-to-year reports of socioeconomic factors throughout childhood provided more detailed information than a single childhood SES measure (such as parental education or income). This allowed us to explore the hypothesis that there is a sensitive period in early life during which environmental exposures have a lasting effect on stress physiology. In addition, for 85% of the current sample, stress-related changes in IL-6 were averaged across two occasions of measurement separated by one month. This provides a more reliable assessment of stable individual differences in inflammatory response than measures from a single session, which may be more influenced by exogenous factors specific to the day of testing.
4.4 Conclusion
In conclusion, our findings suggest that early life socioeconomic factors are associated with the magnitude of IL-6 responses to a brief laboratory stressor in adulthood, with greater socioeconomic disadvantage in the earliest years of life associating with larger IL-6 stress responses. Adult SES does not appear to affect this relationship and childhood socioeconomic exposures at later points in early childhood were not significantly associated with IL-6 responses, indicating that socioeconomic conditions in the first 0-2 years of life may be uniquely associated with later inflammatory responses to acute stress. The implications of these brief inflammatory changes are not yet known, but they may be related to inflammatory disease risk. Our findings add to a growing literature showing that early experiences may impact future psychological and physiological stress responses.
Highlights.
This study examines the link between early life SES and adult IL-6 responses.
Lower SES at age 1-2 associates with greater IL-6 response to acute stress.
This relationship is independent of adult SES and affective responses.
Results support a link between early life disadvantage and adult stress responses.
Acknowledgments
This work was supported by grant NR008237 (ALM), T32 HL07560 (KGL, NJH), and National Science Foundation Graduate Research Fellowship Program DGE-1247842 (KGL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors have no conflicts of interest to declare
References
- Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Brydon L, Steptoe A. Stress-induced increases in interluekin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow up. J Hypertens. 2005;23:1001–1007. doi: 10.1016/j.hjh.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, Marsland AL. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain Behav Immun. 2011a;25:1468–1474. doi: 10.1016/j.bbi.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, Marsland AL. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun. 2011b;25:232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Langer DA, Raphaelson YE, Matthews KA. Socioeconomic status and health in adolescents: the role of stress interpretations. Child Dev. 2004;75:1039–1052. doi: 10.1111/j.1467-8624.2004.00724.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews KA. Socioeconomic Differences in Social Information Processing and Cardiovascular Reactivity. Ann New York Acad Sci. 1999;896:419–419. doi: 10.1111/j.1749-6632.1999.tb08158.x. [DOI] [PubMed] [Google Scholar]
- Cohen S. SC Childhood Interview [WWW Document] 2010 [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66:553–8. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N. Stable individual differences in physiological response to stressors: implications for stress-elicited changes in immune related health. Brain Behav Immun. 2003;17:407–14. doi: 10.1016/s0889-1591(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J, Measelle J, Ostlund B, Ablow J. Association between early life adversity and inflammation during infancy. Dev Psychobiol. 2017;59:696–702. doi: 10.1002/dev.21538. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Critical periods, stimulus input, and emotional reactivity: a theory of infantile stimulation. Psychol Rev. 1964;71:335–351. doi: 10.1037/h0042567. [DOI] [PubMed] [Google Scholar]
- DeRijk RH, Boelen A, Tilders FJ, Berkenbosch F. Induction of plasma interleukin-6 by circulating adrenaline in the rat. Psychoneuroendocrinology. 1994;19:155–63. doi: 10.1016/0306-4530(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Derry HM, Fagundes CP, Andridge R, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Lower subjective social status exaggerates interleukin-6 responses to a laboratory stressor. Psychoneuroendocrinology. 2013;38:2676–85. doi: 10.1016/j.psyneuen.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights Into Causal Pathways for Ischemic Heart Disease: Adverse Childhood Experiences Study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Ellins E, Halcox J, Donald A, Field B, Brydon L, Deanfield J, Steptoe A. Arterial stiffness and inflammatory response to psychophysiological stress. Brain Behav Immun. 2008;22:941–948. doi: 10.1016/j.bbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic programming of stress responses through variations in maternal care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Fries AB, Shirtcliff EA, Pollack SD. Neuroendocrine dysregulation following early social deprivation in children. Dev Psychobiol. 2008;50:588–599. doi: 10.1002/dev.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Davey Smith G. Childhood Socioeconomic Circumstances and Cause-specific Mortality in Adulthood: Systematic Review and Interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Heal. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Frenn K, Wewerka SS, Van Ryzin MJ. Moderate versus severe early life stress: Associations with stress reactivity and regulation in 10-12-year-old children. Psychoneuroendocrinology. 2009;34:62–75. doi: 10.1016/j.psyneuen.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Granja M, Hoball H. Basic Facts About Low Income Children [WWW Document] 2017 URL www.nccp.org.
- John-Henderson NA, Marsland AL, Kamarck TW, Muldoon MF, Manuck SB. Childhood Socioeconomic Status and the Occurrence of Recent Negative Life Events as Predictors of Circulating and Stimulated Levels of Interleukin-6. Psychosom Med. 2016;78:91–101. doi: 10.1097/PSY.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarck TW, Debski TT, Manuck SB. Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology. 2000;37:533–42. [PubMed] [Google Scholar]
- Kamarck TW, Jennings JR, Debski TT, Glickman-Weiss E, Johnson PS, Eddy MJ, Manuck SB. Reliable measures of behaviorally-evoked cardiovascular reactivity from a PC-based test battery: Results from student and community samples. Psychophysiology. 1992;29:17–28. doi: 10.1111/j.1469-8986.1992.tb02006.x. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Weissman NJ, Zhu J, Bonsall RW, Doyle M, Stretch MR, Glaes SB, Krantz DS, Gottdiener JS, Tracy RP. Effects of acute mental stress and exercise on inflammatory markers in patients with coronary artery disease and healthy controls. Am J Cardiol. 2008;101:767–773. doi: 10.1016/j.amjcard.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science (80-) 1957;126:405–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Lockwood KG, Marsland AL, Cohen S, Gianaros PJ. Sex differences in the association between stressor-evoked interleukin-6 reactivity and C-reactive protein. Brain Behav Immun. 2016;58:173–180. doi: 10.1016/j.bbi.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan HL, Georgiades K, Duku EK, Shea A, Steiner M, Niec A, Tanaka M, Gensey S, Spree S, Vella E, Walsh CA, De Bellis MD, Van der Meulen J, Boyle MH, Schmidt LA. Cortisol response to stress in female youths exposed to childhood maltreatment: Results of the youth mood project. Biol Psychiatry. 2009;66:62–68. doi: 10.1016/j.biopsych.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity and susceptibility to infectious disease. Physiol Behav. 2002;77:711–716. doi: 10.1016/s0031-9384(02)00923-x. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Manuck SB, Fazzari TV, Stewart CJ, Rabin BS. Stability of individual differences in cellular immune responses to acute psychological stress. Psychosom Med. 1995;57:295–298. doi: 10.1097/00006842-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnis CM, Thoma MV, Gianferante D, Hanlin L, Chen X, Breines JG, Hong S, Rohleder N. Measures of adiposity predict interleukin-6 responses to repeated psychosocial stress. Brain Behav Immun. 2014;42:33–40. doi: 10.1016/j.bbi.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-κB and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA., III Causal effects of the early caregiving environment on the development of stress response systems in children. PNAS. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- Meaney M, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl J, Plotsky P. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E. The Biological Residue of Childhood Poverty. Child Dev Perspect. 2013;7:67–73. doi: 10.1111/cdep.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011;137:959–997. doi: 10.1037/a0024768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed-Ali V, Flower L, Sethi J, Hotamisligil GS, Gray R, Humphries SE, York DA, Pinkney J. b-Adrenergic regulation of IL-6 release from adipose tissue: In vivo and in vitro studies. J Clin Endocrinol Metab. 2001;86:5864–5869. doi: 10.1210/jcem.86.12.8104. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodric S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Miller GE. Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biol Psychiatry. 2016;80:23–32. doi: 10.1016/j.biopsych.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Petrides JS, Tsigos C, Bina S, KT K, Wilder RL, PW G, PA D, Chrousos GP. Exercise stimulates interleukin-6 secretion: inhibition by glucocorticoids and correlation with catecholamines. Am J Physiol. 1996;271:E601–605. doi: 10.1152/ajpendo.1996.271.3.E601. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur J Epidemiol. 2007;22:55–66. doi: 10.1007/s10654-006-9082-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Ironson G, Siegal S. Stress and health: psychological, behavioral, and biological determinants. Annu Rev Clin Psychol. 2008;1:607–628. doi: 10.1146/annurev.clinpsy.1.102803.144141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. The utility of the cube root of income. J Off Stat. 1985;1:5–19. [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, Molecular Biology, and the Childhood Roots of Health Disparities. JAMA. 2009;301:2252. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Steptoe Hamer, Chida MY. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci U S A. 2010;107:8507–12. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Seeman TE. Early adversity and adult health outcomes. Dev Psychopathol. 2011;23:939–954. doi: 10.1017/S0954579411000411. [DOI] [PubMed] [Google Scholar]
- Usala PD, Hertzog C. Measurement of affective states in adults. Evaluation of an adjective rating scale instrument. Res Aging. 1989;11:403–26. doi: 10.1177/0164027589114001. [DOI] [PubMed] [Google Scholar]


