Summary
Considerable advances have been made in recent years in understanding the generation and function of memory T cells. In this review, we consider new developments in the field and focus on how emerging differences between memory cells with respect to their trafficking, metabolism, epigenetic regulation and longevity may fail to fit into small groups of “memory subsets”, but rather that the properties of individual memory cells fall on a continuum within each of these (and other) parameters. How that influences the way we assess the efficacy of vaccination and suitability (or not) of a memory population for protective immunity is also discussed.
eTOC
Memory T cells are typically parsed into discreet subsets based on phenotypic definitions that connote distinct roles in immunity. This review argues that the conventional subset nomenclature fails to accurately encompass the distribution of functional traits within this diverse population.
What is T cell memory?
This deceptively simple question is hard to answer. At a functional level, immunological memory typically refers to an enhanced immune response upon reencounter with an antigen relative to the first encounter. This definition would encompass “classic” immune memory induced by acute infections or vaccination, but fails to include cells that have important memory traits: Are CD8 T cells responsive to antigens from persistent infections (where there may be no sustained gap between initial and subsequent antigen encounters) not memory cells? There is evidence in mouse models that prolonged maintenance of antigen-primed CD4 T cells following an infection – and sustained protective immunity against reinfection – depends on low-grade persistence of the pathogen (Belkaid et al., 2002; Nelson et al., 2013; Tubo and Jenkins, 2014; Zaph et al., 2004), and CD8+ T cells responding to persistent viruses, despite some features of functional “exhaustion”, are critical for prolonged pathogen control (Paley et al., 2012; Virgin et al., 2009) and maintain numerous properties of memory cells (Utzschneider et al., 2013). Hence it would be misleading to say that these are not functionally “memory” populations. And what about cells that acquire memory-like properties following self-antigen recognition (during normal homeostasis, rather than an autoimmune reaction) such as “virtual” and “innate” memory T cells (Jameson et al., 2015; Sprent and Surh, 2011; White et al., 2017), or the memory cells responding in situations of heterologous immunity (where the priming antigen/pathogen may be quite distinct from the antigens/pathogens that evoke a recall response) (Welsh and Selin, 2002)? Last, our brief definition of memory doesn’t define whether an “enhanced immune response” is appropriate for the host – if a recall response fails to control an infection or results in lethal immunopathology, it would still be classified as immunological memory, but would hardly serve the overall goal of the immune system in protecting the host from harm.
One could continue to refine an all-encompassing definition, but perhaps the overall message of these examples is that T cell memory is heterogeneous and not easily placed in a box – although that is often what immunologists try to do, since the ability to define functionally distinct subsets of memory cells has considerable appeal as a way to quantitatively and qualitatively characterize an immune response. If identification of functionally relevant subsets can be used to predict the likely efficacy of a recall response, this is of great interest for vaccine development or understanding how protective immunity may or may not be sustained following a natural infection or treatment. To do this, the field has long relied on cell surface phenotypic markers, intended to segregate memory cells based on their functional properties. Unfortunately, this can confound characterization of a particular memory cell population, either through not recognizing that functionally distinct groups of cells may share key phenotypic traits, or that there may be overlapping functions in populations with distinct phenotypes. As we had discussed in a previous review (Jameson and Masopust, 2009), this has led to a plethora of proposed “subsets” – a trend that has only increased as more markers are introduced (for example, through use of mass cytometry) (Newell and Cheng, 2016; Newell et al., 2012) and single cell transcriptional and epigenetic analysis becomes more routine.
Most important, assumptions about the properties of a memory cell based on rigid subsetting can be misleading: memory cell populations cover a range of properties within key functional traits – such as trafficking/localization, effector functions and durability – that do not necessarily coordinate with each other. Our developing understanding of T cell trafficking provides a good example of the dangers of conflating phenotypic characteristics with function: CD8+ T cells found in non-lymphoid tissues were found to share phenotypic features – notably, a lack of CD62L and/or CCR7 expression – with “effector memory” (TEM) cells in the blood and spleen, which led researchers to postulate that these cells were a single population at different stages of their trafficking cycle throughout the body. The realization that the vast majority of CD8+ T cells in non-lymphoid sites (and, at least in humans, perhaps a substantive fraction of memory-phenotype CD8+ T cells in secondary lymphoid sites (Sathaliyawala et al., 2013; Thome et al., 2014)) are non-recirculating tissue-resident memory cells (TRM) rather than the quite rare population of tissue-recirculating TEM has profound implications for the way in which they participate in an immune response. Trying to combine the characteristics of these populations into one “subset” becomes increasingly difficult – rather, at least with respect to the trait of recirculation in blood, TEM and central memory (Tcm) have much more in common with each other than either does with TRM.
With the advent of sophisticated methods for defining T cell populations based on detailed descriptions of their phenotypic, metabolic, transcriptional and epigenetic state (increasingly with single-cell resolution), it may be time to reassess the value of trying to shoe-horn memory T cell populations into rigid subsets with coherent functional properties. Rather, perhaps it will be more useful to consider that each memory T cell has a combination of functional properties and potentials that can distinguish it from many of its sisters. This is not to say that every individual memory T cell is its own subset – just to suggest that one phenotypic or functional feature does not automatically imply another. Instead, we propose that there are quintessential characteristics that accurately distinguish T cell populations (including naïve and memory T cell populations) but may not correspond one-to-one with simple phenotypic “subset” designations (Figure 1).
Fig. 1. Traits that distinguish naïve and major memory T cell populations.
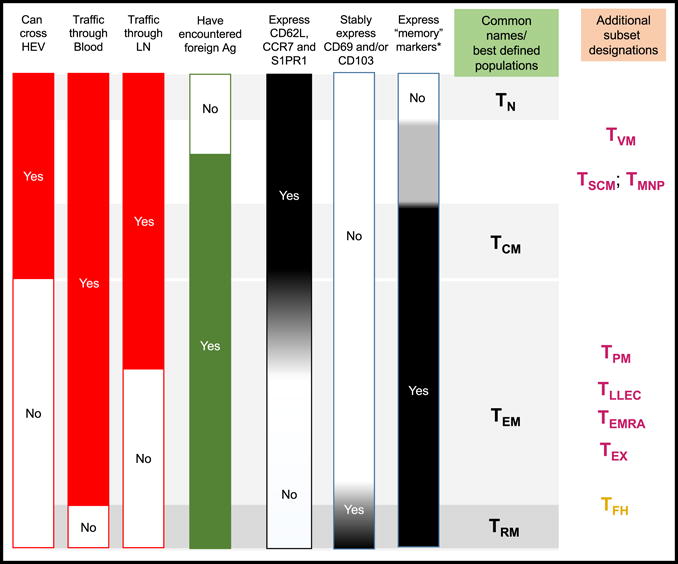
The bars on the left indicate various characteristics, which can be used to distinguish the T cell subsets listed on the right. The first 4 bars indicate trafficking capabilities and stimulation history of the cells, providing rigid distinctions that can be used to define T cell populations – but note that there is only limited concordance between these traits. The other bars indicate gene expression or phenotypic characteristics, focused on molecules associated with trafficking, tissue retention, and “memory markers” (in mice these would include elevated expression of CD44 and CD11a and reduced expression of CD45RB, while in humans this would include elevated expression of CD45RO and CD11a and reduced expression of CD45RA). Grey shading in these bars indicates where phenotypic/gene expression characteristics fail to clearly correlate with the red and green bars on the left. At the far right are T cell subsets typically associated with these combinations of traits and phenotypic/gene expression characteristics – note that the position of these identifiers is inherently vague, since the typical criteria for defining “subsets” use markers that may not faithfully correlate with the cells’ stimulation history or migration potential. Abbreviations for T cells: Naïve, TN; Central Memory, TCM; Effector Memory, TEM; Tissue-Resident Memory, TRM; Virtual Memory (also encompassing “Innate” memory), TVM; Stem Cell Memory, TSCM; Memory T cells with Naïve Phenotype, TMNP; Peripheral Memory, TPM; Long-Lived Effector Cells, TLLEC; CD45RA+ Effector Memory (defined in humans), TEMRA; Exhausted T cells, TEX; TFH Follicular Helpers). Color coding of the T cell subsets indicates whether they have been primarily described for CD8+ T cells (maroon), CD4+ T cells (gold) or both populations (black).
In the sections below, then, we consider traits of memory T cells that may unite or divide currently defined “subsets” and explore how this can help identify functional properties of memory cells that contribute to the success (or failure) of recall immune responses. These sections will address responses by recirculating versus resident memory T cells and the regulation of memory T cell effector functions and durability. We will continue to refer to the familiar memory T cell subset designations in these discussions to serve as a point of reference, though as we’ll see these designations tend to break down upon closer scrutiny. Reflecting the current state of the field, most of our discussion centers on CD8+ T cells, though we highlight similarities and differences in memory CD4+ T cells where known.
Localization and trafficking
A fundamental evolutionary benefit of immunological memory is the ability for the host to respond more quickly and effectively to reencounter with pathogens. The widespread tissue access of antibodies makes this relatively easy for humoral immunity, but T cells act locally, necessitating their localization to sites of infection in order to mediate pathogen control. There are at least three ways to achieve this surveillance (Figure 2): two of these mechanisms build on the efficient lymphocyte recirculation network, such that memory cells trafficking through lymphoid tissues (TCM) or recirculating though non-lymphoid tissues and the blood (TEM) survey the body for sites of infection. The alternative strategy is to embed memory T cells into tissues (including key barrier sites like the skin, lungs and gut) as TRM which are maintained long-term in situ without exchanging with the circulating pool. This latter mechanism sacrifices the ability to distribute memory cells throughout the body, but allows them to bias their surveillance to specific regions where they will be immediately on hand in case of a local pathogen breach. In the next two sections, we consider these alternative strategies and how they can each contribute to achieve robust recall responses. First, however, it is useful to consider how these trafficking patterns are established in T cell populations during an immune response.
Fig. 2. Trafficking characteristics is a key feature for resolving memory T cell subpopulations.
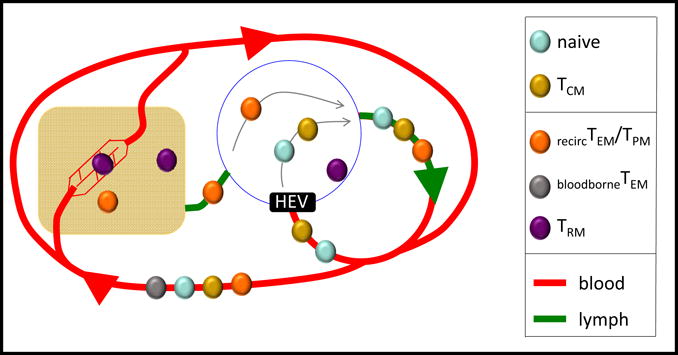
The schematic indicates the trafficking patterns of several T cell populations. Naïve, and TCM cells recirculate through blood and SLOs, and enter LNs via HEVs. Some sub-populations of TEM recirculate from blood to nonlymphoid tissues, and pass through lymphatics and LNs on the way to rejoining the blood supply (recirc TEM, also referred to as TPM), while others are confined to recirculating in blood (bloodborne TEM). TRM do not recirculate, and are parked within nonlymphoid tissues, SLOs, and local vascular compartments.
Should I stay or should I go?
Naïve T cells appear to follow a set routine of trafficking steps, in which the cells access secondary lymphoid organs (SLO) from the blood, percolate through the T cell zones of lymph nodes or the white pulp of the spleen and then reenter the circulation through lymphatic or blood vessels. The transition between each step is regulated by GPCRs recognizing chemokines or the lysosphingolipid S1P (lymph node entry also requiring integrins and, in many cases, selectins) working together in a well-coordinated ballet that results in efficient surveillance of diverse lymphoid sites for cognate antigen. Upon TCR recognition, however, an activated T cell faces a series of decisions about whether they will revert to the nomadic existence of naïve T cells, or whether they will become residents in tissues (which could be lymphoid or non-lymphoid). These decisions are unlikely to be absolute in early stages of the immune response – but altered gene expression characteristics suggest that memory cells may become effectively locked into circulatory versus resident trafficking choices (although antigen reencounter may reopen the opportunities for memory cells to revise their initial choice).
Following activation, the T cell’s first trafficking decision is whether to remain in the lymphoid tissue. This in turn depends on the nature and context of TCR stimulation. For example, CD8 T cells activated by low-affinity peptide/MHC ligands migrate into the blood earlier than cells encountering high-affinity ligands (Zehn et al., 2009), suggesting that stronger and/or longer TCR stimuli extends the time that a T cell receives programming in lymphoid sites. This should not be confused with findings that showed prolonged encounters with antigen-bearing DC are important for “full” T cell activation: truncated DC interactions appear not to compromise CD8+ T cell proliferation or effector differentiation, but do impair memory generation (Henrickson et al., 2013) – in contrast, CD8+ T cell encounter with a lower affinity ligand reduces overall T cell expansion but proportionally enhances Tcm generation (Zehn et al., 2009). Some activated T cells become “resident” in SLOs – one manifestation of this is follicular helper CD4+ T cells (Tfh), which, to be functionally relevant in promoting antigen specific B cell expansion and affinity must stay in the lymph node (though not necessarily a single GC) long-term (Crotty, 2014). In addition, SLO-resident CD8+ T cells and non-Tfh CD4+ T cells have also been reported (Schenkel et al., 2014b; Ugur et al., 2014). Many activated CD4+ and CD8+ T cells, in contrast, emigrate from the SLOs in which they were stimulated, prepared to deploy effector functions at other tissues sites. At this stage, the cells again face a decision as to whether they will adopt the non-lymphoid tissues as a long-term home or remain among the recirculating pool. CD8+ T cells with gene expression and chromatin accessibility characteristics of resident memory appear in non-lymphoid sites quite early during the immune response (Milner et al., 2017), at around the same time as the generation of recirculating memory cells, and both are thought to derive from “memory precursor” phenotype cells (Mackay et al., 2013; Sheridan et al., 2014), suggesting populations with these distinct trafficking characteristics may arise simultaneously.
The mechanisms that regulate all these patterns of migration are still being discovered, but a central element appears to be control of the egress factor S1PR1 (Schwab and Cyster, 2007). This GPCR recognizes S1P, which is found at high concentration in the blood and lymph, essentially allowing lymphocytes to drain from tissue sites. Two distinct mechanisms are known to control S1PR1 expression: first, at the level of gene expression, as revealed by the regulation of S1pr1 transcription during T cell differentiation and trafficking (Carlson et al., 2006; Skon et al., 2013); second, indirectly, through expression of CD69 which binds to the S1PR1 protein and prevents its cell surface expression (Bankovich et al., 2010; Shiow et al., 2006). Either mechanism may suffice to prevent T cells in tissues from accessing the vasculature (perhaps analogous to turning off versus covering the lights leading to an exit), and both processes may operate sequentially in some sites (Mackay et al., 2015a). Expression and reactivity of S1pr1 is important for recirculation of naïve T cells and the ability of effector cells to leave lymphoid sites following activation, while reduced S1pr1 expression appears obligatory for CD8+ T cells to become resident in most non-lymphoid sites (Mackay et al., 2016; Skon et al., 2013), and for CD4+ T cells to generate a mature Tfh population in the germinal center (Lee et al., 2015). At the same time, other receptor/ligand pairs can serve to maintain T cells in tissue sites: CD103 (αE integrin which pairs with β7 integrin to bind E-cadherin) is a well-characterized example. CD103/β7 integrin is highly expressed by many – but not all – resident memory CD8 T cells, and a smaller fraction of CD4 TRM (Iijima and Iwasaki, 2015; Schenkel and Masopust, 2014). CD103 expression is vital for maintaining TRM in some sites (the small intestine IEL and skin epidermis being best defined) (Casey et al., 2012; Mackay et al., 2013), but it is likely that other factors (such as α1 integrin, part of VLA-1) (Ray et al., 2004) are also important, at least in certain tissues. The specialized phenotype and trafficking of TRM reflects altered gene expression profiles. Pioneering work has shown that TRM differ from their recirculating counterparts in expression of critical transcription factors: Blimp1, Hobit (a relative of Blimp1), Nur77 and Runx3 promote generation of TRM, while Eomes, T-bet and KLF2 are favored in the recirculating populations (Boddupalli et al., 2016; Laidlaw et al., 2014; Mackay et al., 2016; Mackay et al., 2015b; Milner et al., 2017; Skon et al., 2013). This is an active area of investigation and the ways in which these and other transcriptional regulators impact differentiation and function of recirculating versus resident cells is not fully clear. Interestingly, other tissue resident lymphocyte populations such as innate lymphoid cells (ILC, including NK cells) share a core transcriptional profile (including expression of HOBIT and/or BLIMP) suggesting that these factors may help imprint the trait of tissue residency (Mackay et al., 2016).
Recirculating memory T cells: Leveraging trafficking flexibility for deployment
Both naïve and central memory T cells are thought to encounter antigen exclusively in lymphoid sites, following antigen acquisition and presentation by professional APC – yet there are important differences in the way these populations enter these responses. At a purely quantitative level, the numbers of central memory T cells typically exceed the numbers of naïve T cells with the same specificity, and this can clearly enhance the likelihood of specific T cell-APC encounters and the magnitude of the response. But recent studies have also revealed important qualitative differences in the way in which naïve and memory CD8+ T cells encounter APC within lymphoid sites. Work from several groups showed that activated CD4+ T cells and memory CD8+ T cells utilize the CXCR3 chemokine receptor to efficiently localize to sites of inflammation within lymph nodes, and leverage this to enhance encounter with antigen presenting cells and stimulatory cytokines (Groom et al., 2012; Hu et al., 2011; Kastenmuller et al., 2013; Sung et al., 2012). In LNs following local viral infection, CXCR3 played a key role in the process by sequentially responding to CXCL10 (induced by IFN-I produced following the infection) and then CXCL9 (induced by IFN-γ produced by the activated CD8 T cells) to further recruit and cement co-localization of memory CD8+ T cells and potential APC. Naïve CD8 T cells also appear to use a chemokine system to enhance T cell-APC interactions, but interesting recent data show that this involves recruitment of DC toward the T cell (via CCL3, CCL4 and Xcl1 induced by CD8 T cell activation) rather than the other way around (Brewitz et al., 2017). Hence, these systems may keep the activation of naïve and central memory CD8 T cells physically and chemotactically separate, and promote more rapid and effective responses by TCM, especially when antigen is delivered in an inflammatory context. Similar processes have been shown to drive localization of Th1 CD4+ T cells (Groom et al., 2012) though whether this extends to CXCR3-expressing memory CD4+ T cells is unclear, nor is it known whether other memory populations (e.g. Th2-polarized memory CD4+ T cells) employ parallel mechanisms involving distinct chemokine/chemokine receptor pairs to efficiently recruit memory cells.
The term “effector memory T cells” needs to be used cautiously, since it has become evident that several distinct populations could be assigned to this group based on the original phenotypic and functional criteria (low expression of CCR7 and/or CD62L and more rapid deployment of effector functions, in comparison with central memory cells). As discussed, TRM would classify as a TEM subset by these criteria, despite radical differences from the circulating TEM pool (Schenkel and Masopust, 2014). Even the circulating effector memory pool can be further subdivided: like Tcm, many cells in the TEM pool express CXCR3 and Eomes, but some express CX3CR1 (and not CXCR3), T-bet (but not Eomes), and retain several phenotypic features of effector cells (such as KLRG1 expression in mice) (Bottcher et al., 2015; Gerlach et al., 2016; Olson et al., 2013). CD8+ T cell populations with these “long-lived effector cell” (“TLLEC”) traits have been identified in both mice (Gerlach et al., 2016; Olson et al., 2013; Ruiz et al., 2014) and humans (Bottcher et al., 2015), and show distinct functional properties from other memory populations, including superior control of systemic bacterial and viral infections (Olson et al., 2013; Ruiz et al., 2014). CX3CR1 is not required for the generation of these cells (Gerlach et al., 2016) but clearly may impact their response to chemokine cues. This pool is especially prominent in the blood and so may be disproportionately represented in the PBMC populations typically sampled in most clinical studies. Intermediate CX3CR1 expression (on a CXCR3+ population) defines yet another subpopulation of TEM – exquisite studies on the trafficking of this pool suggests that they (and not the more numerous CXCR3+CX3CR1− or CXCR3−CX3CR1+ subsets) embody the population that has the “classic TEM” property of being able to traffic from the circulation into non-lymphoid sites and back into the circulation (Gerlach et al., 2016). As a result, this population has been called “TPM”, reflecting their ability to recirculate through peripheral tissues. The fact that these cells express CCR7 may provide an explanation for their ability to successfully egress from non-lymphoid sites, and some TPM also express CD62L, hence this subset overlaps with both TCM and TEM populations (Gerlach et al., 2016). The functional significance of peripheral-recirculating memory T cells is somewhat lessened by growing evidence that resident rather than recirculating memory T cells are dominant numerically, and more pivotal in mediating protective immunity at many barrier tissues (Mueller and Mackay, 2016; Schenkel and Masopust, 2014). On the other hand, very recent reports suggest that the circulating TEM pool may be vital for sustaining the numbers of TRM-like CD8+ T cells in the lung following influenza infection, and preservation of protective heterosubtypic immunity (Slutter et al.), although this was not observed in all studies (Takamura et al., 2016). Hence cells in the circulating TEM pool may well play unique and vital roles in sustaining immune defense. Indeed, while in mouse studies involving single rounds of immunization/infection the circulating TEM pool fades over time (long term memory being embodied in TCM and TRM), repeated boosting leads to a far more sustained representation of TEM in the circulation (Fraser et al., 2013; Masopust et al., 2006). Furthermore, in adult humans the CD8+ TCM pool is rare, and the majority of non-naïve phenotype CD4+ and CD8+ T cells in the blood and lymphoid tissues are TEM or the “terminal, senescent” CD45RA+ TEM pool (TEMRA) (Sathaliyawala et al., 2013; Thome et al., 2014): since most of the people reading this review resist re-infections quite well, it would be reasonable to assume that these TEM–like recirculating populations are playing an important role.
Finally, very recent studies proposed that CD8 T cells that have acquired expression of CXCR5 (enabling them to enter B cell follicles) may be important in control of infections such as HIV (which is thought to physically evade CXCR5− CD8 T cells, in part, by virtue of infected CD4 T cells being sequestered in germinal centers) (He et al., 2016). Intriguingly, CXCR5 expression was also one of the markers identified on a subpopulation of “exhausted” CD8 T cells (TEX: cells with degraded functional properties produced during responses to chronic infections and tumor) that respond optimally to checkpoint blockade (He et al., 2016; Im et al., 2016; Utzschneider et al., 2016). Whether the trafficking characteristics of these cells contributes to them being easier to reinvigorate by PD-1/PD-L1 blockade is not yet clear.
Resident memory T cells: Immigrants that mediate border security
Because T cells are tactile, and must scan the surface of host cells for target recognition, co-localization with infected cells is essential. Identification of memory T cells constitutively positioned within nonlymphoid tissues suggested a mechanism by which protective immunity could be accelerated against secondary infections at barrier sites (Schenkel and Masopust, 2014). Unlike TCM, which typically wait for infection-derived antigens to arrive in a draining LN before anamnestic recognition of an infection event, nonlymphoid memory T cells are anatomically poised for more immediate detection. And because nonlymphoid memory T cells were shown to constitutively maintain enhanced effector-like properties, it further suggested that this population could make rapid contributions to infection control (Masopust et al., 2001; Reinhardt et al., 2001). In support of this concept, numerous reports have demonstrated that CD8+ and CD4+ TRM accelerate protection from reinfection at barrier sites, hastening clearance of viral, bacterial, and parasitic infections in mucosal tissues, skin, and even solid organs (Gebhardt et al., 2009; Glennie et al., 2015; Iijima and Iwasaki, 2014; Jiang et al., 2012; Schenkel et al., 2014a; Schenkel et al., 2013; Shin and Iwasaki, 2012; Stary et al., 2015). TRM may also play important roles in tumor immunosurveillance (Malik et al., 2017; Milner et al., 2017) and the abundance of CD103+ tumor infiltrating lymphocytes (putatively TRM) correlates with favorable prognosis in bladder, ovarian, breast and lung cancers (Djenidi et al., 2015; Wang et al., 2015; Wang et al., 2016; Webb et al., 2014).
The number of antigen-experienced memory T cells that can be successfully isolated from nonlymphoid organs pales in comparison to secondary lymphoid organs such as the lymph nodes or spleen. In light of the protection data, this raised questions of how TRM could physically scan the enormous nonlymphoid real estate in order to rapidly detect rare infected host cells, especially given that the motility rate of TRM in some locations, such as epidermis, is quite slow (Gebhardt et al., 2011) (Ariotti et al., 2012). However, recent reports suggest that isolation of TRM via mechanical and enzymatic approaches may grossly underrepresent their true numbers (Preza et al., 2015; Steinert et al., 2015). Visualization and enumeration of TRM using quantitative immunofluorescence microscopy indicated that TRM may outnumber their counterparts in lymphoid organs. Unfortunately, cell isolation of TRM from some compartments captured as few as 2% of the total cells, and did not faithfully reflect the proportions of unique subsets. Some research indicates that TRM may undergo anoikis or DAMP-driven cell death upon removal (Fernandez-Ruiz et al., 2016), which could explain the poor isolation efficiency and perhaps will inspire strategies to overcome this vexing technical hurdle.
While TRM are much more abundant than initially perceived, they also amplify local immune responses in a process that has been likened to a sensing and alarm function (Schenkel et al., 2013). When CD8 T cells positioned within the female reproductive tract or skin recognize cognate antigen, they broadcast information of a perceived reinfection event to local members of the innate immune system, including dendritic cells, NK cells, fibroblasts, epithelium, and endothelium (Ariotti et al., 2014; Schenkel et al., 2014a; Schenkel et al., 2013). These events can trigger an interferon response and transient induction of an antiviral state that protects host cells from infection. Moreover, TRM activation can induce a local chemokine storm and expression of VCAM-1 on vascular endothelium that achieves local recruitment of recirculating B and T cells. Similar sensing and alarm functions have been demonstrated for CD4+ TRM, resulting in accelerated control of Leishmania parasites (Glennie et al., 2017; Glennie et al., 2015). Nonlymphoid CD8+ TRM have also been shown to undergo in situ antigen-driven proliferation after local rechallenges that, at least in the models tested, contributed substantially more than recirculating populations (including TCM) to the amplification of local effector responses and long-lived boosted TRM (in press – references will be provided by proof stage). These data indicate that TRM are capable of autonomously and locally mediating their own expansion and maintenance without relying on reactivation of TCM within lymphoid organs. Self-regulating resident T cell responses may have relevance for iterative local reinfections, site-specific pathogen recrudescence, and in T cell driven autoimmune and allergic responses. While much of the emphasis and discovery has focused on the role of TRM in protective immunity against infection, resident T cell populations may also be major contributors to disease. Fixed drug eruption, in which an allergic response recurs at the same site each time a drug is taken, has suggested that TRM participate in allergy (Park and Kupper, 2015). Indeed, resident CD4 T cell populations are drivers of disease in a mouse model of allergic airway disease akin to asthma (Hondowicz et al., 2016). TRM are also clearly involved in autoimmune diseases, particularly psoriasis and alopecia areata, and can drive contact hypersensitivity in the skin (Cheuk et al., 2017; Gaide et al., 2015; Park and Kupper, 2015). However, this may be the tip of the iceberg, and a role for Trm could be envisioned for a variety of allergic and autoimmune diseases. Given TRM effector functions, a role should also be considered in transplant rejection, including by passenger graft-associated donor lymphocytes that may help trigger allogeneic responses. Of course, consideration of a role for TRM in disease may present novel therapeutic opportunities and provides rationale for uncovering the mechanisms of maintenance and the elucidation of strategies for site-specific elimination.
Metabolic regulation of memory T cell differentiation and function
The significance of metabolism in the immune system has become increasingly clear in recent years. While it is easy to understand why the transition from quiescent naïve T cells to proliferating effector T cells comes with a series of altered metabolic demands, it was less clear that the differentiation of memory cells from the activated T cell pool requires a carefully orchestrated transition to reverse the cell’s focus on vigorous expansion and to equip nascent memory cells for both sustained maintenance and the enhanced metabolic capability needed to mount a rapid recall response.
Effector differentiation entails dramatic reprogramming of metabolic functions, whereby the demands for increased biosynthesis and energy utilization are met most prominently by induction of aerobic glycolysis (oxidative phosphorylation – characteristic of the catabolic state of naïve cells – is also maintained but plays a proportionately lesser role). To come out of this rapidly expanding state and enter the memory pool, the cells need to reprogram metabolism again (Pearce et al., 2013). This was first demonstrated by pioneering studies showing that inhibition of the mTOR pathway and enhancement of fatty acid oxidation (FAO) induced an increase in the differentiation of memory CD8+ T cells (Araki et al., 2009; Pearce et al., 2009; Rao et al., 2010). But, importantly, the metabolic state of memory cells is not the same as that of naïve cells – changes in mitochondrial mass and FAO capabilities (among other alterations) result in memory cells that are much more capable of undergoing metabolic reprogramming again. For example, compared to naïve cells, memory CD8+ T cells show increased mitochondrial mass and spare respiratory capacity (van der Windt et al., 2012; van der Windt et al., 2013), which can be thought of as the potential of the cells to respond quickly to increased metabolic demands, and memory cells selectively express the glycerol transporter Aquaporin-9 which plays a role in fatty acid storage (Cui et al., 2015). Failure to achieve this state correlates with impaired central memory maintenance and/or reactivity (Cui et al., 2015; van der Windt et al., 2012). Production of metabolically “fit” memory cells appears to be promoted by mitochondrial remodeling (including networks of fused mitochondria)(Buck et al., 2016), and recent findings show that CD28 costimulation drives that process (Klein Geltink et al., 2017). There is much more to learn about how metabolic pathways regulate T cell survival, differentiation, and function – for example the requirement for FAO in CD8+ Tcm is satisfied in a way that is difficult to understand: Tcm require import of glucose and glycerol to synthesize and then metabolize triacylglycerides in what appears to be a futile metabolic cycle (i.e. one that expends as much energy as it produces) (O’Sullivan et al., 2014). Presumably the temporal gap between synthesis/storage (in times of plenty) and lipolysis (in times of need) provides survival continuity for TCM, but why this is preferred to direct uptake of free fatty acids (FFA) is unclear. CD4+ memory T cells also have a requirement for glucose uptake for their sustained maintenance, in a pathway regulated by signaling through Notch (Maekawa et al., 2015), though it is not yet clear how similar the metabolic requirements are for maintenance of CD4 and CD8 Tcm. The transcriptional activation of HIF-1a by hypoxia (or other cues) is associated with promoting effector at the expense of memory differentiation (Doedens et al., 2013; Palazon et al., 2014), but may also mediate epigenetic regulation (Tyrakis et al., 2016) – likewise, regulation of aerobic glycolysis can impact transcription and epigenetic control of gene expression (Chang et al., 2013; Peng et al., 2016).
Interestingly, recent data suggest there are alternative metabolic strategies available to memory T cell populations. Studies in which CD8+ T cells were prevented from reverting from aerobic glycolysis to oxidative phosphorylation during the immune response (through sustained activation of HIF) showed an unexpectedly robust generation of memory cells in lymphoid sites – especially TEM phenotype cells – suggesting that reliance on glycolysis is sufficient to meet the metabolic needs for at least some recirculating memory CD8+ T cells (Phan et al., 2016). TRM appear to employ yet another mechanism to solve their metabolic dilemmas. As mentioned above, Tcm make their own fatty acids in order to drive FAO: skin TRM, in contrast, evidently use the more direct pathway of free fatty acid uptake (involving the fatty acid binding proteins Fabp4 and Fabp5) to satisfy their FAO needs – as indicated by the finding that Fabp4/Fabp5 deficiency selectively affected skin TRM but not recirculating memory CD8+ T cell generation (Pan et al., 2017). Whether this metabolic pathway is also used by TRM in other tissue sites will be important to determine. These findings illustrate the point that there are evidently multiple ways to solve the metabolic requirements for sustained maintenance of memory cells – whether these different solutions will correlate conveniently with current subset designations is unclear.
Epigenetic control of T cell memory differentiation and function
The field has long sought to identify differences in gene expression that distinguish activated T cell sub-populations and predict their fate, as well as the key transcription factors (TFs) that drive those transcriptional profiles. This has met with considerable success, for example, showing that small groups of TFs drive differentiation toward short-lived effector (T-bet, Blimp1, Id2 and Zeb2), versus long-lived memory fates (Eomes, Foxo1 Bcl6, Id3 and Tcf1), sometimes through mutual antagonism of each other’s expression or activity (Chang et al., 2014; Hamilton and Jameson, 2015; Kaech and Cui, 2012; Palazon et al., 2014). But there are important intrinsic limitations to this type of analysis due to epigenetic regulation mechanisms. Analysis of TF expression may be uninformative or misleading if we don’t also define the accessibility of the genetic loci at which these factors can act. Recent work exemplifies the power of pairing transcriptomic and epigenetic analyses in the context of memory CD8+ T cells. For example, Goldrath and colleagues found that expression levels of the T-bet TF are similar in effector and memory CD8+ T cells, yet these populations differ in expression of T-bet regulated genes (Yu et al., 2017). Likewise, Runx3 is expressed to similar levels in resident and recirculating memory CD8+ T cells, yet it’s expression is required for generation of TRM (Milner et al., 2017). This may partly relate to the action of partner TFs (e.g. Zeb2 in the case of T-bet), but also evidently reflects the level of chromatin accessibility for specific T-bet target genes in these cell populations.
On a more general level, transcriptional analysis tells us only about the current status of gene expression, but it is equally (and often more) important to determine which genes can rapidly be induced in these populations, for example following TCR stimulation – understanding whether certain gene loci are poised for expression or are inaccessible could be revealing as to the derivation of these cells as well as their functional potential. Studies measuring chromatin accessibility and DNA methylation have revealed relationships that are difficult if not impossible to determine by phenotypic or transcriptomic analysis. So, while mouse and human memory CD8+ T cells have many gene expression characteristics in common with naïve T cells, recent studies reveal that the epigenetic landscape in memory CD8+ T cells shares many features with effector cells (Akondy et al., 2017; Philip et al., 2017; Youngblood et al., 2017). These studies suggest that differentiation of memory and effector T cells go through similar steps of epigenetic remodeling which is relevant for addressing models of when effector- and memory-destined precursors diverge (discussed later), but also highlight the risks of relying on phenotypic and transcriptional profiles to categorize cells. This latter point is perhaps most strikingly demonstrated in studies on human memory CD8+ T cells, analyzed years after administration of the Yellow Fever vaccine (YFV): these memory cells could be easily identified using peptide/MHC tetramers, yet the standard phenotypic markers used by most investigators (CD45RA+, CD45RO−, HLA-DRlo) would lump this population in with naïve CD8+ T cells, and RNA-seq analysis was only slightly more useful for resolving memory from naïve cells (Akondy et al., 2017). Similarly, human “memory T cells with a naïve phenotype” (TMNP) were observed, especially in aged individuals (Pulko et al., 2016). On the other hand, chromatin accessibility analysis unambiguously demonstrated numerous differences between naïve and memory CD8+ T cells, and some of these were shown to predict the functional potential of the pools (e.g. accessibility of the IFN-γ locus in memory but not naïve CD8+ T cells reflecting the ability of the former to rapidly produce this cytokine upon activation) (Akondy et al., 2017; Pulko et al., 2016). This is not to say that all hope is lost in using cell surface phenotype to distinguish memory cells from effector or naïve T cells: careful review of the scarce gene expression differences between naïve and long-lived memory CD8+ T cells led to identification of a few novel markers that may help distinguish these populations in the future (Akondy et al., 2017). These markers include several features of “memory stem-cells” (TSCM), another antigen-primed population displaying numerous phenotypic features of naïve CD8+ T cells (yet distinguishable from TN by a characteristic CD95hi, CD31lo phenotype) (De Rosa et al., 2001; Gattinoni et al., 2012; Gattinoni et al., 2011). However, while TSCM were proposed to differentiate directly from naïve cells without passing through an effector phase (Restifo and Gattinoni, 2013), this appears not to be the case for the long-term memory cells produced by YFV vaccination. Together, such findings raise substantial concerns about interpretation of previous studies on the characteristics of naïve and memory CD8+ T cells – have long-term memory cells been a contaminant in assays on the function of human “naïve” CD8+ T cells? Have we missed key characteristics of long-lived human memory CD8+ T cells because these were phenotypically excluded along with the naïve pool in previous studies? Before mouse immunologists feel too comfortable about their studies, it’s important to mention that TSCM CD8+ T cells were first described in mice (Zhang et al., 2005). Further work will be needed to flesh out these issues, as well as the broader topic of how epigenetic characteristics distinguish distinct memory subpopulations (TEM, TCM, TRM, TSCM etc.).
Epigenetic “memory” (for lack of a better word), is also reflected in studies on dysfunctional tumor-specific CD8+ T cells: Schietinger’s group found evidence for phases of dysfunction, in which tumor-responsive T cells could initially be “rescued” (e.g. by cytokine induced proliferation) and regain functionality, while prolonged exposure to the tumor environment led to an irreversible tolerant state (Schietinger et al., 2012; Schietinger et al., 2016). Chromatin accessibility analysis showed dramatic remodeling of the epigenetic landscape, suggestive of losses of binding sites that could be occupied by key TFs (Philip et al., 2017). Like the studies on functional memory cells, these findings helped leverage identification of a few novel phenotypic markers that could be valuable for identifying reversibly- versus irreversibly-tolerant tumor specific CD8 T cells. It is unclear whether this and other approaches for “reinvigorating” dysfunctional CD8+ T cells results in establishment of a true memory population (Philip et al., 2017). Elegant work from Wherry and colleagues showed that PD-1/PD-L1 checkpoint blockade had minimal sustained impact on the transcriptional and epigenetic characteristics of exhausted CD8 T cells arising during chronic LCMV infection: although these reinvigorated cells showed re-induction of effector programs that improved viral control, these cells reverted to showing a transcriptional and epigenetic profile distinct from typical effector or memory cells (Pauken et al., 2016). With recognition of distinct subpopulations of exhausted CD8+ T cells which evidently differ in sensitivity to checkpoint blockade, it is will be interesting to revisit this issue. Whether improved therapeutic approaches will be able to drive exhausted/tolerant T cells into an effector- or memory-like epigenetic state may be the most accurate way of estimating whether their salvation is complete.
Unequal opportunities: Asymmetric division and memory T cell generation
The pathways that determine whether an activated T cell will become a long-lived memory cell or die during the effector phase have been intensively studied, and yet the stage during an immune response when this “fate decision” is made is still very controversial. Some unlikely possibilities – such as the idea that short-lived effector and long-lived memory cells derive from different naïve T cell clones – have been experimentally disproven by single cell adoptive transfer or barcoding approaches (Gerlach et al., 2013; Stemberger et al., 2007; Tubo et al., 2013), and the field has largely settled on two models: In one, the fate decision is made very early in the immune response, accompanying the first asymmetric cell division which is proposed to leave one daughter committed to the memory fate, the other to become a short-lived effector cell (Chang et al., 2014). The opposing model is that activated T cells go through many days of proliferation as a somewhat homogenous population before the decision is made (by or for the T cell) about whether it will die as an effector cell or persist as a memory cell. These two models have been proven remarkably resilient, with groups reporting compelling evidence that supports one or the other: accordingly, there is no conclusive resolution of which model better reflects reality (or whether the true biology includes elements of both mechanisms). We will briefly describe recent advances in this area.
Some data supporting an early decision model come from studies exploring the single-cell gene expression characteristics of CD8 T cells that have just gone through the first division, compared with cells that had matured into effector or memory subpopulations (Arsenio et al., 2014; Kakaradov et al., 2017). Interestingly, differences in gene expression were detected among cells that had undergone a single cell division, and these transcriptional characteristics had hallmarks that distinguish effector and memory cells. Using computational models to analyze these (and other intermediate time points), the authors concluded that cells with “pre-effector” or “pre-memory” gene expression traits were present at the first cell division, and that cells sorted from the first division based on some differentially expressed molecules (CD25 and CD62L) showed predicted differences in generation of central memory cells after adoptive transfer. Interestingly, some studies on the consequences of CD8+ T cell asymmetric division suggest that “pre-effector” daughters of the first cell division are not doomed to a short life: instead these cells fairly efficiently gave rise to cells that are retained to memory phase but bear characteristics of “long-lived effector cells” (a population that shares features of TEM and short-lived effector cells), while “pre-memory” cells, on the other hand, appeared better suited to produce TCM and “classic” TEM (Arsenio et al., 2014; Metz et al., 2015). Hence, the fate of the daughters arising from the initial asymmetric division may be more relevant to the types of memory sub-populations that are generated late in the response, rather than dictating a digital decision about whether a daughter cell persists to memory phase or not.
Asymmetric cell division entails an unequal distribution of cellular proteins and structures, which may influence both gene expression (e.g. through control of T-bet protein abundance by asymmetric inheritance of the proteasome (Chang et al., 2011)) but also other functions including metabolism. Recent reports suggest that key elements in metabolic regulation are differentially represented in daughter T cells after the first cell division (Pollizzi et al., 2016; Verbist et al., 2016). These include protein expression levels of key amino acid transporters and of c-Myc, and sustained mTORC1 activity, all of which are thought to be increased in daughter cells that are destined to become effector cells. Interestingly, these changes can potentially be self-sustaining – for example increased amino acid uptake helping sustain mTORC1 activity, promoting c-Myc translation and sustained expression of the amino acid transporters – which is important in determining how the outcome of asymmetric division could potentially be passed on during further cell divisions.
On the other hand, the idea that cells destined to join the memory pool are marked very early in the immune response is not supported by some other findings. The discovery that memory CD8+ T cells go through steps that are characteristic of effector cells, such as transcriptional expression of Granzyme B, was used to suggest a common initial differentiation program for both effector and memory cells (Bannard et al., 2009). Very recent work reexamined such questions at the epigenetic level, showing that DNA methylation patterns in memory precursors and effector cells was very similar, including inhibitory methylation patterns at loci that are then re-expressed in mature memory cells (including, for example, Sell the gene encoding CD62L) (Youngblood et al., 2017). Such findings indicate that memory-precursors initially undergo “effector-like” epigenetic remodeling, some of which is reversed as they complete maturation into long-lived memory cells (Youngblood et al., 2017).
Hence, while we await a resolution to the question of whether asymmetric cell division plays a dominant role in driving subsequent memory T cell differentiation, the field greatly benefits from the knowledge coming out of increasingly sophisticated approaches used to explore this issue.
Memory maintenance
Durability is an important characteristic of T cell memory. CD8+ Tcm are often proposed to be examples of an ideal population, since studies on primary immune responses in mice indicate these cells persist in the circulation estimated half-lives that well exceed the lifespan of a mouse – long after other populations (such as short-lived effector cells, long-lived effector cells and TEM) have died and/or converted into TCM (Kaech and Wherry, 2007) (Gerlach et al., 2016). Yet, some data appear to contradict this model. Boosting an immune response (still a relatively rare thing in experimental immunology, but perhaps more likely to happen in real life when pathogens are reencountered) yields memory populations that include progressively lower percentages of TCM, yet these secondary and tertiary memory cells are long-lived and potent at pathogen control (Masopust et al., 2006; Vezys et al., 2009) (Jabbari and Harty, 2006; Olson et al., 2013). At least in part, this enhanced survival and functionality reflects changes in gene expression and metabolism of the boosted TEM pool (Fraser et al., 2013) – yet another example of where lumping all “TEM phenotype” cells together may be misleading since cells produced in a primary versus, say, a quaternary response may “look” similar but have quite different properties, including long-term maintenance.
The modes of CD4 and CD8 memory maintenance appear to differ in important ways – but this may, again, reflect the focus on specific T cell subsets in reaching these conclusions. Considerable evidence suggests that maintenance of CD8+ T cell memory does not rely on antigen encounter (or perhaps even TCR expression) but is supported by the cytokines IL-7 (which also promotes naïve T cell survival) and IL-15 (Surh and Sprent, 2008). Following acute infection, memory CD8+ T cells in the circulation persist in the absence of TCR ligands but progressively decline in the absence of IL-15. But this doesn’t seem to be a universal mechanism of memory CD8+ T cell maintenance: CD8+ TRM pools may or may not rely on IL-15 depending on the tissues in which they are found (Mackay et al., 2015b; Schenkel et al., 2016; Schenkel et al., 2014b; Verbist et al., 2011) and with repeated boosting, “TEM” CD8+ T cells in the circulation gain independence from IL-15, even as they acquire some phenotypic characteristics of long-lived effector cells (which are especially IL-15 dependent in primary responses) (Schenkel et al., 2016). The typical model for IL-15 function in memory CD8+ T cell maintenance is that it sustains the basal proliferation of the population, and that this balances an underlying death rate (various), while IL-7 may be more important to sustain survival. Intriguingly, however, recent work found that, in a few TRM populations, dependence on IL-15 didn’t appear to correlate with turnover as a mechanism of memory persistence, and that some TRM populations were maintained long-term in the absence of prominent proliferation at all (Schenkel et al., 2016).
With regard to antigen dependence, there is no doubt that CD8+ memory can persist in the absence of foreign antigen, but is this always the case? Antigen drives functionally competent “inflationary memory” in the response to persistent MCMV infection (Karrer et al., 2003; Snyder et al., 2008), and T cells responding to chronic LCMV infection become “addicted” to antigen, yet those “exhausted” cells are competent to provide viral control when tested (Utzschneider et al., 2013) implying that situations in which memory CD8+ T cell maintenance depends on sustained antigen encounter may still promote functionality. Something similar may occur for CD4+ T cell memory. Striking data from Oldstone’s group many years ago revealed that, following acute LCMV infection, antigen specific memory CD4+ T cells steadily declined in numbers while their CD8+ counterparts showed impressive durability (Homann et al., 2001). Initial studies suggested CD4+ memory maintenance was IL-15 independent – and while that is true for some memory-phenotype CD4+ T cells, antigen primed circulating memory CD4+ T cells seem to rely on IL-15 for their maintenance (Purton et al., 2007). Localized antigen persistence, however, seems to promote maintenance of CD4+ memory T cells, at least those with Th1 properties. Studies on Leishmania major infection in the skin show that a strong Th1 response may ensue and prevent secondary infections at distal sites, while the primary site of infection is never fully cleared of the pathogen. The basis for this effect (called concomitant immunity) is still somewhat unclear, but it was found that pathogen persistence at the primary site was necessary for robust maintenance of protective CD4+ “memory” (Belkaid et al., 2002; Zaph et al., 2004). Similarly, Salmonella infections that persist in the intestines correlate with prolonged Th1-polarized antigen-specific CD4+ T cell memory, while attenuated, non-persistent Salmonella might prime a similar response but not induce long-term persistent memory (Nelson et al., 2013). Whether this is a general rule and how those findings translate to humans is harder to know: Studies on the human response to a single immunization with the smallpox vaccine showed slightly longer durability of antigen specific memory CD4+ T cells than CD8+ T cells (Hammarlund et al., 2003), questioning the concept that acute infections favor longer-term CD8+ memory. Both CD4+ and CD8+ measles-specific memory T cells are also relatively abundant ~20 years after recovery from childhood infection (Nanan et al., 2000). Antigen cross-reactivity is hard to rule out as a potential mechanism for memory CD4+ T cell maintenance, especially in humans where uncharacterized sequential infections may occur regularly – indeed, Davis’ group recently proposed that many human CD4+ T cells specific for novel foreign antigens are already of memory-phenotype, due to prior encounter with a cross-reactive antigen (Su et al., 2013) (although generation of memory-like cells by homeostatic mechanisms is hard to rule out). This may not be accurately modelled in typical mouse studies, where microbial exposure is tightly controlled – potentially such populations will be apparent in mice with a more physiological immunological experience (so-called “dirty mice”) (Beura et al., 2016; Reese et al., 2016).
In the CD4+ field, long-term preservation of memory populations appears to differ based on effector differentiation. So, while numerous studies find relatively sustained Th1 memory, Th17 differentiated cells appear to decline quite rapidly (Pepper and Jenkins, 2011; Pepper et al., 2010). This may not always reflect impaired survival of Th17 cells per se, since there is mounting evidence that Th17 cells can convert into populations with different phenotypic and/or cytokine production characteristics (Gagliani et al., 2015; Hirota et al., 2013).
Conclusion
There is considerable debate about the ontogeny and inter-relatedness of memory T cell populations. Part of this lack of resolution may arise from a lack of precise definitions of memory cells and their functional or phenotypic characteristics (a problem that is further exacerbated by the loaded yet vague term “effector T cell”). Perhaps a focus on distinguishing T cell populations with more strictly defined characteristics, including those indicated in Figure 1, can provide an improved framework for identifying key phenotypic or gene expression patterns that unambiguously associate with those traits. This will not be easy – knowing whether a cell can cross an HEV, for example, requires direct functional assessment of this property – but will not be impossible. And the rewards may be well worth taking the associated effort.
Acknowledgments
We thank Drs. Lalit Beura and Henrique Borges da Silva for valuable comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Citations
- Akondy RS, Fitch M, Edupuganti S, Yang S, Kissick HT, Li KW, Youngblood BA, Abdelsamed HA, McGuire DJ, Cohen KW, et al. Origin and differentiation of human memory CD8 T cells after vaccination. Nature. 2017 doi: 10.1038/nature24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Maree AF, Zal T, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song JY, Jacobs H, Haanen JB, Schumacher TN. T cell memory. Skin-resident memory CD8(+) T cells trigger a state of tissue-wide pathogen alert. Science. 2014;346:101–105. doi: 10.1126/science.1254803. [DOI] [PubMed] [Google Scholar]
- Arsenio J, Kakaradov B, Metz PJ, Kim SH, Yeo GW, Chang JT. Early specification of CD8+ T lymphocyte fates during adaptive immunity revealed by single-cell gene-expression analyses. Nat Immunol. 2014;15:365–372. doi: 10.1038/ni.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. The Journal of biological chemistry. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannard O, Kraman M, Fearon DT. Secondary replicative function of CD8+ T cells that had developed an effector phenotype. Science. 2009;323:505–509. doi: 10.1126/science.1166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, Thompson EA, Fraser KA, Rosato PC, Filali-Mouhim A, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddupalli CS, Nair S, Gray SM, Nowyhed HN, Verma R, Gibson JA, Abraham C, Narayan D, Vasquez J, Hedrick CC, et al. ABC transporters and NR4A1 identify a quiescent subset of tissue-resident memory T cells. J Clin Invest. 2016;126:3905–3916. doi: 10.1172/JCI85329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottcher JP, Beyer M, Meissner F, Abdullah Z, Sander J, Hochst B, Eickhoff S, Rieckmann JC, Russo C, Bauer T, et al. Functional classification of memory CD8(+) T cells by CX3CR1 expression. Nature communications. 2015;6:8306. doi: 10.1038/ncomms9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewitz A, Eickhoff S, Dahling S, Quast T, Bedoui S, Kroczek RA, Kurts C, Garbi N, Barchet W, Iannacone M, et al. CD8+ T Cells Orchestrate pDC-XCR1+ Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity. 2017;46:205–219. doi: 10.1016/j.immuni.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, Qiu J, Kretz O, Braas D, van der Windt GJ, et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell. 2016;166:63–76. doi: 10.1016/j.cell.2016.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CM, Endrizzi BT, Wu J, Ding X, Weinreich MA, Walsh ER, Wani MA, Lingrel JB, Hogquist KA, Jameson SC. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature. 2006;442:299–302. doi: 10.1038/nature04882. [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. Journal of immunology. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O’Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Ciocca ML, Kinjyo I, Palanivel VR, McClurkin CE, Dejong CS, Mooney EC, Kim JS, Steinel NC, Oliaro J, et al. Asymmetric proteasome segregation as a mechanism for unequal partitioning of the transcription factor T-bet during T lymphocyte division. Immunity. 2011;34:492–504. doi: 10.1016/j.immuni.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk S, Schlums H, Gallais Serezal I, Martini E, Chiang SC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M, et al. CD49a Expression Defines Tissue-Resident CD8+ T Cells Poised for Cytotoxic Function in Human Skin. Immunity. 2017;46:287–300. doi: 10.1016/j.immuni.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, Kaech SM. IL-7-Induced Glycerol Transport and TAG Synthesis Promotes Memory CD8+ T Cell Longevity. Cell. 2015;161:750–761. doi: 10.1016/j.cell.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nature medicine. 2001;7:245–248. doi: 10.1038/84701. [DOI] [PubMed] [Google Scholar]
- Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Ruiz D, Ng WY, Holz LE, Ma JZ, Zaid A, Wong YC, Lau LS, Mollard V, Cozijnsen A, Collins N, et al. Liver-Resident Memory CD8+ T Cells Form a Front-Line Defense against Malaria Liver-Stage Infection. Immunity. 2016;45:889–902. doi: 10.1016/j.immuni.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Fraser KA, Schenkel JM, Jameson SC, Vezys V, Masopust D. Preexisting High Frequencies of Memory CD8(+) T Cells Favor Rapid Memory Differentiation and Preservation of Proliferative Potential upon Boosting. Immunity. 2013;39:171–183. doi: 10.1016/j.immuni.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T, et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221–225. doi: 10.1038/nature14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, Kupper TS. Common clonal origin of central and resident memory T cells following skin immunization. Nature medicine. 2015;21:647–653. doi: 10.1038/nm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nature reviews. Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Moseman EA, Loughhead SM, Alvarez D, Zwijnenburg AJ, Waanders L, Garg R, de la Torre JC, von Andrian UH. The Chemokine Receptor CX3CR1 Defines Three Antigen-Experienced CD8 T Cell Subsets with Distinct Roles in Immune Surveillance and Homeostasis. Immunity. 2016;45:1270–1284. doi: 10.1016/j.immuni.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C, Rohr JC, Perie L, van Rooij N, van Heijst JW, Velds A, Urbanus J, Naik SH, Jacobs H, Beltman JB, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- Glennie ND, Volk SW, Scott P. Skin-resident CD4+ T cells protect against Leishmania major by recruiting and activating inflammatory monocytes. PLoS pathogens. 2017;13:e1006349. doi: 10.1371/journal.ppat.1006349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J Exp Med. 2015;212:1405–1414. doi: 10.1084/jem.20142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity. 2012;37:1091–1103. doi: 10.1016/j.immuni.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Jameson SC. Effective effector generation of CD8+ T cells and NK cells: A need for T-bet and ZEB-too. J Exp Med. 2015;212:1990. doi: 10.1084/jem.21212insight3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nature medicine. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- Henrickson SE, Perro M, Loughhead SM, Senman B, Stutte S, Quigley M, Alexe G, Iannacone M, Flynn MP, Omid S, et al. Antigen availability determines CD8(+) T cell-dendritic cell interaction kinetics and memory fate decisions. Immunity. 2013;39:496–507. doi: 10.1016/j.immuni.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Turner JE, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 2013;14:372–379. doi: 10.1038/ni.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nature medicine. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, et al. Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity. 2016;44:155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JK, Kagari T, Clingan JM, Matloubian M. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:E118–127. doi: 10.1073/pnas.1101881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima N, Iwasaki A. Tissue instruction for migration and retention of TRM cells. Trends in immunology. 2015;36:556–564. doi: 10.1016/j.it.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Lee YJ, Hogquist KA. Innate memory T cells. Advances in immunology. 2015;126:173–213. doi: 10.1016/bs.ai.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakaradov B, Arsenio J, Widjaja CE, He Z, Aigner S, Metz PJ, Yu B, Wehrens EJ, Lopez J, Kim SH, et al. Early transcriptional and epigenetic regulation of CD8+ T cell differentiation revealed by single-cell RNA sequencing. Nat Immunol. 2017;18:422–432. doi: 10.1038/ni.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer U, Sierro S, Wagner M, Oxenius A, Hengel H, Koszinowski UH, Phillips RE, Klenerman P. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. Journal of immunology. 2003;170:2022–2029. doi: 10.4049/jimmunol.170.4.2022. [DOI] [PubMed] [Google Scholar]
- Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity. 2013;38:502–513. doi: 10.1016/j.immuni.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Geltink RI, O’Sullivan D, Corrado M, Bremser A, Buck MD, Buescher JM, Firat E, Zhu X, Niedermann G, Caputa G, et al. Mitochondrial Priming by CD28. Cell. 2017;171:385–397 e311. doi: 10.1016/j.cell.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity. 2014;41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, Jenkins MK, Rosenfeld MG, Hogquist KA, Jameson SC. The transcription factor KLF2 restrains CD4(+) T follicular helper cell differentiation. Immunity. 2015;42:252–264. doi: 10.1016/j.immuni.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Braun A, Macleod BL, Collins N, Tebartz C, Bedoui S, Carbone FR, Gebhardt T. Cutting edge: CD69 interference with sphingosine-1-phosphate receptor function regulates peripheral T cell retention. J Immunol. 2015a;194:2059–2063. doi: 10.4049/jimmunol.1402256. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science. 2016;352:459–463. doi: 10.1126/science.aad2035. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, Carbone FR. T-box Transcription Factors Combine with the Cytokines TGF-beta and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity. 2015b;43:1101–1111. doi: 10.1016/j.immuni.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Maekawa Y, Ishifune C, Tsukumo S, Hozumi K, Yagita H, Yasutomo K. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nature medicine. 2015;21:55–61. doi: 10.1038/nm.3758. [DOI] [PubMed] [Google Scholar]
- Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, Molodtsov AK, Bowers JS, Angeles CV, Paulos CM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Science immunology. 2017;2 doi: 10.1126/sciimmunol.aam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Metz PJ, Arsenio J, Kakaradov B, Kim SH, Remedios KA, Oakley K, Akimoto K, Ohno S, Yeo GW, Chang JT. Regulation of asymmetric division and CD8+ T lymphocyte fate specification by protein kinase Czeta and protein kinase Clambda/iota. J Immunol. 2015;194:2249–2259. doi: 10.4049/jimmunol.1401652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, et al. Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature. 2017;552:253–257. doi: 10.1038/nature24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. 2016;16:79–89. doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- Nanan R, Rauch A, Kampgen E, Niewiesk S, Kreth HW. A novel sensitive approach for frequency analysis of measles virus-specific memory T-lymphocytes in healthy adults with a childhood history of natural measles. J Gen Virol. 2000;81:1313–1319. doi: 10.1099/0022-1317-81-5-1313. [DOI] [PubMed] [Google Scholar]
- Nelson RW, McLachlan JB, Kurtz JR, Jenkins MK. CD4+ T cell persistence and function after infection are maintained by low-level peptide:MHC class II presentation. J Immunol. 2013;190:2828–2834. doi: 10.4049/jimmunol.1202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell EW, Cheng Y. Mass cytometry: blessed with the curse of dimensionality. Nat Immunol. 2016;17:890–895. doi: 10.1038/ni.3485. [DOI] [PubMed] [Google Scholar]
- Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ T cell phenotypes. Immunity. 2012;36:142–152. doi: 10.1016/j.immuni.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41:75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8(+) T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF transcription factors, inflammation, and immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL, Wherry EJ. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Tian T, Park CO, Lofftus SY, Mei S, Liu X, Luo C, O’Malley JT, Gehad A, Teague JE, et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature. 2017;543:252–256. doi: 10.1038/nature21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CO, Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nature medicine. 2015;21:688–697. doi: 10.1038/nm.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science. 2016;354:1160–1165. doi: 10.1126/science.aaf2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science. 2013;342:1242454. doi: 10.1126/science.1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science. 2016;354:481–484. doi: 10.1126/science.aaf6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Jenkins MK. Origins of CD4(+) effector and central memory T cells. Nat Immunol. 2011;12:467–471. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, Jenkins MK. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology. 2010;11:83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan AT, Doedens AL, Palazon A, Tyrakis PA, Cheung KP, Johnson RS, Goldrath AW. Constitutive Glycolytic Metabolism Supports CD8+ T Cell Effector Memory Differentiation during Viral Infection. Immunity. 2016;45:1024–1037. doi: 10.1016/j.immuni.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature. 2017;545:452–456. doi: 10.1038/nature22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollizzi KN, Sun IH, Patel CH, Lo YC, Oh MH, Waickman AT, Tam AJ, Blosser RL, Wen J, Delgoffe GM, Powell JD. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17:704–711. doi: 10.1038/ni.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preza GC, Yang OO, Elliott J, Anton PA, Ochoa MT. T lymphocyte density and distribution in human colorectal mucosa, and inefficiency of current cell isolation protocols. PLoS One. 2015;10:e0122723. doi: 10.1371/journal.pone.0122723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulko V, Davies JS, Martinez C, Lanteri MC, Busch MP, Diamond MS, Knox K, Bush EC, Sims PA, Sinari S, et al. Human memory T cells with a naive phenotype accumulate with aging and respond to persistent viruses. Nat Immunol. 2016;17:966–975. doi: 10.1038/ni.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J Exp Med. 2007;204:951–961. doi: 10.1084/jem.20061805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, Topham DJ. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- Reese TA, Bi K, Kambal A, Filali-Mouhim A, Beura LK, Burger MC, Pulendran B, Sekaly RP, Jameson SC, Masopust D, et al. Sequential Infection with Common Pathogens Promotes Human-like Immune Gene Expression and Altered Vaccine Response. Cell Host Microbe. 2016;19:713–719. doi: 10.1016/j.chom.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Restifo NP, Gattinoni L. Lineage relationship of effector and memory T cells. Current opinion in immunology. 2013;25:556–563. doi: 10.1016/j.coi.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz AL, Soudja SM, Deceneux C, Lauvau G, Marie JC. NK1.1+ CD8+ T cells escape TGF-beta control and contribute to early microbial pathogen response. Nature communications. 2014;5:5150. doi: 10.1038/ncomms6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, Bickham KL, Lerner H, Goldstein M, Sykes M, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014a doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Casey KA, Beura LK, Pauken KE, Vezys V, Masopust D. IL-15-Independent Maintenance of Tissue-Resident and Boosted Effector Memory CD8 T Cells. J Immunol. 2016;196:3920–3926. doi: 10.4049/jimmunol.1502337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Masopust D. Cutting edge: resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol. 2014b;192:2961–2964. doi: 10.4049/jimmunol.1400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hammerling GJ, et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity. 2016;45:389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrancois L. Oral infection drives a distinct population of intestinal resident memory CD8(+) T cells with enhanced protective function. Immunity. 2014;40:747–757. doi: 10.1016/j.immuni.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutter B, Van Braeckel-Budimir N, Abboud G, Varga SM, Salek-Ardakani S, Harty JT. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Science immunology. 2:XX. doi: 10.1126/sciimmunol.aag2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–659. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, et al. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, Masopust D. Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JH, Zhang H, Moseman EA, Alvarez D, Iannacone M, Henrickson SE, de la Torre JC, Groom JR, Luster AD, von Andrian UH. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Takamura S, Yagi H, Hakata Y, Motozono C, McMaster SR, Masumoto T, Fujisawa M, Chikaishi T, Komeda J, Itoh J, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med. 2016;213:3057–3073. doi: 10.1084/jem.20160938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Yudanin N, Ohmura Y, Kubota M, Grinshpun B, Sathaliyawala T, Kato T, Lerner H, Shen Y, Farber DL. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell. 2014;159:814–828. doi: 10.1016/j.cell.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo NJ, Jenkins MK. CD4+ T Cells: guardians of the phagosome. Clin Microbiol Rev. 2014;27:200–213. doi: 10.1128/CMR.00097-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrakis PA, Palazon A, Macias D, Lee KL, Phan AT, Velica P, You J, Chia GS, Sim J, Doedens A, et al. S-2-hydroxyglutarate regulates CD8+ T-lymphocyte fate. Nature. 2016;540:236–241. doi: 10.1038/nature20165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur M, Schulz O, Menon MB, Krueger A, Pabst O. Resident CD4+ T cells accumulate in lymphoid organs after prolonged antigen exposure. Nature communications. 2014;5:4821. doi: 10.1038/ncomms5821. [DOI] [PubMed] [Google Scholar]
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, et al. T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Utzschneider DT, Legat A, Fuertes Marraco SA, Carrie L, Luescher I, Speiser DE, Zehn D. T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol. 2013;14:603–610. doi: 10.1038/ni.2606. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, O’Sullivan D, Everts B, Huang SC, Buck MD, Curtis JD, Chang CH, Smith AM, Ai T, Faubert B, et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc Natl Acad Sci U S A. 2013;110:14336–14341. doi: 10.1073/pnas.1221740110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist KC, Field MB, Klonowski KD. Cutting edge: IL-15-independent maintenance of mucosally generated memory CD8 T cells. J Immunol. 2011;186:6667–6671. doi: 10.4049/jimmunol.1004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist KC, Guy CS, Milasta S, Liedmann S, Kaminski MM, Wang R, Green DR. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature. 2016;532:389–393. doi: 10.1038/nature17442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature. 2009;457:196–199. doi: 10.1038/nature07486. [DOI] [PubMed] [Google Scholar]
- Virgin HW, Wherry EJ, Ahmed R. Redefining chronic viral infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Wang B, Wu S, Zeng H, Liu Z, Dong W, He W, Chen X, Dong X, Zheng L, Lin T, Huang J. CD103+ Tumor Infiltrating Lymphocytes Predict a Favorable Prognosis in Urothelial Cell Carcinoma of the Bladder. J Urol. 2015;194:556–562. doi: 10.1016/j.juro.2015.02.2941. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. CD103 and Intratumoral Immune Response in Breast Cancer. Clin Cancer Res. 2016;22:6290–6297. doi: 10.1158/1078-0432.CCR-16-0732. [DOI] [PubMed] [Google Scholar]
- Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. 2014;20:434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–426. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- White JT, Cross EW, Kedl RM. Antigen-inexperienced memory CD8+ T cells: where they come from and why we need them. Nat Rev Immunol. 2017;17:391–400. doi: 10.1038/nri.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblood B, Hale JS, Kissick HT, Ahn E, Xu X, Wieland A, Araki K, West EE, Ghoneim HE, Fan Y, et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature. 2017 doi: 10.1038/nature25144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Zhang K, Milner JJ, Toma C, Chen R, Scott-Browne JP, Pereira RM, Crotty S, Chang JT, Pipkin ME, et al. Epigenetic landscapes reveal transcription factors that regulate CD8(+) T cell differentiation. Nat Immunol. 2017;18:573–582. doi: 10.1038/ni.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nature medicine. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nature medicine. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]


