Abstract
Caspase-8 is known as an executioner of apoptosis, but more recent studies have shown that it participates in the regulation of necroptosis and innate immunity. In this study, we show that caspase-8 negatively regulates RIG-I signaling such that, in its absence, stimulation of the RIG-I pathway in dendritic cells produced modestly enhanced activation of IRF3 with correspondingly greater amounts of pro-inflammatory cytokines. In addition, mice lacking DC-specific caspase-8 (dcCasp8−/− mice) develop age-dependent symptoms of autoimmune disease characterized by hyperactive DCs and T cells, spleen and liver immunopathology, and the appearance of Th1 polarized CD4+ T cells. Such mice infected with chronic lymphocytic choriomeningitis virus (LCMV), an RNA virus detected by RIG-I, mounted an enhanced LCMV-specific immune response as measured by increased proportions of antigen-specific CD4+ T cells and multi-cytokine producing CD4+ and CD8+ T cells. These results show that caspase-8 subtly modulates DC maturation, and yet this controls the spontaneous appearance of autoimmune T cells while simultaneously attenuating the acquired immune system and its potential to control a persistent viral infection.
Keywords: Autoimmunity, Apoptosis, Dendritic Cells, Viral infection
Introduction
Dendritic cells (DCs) bridge innate and adaptive immunity by acting as sentries that detect invading pathogens. In addition, they act as professional antigen-presenting cells (APCs) capable of potently activating antigen-specific T cells (1, 2). As a consequence of pathogen recognition, DCs undergo a program of maturation that includes antigen presentation by cell surface MHC molecules and enhanced expression of essential co-stimulatory molecules including CD80 and CD86 (1–3). In the steady state, immature DCs instead contribute to immune tolerance by presenting self-antigens to autoreactive lymphocytes, leading to their anergy, deletion, or conversion to iTreg cells (4, 5). DCs that become mature in the absence of infection can thus provoke an immune response to self antigens, as seen in autoimmune diseases from multiple sclerosis and type 1 diabetes (T1D) to systemic lupus erythematosus (SLE) (6). A role for DCs in human SLE pathogenesis is supported by the finding that DCs from SLE patients have been shown to upregulate CD86 expression independent of activating stimuli (7).
Immature DCs utilize endocytic pathogen recognition receptors (PRRs) to detect infectious agents that bear pathogen associated molecular patterns (PAMPs), and upon uptake and processing, present associated oligopeptides to T cells (8). For example, DCs can detect viral double-stranded RNA using RIG-I-like receptors (RLRs) such as retinoic-acid inducible gene-I (RIG-I) (9, 10). Initiation of RIG-I signaling leads to the formation of a mitochondrial signaling complex (MSC) consisting of MAVS, FADD, TRADD, TANK and RIPK1, activation of interferon regulatory factors (IRFs) 3 and 7, and production of type I interferons (IFN-I), cytokines with pleiotropic effects in immunity and disease (9, 11–15). IFN-I constitute important anti-viral cytokines and are a major stimulus for DC activation, but they are also considered a key driver of disease development in SLE (16, 17).
The cysteine–aspartic acid protease, caspase-8 (CASP8), known for its canonical role in executing death receptor-mediated apoptosis, also inhibits necroptosis, an alternative form of programmed cell death (PCD) that occurs under CASP8 deficient conditions (18, 19). In many cell types, when CASP8 is inhibited, necroptosis can be triggered by the same stimuli that initiate apoptosis, such as death receptor ligation or T cell receptor stimulation, and is mediated by the kinases RIPK1 and RIPK3 (20–30). However, Casp8−/− DCs are not more prone to dying upon treatment with the death receptor ligands TNF-α or FasL, suggesting that DCs lacking CASP8 are not sensitized towards necroptosis (31, 32).
In addition to its inhibitory effect on necroptosis, CASP8 is also a negative regulator of RIG-I signaling. CASP8 inhibits the formation of the RIG-I-dependent MSC by cleaving RIPK1, and a loss of CASP8 in non-hematopoietic cells resulted in enhanced phosphorylation of IRF3 (33). Caspase-8 was also shown to directly cleave IRF3, leading to its degradation (34). Subsequent studies showed that Casp8 ablation in DCs led to increased production of pro-inflammatory cytokines, due to a loss of inhibition of either RIPK3-mediated NLRP3 inflammasome activation, or RIPK1- and MyD88-mediated DC activation (31, 32). Mice with Casp8−/− DCs were also shown to develop an age-dependent autoimmune disease that was not prevented by deleting RIPK3, suggesting a non-necroptotic driver of this autoimmunity (32). Indeed, DCs, as well as macrophages, appear to be unique in that a loss of Casp8 in these cell types does not appear to potentiate necroptosis, unlike in other tissues (26, 28–30, 35). We chose to investigate the role of RIG-I signaling in disease development of these mice, as the overall contribution of the RIG-I pathway to the function of Casp8−/− DCs is unclear.
In this study we addressed the role of CASP8 in specific pathogen-free and disease conditions, and specifically, how the subtle modulation of one aspect of the innate immune response changes the balance between spontaneous autoimmune pathology and the efficacy of viral clearance. We show that mice with Casp8−/− DCs develop an age-dependent hyperactivation of DCs and T cells and associated immunopathology, with a predominance of IFNγ producing Th1 cells. Along with age-dependent autoimmunity, the loss of Casp8 in DCs also allowed mice to mount an enhanced response to chronic viral infection, which was characterized by less exhausted, antigen-specific T cells and lower viral loads. These immunity characteristics correlated with DCs that were more sensitive to RIG-I stimulation in vitro. Thus, by regulating just one aspect of DC activation, CASP8 acts as a rheostat to dial in a well-tempered immune system, albeit one that is less efficacious.
Materials and Methods
Mice
Mice in which exon 3 of Casp8 is flanked by loxP sites (B6.129-Casp8tm1Hed) (backcrossed to C57BL/6J, n > 10) (36) were crossed to Cd11cCre mice (B6.Cg-Tg(Itgax-cre)1-1Reiz/J) (37) to generate Casp8flox/flox Cd11cCre mice. These mice are referred to as dcCasp8−/−. SMARTA transgenic mice (TCR transgenes specific for H2-Ab bound with LCMV glycopeptide 61–80) (38) were a gift from A. Goldrath, University of California, San Diego (UCSD). Animal work was performed according to UCSD guidelines.
Viral Infections
6- to 10-week old mice were infected intravenously (i.v.) via the retroorbital sinus with 2 x 106 pfu LCMV Cl13. Virus was grown, identified, quantified, and titered by plaque assay as previously described (39). LCMV-specific ELISAs were performed as previously described (40).
Flow Cytometry
Spleens were harvested from mice and treated with Collagenase D (Roche) at 37°C for 20 min. Splenic single cell suspensions were made and incubated with the indicated fluorochrome-conjugated antibodies. For intracellular staining of FOXP3 and cytokines (IFNγ, TNF and IL-2), FoxP3 Fix/Perm kit (eBioscience) was used. For intracellular staining of phosphorylated IRF3 (P-IRF3), Cytofix Fixation Buffer and Perm Buffer III (BD) were used. LCMV-specific T cells were examined with MHC class II (H2-Ab GP66) and MHC class I (H2-Db GP33, H2-Db GP276, H2-Db NP396) tetramers obtained from the NIH Tetramer Core Facility (Emory University). All antibodies for surface and intracellular cytokine staining were purchased from eBioscience, BioLegend or BD. P-IRF3 antibody was purchased from Cell Signaling Technology.
Data were collected on an LSRFortessa (BD) and analyzed with FlowJo software (Tree Star).
Flow Cytometry Gating Strategy
For DCs: splenocytes were gated to 1) exclude T cells, B cells, NK cells, macrophages and monocytes (antibodies to CD3, CD19, CD49b, F4/80 and Ly6C, respectively); 2) exclude plasmacytoid DCs (B220+CD11clo) and include conventional DCs (cDCs) (B220-CD11chi); 3) subset CD11b+CD8− (“CD11b+”) vs. CD11b-CD8+ (“CD8+”) cDCs. For P-IRF3 expression studies, CD11b+ cDCs were further subsetted into CD86hi and CD86lo populations.
In Vitro Stimulations
106 splenocytes were isolated and stimulated for one hour with PMA (10 ng/ml) and Ionomycin (1 μM) to assess CD4 T cell skewing, or LCMV peptides GP33-41 (0.5 μM) or GP61-80 (2 μg/ml) to assess cytokine production, after which monensin (eBioscience) was added for an additional 3.5 hours, for a total stimulation of 4.5 hours.
Poly(I:C) Transfections
3–4 spleens per genotype were pooled to isolate 2.7 x 105 CD11c+ cells per well to at least 90% purity according to EasySep CD11c Positive Selection Kit with Spleen Dissociation Medium (Stemcell). For CD86 upregulation and pro-inflammatory cytokine measurements, CD11c+ cells were transfected with short-length poly(I:C)-LyoVec (Invivogen) for 20 hours at the indicated concentrations, according to the manufacturer’s instructions. Lipopolysaccharide was added at 0.1 μg/well. Cytokines were assessed in the cell supernatant by cytometric bead assay (BioLegend). For DC co-culture assay, 0.9 x 105 CD45.1+ CD45.2+ WT CD11c+ cells were co-cultured with 1.8 x 105 CD45.2+ control or Casp8−/− CD11c+ cells in the presence of transfected short-length poly(I:C)-LyoVec at the indicated concentrations for 16 hours. Total viability was assessed with the Zombie Aqua Fixable Viability Kit (Biolegend). For cell death assays, CD11c+ cells were stimulated for 16 hours with anti-Fas, Jo2 clone (0.2 mg/mL; BD Biosciences) or short-length poly(I:C)-LyoVec at the indicated concentrations, with or without pre-treatment for one hour with Necrostatin-1 (30 μM; Enzo Life Sciences), and then stained with Annexin V and propidium iodide (eBioscience), according to the manufacturer’s instructions. For P-IRF3 analysis by flow cytometry, CD11c+ cells were transfected with short-length poly(I:C)-LyoVec (5 μg/ml) for the indicated lengths of time. Poly(I:C)-LyoVec preparations and media used were endotoxin free.
Histopathology
Paraffin-embedded spleen and liver sections were stained with hematoxylin & eosin (H&E). Splenic white and red pulp areas were quantified by ImageJ software (NIH).
SMARTA Adoptive Transfer
LCMV-specific CD45.1+ CD45.2+ transgenic SMARTA CD4+ T cells were isolated by microbeads using negative selection to at least 85% purity (Miltenyi Biotec). 5,000 SMARTAs were then transferred i.v. into recipient mice 1 day prior to LCMV Cl13 infection.
BMDC Differentiation
Bone marrow was harvested from femurs and tibias, resuspended in complete media containing 20ng/ml each of GM-CSF and IL-4 (Peprotech), and placed in 6-well plates at a density of 1x106 cells/ml. Two thirds of the media was replaced on days 3, 5 and 7 with fresh media plus GM-CSF and IL-4. Non-adherent cells were harvested on day 9, and negative selection with microbeads (Miltenyi Biotec) was used to remove F4/80+, Ly6C+ and B220+ cells. BMDCs were then transfected for 0, 1, 2 or 4 hours with short-length poly(I:C)-LyoVec and total cell lysates were isolated.
Immunoblot Analysis
Equal amounts of protein from total BMDC lysates were resolved by 4–12% NU PAGE Bis-Tris gel (Invitrogen) and were transferred to Immobilon-P PVDF membrane (Millipore) by semidry transfer (Bio-Rad). Blots were incubated overnight at 4°C with primary antibodies to P-IRF3 (Cell Signaling), total IRF3 (Cell Signaling) or LaminB (Santa Cruz), then incubated for 1 hour at 25°C with the appropriate horseradish peroxidase-conjugated secondary antibody. Bands were visualized by SuperSignal West Femto Maximum Sensitivity Substrate (ThermoFisher).
Results
Mice with Casp8−/− dendritic cells develop an age-dependent autoimmune phenotype
To investigate the contribution of CASP8 in the regulation of dendritic cells, mice with a Casp8flox/flox allele were crossed with Cd11cCre mice to generate Casp8flox/floxCd11cCre conditional knockout (cKO) mice (hereafter referred to as "dcCasp8−/− mice"). Deletion of exon 3 of Casp8 in CD11c+ splenocytes from dcCasp8−/− mice was validated by PCR (Fig. 1A). These mice displayed higher proportions of hyperactive CD11b+ and CD8+ conventional DCs (cDCs) at three months of age, as assessed by the expression of CD86 (Fig. 1B), and cDCs from dcCasp8−/− mice remained hyperactive through 14-plus months of age. To determine whether the hyperactivation seen in cDCs was reflected in the population of T cells, we assessed the status of T cells by enumerating previously activated (CD44hiCD62lo) vs. naïve (CD44loCD62Lhi) T cells found in the spleen and blood. As shown, CD4+ T cells from dcCasp8−/− mice generated increased proportions of activated T cells at three months of age, while activated CD8+ T cells were not increased until six months of age (Fig. 1C). The proportion of previously activated T cells in control (Casp8flox/flox) mice increased with age; however, this was substantially accelerated in dcCasp8−/− mice for both CD4+ and CD8+ subsets.
Figure 1.
Phenotype of aged dcCasp8−/− mice. A) Deletion of exon 3 of caspase-8 was assessed in CD11c+ splenocytes from Casp8fl/fl (control), Casp8fl/fl Cd11cCre (dcCasp8−/−), and Casp8−/− Ripk3−/− mice by PCR. B) The expression of CD86 in splenic cDCs from control and dcCasp8−/− mice was determined at 3, 6, 10 and 14+ months (left), and the percentage of cDCs expressing high levels of CD86 is depicted (right). C) The expression of CD62L and CD44 in splenic and blood T cells from control and dcCasp8−/− mice was determined at 3, 6, 10 and 14+ months (left), and the percentage of T cells that were naive (CD62LhiCD44lo) or activated (CD62LloCD44hi) is depicted (right). D) Spleens and livers from 10-month old control and dcCasp8−/− mice were sectioned and stained with hematoxylin & eosin (H&E). The percentage of white pulp area per spleen section in control vs. dcCasp8−/− mice is depicted (right). E) The expression of Foxp3 in splenic CD4+ T cells from 10-month-old control and dcCasp8−/− mice was determined by percentage (left) and absolute number per spleen (right). Data for A, B, D and E are representative of three independent experiments for each time point, with n = 4. Data for C are representative of 16 mice. Error bars represent S.E.M.
We next determined whether the hyperactive cDCs and T cells in aged dcCasp8−/− mice had an effect on organ immunopathology. Spleens from aged dcCasp8−/− mice displayed a disrupted splenic architecture, as demonstrated by an expansion of white pulp (Fig. 1D). Livers from aged dcCasp8−/− mice showed an increase in polymorphic infiltrates similar to the infiltrating leukocytes seen in autoimmune mice with Fas-deficient cDCs (41) (Fig. 1D). Since aged dcCasp8−/− mice appeared to display signs of autoimmunity, we examined whether the number of FOXP3+ regulatory T (Treg) cells was diminished. On the contrary, we found increased percentages and numbers of FOXP3+ Treg cells in ten-month-old dcCasp8−/− mice (Fig. 1E).
CD4+ T cells from aged dcCasp8−/− mice skew towards a Th1 phenotype
Many autoimmune diseases are characterized by the emergence of a particular CD4+ T helper (Th) subset characterized by its hallmark cytokine. For example, IFNγ-producing Th1 cells are more prevalent in the peripheral blood of human SLE patients (42). To determine whether CD4+ T cells in aged dcCasp8−/− mice were polarized for a specific Th subset, we stimulated splenocytes in vitro with PMA and ionomycin for 4 hr and analyzed the appearance of diagnostic cytokines. The results showed an increased subset of CD4+ T cells from aged dcCasp8−/− mice that produced IFNγ, when compared to control mice, whereas the proportions of CD4+ T cells producing IL-4 and IL-17 were similar (Fig. 2).
Figure 2.

CD4+ T cells from aged dcCasp8−/− mice skew towards a Th1 phenotype. Splenocytes from 10-month-old control and dcCasp8−/− mice were tested for the expression of IFNγ, IL-4 and IL-17 by intracellular staining. The percentage of CD44+ CD4+ T cells expressing IFNγ, IL-4 or IL-17 is depicted (right). Data are representative of three independent experiments, with n = 4. Error bars represent S.E.M.
Young adult dcCasp8−/− mice mount an enhanced antigen-specific T cell response to chronic viral infection
Since specific pathogen free dcCasp8−/− mice display accelerated development of activated cDCs and T cells over time, we determined how dcCasp8−/− mice would respond to a viral infection. Taking into account our observation that dcCasp8−/− mice developed a detectable increase in activated cDCs and CD4+ T cells by 3 months of age, we infected young adult (6–10 week old) mice before the appearance of phenotypic manifestations of the Casp8 deletion. Specifically, we wished to determine whether dcCasp8−/− mice would mount an exaggerated response to viral infection as a result of enhanced cDC activation and antigen presentation to T cells.
Lymphocytic choriomeningitis virus (LCMV) is an RNA virus that is detected by RIG-I (43). Inoculation of C57BL/6 mice with 106 PFU LCMV clone 13 (LCMV Cl13), the chronic form of LCMV, results in a long and persistent viral infection (39, 44). A characteristic of the CD4+ and CD8+ T cell response is the induction of multiple negative feedback pathways starting day 12 post infection, and these mechanisms appear to prolong the viral infection while lessening the associated immunopathology (45, 46). Previous work has shown that removal of negative regulation can be readily detected (47, 48). In addition, LCMV Cl13 is able to establish a chronic infection partly because it selectively infects and suppresses DCs (49), and thus, enhanced DC function might be expected to mitigate this form of LCMV virulence.
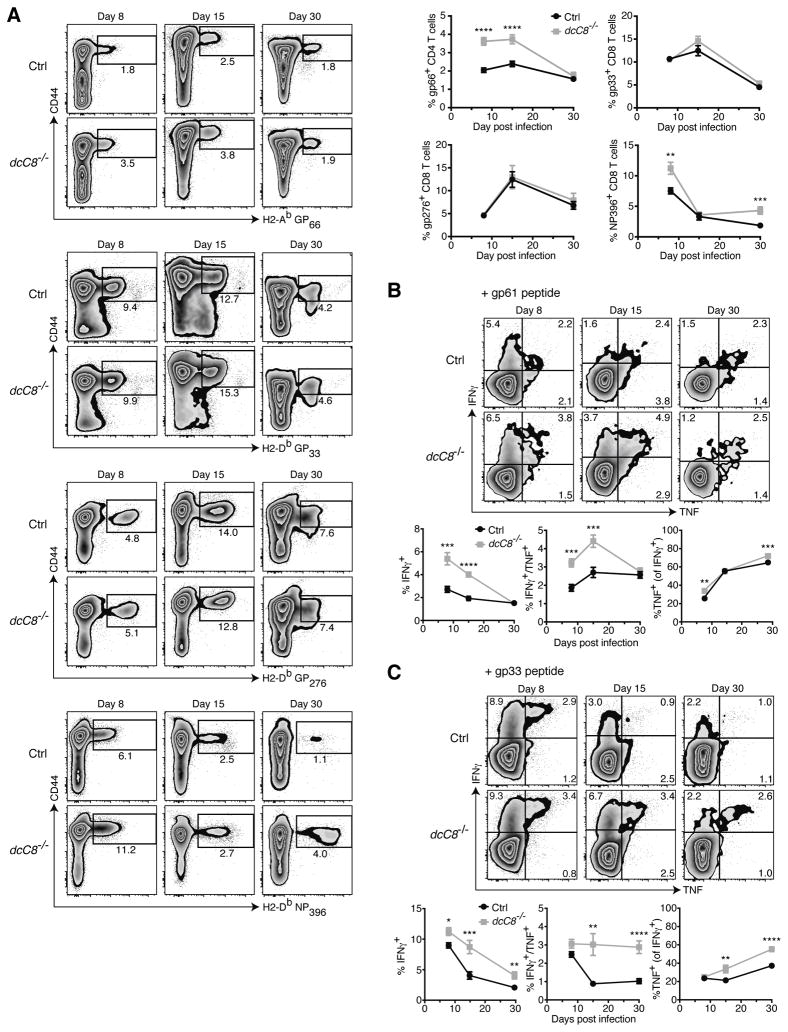
Upon infection of 6–10 week old dcCasp8−/− mice with LCMV Cl13, the expansion of antigen-specific T cell responses was examined using tetramers specific for the major immunodominant CD4+ and CD8+ T cell epitopes. We found that dcCasp8−/− mice had higher proportions of H2-Ab GP66+ CD4+ T cells at days 8 and 15 p.i. (Fig. 3A). dcCasp8−/− mice had similar proportions of H2-Db GP33+ CD8+ and H2-Db GP276+ CD8+ T cells from days 8 to 30 p.i., but interestingly, dcCasp8−/− mice had larger percentages of H2-Db NP396+ CD8+ T cells at days 8 and 30 (but not 15) p.i. (Fig. 3A). Overall, dcCasp8−/− mice possessed substantially higher frequencies of GP66-specific CD4+ T cells compared to control mice early in the infection, and this would be predicted to have an important effect on the expansion of CD8+ T cells and the clearance of virus.
Figure 3.
Young adult dcCasp8−/− mice mount an enhanced T cell response to chronic LCMV. Control and dcCasp8−/− mice were infected with LCMV Cl13. After 8, 15 and 30 days, spleen cells were harvested and analyzed. A) Representative analysis of CD44+ CD4+ T cells with H2-Ab GP66+ tetramers and CD44+ CD8+ T cells with H2-Db GP33, GP276 or NP396 tetramers (left). The accumulation of data is depicted (upper-right). B,C) Splenocytes were stimulated in vitro with either B) GP61-80 (CD4) or C) GP33-41 (CD8) peptide and the expression of IFNγ and TNF was assessed by intracellular staining (top). The percentage of CD44+ CD4+ and CD8+ T cells that produce either IFNγ alone, both IFNγ and TNF, or TNF in addition to IFNγ is depicted (bottom). Data were averaged from 3 independent experiments measuring at least twelve mice per group (A) or 10 mice per group (B). Error bars represent S.E.M.
T cells from chronically infected dcCasp8−/− mice retain effector function
T cells achieve their antiviral effector function, in part, by producing cytokines such as interferon-γ (IFNγ) and tumor necrosis factor (TNF). We assessed the ability of T cells from LCMV Cl13-infected dcCasp8−/− mice to produce IFNγ and TNF upon re-stimulation with either CD4+ or CD8+ T cell LCMV-specific peptides (GP61-80 and GP33-41, respectively). The results showed that, consistent with the increased frequency of LCMV-specific T cells, a higher proportion of CD4+ T cells from dcCasp8−/− mice were polyfunctional and produced both IFNγ and TNF at days 8 and 15 p.i. (Fig. 3B), and a higher proportion of CD8+ T cells from dcCasp8−/− mice were polyfunctional and produced both IFNγ and TNF at days 15 and 30 p.i. (Fig. 3C). Taken together, these results suggest that chronically infected dcCasp8−/− mice have an early (days 8–15 p.i.) enhancement in the CD4+ T cell response, and a late (days 15–30 p.i.) enhancement in the CD8+ T cell response.
The effect of DC-specific Casp8 deletion on T cell exhaustion and viral clearance
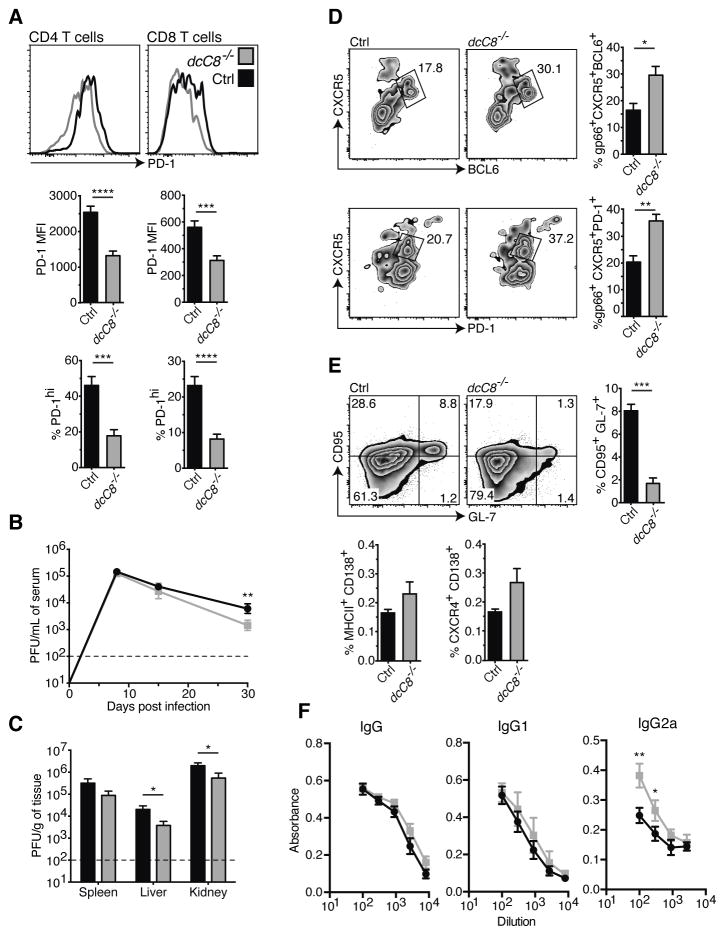
Since we had seen more effector CD4+ and CD8+ T cells from dcCasp8−/− mice compared with control mice, we investigated whether T cells from dcCasp8−/− mice expressed differential levels of the inhibitory receptor PD-1, which is upregulated during T cell exhaustion (45). Analysis showed that H2-Ab GP66+ CD4+ T cells from dcCasp8−/− mice expressed lower amounts of PD-1 by both MFI and the percentage of PD-1hi expressing cells at day 30 p.i. (Fig. 4A). Similar results were found analyzing H2-Db GP33+ CD8+ T cells from dcCasp8−/− mice.
Figure 4.
Phenotype of chronically infected young adult dcCasp8−/− mice. 6- to 10-week old control (Black) and dcCasp8−/− (Grey) mice were infected with LCMV Cl13. After 30 days, various organs and blood were harvested and analyzed. A) The expression of PD-1 in H2-Ab GP66+ CD4+ or H2-Db GP33 CD8+ splenic T cells was assessed. The PD-1 median fluorescence intensity (MFI) and percentage of cells expressing high levels of PD-1 is depicted (bottom). Virus titers were determined (Plaque assays) in B) serum, C) spleen, liver and kidney. D) The expression of CXCR5 and BCL6 or PD-1 in GP66+ CD44+ CD4+ T cells was assessed, and the percentage of CXCR5hiBCL6hi and CXCR5hiPD-1hi cells is depicted (right). E) The expression of CD95 and GL-7 in B cells was assessed, and the percentage of CD95+GL-7+ cells is depicted (right). The percentage of MHCII+CD138+ and CXCR4+CD138+ cells in the spleen is also depicted (bottom). F) ELISAs were performed to determine the amount of LCMV-specific IgG, IgG1 and IgG2a antibodies in serum. Data for A, D and E show the average of at least twelve individual mice per group taken from two independent experiments. Data for B depict eight pooled mice per group (days 8 & 15 p.i.) and twenty pooled mice per group (day 30 p.i.) from 2–3 independent experiments. Data for C depict eight pooled mice per group from two independent experiments. Data for F are representative of two independent experiments, with n = 4. Error bars represent S.E.M. (A–E) and S.D. (F).
Since dcCasp8−/− mice possessed T cells that retained the ability to produce antiviral cytokines and were less exhausted at d30 after infection, there was the possibility that virus load would be correspondingly reduced. Consistent with the time-course of T cell expansion and effector function, dcCasp8−/− mice displayed lower viral loads in serum, liver and kidney at day 30 p.i. as measured by plaque assay (Fig. 4B–C).
dcCasp8−/− mice have more antigen-specific Tfh cells and higher levels of IgG2a antibody a month after chronic infection
While WT mice clear an LCMV Cl13 infection (in lymphoid tissues) in two to three months, mice lacking CD4+ T cells do not clear the virus for over four months, and this appears to be due to the importance of CD4+ T cells both in providing help to CD8+ T cells and in promoting LCMV-specific antibody responses (50–52). Since we saw an enhanced LCMV-specific CD4+ T response in dcCasp8−/− mice, we examined whether the antibody response was likewise enhanced. Analyses showed that dcCasp8−/− mice had greater proportions of H2-Ab GP66+ CD4+ follicular helper T (Tfh) cells at day 30 p.i. (Fig. 4D). Unexpectedly, we found that dcCasp8−/− mice had substantially fewer germinal center (GC) B cells as measured by the expression of GL-7 and Fas (53) (Fig. 4E). GC B cells can differentiate into plasmablasts, which then form antibody-producing plasma cells. Yet, despite the reduction of GC B cells in dcCasp8−/− mice, these mice trended towards having higher frequencies of plasmablasts (MHCII+CD138+) and plasma cells (CXCR4+CD138+) (54). Additionally, at day 30 p.i. dcCasp8−/− mice had higher levels of LCMV-specific IgG2a antibody, which is the isotype preferentially driven by increased IFNγ (55). No differences in the amounts of LCMV-specific total IgG or IgG1 antibodies were found (Fig. 4F).
The discrepancy between increased numbers of Tfh cells, increased antibodies, but reduced GC B cells has not been resolved. Although the increased frequency of plasmablasts and plasma cells in dcCasp8−/− mice was not statistically significant, it could help explain why dcCasp8−/− mice have increased Tfh cells and increased LCMV-specific antibodies. A chronic viral infection could accelerate the disruption of splenic architecture observed in uninfected aged dcCasp8−/− mice (Fig. 1D), leading to a loss of splenic structure required for GC B cell formation. For example, although Lupus-prone MRL.Faslpr mice spontaneously develop GCs, they are lost over time and are no longer detectable in six-month-old mice, perhaps due to the collapse of splenic architecture (56).
LCMV-specific CD4 T cells from chronically infected dcCasp8−/− mice have enhanced expansion and effector function
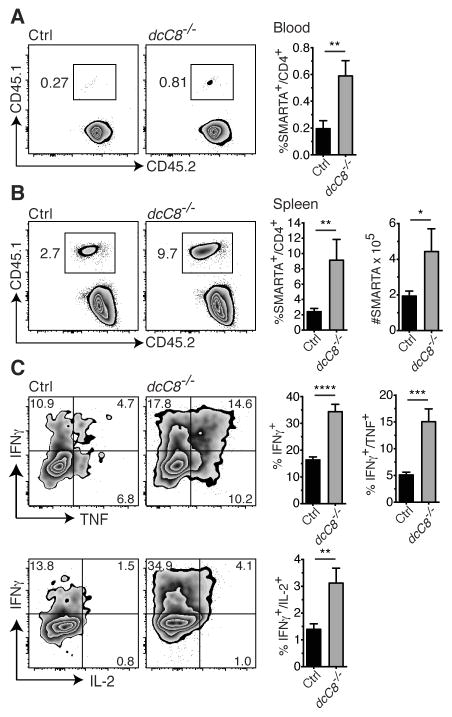
To address the possibility that pre-existing hyperactivated CD4+ T cells in dcCasp8−/− mice contribute to the larger proportion of either H2-Ab GP66+ CD4+ T cells or IFNγ/TNF producing CD4+ T cells in these mice (Fig. 3A and B), we adoptively transferred CD4+ T cells from SMARTA transgenic mice into control and dcCasp8−/− recipients followed by infection with LCMV Cl13. SMARTA mice have CD4+ T cells specific for the gp61-80 epitope of LCMV (38). We found that CD45.1+CD45.2+ SMARTA CD4+ T cells transferred into dcCasp8−/− hosts expanded to a greater degree in the blood at day 5 p.i. (Fig. 5A), and in the spleen at day 8 p.i. (Fig. 5B). When we examined the ability of SMARTA CD4+ T cells from dcCasp8−/− hosts to produce effector cytokines upon re-stimulation with the CD4+ T cell LCMV-specific peptide (GP61-80), we found that a higher proportion of SMARTA CD4+ T cells from dcCasp8−/− mice produced either IFNγ alone, both IFNγ and TNF, or both IFNγ and IL-2, a finding consistent with the enhanced expansion of these cells in dcCasp8−/− hosts (Fig. 5C). These results show that dcCasp8−/− mice promote an enhanced expansion of naïve T cells.
Figure 5.
SMARTA T cells transferred into chronically infected dcCasp8−/− mice display great effector function. LCMV-specific CD45.1+ CD45.2+ transgenic CD4+ T cells (SMARTA) were transferred i.v. 1 day prior to LCMV Cl13 infection. A) The frequency of blood CD4+ T cells that were SMARTA was assessed at day 5 p.i. B) The frequency and number of spleen CD4+ T cells that were SMARTA was assessed at day 8 p.i. C) Splenocytes isolated at day 8 p.i. were stimulated with GP61–80 (CD4) peptide and assessed for the expression of IFNγ, TNF-α and IL-2 by SMARTA CD4+ T cells by intracellular staining (left). The percentage of SMARTA CD4+ T cells that produce either IFNγ alone, IFNγ and TNF-α, or IFNγ and IL-2 is depicted (right). Data for A and B depict at least ten pooled mice per group, and data for C depict at least eight pooled mice per group taken from 2–3 independent experiments. Error bars represent S.E.M.
RIG-I stimulation hyper-activates cDCs lacking Casp8
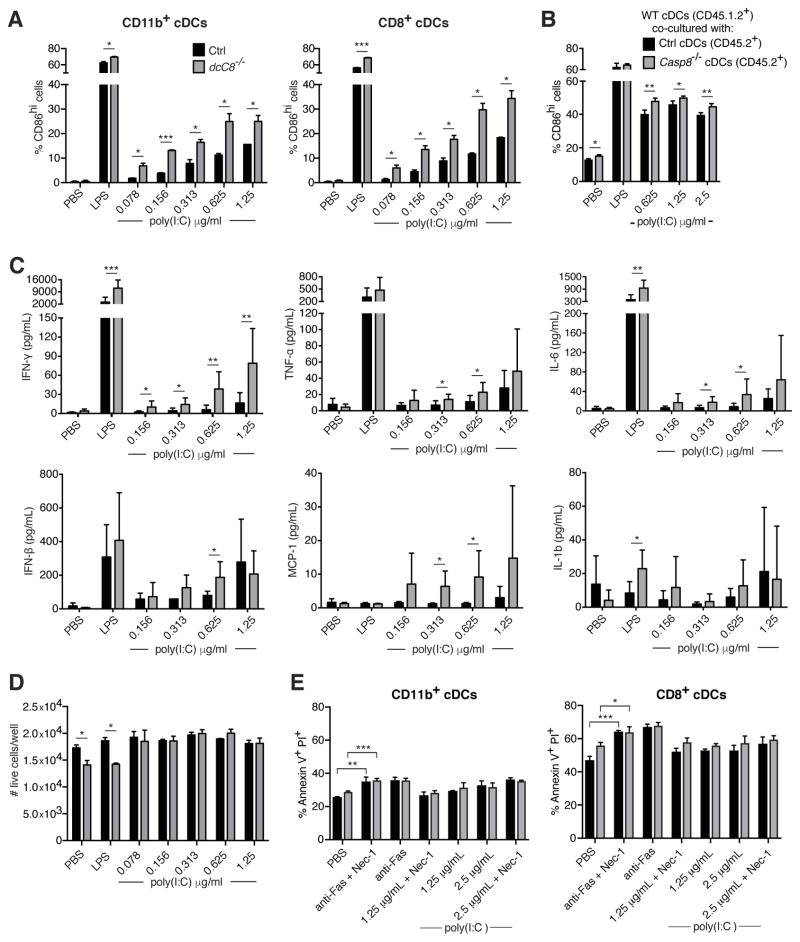
We hypothesized that, in dcCasp8−/− mice, both the age-dependent autoimmune phenotype and the enhanced response to chronic infection resulted from the loss of negative regulation of RIG-I signaling by CASP8 in cDCs. To directly test this, we activated RIG-I in Casp8−/− cDCs by transfecting them with short-length poly(I:C), which is a surrogate for dsRNA and is specifically detected by RIG-I (57). We isolated splenic CD11c+ cells from control and dcCasp8−/− mice and transfected them with increasing doses of poly(I:C), then assessed the upregulation of the co-stimulation molecule CD86. We found that both CD11b+ and CD8+ cDCs upregulated CD86 to a greater degree than control cDCs in a poly(I:C) dose-dependent manner (Fig. 6A).
Figure 6.
Caspase-8 deficient cDCs hyperactivate in response to RIG-I stimulation. A) CD11c+ spleen cells were transfected with short-length poly(I:C) for 20 hours, and the expression of CD86 was assessed in CD11b+ and CD8+ cDCs. B) CD45.1.2+ WT CD11c+ cells were co-cultured with CD45.2+ control or Casp8−/− CD11c+ cells in the presence of transfected short-length poly(I:C), and after 16 hours assessed for the expression of CD86. C) CD11c+ cells were isolated from the spleen and transfected with short-length poly(I:C) for 20 hours, and levels of pro-inflammatory cytokines were measured in the cell supernatant by cytometric bead assay. D) The number of live cDCs from A) was assessed by labeling with an amine-reactive dye. E) CD11c+ cells were isolated from the spleen and either stimulated with anti-Fas or transfected with short-length poly(I:C) for 16 hours, with or without Nec-1 pre-treatment. CD11b+ and CD8+ cDCs were then labeled with Annexin V and PI to measure cell death. Data for A, B and D are representative of three independent experiments, with three replicates per genotype. Data for C are representative of two independent experiments, with two replicates per genotype. Data for E are representative of two independent experiments, with three replicates per genotype. Error bars represent S.D.
To determine whether Casp8−/− cDCs hyperactivate due to a cell-intrinsic or cell-extrinsic mechanism, we co-cultured Casp8−/− and WT CD11c+ cells in the presence of transfected poly(I:C). The results indicated that the hyperactivation of Casp8−/− cDCs was cell-extrinsic, as CD45.1+CD45.2+ WT cDCs co-cultured with CD45.2+ Casp8−/− cDCs upregulated CD86 to a greater degree than when co-cultured with CD45.2+ control cDCs (Fig. 6B).
To directly assess whether this cell-extrinsic effect could be the result of Casp8−/− cDCs producing more IFN-I upon RIG-I stimulation, we measured levels of IFNβ in the supernatant of poly(I:C)-transfected CD11c+ cells. Surprisingly, we found that the supernatant of Casp8−/− CD11c+ cells contained significantly higher levels of IFNβ at only one concentration of transfected poly(I:C) compared to control supernatant (Fig. 6C). However, when we assessed the levels of several other pro-inflammatory cytokines, IFNγ, TNF-α, IL-6, and MCP-1 were all found to be upregulated in the supernatant of Casp8−/− CD11c+ cells in response to increasing doses of transfected poly(I:C) (Fig. 6C), consistent with the cell-extrinsic DC hyperactivation mechanism predicted by Figure 6B.
cDCs lacking Casp8 are not sensitized to necroptosis
Loss of Casp8 in a variety of cell types leads to necroptosis, an alternative mode of PCD that occurs when a cell receives death stimuli in the face of apoptosis inhibition (24, 25, 28). Immature DCs become mature upon co-culture with cells undergoing necrosis or exposure to necrotic cell supernatant, likely due to the release of danger-associated molecular patterns (DAMPs) during necrosis, but they do not mature when cultured with apoptotic cells or apoptotic cell supernatant (58).
To investigate whether necroptosis of Casp8−/− cDCs could contribute to the hyperactivation of these cells, we first used a membrane permeable dye to compare levels of total cell death in control and Casp8−/− poly(I:C)-transfected CD11c+ cells, and found that the numbers of live control or Casp8−/− cDCs per well was comparable (Fig. 6D). Next, we labeled poly(I:C)-transfected CD11c+ cells with Annexin V and propidium iodide (PI) and assessed the frequency of necroptotic cell death (Annexin V+PI+). While both control and Casp8−/− cDCs (CD11b+ or CD8+ subsets) displayed greater numbers of Annexin V+PI+ cells upon ligation of the death receptor Fas (by addition of anti-Fas antibody), neither control nor Casp8−/− cDCs had an increase in the frequency of Annexin V+PI+ cells upon poly(I:C) transfection (Fig. 6E). We infer that the increase in Annexin V+PI+ cells in anti-Fas treated cDCs was due to late apoptosis, not necroptosis, as this cell death was triggered by Fas signaling yet was not inhibited by the addition of Necrostatin-1 (Nec-1), an inhibitor of RIPK1. Thus, Casp8−/− cDCs do not appear to undergo necroptosis when death receptor signaling is triggered, consistent with a previous report (32).
BMDCs lacking Casp8 have enhanced IRF3 activation upon RIG-I stimulation
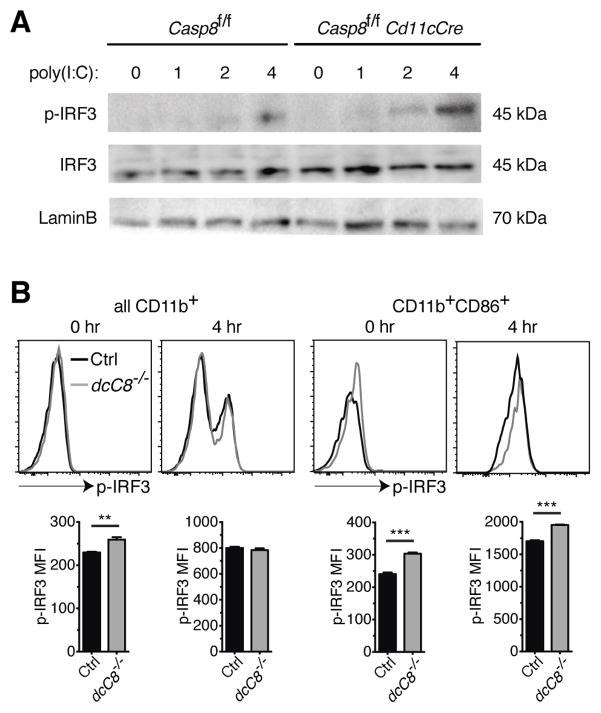
We originally predicted that the hyperactivation phenotype of Casp8−/− DCs was due to a loss of negative regulation of IRF3. Thus, we measured whether levels of phosphorylated IRF3 (P-IRF3) in poly(I:C)-transfected Casp8−/− DCs were higher. Casp8−/− BMDCs transfected with poly(I:C) showed enhanced P-IRF3 expression at 4 hr compared to control BMDCs, while levels of total IRF3 and LaminB were similar between Casp8−/− and control BMDCs at 4 hr (Fig. 7A).
Figure 7.
Enhanced IRF3 activation in RIG-I stimulated caspase-8 deficient BMDCs and splenic cDCs. A) Control and Casp8fl/fl Cd11cCre BMDCs were transfected with short-length poly(I:C) for the indicated lengths of time, then total cellular lysates were immunoblotted for P-IRF3. The membrane was then stripped and reblotted for total IRF3 and LaminB as a loading control. B) CD11c+ cells were isolated from the spleen and transfected with short-length poly(I:C). After 4 hours, the expression of P-IRF3 was assessed in CD11b+ and CD11b+CD86+ cDCs. Data for A are representative of two independent experiments. Data for B are representative of two independent experiments, with three replicates per genotype per experiment. Error bars represent S.D.
To test whether splenic Casp8−/− cDCs also had enhanced P-IRF3 expression upon RIG-I stimulation, we analyzed levels of P-IRF3 in poly(I:C)-transfected CD11c+ cells by flow cytometry. We found that while levels of P-IRF3 were not notably higher in either unstimulated (0 hr) or RIG-I stimulated (4 hr) CD11b+ Casp8−/− cDCs, P-IRF3 levels were higher in a subset of CD11b+ cDCs, those that were also CD86+, under both conditions (Fig. 7B). We infer that the CD11b+ CD86+ cDCs are the DCs that are either endogenously activated (0 hr) or become activated after RIG-I stimulation (4 hr). Thus, it appears that both Casp8−/− BMDCs and the activated subset of Casp8−/− splenic cDCs upregulate P-IRF3 to a greater degree compared to controls.
Of note, control and Casp8−/− CD8+ splenic cDCs expressed similar levels of P-IRF3, regardless of whether or not they were transfected with poly(I:C) (data not shown). One explanation is the very low expression of RIG-I in CD8+ DCs and the lack of IFNα production by this DC subset in response to infection with two RNA viruses, Sendai virus or influenza A virus (59), indicating that CD8+ DCs do not use RIG-I to detect viral RNA.
Discussion
Although DCs represent a small proportion of total immune cells, they are essential for both initiating and preventing adaptive immune responses. These seemingly paradoxical outcomes depend in large part on whether the DCs are activated. Immature, unactivated DCs help prevent autoimmunity by presenting self-antigens to self-reactive T cells that have escaped thymic deletion, and such T cells are either deleted or become anergic (60). If mature DCs present oligopeptides to T cells, T cell activation and proliferation result such that in principle, presentation of a self-peptide by a mature DC could result in an autoimmune response (6). The fact that T cells require activated, mature DCs (induced through exposure to adjuvant or by pathogen-recognition receptor signaling) constitutes the basis for "the immunologist’s dirty little secret" as presciently described by Charles Janeway, Jr. (61). A surprising aspect of the present work is that CASP8 exerts a quantitatively modest effect on the activation status and cytokine production of DCs, and yet, Casp8 deletion in DCs is sufficient to push the immune system into a state of self-reactivity as well as affect the progression of a persistent viral infection. This highlights the notion that immune regulation operates on a knife-edge balanced between self-reactivity and effective immunity. As it is presently evolved, there appears to be little margin for the further enhanced effectiveness of adaptive immunity.
The importance of DCs in mediating self-tolerance is illustrated in mouse models of constitutive DC ablation. CD11c-DTA mice, which primarily lack DCs (but also certain macrophages, NK cells, and activated T cells), display impaired CD4+ and CD8+ T cell responses, and in one study they developed steady-state autoimmunity characterized by CD4+ T cell activation (62). When DCs are constitutively deleted from lupus-prone MRL.Faslpr mice, age-dependent T cell expansion and differentiation into IFNγ producing effector cells was diminished, leading to disease reduction (63). However, when activated DCs are unable to be eliminated via Fas-mediated killing, systemic autoimmunity developed (41).
Several genes have been identified as negative regulators of DC activation; for example, mice with DC-specific ablations of A20 or SHP1 spontaneously developed mature DCs that produced more inflammatory cytokines and drove the increased activation and differentiation of T cells (6). The effect of deleting a DC negative regulator on the viral immune response in vivo is less clear. Caspase-8 is a negative regulator of RIG-I signaling, a key pathway for detection of RNA viruses in DCs (10, 33). Mice with a DC-specific ablation of Casp8 have previously been shown to develop an age-dependent autoimmunity that depends on TLR signaling (32). We show here that stimulated CD4+ T cells from aged dcCasp8−/− mice skew towards a Th1 phenotype, indicating a possible role for IFNγ in disease pathogenesis. We further explored the consequences of hyperactive DCs in the context of a chronic viral infection. These experiments revealed an adjuvant-like effect with the loss of Casp8 in DCs, as dcCasp8−/− mice have an enhanced immune response to LCMV Cl13, an RNA virus detected by RIG-I. The observation that hyperactive DCs can aid in the resolution of chronic viral infection, despite their damaging role in driving steady-state autoimmunity, is consistent with the finding that although mice with Fas-deficient DCs develop systemic autoimmunity, they also clear a chronic LCMV infection more rapidly (41, 64).
Over time, dcCasp8−/− mice develop systemic autoimmunity in the absence of external pathogen challenge. This suggests that peripheral tolerance depends upon the precise regulation of DC activation, and self-tolerance is lost with even a modest imbalance in innate signaling. dcCasp8−/− mice would be expected to display enhanced antigen presentation to naïve T cells, and this was borne out in experiments in which LCMV-specific CD4 T cells (SMARTA) were transferred into either control or dcCasp8−/− mice. This finding supports the idea that the enhanced early CD4+ T cell response in LCMV Cl13 infected dcCasp8−/− mice is likely due to the super-efficient cDCs and not pre-existing hyperactivated T cells. In contrast, the enhanced CD8+ T cell response found later (days 15 & 30 p.i.) may or may not result from Casp8 deficient DCs. Given that a greater proportion of antigen-specific CD4+ T cells in dcCasp8−/− mice are polyfunctional, one possibility is that the CD8+ T cells in infected dcCasp8−/− mice receive more “help” from CD4+ T cells (50, 52).
We predicted that Casp8 deficient cDCs are hypersensitive to RIG-I stimulation, and we found that Casp8−/− cDCs upregulated CD86 to a greater degree than control cDCs upon transfection with poly(I:C). We also predicted that a loss of Casp8 in cDCs would lead to hyperactive IRF3 and increased IFN-I production, and while the increase in IFNβ secretion by RIG-I stimulated Casp8−/− cDCs was only significant at a single concentration of transfected poly(I:C) (Fig. 6C), we found that Casp8−/− BMDCs had enhanced IRF3 activation as measured by P-IRF3 (Fig. 7A). Exposure to IFN-I not only leads to DC activation, including upregulation of co-stimulatory molecules such as CD86 and CD80, but has also been shown to increase IFNγ and TNF-α production by DCs (65–68). Thus, the enhanced activation of IRF3 in poly(I:C)-transfected Casp8−/− DCs appears to lead to slightly higher levels of IFNβ production, which could act in an autocrine fashion on DCs to promote pro-inflammatory cytokine production and T cell stimulation.
If enhanced IRF3 activation in Casp8−/− cDCs were responsible for the observed DC hyperactivation, we might expect that deleting IRF3 in dcCasp8−/− mice would ameliorate this phenotype. Perhaps surprisingly, one group found that IRF3−/− dcCasp8−/− mice instead had exacerbated lymphoproliferation compared to dcCasp8−/− mice (32). However, the IRF3−/− dcCasp8−/− mice analyzed in this study had a germline deletion of IRF3, indicating that the contributions of IRF3-deficient non-DCs to accelerating lymphoproliferation cannot be ruled out.
Unlike in many other cell types, Casp8 deficiency in DCs does not appear to potentiate necroptosis. CASP8 is needed to both prevent necroptosis of proliferating T cells during the initiation of an adaptive immune response, as well as trigger apoptosis of T cells during the contraction of the response (27, 28, 69). However, RIG-I stimulated control and Casp8−/− cDCs underwent comparable levels of AnnV+PI+ cell death regardless of whether Nec-1 was added, indicating a RIPK1-independent root cause of the phenotypes observed in dcCasp8−/− mice. Additionally, while we found that Casp8−/− cDCs secreted increased amounts of IL-1β upon LPS treatment, consistent with two previous studies (31, 32), there was no difference in IL-1β production from poly(I:C)-transfected cDCs. We conclude that our findings are consistent with studies showing that deletion of Casp8 in DCs leads to hyperactivation of downstream elements (31–34). In addition, this hyperactivation would appear to be RIPK1- and IL-1β-independent.
Finally, we found that mice with hyperactive DCs lacking Casp8 have delayed T cell exhaustion during chronic LCMV infection, indicating a possible therapeutic application for these DCs. T cell exhaustion is characterized by a loss of effector cytokine production and upregulation of inhibitory receptors such as PD-1 and CTLA-4, and it plays a major role in disease progression of many chronic infections and cancer (45, 46). Given the role of therapeutic checkpoint inhibitors that block these T cell inhibitory pathways to enhance anti-tumor immunity, we speculate that enhancing DC function, even modestly, could have a major impact on the course of an immune response.
Acknowledgments
We thank Arnaud Delpoux, Carol Katayama, Daniel Utzschneider and Brittney Wellisch for experimental support and animal management.
References
- 1.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 2.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson NS, El-Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, Villadangos JA. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187–2194. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 4.Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic Cells Induce Peripheral T Cell Unresponsiveness under Steady State Conditions in Vivo. Journal of Experimental Medicine. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct Expansion of Functional CD25 +CD4 +Regulatory T Cells by Antigen-processing Dendritic Cells. Journal of Experimental Medicine. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crispín JC, Vargas-Rojas MI, Monsiváis-Urenda A, Alcocer-Varela J. Phenotype and function of dendritic cells of patients with systemic lupus erythematosus. Clinical Immunology. 2012;143:45–50. doi: 10.1016/j.clim.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 10.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao SM, Maniatis T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, tenOever BR, Grandvaux N, Zhou GP, Lin R, Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 14.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 15.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Blanco P, Banchereau J. Induction of Dendritic Cell Differentiation by IFN-α in Systemic Lupus Erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 17.González-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nat Rev Immunol. 2012;12:125–135. doi: 10.1038/nri3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vercammen D, Beyaert R, Denecker G, Goossens V, Van Loo G, Declercq W, Grooten J, Fiers W, Vandenabeele P. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J Exp Med. 1998;187:1477–1485. doi: 10.1084/jem.187.9.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 20.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FKM. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an Energy Metabolism Regulator That Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberst A, Dillon CP, Weinlich R, Mccormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varfolomeev EE, Schuchmann M, Luria V, Chiannilkulchai N, Beckmann JS, Mett IL, Rebrikov D, Brodianski VM, Kemper OC, Kollet O, Lapidot T, Soffer D, Sobe T, Avraham KB, Goncharov T, Holtmann H, Lonai P, Wallach D. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 27.Chen I, Beisner D, Degterev A, Lynch C, Yuan J, Hoffmann A, Hedrick SM. Antigen-mediated T cell expansion regulated by parallel pathways of death. PNAS. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ch’en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. Journal of Experimental Medicine. 2011;208:633–641. doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Günther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, Geng J, Py B, Zhou W, Amin P, Lima JB, Qi C, Yu Q, Trapp B, Yuan J. Activation of Necroptosis in Multiple Sclerosis. Cell Rep. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 Blocks Kinase RIPK3-Mediated Activation of the NLRP3 Inflammasome. Immunity. 2012;38:1–14. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Cuda CM, Misharin AV, Gierut AK, Saber R, Haines GK, Hutcheson J, Hedrick SM, Mohan C, Budinger GS, Stehlik C, Perlman H. Caspase-8 Acts as a Molecular Rheostat To Limit RIPK1- and MyD88-Mediated Dendritic Cell Activation. The Journal of Immunology. 2014;192:5548–5560. doi: 10.4049/jimmunol.1400122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajput A, Kovalenko A, Bogdanov K, Yang SH, Kang TB, Kim JC, Du J, Wallach D. RIG-I RNA Helicase Activation of IRF3 Transcription Factor Is Negatively Regulated by Caspase-8-Mediated Cleavage of the RIP1 Protein. Immunity. 2011;34:340–351. doi: 10.1016/j.immuni.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Sears N, Sen GC, Stark GR, Chattopadhyay S. Caspase-8-mediated Cleavage Inhibits IRF-3 Protein by Facilitating Its Proteasome-mediated Degradation. Journal of Biological Chemistry. 2011;286:33037–33044. doi: 10.1074/jbc.M111.257022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cuda CM, Misharin AV, Khare S, Saber R, Tsai F, Archer AM, Homan PJ, Haines GK, Hutcheson J, Dorfleutner A, Budinger GRS, Stehlik C, Perlman H. Conditional deletion of caspase-8 in macrophages alters macrophage activation in a RIPK-dependent manner. Arthritis Res Ther. 2015;17:291–307. doi: 10.1186/s13075-015-0794-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beisner DR, I, Ch’en L, Kolla RV, Hoffmann A, Hedrick SM. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J Immunol. 2005;175:3469–3473. doi: 10.4049/jimmunol.175.6.3469. [DOI] [PubMed] [Google Scholar]
- 37.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harker JA, Lewis GM, Mack L, Zuniga EI. Late Interleukin-6 Escalates T Follicular Helper Cell Responses and Controls a Chronic Viral Infection. Science. 2011;334:825–829. doi: 10.1126/science.1208421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, Fink PJ, Chervonsky AV. Elimination of Antigen-Presenting Cells and Autoreactive T Cells by Fas Contributes to Prevention of Autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive Production of IFN-γ in Patients with Systemic Lupus Erythematosus and Its Contribution to Induction of B Lymphocyte Stimulator/B Cell-Activating Factor/TNF Ligand Superfamily-13B. The Journal of Immunology. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 43.Clingan JM, Ostrow K, Hosiawa KA, Chen ZJ, Matloubian M. Differential roles for RIG-I-like receptors and nucleic acid-sensing TLR pathways in controlling a chronic viral infection. The Journal of Immunology. 2012;188:4432–4440. doi: 10.4049/jimmunol.1103656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Crawford A, Angelosanto JM, Kao C, Doering TA, Odorizzi PM, Barnett BE, Wherry EJ. Molecular and Transcriptional Basis of CD4+ T Cell Dysfunction during Chronic Infection. Immunity. 2014;40:289–302. doi: 10.1016/j.immuni.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 48.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, Yang E, Johnson RS, Goldrath AW. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MBA. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. Journal of Clinical Investigation. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elsaesser H, Sauer K, Brooks DG. IL-21 is required to control chronic viral infection. Science. 2009;324:1569–1572. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fröhlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–1580. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 52.Yi JS, Du M, Zajac AJ. A Vital Role for Interleukin-21 in the Control of a Chronic Viral Infection. Science. 2009;324:1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumjohann D, Preite S, Reboldi A, Ronchi F, Ansel KM, Lanzavecchia A, Sallusto F. Persistent antigen and germinal center B cells sustain T follicular helper cell responses and phenotype. Immunity. 2013;38:596–605. doi: 10.1016/j.immuni.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015;15:160–171. doi: 10.1038/nri3795. [DOI] [PubMed] [Google Scholar]
- 55.Tishon A, Lewicki H, Rall G, Von Herrath M, Oldstone MB. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology. 1995;212:244–250. doi: 10.1006/viro.1995.1477. [DOI] [PubMed] [Google Scholar]
- 56.Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, Handwerger BS. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–584. [PubMed] [Google Scholar]
- 57.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid–inducible gene-I and melanoma differentiation–associated gene 5. Journal of Experimental Medicine. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luber CA, Cox J, Lauterbach H, Fancke B, Selbach M, Tschopp J, Akira S, Wiegand M, Hochrein H, O’Keeffe M, Mann M. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 2010;32:279–289. doi: 10.1016/j.immuni.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 61.Janeway CA. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 62.Ohnmacht C, Pullner A, King SBS, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. Journal of Experimental Medicine. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teichmann LL, Ols ML, Kashgarian M, Reizis B, Kaplan DH, Shlomchik MJ. Dendritic Cells in Lupus Are Not Required for Activation of T and B Cells but Promote Their Expansion, Resulting in Tissue Damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varanasi V, Khan AA, Chervonsky AV. Loss of the death receptor CD95 (Fas) expression by dendritic cells protects from a chronic viral infection. Proceedings of the National Academy of Sciences. 2014;111:8559–8564. doi: 10.1073/pnas.1401750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 66.Parlato S, Santini SM, Lapenta C, Di Pucchio T, Logozzi M, Spada M, Giammarioli AM, Malorni W, Fais S, Belardelli F. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–3029. doi: 10.1182/blood.v98.10.3022. [DOI] [PubMed] [Google Scholar]
- 67.Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, Tough DF. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 68.Montoya M, Edwards MJ, Reid DM, Borrow P. Rapid Activation of Spleen Dendritic Cell Subsets following Lymphocytic Choriomeningitis Virus Infection of Mice: Analysis of the Involvement of Type 1 IFN. The Journal of Immunology. 2005;174:1851–1861. doi: 10.4049/jimmunol.174.4.1851. [DOI] [PubMed] [Google Scholar]
- 69.Hedrick SM, I, Ch’en L, Alves BN. Intertwined pathways of programmed cell death in immunity. Immunological Reviews. 2010;236:41–53. doi: 10.1111/j.1600-065X.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]