Abstract
Cognitive bias and physiological arousal are two putative markers that may underlie youth anxiety. However, data on relationships between cognitive bias and arousal are limited, and typically do not include behavioral measurement of these constructs in order to tap real-time processes. We aimed to examine the relationship between performance-based cognitive bias and sympathetic arousal during stress in clinically anxious and typically-developing youth. The sample included children and adolescents ages 9 to 17 (Mean age = 13.18, SD = 2.60) who either met diagnostic criteria for primary generalized anxiety, social phobia, or separation anxiety (N=24) or healthy controls who had no history of psychopathology (N=22). Youth completed performance-based measures of attention and interpretation bias. Electrodermal activity was assessed while youth participated in the Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum, Jobst, & Wustmans, 1997). A mixed models analysis indicated significant linear and non-linear changes in skin conductance, with similar slopes for both groups. Interpretation bias, but not attention bias, moderated the relationship between group status and sympathetic arousal during the TSST-C. Arousal trajectories did not differ for anxious and healthy control youth who exhibited high levels of threat interpretation bias. However, for youth who exhibited moderate and low levels of interpretation bias, the anxious group demonstrated greater arousal slopes than healthy control youth. Results provide initial evidence that the relationship between anxiety status and physiological arousal during stress may be moderated by level of interpretation bias for threat. These findings may implicate interpretation bias as a marker of sympathetic reactivity in youth. Implications for future research and limitations are discussed.
Keywords: anxiety, child, adolescent, physiological arousal, interpretation bias
Anxiety disorders are distressing, disabling, and the most common mental health problem in children and adolescents (up to 25%; Beesdo, Knappe, & Pine, 2009). Core features of anxiety include hyper-reactivity to environmental stimuli across cognitive, physiological, and behavioral domains. Indeed, information processing (e.g., Daleiden, 1997; Field & Lester, 2010) and tripartite (e.g., Chorpita, Plummer, & Moffitt, 2000) theories of youth anxiety suggest that children and adolescents selectively attend to threat and interpret threat from ambiguity, and also experience physiological arousal across autonomic processes in response to stress. It follows that our current gold-standard psychosocial intervention for youth anxiety, cognitive behavioral therapy, targets threat-based and/or negative thinking with cognitive restructuring and physiological arousal/somatic symptoms with relaxation training (Kendall & Hedtke, 2006). However, relationships between these potential markers of disorder are understudied. A better understanding of potential relationships between these implicated processes would provide more direct cognitive and biological prevention and treatment targets.
Cognitive biases and youth anxiety
Anxious youth have also been shown to demonstrate an attention bias, or selectively attend to threat (e.g., Bar-Haim, Lamy, & Pergamin, 2007) and an interpretation bias, or interpret threat from ambiguous information (e.g., Cannon & Weems, 2010; Rozenman, Amir, & Weersing, 2014), using performance-based tasks. The attention bias literature in youth is mixed, suggesting that selective attention to threat may be quite heterogeneous and not necessarily a ubiquitous phenomenon in youth (e.g., Bar-Haim, Kerem, Lamy, & Zakay, 2010; Eldar, Apter, Lotan, & Edgar, 2012; Waters, Bradley, & Mogg, 2014). In contrast, a growing body of research supports the notion that anxious youth consistently demonstrate an interpretation bias, or resolve ambiguity with threatening appraisals (Creswell, Schniering, & Rapee, 2005; Muris, Huijding, Mayer, & Remmerswaal, 2009; Rozenman et al., 2014; Vassilopoulos, Banerjee, & Prantzalou, 2009).
Of note, in the last two decades, cognitive bias modification (CBM) interventions have been used to directly intervene upon attention and interpretation biases with the goal of reducing anxiety symptoms (for reviews, see Beard, Sawyer, & Hofmann, 2012; Hakamata, Lissek, Bar- Haim, & Britton, 2010; Hallion & Ruscio, 2011; Menne-Lothmann, Viechtbauer, & Höhn, 2014). In addition to reducing anxiety symptoms in clinically anxious adolescents (Reuland & Teachman, 2014), CBM for interpretation bias in particular has been shown to reduce self-reported anxiety during psychological challenge (Lau, Belli, & Chopra, 2013) and life stress (Telman, Holmes, & Lau, 2013) in non-diagnosed youth samples.
Autonomic arousal and anxiety
Physiological arousal, or the nervous system’s response to real or perceive threat, is also an associated feature of anxiety. Psychophysiology can be assessed with several measures, although these differ in the information provided about autonomic nervous system activity. Heart rate and respiratory sinus arrhythmia reflect an interplay between sympathetic and parasympathetic nervous system activity (e.g., Appelhans & Luecken, 2006), which may reflect both initial arousal and the attempt to downregulate or return to homeostasis and occurs several to many seconds after stimulus onset. Conversely, electrodermal activity (EDA) provides a relatively quick and undiluted representation of sympathetic activity (e.g., Cacioppo, Tassinary, & Berntson, 2007), which may be more reflective of an immediate physiological response to threat. EDA has been demonstrated as a validated physiological marker of anxiety in threatening situations (Beauchiane, 2001; Erath, Tu, & El-Sheikh, 2012; Fowles, Kochanska, & Murray, 2000) and, because it directly reflects sympathetic fear response, has been used as a proxy for fear acquisition in Pavlovian conditioning tasks (e.g., Craske, Waters, Bergman, & Naliboff, 2008). Interestingly, behavioral measures and self-reports of arousal do not always converge in youth samples (De Los Reyes et al., 2012; Kristensen, Oerbeck, & Torgersen, 2014; Miers, Blote, Sumter, & Kallen, 2011) suggesting that psychophysiological measures may provide distinct information about fearful autonomic responding that might not be acquired by self-report alone.
Several studies have found that youth with elevated anxiety symptoms and anxiety disorders exhibit autonomic nervous system hyper-arousal, including elevated resting heart rate, increased sympathetic and parasympathetic activity during stress, and impaired autonomic recovery following stress (Blom, Olsson, & Serlachius, 2010; Boyce, 2001; Krämer, Seefeldt, & Heinrichs, 2012; Monk, Kovelenko, & Ellman, 2001; Schmitz & Krämer, 2011; Sharma, Balhara, Sagar, Deepak, & Mehta, 2011). Other investigations have found that, depending on the task and measure of arousal, anxious and typically-developing youth demonstrate comparable levels of arousal (Alkozei, Creswell, Cooper, & Allen, 2015; Anderson & Hope, 2009; Gonzalez, Moore, & Garcia, 2011). These seemingly discrepant findings may be accounted for by cognitive bias, as theoretical models suggest an interplay between cognitive and physiological features of anxiety. We now turn to a brief description of the literature on relationships between cognitive bias and physiological arousal.
Cognitive bias and autonomic arousal: interactive processes?
Anxiety theories propose aberrant hyper-arousal in cognitive and physiological processes (Barlow, 2000) and, as described above, the literature supports links between each of these processes and anxiety symptoms and avoidance behavior. However, links between these processes have not been well-studied. This is surprising, given that our theoretical models (and the resultant rationale for targeting thoughts and feelings in CBT) clearly specify that the interactions between deficits in cognition and psychophysiology play a causal role in the development and maintenance of anxiety over time. Yet empirical studies typically only test relationships between anxiety symptoms and either cognitive bias or physiological arousal, but not both. While it is important to test relationships between proposed underlying mechanism and resultant psychopathology, it is equally important to understand relationships between these mechanisms in order to understand how they influence the mental health construct under study (Garvey, Avenevoli, & Anderson, 2016). Of particular relevance to anxiety, cognitive and physiological responses do not always correlate or move in synchronicity (e.g., Hodgson & Rachman, 1974; Lang, 1968; Zinbarg, 1998), suggesting that cognitive bias may exacerbate or interact with autonomic reactivity to produce anxiety (Alfano, Beidel, & Turner, 2006). Thus, the influence of cognition on psychophysiology should be examined in youth.
Only a handful of studies have examined both physiological arousal and cognitive bias in children and adolescents, finding each to predict anxious symptoms and avoidance (Dalrymple-Alford & Salmon, 2015; Field & Lawson, 2003; Weems, Zakem, & Costa, 2005). Moreover, when researchers provide verbal threat information (with the intent of inducing a threat bias) to community children, these youth exhibit elevated physiological arousal during behavioral approach toward the stimuli about which they were provided threat information (Askew, Hagel, & Morgan, 2015; Field & Price-Evans, 2009; Field & Schorah, 2007; Reynolds, Field, & Askew, 2014). These data provide preliminary evidence that cognitive bias may influence physiological arousal, which in turn may lead to anxious avoidance or symptoms. The above-described studies that have looked at relationships between cognitive bias and autonomic arousal are almost exclusively limited to typically-developing samples of youth, despite the fact that both constructs are considered core features of anxiety disorder. It is yet unclear whether cognition and psychophysiology may influence one another similarly in anxious versus typically-developing youth; such work is important for both our conceptualization of pediatric anxiety and maximizing intervention and prevention efforts to target underlying mechanisms.
As the field moves towards examination of these cognitive and physiological markers of anxiety in order to directly target these processes with translational, novel, and personalized intervention (March, 2011), it is critical to understand the respective and interactive contributions of autonomic arousal and cognitive bias to anxiety. We conducted this study in order to begin answering the question: does cognitive bias moderate the relationship between anxiety disorder status and sympathetic arousal in youth? Given prior theories suggesting that level of cognitive bias may influence degree of arousal (Alfano et al., 2006), and the findings in typically-developing youth that increasing cognitive bias leads to autonomic reactivity, we specifically hypothesized that anxious and typically-developing youth would have similar physiological arousal slopes over the course of stress at higher levels of cognitive bias. In contrast, we predicted that anxious youth would demonstrate greater arousal trajectories than their non-anxious counterparts at lower levels of cognitive bias, with the rationale that low levels of cognitive bias for typically-developing youth may serve as a protective factor and their thinking patterns would not necessarily negatively influence their arousal during stress. As prior studies have not yet examined more than one type of cognitive bias, we also were interested in exploring whether attention bias or interpretation bias for threat would moderate the relationship between diagnostic status and arousal similarly, or whether one of these cognitive bias variables would exhibit stronger effects over the other. We chose performance-based cognitive processing measures as our potential moderators in order to target less controlled, more automatic thinking patterns in youth. Moreover, as we were specifically interested in relationships between cognitive bias and sympathetic, rather than parasympathetic, arousal, we chose EDA as our psychophysiological outcome.
Method and Materials
Participants
Anxious Sample
The final anxious (ANX) sample included 24 children and adolescents (46% male, Mean age = 13.57, SD = 2.70, 54% racial/ethnic minority). Children were included if they met for a current primary diagnosis of Generalized Anxiety Disorder (n=15), Social Phobia (n=6), or Separation Anxiety Disorder (n=3) as assessed by the ADIS-C/P. Comorbidity in the sample was high; 42% of the sample met for secondary anxiety disorder, 8% met for Major Depressive Disorder, and 13% met for ADHD. Clinical Global Impressions – Severity (CGI-S) ranged from 4 (“moderately ill”) to 6 (“severely ill”) with a Mean CGI-S of 4.67 (SD = 0.56).
Healthy Control Sample
The final healthy control (HC) sample included 22 children and adolescents (55% male, Mean age = 12.75, SD = 2.28, 46% racial/ethnic minority). Children were included if they did not meet for any current or past diagnoses as assessed by the ADIS-C/P, and if the family denied any history of emotional or behavioral difficulties or receipt of any mental health services for the child and any first-degree relatives.
Other inclusion/exclusion criteria
Eligible for inclusion were those youth who (a) were aged 9 to 17, inclusive, at the time of study participation, (b) spoke fluent English, (c) read at age level, (d) no cognitive, vision or other problems that would interfere with completion of cognitive bias tasks, (e) no physical health problems (e.g., heart condition) that would interfere with completion of physiological data acquisition, and (f), lived with a legal guardian who was able to provide consent for study participation. Youth recruited for the ANX group also had to meet for diagnosis of primary generalized anxiety, social phobia, and/or separation anxiety. Prior to recruitment, we decided upon diagnostic exclusion criteria for the ANX group, which included diagnosis for any other mental health condition that required immediate intervention (i.e., ADHD, OCD, bipolar disorder, psychosis, PTSD, substance dependence) and might affect performance/arousal during our study tasks. One youth was screened and excluded from the ANX group for primary OCD. Additional inclusion criteria for the HC group was child or parent report during telephone screen or diagnostic interview that the child and all first-degree relatives had never had difficulties with emotional and/or behavioral problems and had never received any mental health services (e.g., psychotherapy, medication, counseling sessions, etc.). No HC youth who completed consent and study activities were excluded on the basis of report of prior or current mental health difficulties. Three additional youth (two ANX, one HC) were excluded from the current study because they became upset during the stressor task and asked to discontinue the task partway through (and therefore, physiological data for all task components were not acquired.
Equipment and materials
Anxiety Disorders Interview Schedule for Children, Version IV (ADIS-IV; Silverman, Saavedra, & Pina, 2001). The ADIS-IV is a semi-structured diagnostic interview that assesses the major DSM-IV disorders, including anxiety. It has demonstrated strong psychometric properties (ADIS-IV; Silverman, Saavedra, & Pina, 2001; Wood, Piacentini, Bergman, McCracken, & Barrios, 2002) and is considered the gold-standard diagnostic tool in large-scale studies of pediatric anxiety (e.g., Walkup et al., 2008). The ADIS was used in this study to confirm primary anxiety diagnosis and to assess for other comorbidities for characterization purposes in the anxious group, and ensure that youth in the healthy control group did not meet diagnostic criteria for any psychopathology.
Clinical Global Impressions – Severity (CGI-S; Guy, 1976) is a clinician-rated dimensional measure of psychopathology severity. The CGI-S ranges from 1(“not at all ill”) to 7 (“extremely ill”). The CGI-S was used to characterize the severity of anxiety in the ANX group.
Wide-Range Achievement Test – 4th Edition Reading Subtest (WRAT-4; Wilkinson & Robertson, 2006) is a well-established academic achievement test with validated standardized scores for youth according to age and grade. The Reading subtest was administered to all youth to ensure that they could read at age-level, as the ability to read is critical to completing the interpretation task (i.e., reading word stimuli presented at 500 ms).
Attention bias: Faces Dot Probe is a modification of that developed by MacLeod, Mathews, & Tata (1986), with eight threatening (i.e., disgust) and eight neutral faces selected from a standard set of emotional expressions (Matsumoto & Ekman, 1989). A total of 200 trials were administered, with 160 threat-neutral and 40 neutral-neutral trials in block-randomized combinations for each youth. Each trial begins with the presentation of a fixation cross (“+”) in the center of the monitor for 500 ms, immediately followed by two faces of the same individual on the top and bottom portions of the screen. Faces were centered horizontally and positioned 3.0 centimeters from the top of the screen and separated by 1.5 centimeters between the bottom of the top image and top of the bottom image. After 500 ms, a probe (either letter E or F) appeared in the location of one of the two faces until the participant indicated with button press whether they saw the letter E or F. After the participant responded, the next fixation cross was presented. During the task, for trials in which a threat face was paired with a neutral face, the probe appeared in the same location as the threat face on 50% of trials. Participants were instructed to respond as quickly and accurately as possible to the probe (E or F), and were not provided any information or instructions in regards to the face stimuli. The program monitored accuracy in identifying the probe type (E or F), as well as reaction time for each trial. On average, the task took approximately 10 to 15 minutes to complete. The task provides a threat bias reaction time index utilizing the Mathews & MacLeod (2005) formula for speed of responding on threat versus neutral faces, whereby larger numbers indicate greater attention bias to threat as compared to neutral faces (Mathew & MacLeod, 2005).
Interpretation bias assessment is a performance-based task developed by Beard & Amir (2008) and adapted by the first author for use in children (Rozenman et al., 2014). The task assesses whether youth exhibit biases in their selection of threat versus benign interpretations of ambiguous sentences). A total of 320 trials were administered, with each sentence presented twice, once following a threat word and once following a benign word for a total of 160 threat trials and 160 benign trials. For example, a youth might see the word “robber” (threat word) or “cat” (neutral word), then see the sentence “You hear a noise outside at night, and then would be asked “Was the word related to the sentence?” Instructions for the task to youth were to indicate as quickly as possible with button press whether they thought word-sentence pairs were related or unrelated. Each trial begins with the presentation of a fixation cross (“+”) in the center of the screen for 500 ms, immediately followed by either a threat word or a benign word in the center of the screen for 500 ms. After 500 ms, an ambiguous sentence appeared in the center of the screen until the participant indicated with a spacebar press that they had finished reading the sentence. Once the participant pushed the spacebar, the computer prompted “Was the word related to the sentence?” and the participant indicated with a button press whether they believed the word and sentence were related. After the participant responded, the next fixation cross was presented. The program monitored the number of threat versus benign word-sentence pairs that were endorsed as related or not related, as well as reaction time for each trial. On average, the task took approximately 15 minutes to complete. For this study, as in other studies of interpretation bias using the same task (Beard & Amir, 2008; Rozenman et al., 2014)., the interpretation bias index was calculated by subtracting reaction times for threat endorsement from reaction times for threat rejection, whereby larger positive numbers indicate greater interpretation bias towards threat. We decided a priori to test the percentage of threat interpretations endorsed and the threat reaction time index in our statistical models, given that these have each been previously found to correlate with anxiety (e.g., Rozenman et al., 2014).
Trier Social Stress Test for Children (TSST-C; Buske-Kirschbaum, Jobst, & Wustmans, 1997) is a standardized stressor task that has been used in numerous studies to assess stress response in community (Aldao, McLaughlin, Hatzenbuehler, & Sheridan, 2014; Gilissen & Bakermans-Kranenburg, 2008; Reynolds, Schreiber, Geisel, MacPherson, Ernst, & Lejuez, 2013) and clinically-anxious youth (Dorn, Campo, Thato, Dahl, & Lewin, 2003; Gerra, Zaimovic, Zambelli, & Timpano, 2000; Krämer et al., 2012; Schmitz & Krämer, 2011). The TSST-C requires youth to prepare and deliver the ending of a story, and verbally respond to challenging arithmetic during which time they are provided with standard prompts that they made errors, in the presence of an audience and with the expectation that they will be evaluated. The TSST-C includes five phases: (1) resting baseline (3 min), (2) story preparation (2 min), (3) story presentation (5 min), math task (5 min), and a recovery period (3 min).
Electrodermal activity (EDA) is the variation in skin conductance levels due to emotional and sympathetic arousal. EDA was measured with an MP150 Biopac Systems and Acknowledge III software, with continuous recordings from two Ag/AgCl electrodes, positioned on the distal phalanges of the forefinger and middle finger of the non-dominant hand using medical tape. Hypoallergenic gel (G100) was used as contact medium between the skin and electrode. Our measure of EDA was the average skin conductance level (SCL) for each of the five phases of the TSST-C, and sent telemetrically to the MP150 data acquisition system.
Procedure
The university Institutional Review Board provided approval, and informed consent was obtained verbally from parents prior to phone screen, and written consent was obtained from parents and assent obtained from children prior to study entry. Youth were recruited from the community with flyers and online advertisement. Families who answered advertisements were phone-screened for the current study to determine initial inclusion/exclusion and broadly describe study procedures. During this phone screen, families calling in response to the healthy control flyer were carefully queried to ensure that the target child, parents, and any other first-degree relatives had never received mental health services or appeared to have emotional or behavioral difficulties. This was done in attempts to recruit a truly “healthy” control sample of youth, and exclude youth who may have other psychopathology or were at risk for psychopathology due to family history. Study participation occurred over the course of a single laboratory visit. Both youth and parent participated in the diagnostic interview. The ADIS was administered by the first author, and 10% of tapes for each ANX and HC groups were rated by another doctoral-level psychologist, with 100% inter-rater agreement for all diagnoses. Parents completed a demographic information sheet, while youth completed a brief reading screener to ensure reading at age-level, as well as performance-based attention and interpretation bias assessments. Finally, youth completed the TSST-C with two research assistants. It should be noted that the cognitive processing tasks were administered prior to the TSST-C in order to obtain baseline measures of attention and interpretation bias that would not be affected by any physiological, cognitive, or emotional processes that might occur during the stressor task. Following the completion of all study procedures, the PI debriefed each participant about the TSST-C.
Data Preparation and Statistical Analyses
Cleaning and screening of performance-based data
Per standardized convention, reaction times on computerized performance-based tasks were cleaned for attention reaction times between 250ms and 2000 ms and interpretation reaction times between 250ms and 3500 ms, with attention (Mathews & MacLeod, 2005; Price et al., 2015) and interpretation (Beard & Amir, 2008; Rozenman et al., 2014) threat bias indices calculated. Across the sample, less than 8% of attention data and less than 4% of interpretation data were lost during cleaning/screening procedures, with less than 10% data lost for any individual youth on either task.
Physiological data
EDA data was scored offline with Mindware 2.1. Skin conductance response was defined as an increase of 0.05 microSiemens. Data are presented in microSiemens and represent the average skin conductance response for each of the 5 TSST-C phases.
Analyses
Descriptive statistics, including frequencies and chi-square, were conducted to examine potential group differences in age, gender, minority status, and reading level in order to characterize the sample. T-tests were used to examine group differences on attention and interpretation bias.
To examine our primary aims of the potential predictive value of anxiety status and cognitive bias on physiological arousal over the course of stress, mixed models analysis of repeated measures were tested in Stata 14. The mixed procedure was chosen for its ability to examine non-linear relationships, account for repeated measures, and handle missing data (e.g., West, Welch, & Galecki, 2007). Initial fixed factors examined as predictors included group (categorical) and attention (continuous and categorical models were tested) and interpretation indices (continuous, including percent threat interpretations endorsed and interpretation bias index). Attention and interpretation biases were tested in separate models due to the relatively small sample size and number of interaction terms tested. Because attention bias has been previously tested as a categorical variable in youth (attention bias towards versus away from threat, (Waters, Mogg, & Bradley, 2012), we also ran a model testing attention bias as a categorical factor. Age and gender were examined as a priori potential covariates in models. Time (EDA during the 5 phases of the TSST) was included as a repeated measures effect and was centered with the first time point (resting baseline) set to zero. As we predicted a priori interactions between cognitive bias and group, these were added into the model in a sequential fashion to examine their respective predictive values. Additionally, as the TSST-C includes a recovery phase, we hypothesized and tested both linear and non-linear (quadratic) effects for physiological arousal over time. Only factors, covariates and repeated time measures that demonstrated significant main and/or interaction effects were retained in the final model. Post hoc comparisons of estimated marginal means (EM) and simple slopes were used to compare significant effects of group and interpretation bias on EDA over time.
Results
Descriptive Characteristics
As shown in Table 1, there were no group differences for demographic (age, gender, minority status) or WRAT-4 reading scores. As expected, there were significant group differences on CGI-S ratings (t(44) = −25.18, p < .001); the ANX group had significantly higher CGI-S scores (M = 4.67, SD = 0.56) than the HC group (M = 1.13, SD = 0.35). There was also a significant group difference for interpretation bias index (t(44) = −4.25, p < .001), such that the ANX group (M = 78.45, SD = 108.60) had faster reaction times for threat endorsement than threat rejection as relevant to ambiguity than the HC group (M = −46.49, SD = 88.71). Group differences on the attention bias index approached significance (t(44) = −1.87, p = .069).
Table 1.
Demographic and clinical characteristics of full sample and by group
| Measure | Full Sample | Anxious | Healthy Control |
|---|---|---|---|
| N | 46 | 24 | 22 |
| Gender (N, % Male) | 50% | 46% | 55% |
| Ethnicity (N, % Minority) | 59% | 54% | 64% |
| Mean Age (SD) | 13.18 (2.60) | 13.57 (2.66) | 12.75 (2.63) |
| WRAT-4 Reading Standard Score (SD) | 111.54 (12.00) | 113.13 (11.37) | 109.82 (12.48) |
| Attention bias index (SD) | 1.37 (53.92) | 15.03 (52.13) | −14.24 (52.83) |
| Interpretation bias index (SD)* | 18.70 (116.97) | 78.45 (108.60) | −46.49 (88.71) |
Group difference *p < .01
Prediction of Sympathetic Arousal Trajectories from Group, Cognitive Bias, and Time
Examining the relationships between hypothesized predictors of group (ANX, HC) and cognitive bias, the best fit model included both linear and quadratic terms for time, interpretation bias as a significant covariate, and group × time and group × interpretation bias interactions. Table 2 provides parameter estimates of regression coefficients, t-test statistics, significance level, and confidence intervals for the final model. Attention bias (as a continuous or categorical variable), percentage of threat interpretations endorsed, age, gender, minority status, and WRAT reading standard scores were not significant covariates and therefore were not included in the final model.
Table 2.
Final mixed effects model
| Independent variables | β | SE | p | 95% CI | |
|---|---|---|---|---|---|
| Time | 81.17 | 6.72 | < .001 | 68.00 | 94.35 |
| Time2 | −15.10 | 1.61 | < .001 | −18.26 | −11.94 |
| Group* | −173.78 | 85.42 | .037 | −341.19 | −6.37 |
| Interpretation bias index | 0.32 | 0.14 | .025 | 0.041 | 0.60 |
| Group × Interpretation bias | 0.54 | 0.19 | 0.004 | 0.17 | 0.90 |
Healthy control group serves as the reference category for group effects
As shown in Table 2, there was a significant main effect for time on both linear and quadratic terms, such that skin conductance over the course of the TSST-C demonstrated a non-linear trend across participants over time. Estimates of fixed effects indicated an overall initial increase in SCL across the sample from resting baseline to story preparation (linear term: β = 81.17, SE = 6.72, CI: 68.00, 94.35, p < .001)), with an average decrease in SCL over the remaining phases of the task (quadratic term: β = −15.10, SE = 1.61, CI: −18.26, 11.94, p < .001). As shown in Figure 1, a sharp increase in SCL from baseline to story preparation was followed by slower increase during story and math portions of the task. The group × time interaction was not significant, suggesting comparable arousal trajectories for ANX and HC groups during the TSST-C.
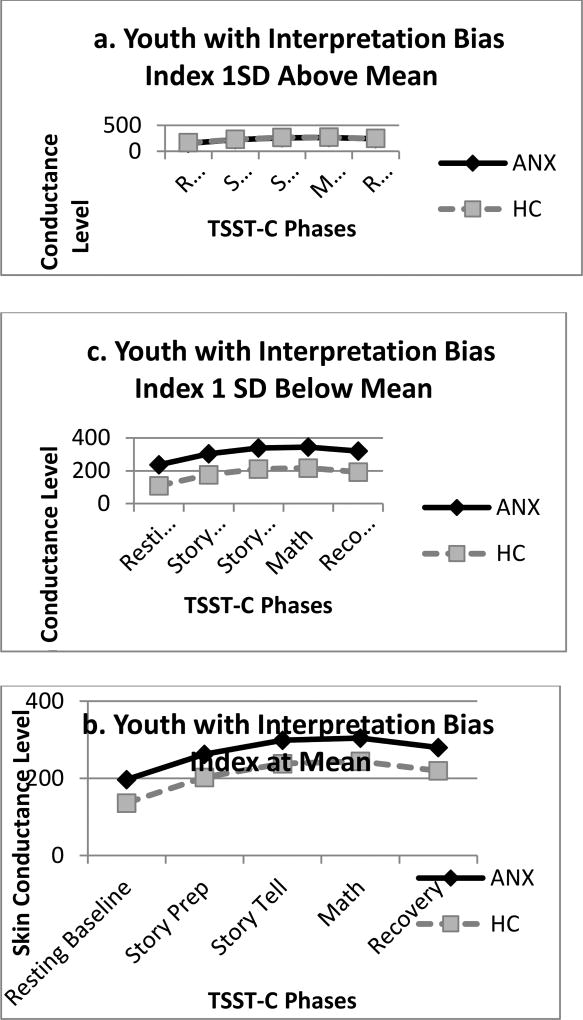
Figure 1.
Mean Skin Conductance Level across TSST-C Phases: Group × Interpretation Bias Interaction
Interaction between Group and Cognitive Bias in Predicting Sympathetic Arousal
There was a significant interaction for group × interpretation bias (β = 0.53, SE = 0.19, CI: 101.42, 360.74, p = .004). Follow-up examination of simple slopes indicated that, at the mean level of interpretation bias for threat, the change in SCL slopes from baseline was significantly greater for the ANX group than the HC group at speech prep (β = 50.97, SE = 3.75, CI: 43.63, 58.31, p < .001) and speech give (β = 20.77, SE = 1.91, CI: 17.04, 24.51, p < .001), and significantly smaller for the ANX group than the HC group during math (β = −9.42, SE = 3.75, CI: −16.77, −2.08, p = .012 and recovery (β = −39.62, SE = 6.72, CI: −52.80, −26.45, p < .001) phases.
We further examined this interaction with follow-up tests of group comparisons at the mean of interpretation bias for threat, as well as at 1 SD above (indicating greater threat interpretation bias) and below (indicating less interpretation bias) the mean. As shown in Figure 1, ANX and HC groups did not significantly differ on average slope of SCL at 1 SD above the mean of interpretation bias. However, groups did significantly differ on average slope of SCL at the mean (β = 60.311, SE = 23.57, CI: 14.11, 106.52, p = .011) and 1 SD below (β = 127.95, SE = 33.06, CI: 63.16, 192.74, p < .001) the mean of the threat interpretation bias index. As shown in Figure 1, ANX and HC youth with a high threat interpretation bias (1 SD above mean, Figure 1a) demonstrated comparable levels of sympathetic activity during the TSST-C, while at the mean of interpretation bias (Figure 1b) and 1 SD below the mean (Figure 1c), ANX youth demonstrated greater sympathetic activity than their HC counterparts.
Discussion
The primary aim of this study was to examine performance-based cognitive bias as a potential moderator of the relationship between anxiety disorder status and sympathetic arousal during a stressor task in youth. This work follows prior investigations which have identified each cognitive bias and physiological arousal as markers of anxiety disorder, mixed findings on whether autonomic reactivity during stress differs between anxious and non-anxious children and adolescents, and theoretical models suggesting that the interplay between cognitive and psychophysiological processes may lead to anxiety. In the current study, anxious and healthy control youth all completed performance-based attention and interpretation bias tasks, providing behavioral (i.e., reaction time) indices of cognitive bias, and also participated in the Trier Social Stress Task for Children (TSST-C; Buske-Kirschbaum et al., 1997) while sympathetic arousal (measured by electrodermal activity) was acquired. Cognitive bias was examined as a putative moderator between anxiety disorder status and sympathetic reactivity during the TSST-C.
Findings provide preliminary support for the hypothesis that cognitive bias moderates the relationship between anxiety disorder status and sympathetic arousal during stress. First, we found comparable arousal slopes (with evidence for both linear and quadratic effects) for SCL level over time for anxious and healthy control youth. This suggests that anxious and non-anxious youth experience similar changes in sympathetic reactivity from resting baseline to the start of the TSST-C, and over the course of the TSST-C to recovery. This also suggests that both linear and non-linear effects should be examined when using tasks that involve a recovery period and/or multiple task components (e.g., story and math in the TSST-C) to fully explore how sympathetic reactivity may change over the course of a stressor.
Second, we found that performance-based interpretation bias for threat, but not attention bias, moderated the relationship between anxiety disorder status and sympathetic reactivity over the course of the TSST-C. More specifically, anxious and healthy control youth exhibiting high levels of threat interpretation bias demonstrated comparable arousal trajectories during the stressor. However, for youth exhibiting moderate levels of threat interpretation bias, the anxious group demonstrated greater arousal slopes than healthy control group. This effect was even more pronounced for youth exhibiting low levels of threat interpretation bias. Broadly, these findings suggest that the relationship between anxiety status and physiological arousal in youth may, at least in part, depend upon the degree to which youth exhibit behavioral (i.e., reaction time) biases in their appraisals of ambiguity as either threatening or neutral.
These results are consistent with prior findings that support a relationship between self-reported cognitive bias, physiological arousal, and anxiety in youth (Dalrymple-Alford & Salmon, 2015; Field & Price-Evans, 2009; Field & Schorah, 2007; Reynolds et al., 2014; Weems et al., 2005). Our findings are also consistent with prior studies showing that threat bias may be a risk marker for anxious processes in non-anxious youth. In our non-anxious sample, sympathetic reactivity increased as threat interpretation bias increased. Indeed, interpretation bias has been implied as a prospective risk marker for anxiety in children as young as preschool-aged (Dodd, Hudson, Morris, & Wise, 2012; Waters, Craske, Bergman, & Treanor, 2008). It may be that interpretation bias and physiological reactivity are processes that are relevant to some, but not all youth; identification of those youth who exhibit these aberrations in core processes underlying anxiety may lead to future intervention personalization efforts. For example, should prospective longitudinal studies find interpretation bias to be a behavioral marker that predicts future anxiety disorder, cognitive bias modification targeting interpretation bias may be done in a personalized, targeted manner for those youth who exhibit the bias. This in turn may help to reduce interpretation bias and subsequently reduce or prevent anxiety.
These current results also extend the literature by examining behavioral measures of cognitive bias (i.e., reaction time) and psychophysiology rather than using self- or parent-reported measures, and provide two potential explanations for why the extant studies on physiological arousal yield mixed results in regards to stress response in anxious and typically-COGNITIVE developing youth. First, in regards to the time course of autonomic reactivity, our results suggest that anxious and non-anxious youth exhibit similar arousal trajectories. Prior mixed findings to date may be, at least in part, due to statistical approaches that compare arousal at one point at a time, which may not reflect the real-time changes that occur over the course of reactivity to stress. Second, we found cognitive bias to moderate the relationship between anxiety disorder status and arousal. Examination of this and other indices of sustained threat, one of the proposed constructs (Cuthbert, 2014) that is likely involved in anxiety disorders, may improve our understanding of psychophysiological arousal as a feature of youth anxiety.
This study may also have implications for the study of anxious arousal as an acute and sustained threat response during fear learning. Studies across the developmental continuum have found that clinically anxious individuals demonstrate elevated sympathetic reactivity during fear learning, as well as difficulties inhibiting autonomic arousal during fear inhibition and learning of new non-threatening associations (Blechert, Michael, Williams, Purkis, & Wilhelm, 2008; Craske, Kircanski, Zelikowsky, Mystkowski, Chowdhury, & Baker, 2008; Lau et al., 2008; Waters et al., 2012). Moreover, recent work by Allison Waters suggests that cognitive avoidance of threat predicts the degree of startle during fear acquisition and extinction (Waters & Kershaw, 2015) and Field and colleagues have consistently found that verbal learning about threat information impacts physiological arousal and behavioral avoidance (e.g., Field & Price-Evans, 2009; Field & Schorah, 2007). Together, these studies provide preliminary support for the hypothesis that threat interpretations may be directly tied to fear learning (Britton, Lissek, Grillon, Norcross, & Pine, 2011). Thus, it may be possible that threat interpretations are learned fear associations that lead to excitatory sympathetic nervous system responding. Future studies in this area might examine whether cognitive biases develop and are maintained by initial autonomic reactivity during associative learning about fear.
This investigation has several limitations that should be addressed in future studies. First, although our sample size was comparable to prior studies comparing autonomic arousal between anxious and non-anxious youth (Alkozei et al., 2015; Gonzalez et al., 2011; Monk et al., 2001; Schmitz, Blechert, Krämer, Asbrand, & Tuschen-Caffier, 2012; Schmitz & Krämer, 2011; Sharma et al., 2011), investigations with larger samples are needed to replicate these results. Replication of the current study with a larger sample would allow for sufficiently powered post hoc analyses that might further clarify the moderating role of cognitive bias on psychophysiological reactivity during stress. Second, we specifically recruited our healthy control group for no history of mental health problems in the participating youth or in their immediate family members, which may limit generalization of findings to other community youth with varying family histories of psychopathology. Additionally, as we were specifically interested in sympathetic reactivity, we used electrodermal activity as our primary outcome measure. Inclusion of other measures of psychophysiology (e.g., cardiac arousal) may provide additional information about emotional responding and parasympathetic influences. Related to this, our recovery period of three minutes may have not provided sufficient time for youths to recover following the TSST-C; future studies might examine how cognitive biases may play a mediating role in length of time to autonomic recovery in anxious versus non-anxious youth. Next, we selected the TSST-C as our stressor task because it has been used in many prior studies with youth to examine psychophysiological arousal. However, this task may have social components that may be more relevant to some anxious youth (e.g., social anxiety disorder) than others, and does not necessarily represent acute fear or sustained threat reactions. More work in this area is needed to understand the overlap between stress and acute/sustained fear responses in youth. In regards to our cognitive bias measures, the reliability of the dot probe task has been questioned in recent years (e.g., Schmukle, 2005), and a potential lack of reliability in the task may have resulted in a null finding herein. Further, we only included disgust faces for the dot probe task, and inclusion of a broader range of faces (i.e., disgust, angry, and sad) may have resulted in different findings. Finally, as the current study was cross-sectional, we cannot examine cognitive and physiological constructs as mechanisms that may underlie the development and maintenance of anxiety. Prospective studies may explore whether either or both of these constructs are consistent traits that present prior to the onset of significant anxiety symptoms, whether they change over the course of development for each anxious and non-anxious youth, or whether they occur as a consequence, rather than an underlying marker, of anxiety. In addition, theory-driven, task-based paradigms may be developed to examine how cognitive bias and autonomic arousal interact in real time during stress, which may provide valuable information about how these constructs should be addressed during exposure therapy and also which youth may benefit from cognitive bias modification interventions. If such work continues to support these relationships, both cognitive bias and physiological arousal might be direct markers of risk and targets for prevention and intervention efforts.
To summarize, the current investigation found preliminary evidence to support performance-based interpretation bias for threat as a moderator of the relationship between anxiety disorder status and sympathetic reactivity during stress. As anxiety is hypothesized to result from a complex interplay between cognitive, physiological, and behavioral processes, more research is needed to understand these dynamic relationships, and identify behavioral markers for future intervention and prevention targets. Such work is especially compelling within pediatric samples, given that anxiety is a childhood-onset disorder and that cognitive processes may be more malleable within this developmental window (Pine, 2007). Future studies, particularly those with a prospective component to track anxiety symptoms over the course of development, as well as those which incorporate the study of immediate and sustained threat in anxiety-driven behavior, may further elucidate how cognitive bias and psychophysiological arousal may be involved in the development and maintenance of anxiety disorders in youth.
Highlights.
Cognitive bias and physiological arousal have each been linked to anxiety
Youth cognitive bias moderates the relationship between anxiety and arousal
Anxious and healthy youth do not differ in arousal when they have high threat interpretation bias
Anxious youth demonstrate greater arousal than healthy youth at moderate and low levels of interpretation bias
Threat interpretation bias may be a marker of physiological arousal
Acknowledgments
This work was supported by the UCLA Research Program for Psychobiological Sciences T32 MH 17140 (Leuchter) and the UCLA Clinical Translational Science Institute UL1TR000124 (Rozenman). The authors would like to acknowledge and thank the children and their parents who participated in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldao A, McLaughlin K, Hatzenbuehler M, Sheridan M. The relationship between rumination and affective, cognitive, and physiological responses to stress in adolescents. Journal of Experimental Psychopathology. 2014;5(3):272–288. doi: 10.5127/jep.039113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano C, Beidel D, Turner S. Cognitive correlates of social phobia among children and adolescents. Journal of Abnormal Child Psychology. 2006;34(2):189–201. doi: 10.1007/s10802-005-9012-9. [DOI] [PubMed] [Google Scholar]
- Alkozei A, Creswell C, Cooper P, Allen J. Autonomic arousal in childhood anxiety disorders: associations with state anxiety and social anxiety disorder. Journal of Affective Disorders. 2015;175:25–33. doi: 10.1016/j.jad.2014.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E, Hope D. The relationship among social phobia, objective and perceived physiological reactivity, and anxiety sensitivity in an adolescent population. Journal of Anxiety Disorders. 2009;23(1):18–26. doi: 10.1016/j.janxdis.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelhans B, Luecken L. Heart rate variability as an index of regulated emotional responding. Review of General Psycholog. 2006;10(3):229–240. doi: 10.1037/1089-2680.10.3.229. [DOI] [Google Scholar]
- Askew C, Hagel A, Morgan J. Vicarious learning of children’s social-anxiety-related fear beliefs and emotional Stroop bias. Emotion. 2015;15(4):501–510. doi: 10.1037/emo0000083. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Kerem A, Lamy D, Zakay D. When time slows down: The influence of threat on time perception in anxiety. Cognition and Emotion. 2010;24(2):255–263. doi: 10.1080/02699930903387603. [DOI] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. American Psychologist. 2000;55(11):1247–1263. doi: 10.1037/0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Beard C, Amir N. A multi-session interpretation modification program: Changes in interpretation and social anxiety symptoms. Behaviour Research and Therapy. 2008;46(10):1135–1141. doi: 10.1016/j.brat.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, Sawyer A, Hofmann S. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43(4):724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine D. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Williams SL, Purkis HM, Wilhelm FH. When two paradigms meet: Does evaluative learning extinguish in differential fear conditioning? Learning and Motivation. 2008;39(1):58–70. doi: 10.1016/j.lmot.2007.03.003. [DOI] [Google Scholar]
- Blom EH, Olsson E, Serlachius E. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatrica. 2010;99(4):604–611. doi: 10.1111/j.1651-2227.2009.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT. Autonomic reactivity and psychopathology in middle childhood. The British Journal of Psychiatry. 2001;179(2):144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: The role of threat appraisal and fear learning. Depression and Anxiety. 2011;28(1):5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosomatic. 1997;59(4):419–426. doi: 10.1087/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Tassinary L, Berntson G. Psychophysiological Science: Interdisciplinary Approaches to Classic Questions About the Mind. Handbook of Psychophysiology. 2007;3:1–16. doi: 10.1017/CBO9780511546396.001. [DOI] [Google Scholar]
- Cannon M, Weems C. Cognitive biases in childhood anxiety disorders: Do interpretive and judgment biases distinguish anxious youth from their non-anxious peers? Journal of Anxiety Disorders. 2010;24(7):751–758. doi: 10.1016/j.janxdis.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Plummer CM, Moffitt CE. Relations of tripartite dimensions of emotion to childhood anxiety and mood disorders. Journal of Abnormal Child Psychology. 2000;28(3):299–310. doi: 10.1023/A:1005152505888. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46(1):5–27. doi: 10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Craske M, Waters A, Bergman R, Naliboff B. Is aversive learning a marker of risk for anxiety disorders in children? Behaviour Research and Therapy. 2008;46(8):954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell C, Schniering CA, Rapee RM. Threat interpretation in anxious children and their mothers: Comparison with nonclinical children and the effects of treatment. Behaviour Research and Therapy. 2005;43(10):1375–1381. doi: 10.1016/j.brat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. Translating intermediate phenotypes to psychopathology: the NIMH Research Domain Criteria. Psychophysiology. 2014;51(12):1205–6. doi: 10.1111/psyp.12342. [DOI] [PubMed] [Google Scholar]
- Daleiden E. An information-processing perspective on childhood anxiety. Clinical Psychology Review. 1997;17(4):407–429. doi: 10.1016/S0272-7358(97)00010-X. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford S, Salmon K. Ambiguous information and the verbal information pathway to fear in children. Journal of Child and Family Studies. 2015;24(3):679–686. doi: 10.1007/s10826-013-9878-z. [DOI] [Google Scholar]
- De Los Reyes A, Aldao A, Thomas SA, Daruwala S, Swan AJ, Van Wie M, Lechner WV. Adolescent self-eports of social anxiety: Can they disagree with objective psychophysiological measures and still be valid? Journal of Psychopathology and Behavioral Assessment. 2012;34(3):308–322. doi: 10.1007/s10862-012-9289-2. [DOI] [Google Scholar]
- Dodd HF, Hudson JL, Morris TM, Wise CK. Interpretation bias in preschool children at risk for anxiety: A prospective study. Journal of Abnormal Psychology. 2012;121:28–38. doi: 10.1037/a0024589. [DOI] [PubMed] [Google Scholar]
- Dorn L, Campo J, Thato S, Dahl R, Lewin D. Psychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42(1):66–75. doi: 10.1097/00004583-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Eldar S, Apter A, Lotan D, Edgar K. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. American Journal of Psychiatry. 2012;169(2):213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erath S, Tu K, El-Sheikh M. Socially anxious and peer-victimized preadolescents:“Doubly primed” for distress? Journal of Abnormal Child Psychology. 2012;40(5):837–848. doi: 10.1007/s10802-011-9600-9. [DOI] [PubMed] [Google Scholar]
- Field A, Lawson J. Fear information and the development of fears during childhood: Effects on implicit fear responses and behavioural avoidance. Behaviour Research and Therapy. 2003;41(11):1277–1293. doi: 10.1016/S0005-7967(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Field AP, Lester KJ. Is there room for “development” in developmental models of information processing biases to threat in children and adolescents? Clinical Child and Family Psychology Review. 2010;13(4):315–32. doi: 10.1007/s10567-010-0078-8. [DOI] [PubMed] [Google Scholar]
- Field A, Price-Evans K. Temperament moderates the effect of the verbal threat information pathway on children’s heart rate responses to novel animals. Behaviour Research and Therapy. 2009;47(5):431–436. doi: 10.1016/j.brat.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Field AP, Schorah H. The verbal information pathway to fear and heart rate changes in children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(11):1088–1093. doi: 10.1111/j.1469-7610.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Field A, Schorah H. The verbal information pathway to fear and heart rate changes in children. Journal of Child Psychology and Psychiatry. 2007;48(11):1088–1093. doi: 10.1111/j.1469-7610.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Fowles D, Kochanska G, Murray K. Electrodermal activity and temperament in preschool children. Psychophysiology. 2000;37(6):777–787. doi: 10.1017/S0048577200981836. [DOI] [PubMed] [Google Scholar]
- Garvey M, Avenevoli S, Anderson K. The National Institute of Mental Health Research Domain Criteria and clinical research in child and adolescent psychiatry. Journal of the American Academy of Child & Adolescent Psychiatry. 2016;55(2):93–98. doi: 10.1016/j.jaac.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Zambelli U, Timpano M. Neuroendocrine responses to psychological stress in adolescents with anxiety disorder. Neuropsychobiology. 2000;42(2):82–92. doi: 10.1159/000026677. [DOI] [PubMed] [Google Scholar]
- Gilissen R, Bakermans-Kranenburg M. Electrodermal reactivity during the Trier Social Stress Test for children: Interaction between the serotonin transporter polymorphism and children’s attachment. Developmental Psychobiology. 2008;50(6):615–625. doi: 10.1002/dev.20314. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Moore P, Garcia A. Activation during observed parent–child interactions with anxious youths: A pilot study. Journal of Psychopathology and Behavioral Assessment. 2011;33(2):159–170. doi: 10.1007/s10862-011-9216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W. Clinical Global Impressions ECDEU assessment manual for psychopharmacology. Rockville, MD: National Institute for Mental Health; 1976. [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton J. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion L, Ruscio A. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hodgson R, Rachman S. II. Desynchrony in measures of fear. Behaviour Research and Therapy. 1974;12(4):319–326. doi: 10.1016/0005-7967(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Kendall P, Hedtke K. Cognitive-behavioral therapy for anxious children: Therapist manual. Vol. 3. Ardmore, PA: Workbook Publishing; 2006. [Google Scholar]
- Krämer M, Seefeldt W, Heinrichs N. Subjective, autonomic, and endocrine reactivity during social stress in children with social phobia. Journal of Abnormal Child Psychology. 2012;40(1):95–104. doi: 10.1007/s10802-011-9548-9. [DOI] [PubMed] [Google Scholar]
- Kristensen H, Oerbeck B, Torgersen H. Somatic symptoms in children with anxiety disorders: An exploratory cross-sectional study of the relationship between subjective and objective measures. European Child & Adolescent Psychiatry. 2014;23(9):795–803. doi: 10.1007/s00787-013-0512-9. [DOI] [PubMed] [Google Scholar]
- Lang P. Research in Psychotherapy Conference, 3rd, May–Jun, 1966. Chicago, IL: US. American Psychological Association; 1968. Fear reduction and fear behavior: Problems in treating a construct; pp. 795–803. [DOI] [Google Scholar]
- Lau J, Belli S, Chopra R. Cognitive bias modification training in adolescents reduces anxiety to a psychological challenge. Clinical Child Psychology and Psychiatry. 2013;18(3):322–333. doi: 10.1177/1359104512455183. [DOI] [PubMed] [Google Scholar]
- Lau JYF, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Pine DS. Fear conditioning in adolescents with anxiety disorders: results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037/0021-843X.95.1.15. [DOI] [PubMed] [Google Scholar]
- March JS. Looking to the future of research in pediatric anxiety disorders. Depression and Anxiety. 2011;18(3):322–333. doi: 10.1002/da.20754. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:322–333. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Matsumoto D, Ekman P. American-Japanese cultural differences in intensity ratings of facial expressions of emotion. Motivation and Emotion. 1989;13(2):143–157. doi: 10.1007/BF00992959. [DOI] [Google Scholar]
- Menne-Lothmann C, Viechtbauer W, Höhn P. How to boost positive interpretations? A meta-analysis of the effectiveness of cognitive bias modification for interpretation. PloS One. 2014;9(6):e100925. doi: 10.1371/journal.pone.0100925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miers A, Blote A, Sumter S, Kallen V. Subjective and objective arousal correspondence and the role of self-monitoring processes in high and low socially anxious youth. Journal of Experimental Psychopathology. 2011;18(3):322–333. doi: 10.5127/jep.019411. [DOI] [Google Scholar]
- Monk C, Kovelenko P, Ellman L. Enhanced stress reactivity in paediatric anxiety disorders: implications for future cardiovascular health. The International Journal of Neuropyschopharmacology. 2001;4(2):199–206. doi: 10.1017/S146114570100236X. [DOI] [PubMed] [Google Scholar]
- Muris P, Huijding J, Mayer B, Remmerswaal D. Ground control to Major Tom: Experimental manipulation of anxiety-related interpretation bias by means of the “space odyssey” paradigm and effects on avoidance. Journal of Anxiety Disorders. 2009;23(3):333–340. doi: 10.1016/j.janxdis.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(7):631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, Amir N. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychological Assessment. 2015;27(2):365–376. doi: 10.1037/pas0000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuland MM, Teachman BA. Interpretation bias modification for youth and their parents: A novel treatment for early adolescent social anxiety. Journal of Anxiety Disorders. 2014;28(8):851–864. doi: 10.1016/j.janxdis.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E, Schreiber W, Geisel K, MacPherson L, Ernst M, Lejuez C. Influence of social stress on risk-taking behavior in adolescents. Journal of Anxiety Disorders. 2013;27(3):272–277. doi: 10.1016/j.janxdis.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G, Field A, Askew C. Effect of vicarious fear learning on children’s heart rate responses and attentional bias for novel animals. Emotion. 2014;14(5):995–1006. doi: 10.1037/a0037225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenman M, Amir N, Weersing VR. Performance-based interpretation bias in clinically anxious youths: Relationships with attention, anxiety, and negative cognition. Behavior Therapy. 2014;45(5):594–605. doi: 10.1016/j.beth.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Blechert J, Krämer M, Asbrand J, Tuschen-Caffier B. Biased perception and interpretation of bodily anxiety symptoms in childhood social anxiety. Journal of Clinical Child and Adolescent Psychology. 2012;41(1):92–102. doi: 10.1080/15374416.2012.632349. [DOI] [PubMed] [Google Scholar]
- Schmitz J, Krämer M. Restricted autonomic flexibility in children with social phobia. Journal of Child Psychology and Psychiatry. 2011;52(11):1203–1211. doi: 10.1111/j.1469-7610.2011.02417.x. [DOI] [PubMed] [Google Scholar]
- Schmukle SC. Unreliability of the dot probe task. European Journal of Personality. 2005;19(7):595–605. doi: 10.1002/per.554. [DOI] [Google Scholar]
- Sharma RK, Balhara YPS, Sagar R, Deepak KK, Mehta M. Heart rate variability study of childhood anxiety disorders. Journal of Cardiovascular Disease Research. 2011;2(2):115–22. doi: 10.4103/0975-3583.83040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Albano A. Anxiety Disorders Interview Schedule for Children-IV. San Antonio, TX: Psychological Corporation; 1996. 1996. [Google Scholar]
- Silverman W, Saavedra L, Pina A. Test-retest reliability of anxiety symptoms and diagnoses with the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent versions. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(8):937–944. doi: 10.1097/00004583-200108000-00016. [DOI] [PubMed] [Google Scholar]
- Telman M, Holmes E, Lau J. Modifying adolescent interpretation biases through cognitive training: Effects on negative affect and stress appraisals. Child Psychiatry & Human Development. 2013;44(5):602–611. doi: 10.1007/s10578-013-0386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulos S, Banerjee R, Prantzalou C. Experimental modification of interpretation bias in socially anxious children: Changes in interpretation, anticipated interpersonal anxiety, and social anxiety symptoms. Behaviour Research and Therapy. 2009;47(12):1085–1089. doi: 10.1016/j.brat.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Walkup J, Albano A, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Kendall PC. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A, Bradley B, Mogg K. Biased attention to threat in paediatric anxiety disorders (generalized anxiety disorder, social phobia, specific phobia, separation anxiety disorder) as a function of ‘distress’ versus ‘fear’ diagnostic categorization. Psychological Medicine. 2014;44(3):607–616. doi: 10.1017/S0033291713000779. [DOI] [PubMed] [Google Scholar]
- Waters AM, Craske MG, Bergman RL, Treanor M. Threat interpretation bias as a vulnerability factor in childhood anxiety disorders. Behaviour Research and Therapy. 2008;46(1):39–47. doi: 10.1016/j.brat.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Waters AM, Kershaw R. Direction of attention bias to threat relates to differences in fear acquisition and extinction in anxious children. Behaviour Research and Therapy. 2015;64:56–65. doi: 10.1016/j.brat.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Waters A, Mogg K, Bradley B. Direction of threat attention bias predicts treatment outcome in anxious children receiving cognitive-behavioural therapy. Behaviour Research and Therapy. 2012;50(6):428–434. doi: 10.1016/j.brat.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Weems C, Zakem A, Costa N. Physiological response and childhood anxiety: Association with symptoms of anxiety disorders and cognitive bias. Journal of Clinical Child and Adolescent Psychology. 2005;34(4):712–723. doi: 10.1207/s15374424jccp3404_13. [DOI] [PubMed] [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. Second. Chapman & Hall; Boca Raton, FL: 2007. [Google Scholar]
- Wilkinson GS, Robertson GJ. Wide Range Achievement Test – Fourth Edition. Psychological Assessment Resources; Lutz, FL: 2006. [Google Scholar]
- Wood JJ, Piacentini JC, Bergman RL, McCracken J, Barrios V. Concurrent validity of the anxiety disorders section of the Anxiety Disorders Interview Schedule for DSM-IV: Child and parent versions. Journal of Clinical Child & Adolescent Psychology. 2002;31(3):335–342. doi: 10.1207/S15374424JCCP3103_05. [DOI] [PubMed] [Google Scholar]
- Zinbarg R. Concordance and synchrony in measures of anxiety and panic reconsidered: A hierarchical model of anxiety and panic. Behavior Therapy. 1998;29(2):301–323. doi: 10.1016/S0005-7894(98)80009-9. [DOI] [Google Scholar]