Abstract
BACKGROUND
A meta-analysis reported an 8% increased risk of cancer with the use of angiotensin receptor blockers (ARBs), but subsequent meta-analyses and observational studies did not confirm this risk. However, telmisartan comprised 85% of the data in the original meta-analysis. Thus, the objective of this study was to determine whether the use of telmisartan, compared with other ARBs, is associated with an increased risk of cancer.
METHODS
We used the United Kingdom Clinical Practice Research Datalink to assemble a cohort of all patients newly treated with ARBs between 2000 and 2008, and followed until December 2010. Time-dependent cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of cancer associated with telmisartan, compared with other ARBs, adjusted for potential confounders. Secondary analyses assessed the risk with each of the 4 most common cancers (lung, breast, prostate, colorectal).
RESULTS
The cohort consisted of 62,109 new ARB users, which included 3,438 telmisartan and 58,671 other ARB users. Compared with other ARBs, telmisartan use was not associated with an increased risk of cancer overall (16.3 vs. 15.0 per 1,000 person-years, respectively; adjusted HR: 0.93, 95% CI: 0.81–1.06) or by cancer site (lung, HR: 0.91, 95% CI: 0.55–1.51; breast, HR: 1.28, 95% CI: 0.90–1.82; prostate, HR: 0.79, 95% CI: 0.53–1.18; colorectal, HR: 1.41, 95% CI 0.95–2.10).
CONCLUSIONS
Compared with other ARBs, telmisartan is not associated with an increased risk of cancer. This study provides reassurance as to the short-term safety of telmisartan.
Keywords: angiotensin receptor blockers, blood pressure, cancer, drug safety, hypertension, telmisartan.
Angiotensin receptor blockers (ARBs) are widely prescribed drugs for the treatment of hypertension, diabetic nephropathy, and heart failure. Despite their popular use, concerns were raised after a meta-analysis of randomized clinical trials had associated the use of ARBs with an 8% increased risk of cancer.1 However, this finding was not confirmed in subsequent meta-analyses,2,3 as well as in a meta-analysis conducted by the US Food and Drug Administration.4 Observational studies also did not unequivocally confirm an overall increased risk of cancer with ARBs.
While the studies above provided some reassurance that ARBs, as a pharmacological class, are not likely to be associated with an increased risk of cancer, uncertainty remains with respect to individual drugs. Specifically, in the original meta-analysis reporting an increased risk of cancer, 85% of the subjects were from randomized trials of telmisartan.1 In contrast, telmisartan constituted only a minority (approximately 5%) of the total ARB exposure in the published observational studies5–10 meaning that a true increase in the risk of cancer associated with telmisartan use might have remained undetected due to the large weight of the null effect conferred by other compounds. Three of these studies assessed the risk of cancer with specific ARBs, but these were exploratory subanalyses where the comparator consisted of angiotensin-converting enzyme inhibitors (ACEIs).8–10 Given the limitations in the previous observational studies, data on the safety of telmisartan remains limited. Therefore, additional studies are needed to assess whether telmisartan is associated with an increased incidence of cancer.
Thus, the primary objective of this large population-based study was to determine whether the use of telmisartan, when compared with other ARBs, is associated with an increased risk of any cancer. A secondary objective was to assess the risk associated with the 4 most common cancers, namely, lung, breast, prostate, and colorectal cancer.
DESIGN AND METHODS
Data sources
This study was conducted using the United Kingdom (UK) Clinical Practice Research Datalink (CPRD). The CPRD is a research database that records primary care encounters, diagnoses, prescriptions, referrals, and vaccinations for approximately 13 million individuals representative of the UK population.11 Clinical data are encoded using the Read classification and all drugs and medical devices written by general practitioners are recorded using the UK Prescription Pricing Authority Dictionary. Accuracy of the diagnoses in the CPRD has been subject to several studies and is considered to be of high quality.12 All practices contributing data to the CPRD are under continuous data-quality surveillance.
The study protocol (13_024RAR) was approved by the Independent Scientific Advisory Committee of the CPRD in the UK and by the Research Ethics Board of the Jewish General Hospital in Montreal, Canada.
COHORT DEFINITION
Base-cohort
Using the CPRD population, we assembled a base-cohort composed of all patients newly treated with antihypertensive agents (i.e., diuretics, calcium channel blockers, ARBs, ACEIs, beta-blockers, alpha-blockers, centrally acting antihypertensive drugs, ganglion blockers, and other antihypertensive drugs), starting from 1 January 1995, the year the first ARB (losartan) entered the UK market to 31 December 2008. All patients were required to be at least 18 years of age at the time of the first antihypertensive prescription and have at least 2 years of registration prior to that first prescription.
Study cohort
Within the base-cohort defined above, we assembled a study cohort of all patients newly prescribed an ARB on or after 1 January 2000, the year telmisartan entered the UK market, through 31 December 2008. Study cohort entry was defined by the date of the first ARB prescription. Thus, patients with a history of ARB use before study cohort entry and those with a previous history of cancer (other than nonmelanoma skin cancer) at any time before study cohort entry were excluded. Finally, all cohort members were required to have at least 1 year of follow-up after entering the study cohort, necessary for latency purposes as short duration exposures are unlikely to be associated with cancer incidence. Thus, follow-up started the year after study cohort entry, until an incident diagnosis of cancer (other than nonmelanoma skin cancer), death from any cause, end of registration with the general practice, or end of study period; 31 December 2010, whichever came first.
Exposure to telmisartan
The use of telmisartan was entered as a time-dependent variable in the models, allowing patients to move from a period of nonexposure to a period of exposure. Since some patients could switch to telmisartan after using another ARB for a period after cohort entry, patients were considered unexposed up until the time of a first telmisartan prescription, and exposed thereafter for the remainder of follow-up. Furthermore, telmisartan exposure was lagged by 1 year in order to take into account a biologically meaningful latency time window given that short duration exposures are unlikely to be associated with cancer incidence. The lag was contributed as unexposed person time upon switch to telmisartan from another ARB.
Based on the above time-dependent exposure definition, exposure to telmisartan was defined in 3 ways: use, cumulative duration of use, and cumulative dose. For the first approach, use of telmisartan was compared with use of other ARBs up until the time of the event. For the second and third approaches, we assessed whether there was duration- and dose-response relationship between the use of telmisartan and the risk of cancer. Cumulative duration of use was defined, in a time-dependent fashion, as the total number of years of telmisartan exposure, calculated by summing the durations of all prescriptions between study cohort entry and the time of the event. This variable was then classified into the following 4 categories: <2 years, 2–4 years, 4–6 years, and >6 years. As for cumulative dose, it was expressed in defined daily doses (DDD) using the 40mg formulation of telmisartan as the reference. Thus, cumulative dose was calculated as a time-dependent variable by summing all DDDs up until the time of the event. This variable was then classified into the following 4 categories: <730 DDDs; 730–1,460 DDDs; 1,460–2,190 DDDs; and >2,190 DDDs.
Potential confounders
All models were adjusted for the following variables measured at study cohort entry: year of study cohort entry, age, sex, body mass index, smoking status, excessive alcohol use, type 2 diabetes, ever use of aspirin, nonsteroidal anti-inflammatory drugs, and statins. The models were further adjusted for previous use of ACEIs, beta-blockers, calcium channel blockers, diuretics, and other antihypertensives, all measured between base-cohort entry and study cohort entry. In the analyses by cancer type, the models were additionally adjusted for the following variables: colorectal cancer: cholecystectomy, inflammatory bowel disease, and history of polyps; breast cancer: oophorectomy, history of oral contraceptives, and hormone replacement therapy; prostate cancer: benign prostatic hyperplasia, ever use of 5-alpha reductase inhibitors, and number of prostate-specific antigen tests (measured in the 2 years prior to study cohort entry). Variables with missing information were coded with an ‘unknown’ category.
Statistical analysis
Descriptive statistics were used to summarize the characteristics of the study cohort. Crude incidence rates and 95% confidence intervals (CIs) based on the Poisson distribution for all cancers and separately for the 4 most common cancers (i.e., lung, breast, prostate, and colorectal) were calculated by cumulating person-years of follow-up separately for telmisartan users and other ARB users. Cox proportional hazards models were used to estimate adjusted hazard ratios (HRs) and 95% CIs of cancer incidence associated with the use of telmisartan compared with the use of other ARBs. This was considered the primary analysis.
We performed 2 secondary analyses. First, we assessed whether there was a duration-response relationship between the use of telmisartan and the incidence of cancer, both overall and according to cancer type. For this analysis, telmisartan cumulative duration of use was entered as a time-dependent variable in the models. Second, we also assessed whether there was a dose-response relationship, with cumulative dose expressed in DDDs entered as a time-dependent variable in the models. Finally, given uncertainties related to the length of the latency time window, we conducted a sensitivity analysis varying the length of the lag period from 1 year to 2 years. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
A total of 62,109 patients newly treated with ARBs were included in the study cohort, which included 3,438 (5.5%) users of telmisartan and 58,671 (94.5%) users of other ARBs at cohort entry (Figure 1). The baseline characteristics of the cohort are presented in Table 1. Overall, users of telmisartan and other ARBs were similar on nearly all characteristics, with the exception of the use of aspirin, statins, antidiabetics, and antihypertensives which were less prevalent in telmisartan users. A total of 547 (0.9%) patients initially prescribed other ARBs eventually switched to telmisartan after a mean (SD) follow-up of 2.0 (1.8) years; their baseline characteristics are presented in a supplementary table (Supplementary Data). During a mean (SD) follow-up of 3.9 (2.2) years, generating 244,039 person-years, 3,947 patients were diagnosed with cancer (crude incidence rate: 16.2, 95% CI: 15.7–16.7 per 1,000 person-years).
Figure 1.
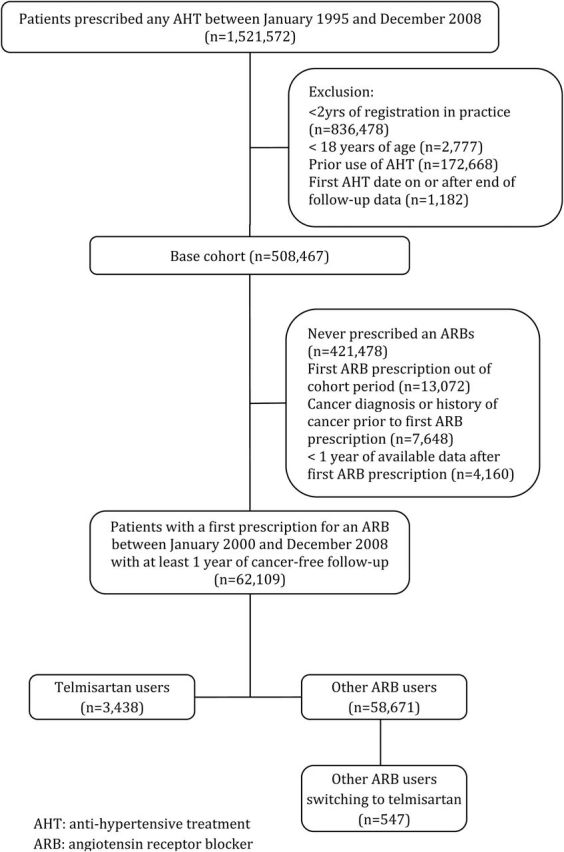
Study flow chart. Abbreviations: AHT, antihypertensive treatment; ARB, angiotensin receptor blocker.
Table 1.
Baseline characteristics of telmisartan and other angiotensin receptor blockers users
| Telmisartan (n = 3,438) | Other ARBs (n = 58,671) | |
|---|---|---|
| Age (years), mean (SD) | 62.5 (12.7) | 62.5 (12.7) |
| Male, n (%) | 1,746 (50.8) | 29,514 (50.3) |
| Follow-up time (years), mean (SD) | 5 (2.2) | 4.9 (2.2) |
| Time at risk (years), mean (SD) | 4 (2.2) | 3.9 (2.2) |
| Year of cohort entry, n (%) | ||
| 2000 | 106 (3.1) | 2,140 (3.6) |
| 2001 | 220 (6.4) | 3,101 (5.3) |
| 2002 | 299 (8.7) | 5,474 (9.3) |
| 2003 | 385 (11.2) | 6,133 (10.5) |
| 2004 | 518 (15.1) | 8,319 (14.2) |
| 2005 | 523 (15.2) | 8,296 (14.1) |
| 2006 | 634 (18.4) | 8,935 (15.2) |
| 2007 | 441 (12.8) | 8,765 (14.9) |
| 2008 | 312 (9.1) | 7,508 (12.8) |
| Excessive alcohol use, n (%) | 125 (3.6) | 2,169 (3.7) |
| Body mass index, n (%) | ||
| <30 | 1,399 (40.7) | 22,931 (39.1) |
| ≥30 | 787 (22.9) | 13,245 (22.6) |
| Unknown | 1,252 (36.4) | 22,495 (38.3) |
| Smoking status, n (%) | ||
| Ever | 1,655 (48.1) | 28,965 (49.4) |
| Never | 1,620 (47.1) | 27,110 (46.2) |
| Unknown | 125 (3.6) | 2,169 (3.7) |
| Aspirin, n (%) | 908 (26.4) | 18,296 (31.2) |
| NSAIDs, n (%) | 2,123 (61.8) | 38,158 (65.0) |
| Statins, n (%) | 1,084 (31.5) | 21,157 (36.1) |
| Drugs used in diabetes, n (%) | ||
| Metformin | 270 (7.9) | 5,736 (9.8) |
| Sulfonylureas | 182 (5.3) | 4,163 (7.1) |
| Insulins | 88 (2.6) | 2,297 (3.9) |
| Other oral antidiabetic drugs | 80 (2.3) | 1,673 (2.9) |
| History of AHT, n (%) | 2,758 (80.2) | 50,026 (85.3) |
| Duration of previous AHT, years (SD) | 2.7 (2.8) | 2.6 (2.7) |
| Drugs used in hypertension, n (%) | ||
| ACEIs | 1,830 (53.2) | 37,593 (64.1) |
| Beta-blockers | 1,204 (35) | 22,070 (37.6) |
| Diuretics | 1,877 (54.6) | 31,079 (53) |
| CCBs | 1,108 (32.2) | 20,079 (34.2) |
| Other antihypertensives | 338 (9.8) | 5,502 (9.4) |
| Colorectal cancer-related variables, n (%) | ||
| Inflammatory bowel disease | 37 (1.1) | 709 (1.2) |
| History of polyps | 30 (0.9) | 666 (1.1) |
| Cholecystectomy | 126 (3.7) | 2,358 (4) |
| Prostate cancer-related variablesa, n (%) | ||
| Benign prostatic hyperplasia | 46 (2.6) | 938 (3.2) |
| Number of PSA test in the 2 years prior to cohort entry | ||
| None | 1,478 (84.7) | 25,576 (86.7) |
| One | 185 (10.6) | 2,969 (10.1) |
| Two | 56 (3.2) | 700 (2.4) |
| Three or more | 27 (1.5) | 269 (0.9) |
| 5-Alpha reductase inhibitors | 36 (1.0) | 691 (1.2) |
| Breast cancer-related variablesb, n (%) | ||
| Oophorectomy | 36 (2.1) | 793 (2.7) |
| Oral contraceptive | 189 (11.2) | 3,413 (11.7) |
| Hormone replacement therapy | 470 (27.8) | 8,344 (28.6) |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AHT, antihypertensive treatment; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; NSAIDs, nonsteroidal anti-inflammatory drug; PSA, prostate-specific antigen.
aPercentages calculated among males.
bPercentages calculated among females.
Table 2 presents the results of the primary and secondary analyses for all cancers combined. Compared with other ARBs, the use of telmisartan was not associated with an increased risk of any cancer (16.3 vs. 15.0 per 1,000 person-years, respectively; adjusted HR: 0.93, 95% CI: 0.81–1.06). Similarly, there was no evidence of a duration- or dose-relationship between telmisartan use and the incidence of all cancers combined.
Table 2.
Crude and adjusted HRs of all cancers associated with telmisartan use compared with other ARBs
| Events | Person-years | Rate per 1,000/year (95% CI) | Crude HR | Adjusted HR (95% CI) | |
|---|---|---|---|---|---|
| All cancers | |||||
| Other ARBs, n (%) | 3,712 | 228,355 | 16.3 (15.7–16.8) | 1.00 | 1.00 (Reference) |
| Telmisartan, n (%) | 235 | 15,684 | 15.0 (13.2–17.0) | 0.92 | 0.93 (0.81–1.06) |
| Cumulative dose* | |||||
| ≤730 DDD | 117 | 7,543 | 15.5 (12.9–18.6) | 0.97 | 0.96 (0.80–1.16) |
| 730–1,460 DDD | 56 | 3,889 | 14.4 (11.1–18.7) | 0.89 | 0.90 (0.69–1.18) |
| 1,460–2,190 DDD | 24 | 1,943 | 12.4 (8.3–18.4) | 0.74 | 0.78 (0.52–1.17) |
| >2,190 DDD | 38 | 2,309 | 16.5 (12.0–22.6) | 0.94 | 0.96 (0.69–1.32) |
| Cumulative duration* | |||||
| ≤2 years | 114 | 7,952 | 14.3 (11.9–17.2) | 0.90 | 0.90 (0.75–1.09) |
| 2–4 years | 71 | 4,878 | 14.6 (11.5–18.4) | 0.88 | 0.89 (0.71–1.13) |
| 4–6 years | 34 | 2,052 | 16.6 (11.8–23.2) | 0.98 | 1.01 (0.72–1.42) |
| >6 years | 16 | 802 | 20.0 (12.2–32.6) | 1.06 | 1.09 (0.66–1.80) |
Abbreviations: ARB, angiotensin receptor blocker; CI, confidence interval; DDD, defined daily dose; HR, hazard ratio.
*P for trend >0.05 for both analyses.
Table 3 presents the results stratified according to cancer type. Overall, compared with other ARBs, the use of telmisartan was not associated with a statistically significant increased risk of lung, breast, prostate, or colorectal cancer. Adjusted HRs ranged between 0.79 and 1.41 with all CIs spanning the null value. In contrast, telmisartan was associated with 17% decreased risk of other cancers (adjusted HR: 0.83, 95% CI: 0.70–0.99). In secondary analyses (Supplementary Data–Supplementary Data), a cumulative duration of less than 2 years and a cumulative dose less than 730 DDDs were both associated with an increased risk of colorectal cancer (Supplementary Data), but there was no clear duration- and dose-response relationship. Varying the latency time window from 1 to 2 years did not materially change the results for all cancers combined and according to cancer type (Supplementary Data).
Table 3.
Crude and adjusted HRs of lung, breast, prostate, and colorectal cancers associated with telmisartan use compared with other ARBs
| Events | Person-years | Rate per 1,000/year (95% CI) | Crude HR | Adjusted HR (95% CI)a | |
|---|---|---|---|---|---|
| Lung cancer | |||||
| Other ARBs, n (%) | 264 | 228,355 | 1.2 (1.0–1.3) | 1.00 | 1.00 (Reference) |
| Telmisartan, n (%) | 16 | 15,684 | 1.0 (0.6–1.7) | 0.87 | 0.91 (0.55–1.51) |
| Breast cancer | |||||
| Other ARBs, n (%) | 385 | 114,388 | 3.4 (3.1–3.7) | 1.00 | 1.00 (Reference) |
| Telmisartan, n (%) | 34 | 7,829 | 4.3 (3.1–6.1) | 1.29 | 1.28 (0.90–1.82) |
| Prostate cancer | |||||
| Other ARBs, n (%) | 459 | 113,967 | 4.0 (3.7–4.4) | 1.00 | 1.00 (Reference) |
| Telmisartan, n (%) | 26 | 7,855 | 3.3 (2.3–4.9) | 0.82 | 0.79 (0.53–1.18) |
| Colorectal cancer | |||||
| Other ARBs, n (%) | 274 | 228,355 | 1.2 (1.1–1.4) | 1.00 | 1.00 (Reference) |
| Telmisartan, n (%) | 27 | 15,684 | 1.7 (1.2–2.5) | 1.41 | 1.41 (0.95–2.10) |
| Other cancers | |||||
| Other ARBs, n (%) | 2,330 | 228,355 | 10.2 (9.8–10.6) | 1.00 | 1.00 (Reference) |
| Telmisartan, n (%) | 132 | 15,684 | 8.4 (7.1–10.0) | 0.82 | 0.83 (0.70–0.99) |
Abbreviations: ARB, angiotensin receptor blocker; CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen.
aAdjusted for the variables listed in Table 1. In addition, cholecystectomy, inflammatory bowel disease and history of polyps for colorectal cancer; benign prostatic hyperplasia, 5-alpha reductase inhibitors, and number of PSA tests for prostate cancer; oophorectomy, use of hormone replacement therapy, and prior use of oral contraceptives for breast cancer.
DISCUSSION
In this large population-based study, the use of telmisartan was neither associated with an overall increased risk of cancer nor with 1 of the 4 most common cancers (lung, breast, prostate, and colorectal) in comparison with other ARBs. In secondary analyses, short durations and low cumulative doses of telmisartan were associated with an increased risk of colorectal cancer. However, the results of these secondary analyses need to be interpreted with caution, especially given the absence of duration- and dose-response relationships.
To our knowledge, this population-based observational study is the first to specifically assess the risk of cancer associated with the use of telmisartan. While the initial meta-analysis reporting an increased risk of cancer with ARBs included predominantly telmisartan,1 in the subsequent observational studies that followed, telmisartan constituted only a minority of total ARB exposure. Furthermore, among the 7 previous observational studies that investigated the risk of cancer with the use of ARBs,5–10,13 38–10 conducted analyses on the individual drugs. The results of our study are consistent with those of a Danish study, that reported no association of any cancer with telmisartan when compared with ACEIs (HR: 0.97, 95% CI: 0.72–1.29).10 Our results are also consistent with those of a previous CPRD study in which telmisartan was not associated with an increased risk of any cancer in comparison with ACEIs (HR: 0.99, 95% CI: 0.87–1.14).8 While the authors also conducted an analysis for specific ARBs, the analysis was restricted to the initial ARB prescribed, disregarding switches between different ARBs. In a Taiwanese study comparing incident cancer cases and controls nested in a cohort of individuals with type 2 diabetes, the use of telmisartan was associated with an increased risk of any cancer9 compared with no ARB use. However, the results were based on few events (n = 29; odds ratio: 1.58, 95% CI: 1.01–2.45) and were no longer significant after statistical adjustment (odds ratio: 1.54, 95% CI: 0.97–2.43). In all of the observational studies described above,8–10 the individual drug-specific analyses were secondary and exploratory in nature, and importantly, CIs of the reported effect estimates did not exclude an 8% increase in the risk of cancer that was reported in the initial meta-analysis.1 This is in contrast to our study that was specifically designed to assess the risk with telmisartan in comparison to other ARBs. In addition, the previous studies compared the different ARBs with ACEIs. As there are differences between ARB and ACE users, residual confounding by indication remains a possibility. In this study, telmisartan was compared with other ARBs, the latter being a group for which there is clinical equipoise. Indeed, the baseline characteristics were similar between the groups suggesting that residual confounding was likely minimal.
Our study has a number of strengths. The use of the CPRD allowed us to assemble a large real-world cohort of new ARB users for whom data collection was independent of the outcomes and exposures, thus minimizing the risk of selection and information bias. The new-user design and use of a time-dependent exposure definition also allowed us to avoid immortal time bias that had been a concern in some previous studies.7,13,14 Residual confounding was likely minimized by using a similar active comparator consisting of other ARB users. Indeed, these patients were very similar to telmisartan users in terms of their baseline characteristics.
Assuming an average follow-up duration of 5 years and a cancer incidence rate of 1.5% per year for the 65- to 69-year-old age group, we had estimated that with a 2-tailed alpha error rate of 5%, 56,660 new ARB users would be sufficient to provide 80% power to detect a cancer incidence of 1.8% per year in telmisartan users—corresponding to a HR of 1.2, assuming that other ARB users would have an incidence similar to the general population. With a larger sample size than our initial plan, the precision of our effect estimate suggests that more than a 6% increased risk of cancer with telmisartan use is very unlikely, which is below the 8% risk reported in the first meta-analysis.
This study also has some limitations. First, drug records in the CPRD represent prescriptions written by the general practitioners, and thus, it is unknown if these prescriptions were filled and whether patients complied with the treatment. However, it is unlikely that this was differential between telmisartan and other ARB users. Second, although we have taken into account the major lifestyle risk factors influencing cancer risk (such as smoking status, body mass index, and excessive alcohol use), these variables were at times missing and additional important cancer risk factors such as family history, ethnicity or genetic susceptibility, environmental and occupational exposures were not available. However, misclassification or missing covariate information is unlikely to be differential between the exposure groups. Finally, the average follow-up was relatively short (3.9 years), and thus, this study provides some reassurance on the relative short-term use of telmisartan. That being said, this duration is comparable to those in the longer ARB trials (LIFE,15 CHARM,16 TRANSCEND,17 and ONTARGET18), which were all included the meta-analysis that generated the increased risk of cancer.
In summary, the results of this large population-based study provides evidence that risk of cancer overall, and of 4 most common cancers, is not increased with the use of telmisartan in comparison with other ARBs. These results provide important evidence regarding the safety of telmisartan, and together with previous observational studies on the association between ARBs and cancer incidence, provide reassurance as to the safety of these drugs.
SUPPLEMENTARY MATERIAL
Supplementary Data are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
Dr Suissa has received research grants, participated in advisory board meetings or as a speaker, supported by AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers-Squibb, Merck, and Novartis. Dr Bartels is an employee of Boehringer Ingelheim GmbH. Other authors declared no conflict of interest. The funding sources had no role in the design, analysis, or interpretation of the results, and thus the authors were independent from the funding sources.
Supplementary Material
ACKNOWLEDGMENTS
Study concept and design was done by Dr Tascilar, Dr Azoulay, Dr Bartels and Dr Suissa. Acquisition of data: Dr Bartels and Dr Suissa. Analysis and interpretation of data: Dr Tascilar, Dr Azoulay, and Dr Suissa. Drafting of the manuscript: Dr Tascilar. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr Tascilar, Dr Azoulay, Dr Dell’Aniello, and Dr Suissa. Obtained funding: Dr Bartels and Dr Suissa. Study supervision: Dr Suissa. This research was funded by grants from the Canadian Institutes of Health Research, Boehringer Ingelheim, and the Canadian Foundation for Innovation. Dr Suissa is the recipient of the James McGill Chair award and Dr Azoulay is the recipient of a Chercheur Boursier award from the Fonds de recherche du Québec – Santé (FRQS). Author contributions: Dr. Suissa had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. There are no previous presentation of the information reported in the manuscript.
REFERENCES
- 1. Sipahi I, Debanne SM, Rowland DY, Simon DI, Fang JC. Angiotensin-receptor blockade and risk of cancer: meta-analysis of randomised controlled trials. Lancet Oncol 2010; 11:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bangalore S Kumar S Kjeldsen SE Makani H Grossman E Wetterslev J, Gupta AK Sever PS Gluud C Messerli FH. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol 2011; 12:65–82. [DOI] [PubMed] [Google Scholar]
- 3. ARB Trialists Collaboration. Effects of telmisartan, irbesartan, valsartan, candesartan, and losartan on cancers in 15 trials enrolling 138,769 individuals. J Hypertens 2011; 29:623–635. [DOI] [PubMed] [Google Scholar]
- 4. FDA. FDA Drug Safety Communication: No increase in risk of cancer with certain blood pressure drugs—Angiotensin Receptor Blockers (ARBs) <http://www.fda.gov/Drugs/DrugSafety/ucm257516.htm> 2011.
- 5. Azoulay L, Assimes TL, Yin H, Bartels DB, Schiffrin EL, Suissa S. Long-term use of angiotensin receptor blockers and the risk of cancer. PLoS One 2012; 7:e50893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hallas J, Christensen R, Andersen M, Friis S, Bjerrum L. Long term use of drugs affecting the renin-angiotensin system and the risk of cancer: a population-based case-control study. Br J Clin Pharmacol 2012; 74:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang CC, Chan WL, Chen YC, Chen TJ, Lin SJ, Chen JW, Leu HB. Angiotensin II receptor blockers and risk of cancer in patients with systemic hypertension. Am J Cardiol 2011; 107:1028–1033. [DOI] [PubMed] [Google Scholar]
- 8. Bhaskaran K, Douglas I, Evans S, van Staa T, Smeeth L. Angiotensin receptor blockers and risk of cancer: cohort study among people receiving antihypertensive drugs in UK General Practice Research Database. BMJ 2012; 344:e2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang CH, Lin JW, Wu LC, Lai MS. Angiotensin receptor blockade and risk of cancer in type 2 diabetes mellitus: a nationwide case-control study. J Clin Oncol 2011; 29:3001–3007. [DOI] [PubMed] [Google Scholar]
- 10. Pasternak B, Svanström H, Callréus T, Melbye M, Hviid A. Use of angiotensin receptor blockers and the risk of cancer. Circulation 2011; 123:1729–1736. [DOI] [PubMed] [Google Scholar]
- 11. Williams T, van Staa T, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf 2012; 3:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiang YY, Chen KB, Tsai TH, Tsai WC. Lowered cancer risk with ACE inhibitors/ARBs: a population-based cohort study. J Clin Hypertens (Greenwich) 2014; 16:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Assimes TL, Suissa S. Immortal person time bias in pharmacoepidemiological studies of antihypertensive drugs. Am J Cardiol 2011; 108:902–903. [DOI] [PubMed] [Google Scholar]
- 15. Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002; 359:995–1003. [DOI] [PubMed] [Google Scholar]
- 16. Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S; CHARM Investigators and Committees Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766. [DOI] [PubMed] [Google Scholar]
- 17. Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P; Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet 2008; 372:1174–1183. [DOI] [PubMed] [Google Scholar]
- 18. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C; ONTARGET Investigators Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 2008; 358:1547–1559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


