Abstract
BACKGROUND
Obesity has been linked with abnormal nocturnal dipping of blood pressure (BP) in adults, which in turn is associated with poor cardiovascular outcomes. There are few data regarding abnormal dipping status in the obese pediatric population. The goal of this study was to further describe the relationship between obesity and non-dipping status on ambulatory blood pressure monitor (ABPM) in children.
METHODS
We conducted a cross-sectional study using a database of patients aged 5–21 years who had undergone 24-hour ABPM at Seattle Children’s Hospital from January 2008 through May 2014. Subjects were grouped by body mass index (BMI) into lean (BMI 15th–85th percentile) and obese (BMI >95th percentile) groups.
RESULTS
Compared to lean subjects ( n = 161), obese subjects ( n = 247) had a prevalence ratio (PR) for non-dipping of 2.15, adjusted for race (95% confidence interval (CI) = 1.25–3.42). Increasing severity of obesity was not further associated with nocturnal non-dipping. Nocturnal non-dipping was not associated with left ventricular hypertrophy (PR = 1.01, 95% CI = 0.71–1.44).
CONCLUSIONS
These results suggest that in children, just as in adults, obesity is related to a relatively decreased dipping in nocturnal BP.
Keywords: ambulatory blood pressure monitor, ABPM, blood pressure, hypertension, nocturnal dipping, obesity, pediatrics.
The prevalence of hypertension in pediatrics is increasing 1 particularly in the obese. 2 Obese children with hypertension are likely to remain hypertensive as they reach adulthood 3 and hypertension has been shown to have significant effects on future cardiovascular health 4 as well as development of chronic kidney disease. 5
Twenty-four-hour ambulatory blood pressure monitoring (ABPM) has been immensely helpful in diagnosing hypertension in children, allowing physicians to identify white coat hypertension, masked hypertension, and evaluate nocturnal dipping status. 6 In adults, obesity is associated with nocturnal hypertension and abnormal dipping status on 24-hour ABPM. 7 Nocturnal hypertension specifically has been shown to significantly affect prognosis in adults 8 and children. 9 In adults, abnormal dipping status noted on ABPM has likewise been independently associated with development of chronic kidney disease 10 and worsening kidney injury, 11 elevated left ventricular mass and impaired left ventricular function, 12 and increased risk of cardiovascular disease in general. 13
There are few data regarding BP dipping status in the obese pediatric population. The studies that have been performed to date have either been limited by small numbers of subjects 14–16 or were conducted in patient populations with significant medical conditions such as diabetes. 17 The goal of this study was to further describe the relationship between obesity and non-dipping status on ABPM, which may eventually lead to better treatments and prevention methods.
METHODS
Data source
We conducted a cross-sectional study using a database of patients aged 5–21 years who had undergone a first-time 24-hour ABPM at Seattle Children’s Hospital from January 2008, through May 2014. Retrospective data collection was approved by the Institutional Review Board at Seattle Children’s Hospital.
Subject selection/methods
Subjects were selected by querying the electronic medical record to identify all patients who had undergone 24-hour ABPM during the study period. Demographic information collected included age, race, gender, past medical history, and current medications at time of ABPM study. Exclusion criteria included conditions that could affect BP (chronic kidney disease, thyroid disease, congenital heart disease, history of prematurity <34 weeks of gestation, sleep disordered breathing diagnosed by no later than 90 days after ABPM study, etc.), and medications that could affect BPs (antihypertensives, glucocorticoids, immunosuppressants, stimulants).
All ABPMs were performed using a Spacelabs (Issaquah, WA) 90217 monitor. The monitor was placed by nursing staff in our office at the time of clinic visit. Appropriate cuff size was determined using guidelines from the 4th report on diagnosis, evaluation, and treatment of pediatric hypertension. 18 Readings were taken every 20 minutes while awake and every 30 minutes while asleep. Patients were asked to keep a diary showing sleep and wake times. The adequacy of the ABPM study was determined by the interpreting physician at the time of ABPM evaluation according to AHA criteria. 6
Subjects were grouped into categories based on body mass index (BMI): lean (15th–85th BMI percentile), overweight (>85th–95th percentile), and obese (≥95th percentile). 19 The primary outcome was the prevalence of adequate nocturnal dipping in the lean group vs. the obese group. Adequate nocturnal dipping was defined as a decrease in both systolic and diastolic mean nocturnal BP of at least 10% from mean systolic and diastolic awake BP.
A secondary analysis was performed examining the relationship between the severity of obesity and prevalence of non-dipping status. Obesity categories generated using BMI z -scores, based on previous studies 20 : obesity category I (BMI z -score 1.6449 to <2), obesity category II (BMI z -score 2 to <2.5), obesity category III (BMI z -score ≥2.5). Lastly, we evaluated the relationship between obesity and the prevalence of isolated nocturnal hypertension or prehypertension, and the relationship between nocturnal non-dipping and the prevalence of left ventricular hypertrophy.
Hypertension was defined as a mean systolic or diastolic ambulatory BP ≥95th percentile for age, sex, and height 21 during either the awake or sleep period. Prehypertension was defined as mean ambulatory BP less than the 95th percentile for age, sex, and height, but with a BP load (percentage of individual BP readings >95th percentile) between 25% and 50%. Elevated mean BPs that met criteria for hypertension or prehypertension but occurred only during sleep hours were designated nocturnal hypertension and prehypertension, respectively. Left ventricular hypertrophy was defined as LV mass indexed to height in meters to the 2.7 power that is greater than the 95th percentile for age and sex, as defined in 2009 by Khoury et al . 22
Statistical analysis
Continuous variables were expressed as the mean ± SD. Differences in means between continuous outcomes were determined using 2-sample t -tests. Our primary analysis of the association of obesity with nocturnal non-dipping was performed using a log-binomial regression to calculate prevalence ratio (PR) estimates and test-based 95% confidence intervals (CIs). Only factors that altered estimates substantially (10% or more) were considered to be confounders and adjusted for in our analysis. Variables that were considered for adjustment were subject age, race, gender, and daytime BP status. Assessment of effect modification was based on biological plausibility and clinically relevant differences in PRs across strata. Secondary analyses were also evaluated by stratified analysis using PR estimates. A P -value of <0.05 was considered significant. STATA (Stata, College Station, TX) version 13.1 was used for statistical analysis.
RESULTS
General characteristics of subjects
A total of 1,620 ABPMs were completed during the study period; 248 of these were repeat ABPMs on an individual subject, thus 1,372 “first-time” ABPMs were identified. Of these, 98 were of inadequate quality for interpretation. Following application of exclusion criteria, there were 408 subjects included in the primary analysis, of whom 161 were lean and 247 obese ( Figure 1 ). Ninety subjects in the overweight group were not included in the analysis as we were interested in comparing only obese to lean subjects. Distributions were generally similar for age and gender between the 2 groups ( Table 1 ). There were differences noted in race, with 49% of subjects in the lean group being Caucasian, compared to 36% in the obese group. There were 15% of subjects in the lean group classified as “Other,” compared to 28% in the obese group. All other racial backgrounds were distributed evenly between the 2 groups.
Figure 1. .
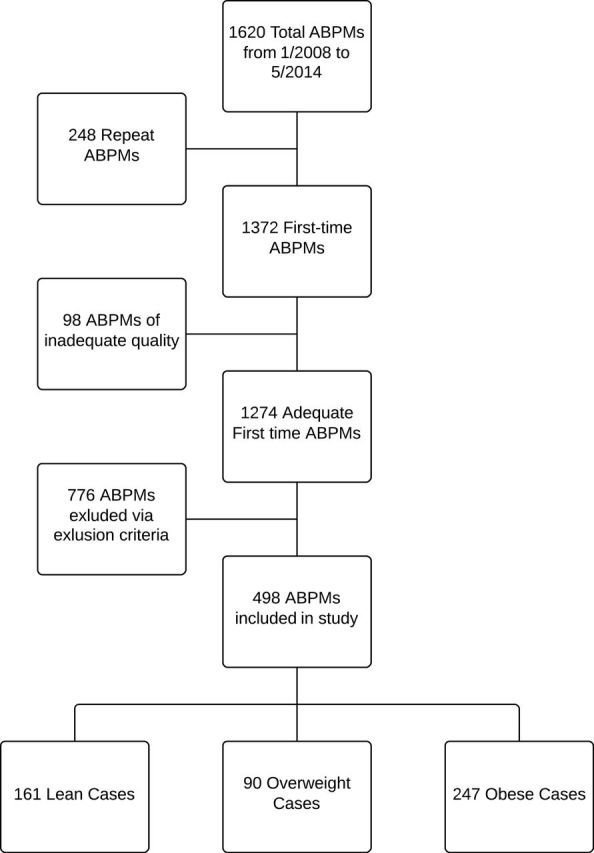
A total of 1,620 ABPMs were performed between January 2008 and May 2014, of which 1,372 were first-time ABPMs. Ninety eight of these were of inadequate quality to be interpretable. Following application of exclusion criteria, 408 first-time ABPMs were eligible for analysis including 161 in lean subjects and 247 in obese subjects. Ninety overweight subjects were not included. Abbreviations: ABPM, ambulatory blood pressure monitor.
Table 1.
Characteristics of lean and obese subjects
| Lean a | Obese a | |||
|---|---|---|---|---|
| N = 161 | % | N = 247 | % | |
| Race | ||||
| White | 79 | 49.1 | 89 | 36.0 |
| Black | 10 | 6.2 | 13 | 5.3 |
| Native American/Alaskan | 0 | 0 | 4 | 1.6 |
| Asian | 19 | 11.8 | 22 | 8.9 |
| Hawaiian/Pacific Islander | 0 | 0 | 2 | 0.8 |
| Other | 24 | 14.9 | 70 | 28.3 |
| Unknown | 29 | 18.0 | 47 | 19.0 |
| Gender | ||||
| Male | 107 | 66.5 | 178 | 72.1 |
| Female | 54 | 33.5 | 69 | 27.9 |
| Age (years; mean ± SD) | 14.6±3.3 | 14.2±2.9 | ||
| Height (cm; mean ± SD) | 164.2±18.2 | 165.4±16.0 | ||
| BMI (kg/m 2 , mean ± SD) | 20.8±2.7 | 33.4±6.4 | ||
Abbreviation: BMI, body mass index.
a Lean group defined as 15th-85th BMI percentile, obese group defined as ≥95th BMI percentile.
Prevalence of non-dipping
Mean awake and asleep systolic and diastolic BPs and dipping status are shown in Table 2 . The prevalence of hypertension was 40.3% in the lean group and 43.7% in the obese group, which is not significantly different (PR = 1.14, 95% CI = 0.92–1.42). Both systolic and diastolic nocturnal dipping were significantly blunted ( Figure 2 ) in the obese group compared to the lean group ( P < 0.0001).
Table 2.
ABPM findings in lean and obese subjects, adjusted for race a
| Lean b | Obese b | P -value | |
|---|---|---|---|
| 24-hour SBP (mm Hg) | 123 ± 11 | 124 ± 9 | 0.305 |
| 24-hour DBP (mm Hg) | 71 ± 7 | 69 ± 6 | 0.005 |
| Awake SBP (mm Hg) | 130 ± 12 | 130 ± 10 | 0.644 |
| Awake DBP (mm Hg) | 77 ± 8 | 74 ± 7 | <0.001 |
| Asleep SBP (mm Hg) | 110 ± 10 | 113 ± 9 | 0.006 |
| Asleep DBP (mm Hg) | 60 ± 7 | 60 ± 6 | 0.976 |
| SBP Noct. Dipping (%) | 15 ± 5 | 12 ± 6 | <0.001 |
| DBP Noct. Dipping (%) | 22 ± 6 | 19 ± 7 | <0.001 |
Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure.
a Data displayed as mean ± SD. b Lean group defined as 15th–85th BMI percentile, obese group defined as ≥95th BMI percentile.
Figure 2.
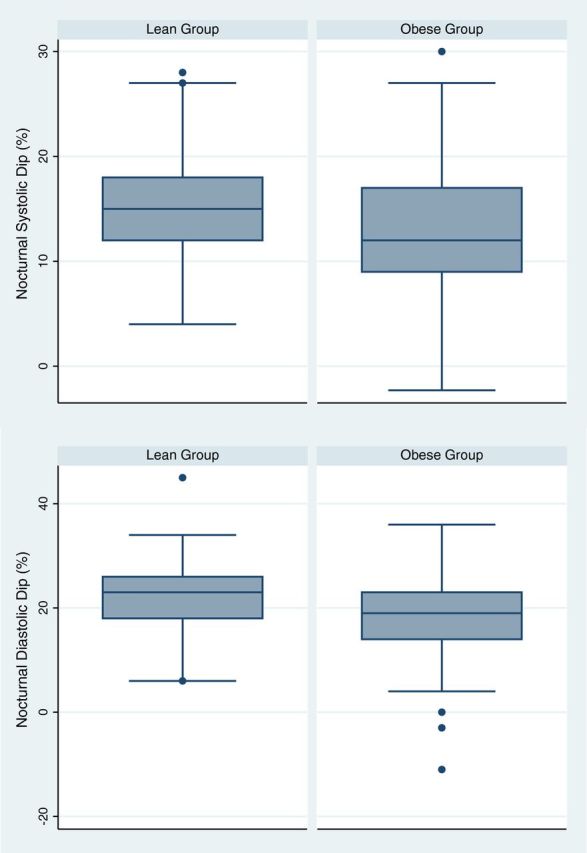
Mean nocturnal dipping was decreased in the obese group compared to the lean group. ( A ) Mean systolic dip was 15.1% ± 4.6% in lean subjects compared to 12.4 ± 5.6% in obese subjects ( P -value < 0.001). (B) Mean diastolic dip was 22.2 ± 6.0% in lean subjects compared to 18.5 ± 7.0% in obese subjects ( P -value < 0.001).
Of the 161 subjects in the lean group, 22 were classified as non-dippers (13.6%) ( Table 3 ). Of the 247 subjects in the obese group, 85 were classified as non-dippers (34.4%). The crude PR of non-dipping was 2.51 (95% CI = 1.65–3.85) in obese subjects compared to lean subjects. The PR adjusted for race was 2.15 (95% CI = 1.25–3.42). The PR was significantly increased in the obese group for both systolic dipping cases ( N = 313, PR = 2.28, 95% CI = 1.40–3.74) and diastolic dipping cases ( N = 113, PR = 3.35, 95% CI = 1.17–9.59). The PR was not affected by adjustment for daytime BP, age, or gender of the subjects, although there was a slightly higher PR in females (PR = 2.37, 95% CI = 1.10–5.11) compared to males (PR = 2.08, 95% CI = 1.16–3.74), which is presented in Table 4 .
Table 3.
Prevalence ratio of the association between obesity and non-dipping Status, adjusted for race
| Non-dipping | PR | 95% CI | ||||
|---|---|---|---|---|---|---|
| Yes | % | No | % | |||
| Lean a | 22 | 13.7 | 139 | 86.3 | 1.00 | Ref |
| Obese a | 85 | 34.4 | 162 | 65.6 | 2.15 | 1.25–3.42 |
Abbreviation: CI, confidence interval; PR, prevalence ratio.
a Lean group defined as 15th–85th BMI percentile, obese group defined as ≥95th BMI percentile.
Table 4.
Prevalence ratio of non-dipping by gender and age, adjusted for race
| Lean a non-dippers | Obese a non-dippers | |||||
|---|---|---|---|---|---|---|
| N | % | N | % | PR | 95% CI | |
| By sex | ||||||
| Male | 14 | 13.1 | 58 | 32.6 | 2.08 | 1.16–3.74 |
| Female | 8 | 14.8 | 27 | 39.1 | 2.37 | 1.10–5.11 |
| By age (years) | ||||||
| 5–8 | 1 | 5.9 | 7 | 46.7 | 5.18 | 0.69–38.9 |
| 9–13 | 8 | 22.2 | 34 | 40.0 | 1.50 | 0.72–3.11 |
| 14–17 | 11 | 11.6 | 38 | 28.6 | 2.14 | 1.11–4.11 |
| 18–21 | 2 | 15.4 | 6 | 42.9 | 4.35 | 0.57–32.77 |
a Lean group defined as 15th–85th BMI percentile, obese group defined as ≥95th BMI percentile.
Secondary analyses
Obesity was found to be significantly associated with isolated nocturnal hypertension; 44 of 247 subjects in the obese group had isolated nocturnal hypertension or prehypertension, compared to 12 of 161 subjects in the lean group. This resulted in a crude PR of 2.37 (95% CI = 1.51–3.73). This was not affected by adjustment for age, race, or gender.
Increasing severity of obesity was not significantly associated with nocturnal non-dipping status. Compared to the reference group of obese category I (BMI z -score 1.65 to <2), subjects in obese category II (BMI z -score 2 to <2.5) had a non-dipping PR of 1.28 (95% CI = 0.66–2.43) and subjects in obese category III (BMI z -score ≥2.5) had a PR of 1.16 (95% CI = 0.61–1.65).
Nocturnal dipping status was not found to be significantly associated with left ventricular hypertrophy (LVH). Although there was a crude PR of 1.26 (95% CI = 0.88–1.81), this decreased to 1.01 (95% CI = 0.71–1.44) when adjusted for obesity status.
DISCUSSION
In this large study of children referred for evaluation of suspected hypertension, obesity was significantly associated with non-dipping status and nocturnal hypertension. We did not find a significant association between non-dipping status and increasing severity of obesity, or nocturnal dipping status and the prevalence of LVH.
Non-dipping status has been shown to have significant effects on health outcomes. In adults, specifically, non-dipping has been associated with poor cardiovascular, 12 , 13 renal, 10 , 11 and diabetic outcomes. 23 The data in the pediatric population are not as strong. Nocturnal hypertension and non-dipping have been associated in adolescent diabetics with diabetic nephropathy, 24 LVH, 25 and increased carotid intimal-media thickness. 9 Non-dipping has also been associated with worsening GFR in children with CKD. 26
There are few available data on the prevalence of dipping status in the general pediatric population. Previous studies that have looked at the association between obesity and nocturnal dipping have been much lesser. Framme et al . found a similar association as ours, 14 with a total of 80 subjects (25 in the lean group and 55 in the obese group). Their study, however, found that that obesity only had a significant effect on nocturnal dipping in females, whereas we saw a strong association between obesity and nocturnal dipping in both genders. A recent 2014 study by Westerstahl et al . looked at the prevalence of non-dipping in 76 obese children and adolescents. 27 Westerstahl et al. found the prevalence of non-dipping in obese children to be about twice that in children overall. We found a similar prevalence of non-dipping in the obese group compared to this study: 34% in our obese group compared to 40% in the Westerstahl et al .’s study. The Westerstahl et al .’s study did not have lean subjects as a control, however, instead using established normative pediatric ABPM data as their comparison group. 28
The lack of significance in the relationship between increasing severity of obesity and prevalence of nocturnal dipping demonstrated here could be due to a lack of statistical power, as the PR for both more obese categories was greater than 1. In addition, it is quite possible that the relationship was underestimated secondary to undiagnosed obstructive sleep apnea (OSA). OSA is positively associated with both obesity and nocturnal hypertension. 29 There seems to be an association between OSA and nocturnal non-dipping, 30 although the data are inconsistent. 31 Poor sleep quality has also been linked with non-dipping. 32 It is likely that there were subjects with undiagnosed OSA in our study.
One surprising finding of this study was the significantly higher awake and 24-hour DBP in lean subjects compared to obese subjects. In addition, there were no significant differences between the systolic BPs of the 2 groups. This may reflect selection bias, as all subjects were referred to Seattle Children’s Hospital for evaluation of elevated BP, which may decrease the generalizability of our results to a general pediatric population. However, our findings would pertain to children referred to other pediatric hypertension clinics. These results also raise the question of whether the higher prevalence of non-dipping in the obese population was due to the lower mean awake DBP in the obese group compared to the lean group. The higher prevalence of non-dipping in the obese group was seen with both systolic and diastolic dipping, however. In addition, there were almost 3 times as many cases of systolic non-dipping ( N = 313) as diastolic non-dipping ( N = 113). The increased prevalence of systolic non-dipping in the obese group cannot be attributed to BP differences in the 24-hour ABPM findings, as the lean and obese groups did not have significant different awake mean SBPs.
The causal mechanism between obesity and nocturnal non-dipping is still a subject of active research and is likely to be complex. 33 Activation of the sympathetic nervous system is thought to play a significant role. Compared to lean children, obese children have reduced cardiac vagal function and overall increased sympathetic activity. 34 Insulin resistance and impaired glucose tolerance are possible mechanisms by which sympathetic activation occurs. 35 Obesity-associated elevation of leptin, 36 reduction of adiponectin, 37 and alteration of other neuropeptides 33 have also been associated with hypertension. Nocturnal natriuresis, which is increased by hyperinsulinemia and elevated leptin levels, is elevated in non-dippers compared to normal dippers. 38 Finally, the renin-angiotensin-aldosterone system (RAAS) activity, which is upregulated in obesity, plays a role in circadian BP patterns. 39
Our study had several limitations. Given the cross-sectional design, we cannot make inference about causality. We do not have information regarding Hispanics subjects, as that was not recorded in our database. This is important as the Hispanic population has been shown to have increased obesity-associated hypertension 20 and may respond to treatment of nocturnal hypertension differently than Caucasian populations. 40 We also did not have information regarding sodium intake or exercise habits of subjects, both of which could affect BP results. Finally, as mentioned above, our subjects were all referred for hypertension evaluations, which may decrease the generalizability of our results. Despite these weaknesses, we feel that this analysis adds significantly to this literature regarding obesity and non-dipping in the pediatric population. Strengths of this study include its size, as it is one of the largest ABPM studies performed in the pediatric population, and the fact that we were able to clearly separate lean and obese groups according to BMI percentile.
In conclusion, we found a significant and robust association between obesity and nocturnal non-dipping. This provides a basis for further prospective study into this relationship, as well as adds to the public health importance of childhood obesity. As non-dipping is so clearly linked to worse health outcomes in adults, it is important for us to continue to describe the relationship between obesity and non-dipping, as well as continue to investigate health outcomes in the pediatric population. Our data also further support the benefit of utilizing ABPM in the evaluation of childhood hypertension, as it offers valuable information that cannot be ascertained from office or home BP measurements.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We thank the National Institute of Diabetes and Digestive and Kidney Diseases for providing fellowship funding (Grant # T32 DK007662) and Data Information Services at Seattle Children’s Hospital for technical support.
REFERENCES
- 1. McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ . Prevalence of hypertension and pre-hypertension among adolescents . J Pediatr 2007. ; 150 : 640 – 644 .e1. [DOI] [PubMed] [Google Scholar]
- 2. Sorof JM, Lai D, Turner J, Poffenbarger T, Portman RJ . Overweight, ethnicity, and the prevalence of hypertension in school-aged children . Pediatrics 2004. ; 113 : 475 – 482 . [DOI] [PubMed] [Google Scholar]
- 3. Chen X, Wang Y . Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis . Circulation 2008. ; 117 : 3171 – 3180 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayman LL . The cardiovascular impact of the pediatric obesity epidemic: is the worst yet to come? J Pediatr 2011. ; 158 : 695 – 696 . [DOI] [PubMed] [Google Scholar]
- 5. Flynn JT, Urbina EM . Pediatric ambulatory blood pressure monitoring: indications and interpretations . J Clin Hypertens 2012. ; 14 : 372 – 382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association . Hypertension 2014. ; 63 : 1116 – 1135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kotsis V, Stabouli S, Bouldin M, Low A, Toumanidis S, Zakopoulos N . Impact of obesity on 24-hour ambulatory blood pressure and hypertension . Hypertension 2005. ; 45 : 602 – 607 . [DOI] [PubMed] [Google Scholar]
- 8. Fan H-Q, Li Y, Thijs L, Hansen TW, Boggia J, Kikuya M, et al. Prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations . J Hypertens 2010. ; 28 : 2036 – 2045 . [DOI] [PubMed] [Google Scholar]
- 9. Lee SH, Kim JH, Kang MJ, Lee YA, Won Yang S, Shin CH . Implications of nocturnal hypertension in children and adolescents with type 1 diabetes . Diabetes Care 2011. ; 34 : 2180 – 2185 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. An HR, Park S, Yoo T-H, Kang S-W, Ryu J-H, Lee YK, et al. Non-dipper status and left ventricular hypertrophy as predictors of incident chronic kidney disease . J Korean Med Sci 2011. ; 26 : 1185 – 1190 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang C, Zhang J, Liu X, Li C, Ye Z, Peng H, et al. Reversed dipper blood-pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease . PloS one 2013. ; 8 : e55419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, et al. Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure . J Hypertens 2004. ; 22 : 273 – 280 . [DOI] [PubMed] [Google Scholar]
- 13. Hermida RC, Ayala DE, Mojon A, Fernandez JR . Blunted sleep-time relative blood pressure decline increases cardiovascular risk independent of blood pressure level--the “normotensive non-dipper” paradox . Chronobiol Int 2013. ; 30 : 87 – 98 . [DOI] [PubMed] [Google Scholar]
- 14. Framme J, Dangardt F, Mårild S, Osika W, Währborg P, Friberg P . 24‐h Systolic blood pressure and heart rate recordings in lean and obese adolescents . Clin Physiol Funct Imaging 2006. ; 26 : 235 – 239 . [DOI] [PubMed] [Google Scholar]
- 15. Török K, Pálfi A, Szelényi Z, Molnár D . Circadian variability of blood pressure in obese children . Nutr Metab Cardiovasc Dis 2008. ; 18 : 429 – 435 . [DOI] [PubMed] [Google Scholar]
- 16. Shatat IF, Freeman KD, Vuguin PM, Dimartino-Nardi JR, Flynn JT . Relationship between adiponectin and ambulatory blood pressure in obese adolescents . Pediatr Res 2009. ; 65 : 691 – 695 . [DOI] [PubMed] [Google Scholar]
- 17. Westerståhl M, Marcus C . Association between nocturnal blood pressure dipping and insulin metabolism in obese adolescents . Int J Obes 2009. ; 34 : 472 – 477 . [DOI] [PubMed] [Google Scholar]
- 18. National High Blood Pressure Education Program . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents . Pediatrics 2004. ; 114 : 555 – 576 . [PubMed] [Google Scholar]
- 19. Barlow SE, Dietz WH . Obesity evaluation and treatment: expert committee recommendations . Pediatrics 1998. ; 102 : e29 . [DOI] [PubMed] [Google Scholar]
- 20. Puri M, Flynn JT, Garcia M, Nussbaum H, Freeman K, DiMartino‐Nardi JR . The frequency of elevated blood pressure in obese minority youth . J Clin Hypertens 2008. ; 10 : 119 – 124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lurbe E, Sorof JM, Daniels SR . Clinical and research aspects of ambulatory blood pressure monitoring in children . J Pediatr 2004. ; 144 : 7 – 16 . [DOI] [PubMed] [Google Scholar]
- 22. Khoury PR, Mitsnefes M, Daniels SR, Kimball TR . Age-specific reference intervals for indexed left ventricular mass in children . J Am Soc Echocardiogr 2009. ; 22 : 709 – 714 . [DOI] [PubMed] [Google Scholar]
- 23. Sturrock N, George E, Pound N, Stevenson J, Peck G, Sowter H . Non‐dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus . Diabetic Med 2000. ; 17 : 360 – 364 . [DOI] [PubMed] [Google Scholar]
- 24. Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes . N Engl J Med 2002. ; 347 : 797 – 805 . [DOI] [PubMed] [Google Scholar]
- 25. Karavanaki K, Kazianis G, Konstantopoulos I, Tsouvalas E, Karayianni C . Early signs of left ventricular dysfunction in adolescents with type 1 diabetes mellitus: the importance of impaired circadian modulation of blood pressure and heart rate . J Endocrinol Invest 2008. ; 31 : 289 – 296 . [DOI] [PubMed] [Google Scholar]
- 26. Mitsnefes MM, Kimball TR, Daniels SR . Office and ambulatory blood pressure elevation in children with chronic renal failure . Pediatr Nephrol 2003. ; 18 : 145 – 149 . [DOI] [PubMed] [Google Scholar]
- 27. Westerståhl M, Hedvall Kallerman P, Hagman E, Ek AE, Rössner SM, Marcus C . Nocturnal blood pressure non-dipping is prevalent in severely obese, prepubertal and early pubertal children . Acta Paediatr 2014. ; 103 : 225 – 230 . [DOI] [PubMed] [Google Scholar]
- 28. Soergel M . Development of normative ambulatory blood pressure data in children. Arbeitsgruppe Padiatrische Hypertonie . Blood Press Monit 1998. ; 4 : 121 – 126 . [PubMed] [Google Scholar]
- 29. Marcus CL, Greene MG, Carroll JL . Blood pressure in children with obstructive sleep apnea . Am J Respir Crit Care Med 1998. ; 157 : 1098 – 1103 . [DOI] [PubMed] [Google Scholar]
- 30. Weber SA, Santos VJ, Semenzati Gde O, Martin LC . Ambulatory blood pressure monitoring in children with obstructive sleep apnea and primary snoring . Int J Pediatr Otorhinolaryngol 2012. ; 76 : 787 – 790 . [DOI] [PubMed] [Google Scholar]
- 31. Horne RS, Yang JS, Walter LM, Richardson HL, O’Driscoll DM, Foster AM, et al. Nocturnal dipping is preserved in children with sleep disordered breathing regardless of its severity . Pediatr Pulmonol 2013. ; 48 : 1127 – 1134 . [DOI] [PubMed] [Google Scholar]
- 32. Yilmaz MB, Yalta K, Turgut OO, Yilmaz A, Yucel O, Bektasoglu G, et al. Sleep quality among relatively younger patients with initial diagnosis of hypertension: dippers versus non-dippers . Blood Pressure 2007. ; 16 : 101 – 105 . [DOI] [PubMed] [Google Scholar]
- 33. Kotsis V, Stabouli S, Papakatsika S, Rizos Z, Parati G . Mechanisms of obesity-induced hypertension . Hypertens Res 2010. ; 33 : 386 – 393 . [DOI] [PubMed] [Google Scholar]
- 34. Altuncu ME, Baspinar O, Keskin M . The use of short-term analysis of heart rate variability to assess autonomic function in obese children and its relationship with metabolic syndrome . Cardiology Journal 2012. ; 19 : 501 – 506 . [DOI] [PubMed] [Google Scholar]
- 35. Landsberg L . Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension (or, how insulin affects blood pressure, and why) . J Hypertens 2001. ; 19 : 523 – 528 . [DOI] [PubMed] [Google Scholar]
- 36. Korda M, Kubant R, Patton S, Malinski T . Leptin-induced endothelial dysfunction in obesity . Am J Physiol Heart Circ Physiol 2008. ; 295 : H1514 - H1521 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brambilla P, Antolini L, Street ME, Giussani M, Galbiati S, Valsecchi MG, et al. Adiponectin and hypertension in normal-weight and obese children . Am J Hypertens 2012. ; 26 ( 2 ): 257 – 264 . [DOI] [PubMed] [Google Scholar]
- 38. Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, et al. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension . Am J Kidney Dis 1999. ; 33 : 29 – 35 . [DOI] [PubMed] [Google Scholar]
- 39. Karas M, Lacourcière Y, LeBlanc A-R, Nadeau R, Dubé B, Florescu M, et al. Effect of the renin–angiotensin system or calcium channel blockade on the circadian variation of heart rate variability, blood pressure and circulating catecholamines in hypertensive patients . J Hypertens 2005. ; 23 : 1251 – 1260 . [DOI] [PubMed] [Google Scholar]
- 40. Hyman DJ, Ogbonnaya K, Taylor AA, Ho K, Pavlik VN . Ethnic differences in nocturnal blood pressure decline in treated hypertensives . Am J hypertens 2000. ; 13 : 884 – 891 . [DOI] [PubMed] [Google Scholar]


