Abstract
The dorsal cochlear nucleus is the first site of multisensory convergence in mammalian auditory pathways. Principal output neurons, the fusiform cells, integrate auditory-nerve inputs from the cochlea with somatosensory inputs from the head and neck. In previous work, we developed a guinea pig model of tinnitus produced by noise exposure and showed that the fusiform cells in these animals exhibited increased spontaneous activity and cross-unit synchrony, which are physiological correlates of tinnitus. Here, we delivered repeated bimodal auditory-somatosensory stimulation to the dorsal cochlear nucleus of guinea pigs with tinnitus, choosing a stimulus interval known to induce long-term depression (LTD). Twenty minutes per day of LTD-targeting bimodal (but not unimodal) stimulation reduced physiological and behavioral evidence of tinnitus in the guinea pigs after 25 days. Next, we applied the same bimodal treatment to 20 human subjects with tinnitus using a double-blinded, sham-controlled, crossover study. Twenty-eight days of LTD-targeted bimodal stimulation reduced tinnitus loudness and intrusiveness. Unimodal auditory stimulation did not deliver either benefit. Bimodal auditory-somatosensory stimulation that targets LTD in the dorsal cochlear nucleus may hold promise for suppressing chronic tinnitus, which reduces quality of life for millions of tinnitus sufferers worldwide.
Introduction
Tinnitus, the phantom perception of sound in the absence of external stimuli, is a disorder that affects 15% of the population in the United States (1) and is the most prevalent service-connected disability for military personnel (2). Whereas some individuals are minimally disturbed by their tinnitus, about 10% are bothered by it, and ~2 million individuals are debilitated (1). Negative impacts of tinnitus include sleep disturbance, poor concentration, distress, depression and anxiety (1, 3). Current tinnitus therapies are more successful at managing a patient’s reaction to their percept rather than addressing the tinnitus, and no one therapy is effective for all patients. Even when improving quality of life, none of the available tinnitus therapies treat the underlying pathology, and few have reported reductions in tinnitus loudness (4). A treatment that targets the underlying tinnitus mechanisms would greatly improve clinical outcomes for patients.
Whereas tinnitus is commonly associated with acoustic overexposure, many patients with tinnitus have clinically normal audiometric thresholds (5, 6) and ~12% report a triggering event such as a tooth abscess, or head and neck injury precipitating their tinnitus (7), indicating that events in addition to acoustic trauma can modify neural activity in auditory pathways. Indeed, 60-80% of tinnitus sufferers display a somatosensory component to their tinnitus, evident in their ability to modulate their tinnitus pitch or loudness by moving or applying pressure to their head or neck (8).
Tinnitus is thought to arise from dysregulated neural synchrony across neural ensembles along the auditory pathway (9), beginning in the dorsal cochlear nucleus (DCN) (10). The DCN is the first central site for multisensory integration, receiving input from the auditory nerve, auditory midbrain, auditory cortex, trigeminal and cervical ganglia, spinal trigeminal nucleus and dorsal column nuclei (11-13). Following noise exposure sufficient to temporarily elevate hearing thresholds, spontaneous activity and cross-neural synchrony of DCN output neurons, the fusiform cells, are increased in animals showing behavioral evidence of tinnitus. Animals without behavioral evidence of tinnitus do not show these neural correlates (14). Further, the tinnitus-related neural changes can occur even in the absence of permanent shifts in behavioral audiometric thresholds or electrophysiological measures of peripheral hearing status (14, 15).
The DCN produces hypersynchronous output through its unique, cerebellar-like circuit (Fig. S1). In this circuit, auditory nerve fibers from the cochlea form synapses with the fusiform-cell basal dendrites, while the non-auditory (e.g. somatosensory) inputs are relayed by granule-cell axons that form synapses with the fusiform-cell apical dendrites (16). The apical-dendritic synapses display spike-timing-dependent plasticity in which repeated elicitation of presynaptic excitatory post synaptic potentials (EPSPs) followed by postsynaptic spikes produce long-term potentiation (LTP), whereas postsynaptic spikes followed by presynaptic EPSPs produce long term depression (LTD) in vitro (17). In vivo, auditory (sound) stimulation can be used to evoke postsynaptic spikes and somatosensory stimulation can be used to evoke presynaptic activity in fusiform cells, such that paired auditory-somatosensory stimulation produces long-term changes in fusiform cell firing rates. In vivo, the resulting long-term effects are termed ‘stimulus-timing dependent plasticity’ (STDP). Whether LTP or LTD occurs depends on the precise order and timing between the bimodal stimuli (15). Importantly, these ‘learning rules’ are altered following noise exposure so that animals with tinnitus show a broader range of stimulus intervals that evoke LTP, whereas noise-exposed animals without tinnitus have broader range intervals that evoke LTD (18). Theoretical models of feedforward networks predict that LTP-driven synaptic strengthening will increase circuit connectivity and result in hypersynchrony (19). Hypersynchrony can also be driven by inhibitory network components (20), such as the cartwheel cells in the DCN (Fig. S1), which are also subjected to spike-timing-dependent synaptic modulation (17). Thus, increased LTP in the fusiform cell circuit could contribute to the hypersynchrony and increased spontaneous activity that are considered neural correlates of tinnitus (14).
Here, using a guinea pig model, we asked whether enhanced LTP and reduced LTD in the fusiform-cell circuit initiated hypersynchrony resulting in behavioral evidence of tinnitus. We show, in vivo, that auditory-somatosensory stimulation strengthened or weakened neural synchrony between fusiform cells, depending on the bimodal-stimulus order and timing. Furthermore, in animals with tinnitus, enhanced LTP correlated with increased synchrony and spontaneous activity in fusiform cells. To counteract tinnitus we stimulated guinea pigs with repeated auditory-somatosensory bimodal stimulation for 20 minutes/day for 25 days, choosing a bimodal interval shown to produce LTD in the fusiform-cell circuit. This non-invasive approach resulted in decreased synchrony and spontaneous activity in fusiform cells and reduced behavioral evidence of tinnitus. Furthermore, neither unimodal sound, nor unimodal somatosensory stimulation reliably decreased behavioral or physiological evidence of tinnitus in these animals. These findings demonstrated that fusiform-cell spike-timing-dependent plasticity may play a fundamental role in regulating neural synchrony and perception, and that LTD could be harnessed to reverse pathological hypersynchrony to reduce tinnitus.
Then, using stimulus protocols determined by the preclinical animal experiments, we conducted a similar study in 20 human participants with somatic tinnitus using a double-blinded, sham-controlled, crossover design. We reasoned that, because the human cochlear nucleus contains the cellular elements present in the DCN of rodents (21), similar learning rules should be present in humans and guinea pigs. We demonstrated that bimodal auditory-somatosensory stimulation, but not unimodal auditory stimulation, effectively reduced tinnitus loudness and intrusiveness cumulatively over the four weeks of treatment.
Results
STDP regulates synchrony among DCN fusiform cells in guinea pigs
To test the role of STDP in regulating synchronous firing among fusiform cells in the DCN, we recorded spontaneous-spiking activity from single fusiform cells in anesthetized normal-hearing guinea pigs before and 15 minutes after bimodal stimulation (Fig. 1A). Bimodal stimulation consisted of sounds (tone-bursts near the unit best frequency) and transcutaneous-electrical stimulation of the neck, presented within a ± 20 ms inter-stimulus window (Fig. 1A). Six bimodal intervals were studied (sound preceding electrical stimulus by 5, 10 or 20 ms, or electrical stimulus preceding sound by 5, 10 or 20 ms) in a separate series in a randomized order; physiological measurements preceded and followed each series (see Table S1 for STDP learning rule types across unit/unit-pairs). To quantify synchronous firing, we measured peak cross-correlation coefficients between spontaneous spike trains from fusiform-cell pairs (Fig. 1B). In one representative unit-pair, the peak cross-correlation coefficient decreased (Fig. 1C, top panel) after auditory-preceding-somatosensory stimulation (−10 ms interval), but increased after somatosensory-preceding-auditory stimulation (10 ms interval; Fig. 1C, lower panel). This unit-pair exhibited a Hebbian-like learning rule (Fig. 1D) in which presynaptic, subthreshold activation of the parallel fibers by somatosensory stimulation followed by postsynaptic activation of the basal dendrites by auditory stimulation (sound) strengthened neural synchrony. In other unit-pairs (e.g. Fig. 1E and 1F) the learning rule was anti-Hebbian-like, where the same bimodal inter-stimulus interval as Fig. 1C produced neural synchrony changes in the opposite direction. Other unit pairs exhibited LTP-only learning rules (where all bimodal intervals strengthened synchrony) or LTD-only learning rules (where all bimodal intervals weakened synchrony).
Fig. 1. STDP regulates synchrony in fusiform cells of the guinea pig DCN.

(A) Spontaneous activity (SA) was recorded across the fusiform-cell (FC) population in ?? guinea pigs for 150s, followed by 60s (5 Hz) of bimodal stimulation (BIS) with bimodal intervals (BI) from −20 to + 20ms. Spontaneous activity was recorded again 15 minutes after BIS for 150 sec. (B) Synchrony was assessed by cross-correlations (x-corr) of spikes in FC pairs (FC1, FC2). Spontaneous activity (SA) of FCs shows Poisson-distributions in interspike interval histograms (ISIH). Synchronous unit-pairs are defined by threshold cross-correlation coefficients (x-corr coef) of 4 SD (dashed line). (C) In one representative FC unit-pair, BI = −10 ms (auditory preceding somatosensory stimulus by 10 ms) reduced the peak x-corr coef (top panel), whereas BI = 10 ms (somatosensory preceding auditory stimulus by 10 ms) increased the peak x-corr coef 15 minutes after BIS (bottom panel). (D) Changes in peak x-corr coef for the FC unit pair in panel C are plotted as a function of BI (learning rule). (E) In a different FC unit pair, BI = 10 increased peak x-corr coef (top panel), whereas BI = 10 ms decreased peak x-corr coef 15 minutes after BIS (bottom panel). (F) For the FC unit pair in panel E changes in x-corr coef after BIS were opposite to that for the FC unit pair in panel D.
STDP regulates tinnitus-related increases in synchrony and spontaneous activity
Increased synchrony, bursting and spontaneous activity are established neural correlates of tinnitus (14). To determine whether dysregulated STDP contributes to tinnitus-related hypersynchrony, we induced tinnitus in guinea pigs using noise exposure, and assessed tinnitus using gap-prepulse inhibition of the acoustic startle (GPIAS) response.). GPIAS measures the acoustic startle response in the presence of a background narrow-band noise. When a gap is inserted into the background noise prior to the startle stimulus, the startle response is reduced in normal animals. However, in animals with tinnitus, the tinnitus obscures the gap and there is no decrement in the startle response. By plotting the amplitude of the gap trials versus the no-gap trials, an estimate of the animals’ tinnitus is obtained (15, 22, 23 (See experimental timeline, Fig. S2). Noise exposure produced only temporary hearing threshold elevations, which recovered after a few days (Fig. S3), but resulted in chronic tinnitus in 16 of 22 (72.7%) guinea pigs after 8 weeks (Fig. S4). Noise-exposed animals with tinnitus (ET) exhibited significant increases in synchrony (One-way ANOVA, F(2)=14.9, P=2.3e-6; post-hoc P<0.05; Fig. 2A) and spontaneous activity (F(2)=17.5,P=3.0e-7; post-hoc P<0.05; Fig. 2B) across the fusiform-cell population compared to normal-hearing animals and the 27.3% of noise-exposed animals that did not develop tinnitus (ENT). STDP for synchrony was assessed and compared across the tinnitus, no-tinnitus, and normal-hearing groups. The tinnitus group exhibited a greater proportion of unit-pairs with LTP-only learning rules, whereas the normal-hearing and the non-tinnitus groups exhibited greater proportions of anti-Hebbian-like and LTD-only learning rules (Fig. 2C; Table S1) (χ2(3)=15.8, P=0.0013). STDP for spontaneous activity of single-units followed a similar trend (Fig. 2D) (χ2(3)=23.4, P=3.3e-5). To further quantify the learning rule distribution shift from LTD towards LTP, we compared the LTD-LTP index (Fig. 2E inset), which sums all positive/LTP integration phases and all negative/LTD phases across all unit-pairs or single-units (Fig. 2E for synchrony; Fig. 2F for spontaneous activity). The tinnitus group showed more LTP across all learning rule types, whereas the no-tinnitus group showed more LTD compared to the normal-hearing group (F(2)=10.33, P=5.3e-5 for synchrony; F(2)=91.7, P=1.6e-37 for spontaneous activity). These findings indicated that the tinnitus-driven circuit had a high probability for LTP and strengthened neural synchrony.
Fig. 2. STDP shifts towards LTP in guinea pigs with tinnitus.
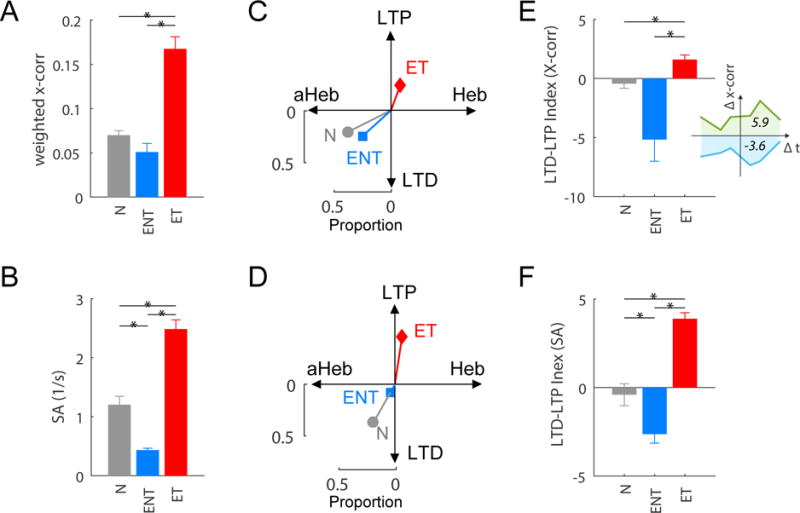
(A) Increased mean cross-correlation coefficient (x-corr; weighted by the proportion of synchronous unit-pairs) and (B) increased mean spontaneous activity (SA) compared to the normal-hearing (N) and exposed-but-no-tinnitus (ENT) groups of animals. * P< 0.05; data shown are mean ± SEM. Spontaneous activity for the N, ENT, and ET groups was 116, 93, 167 unit-pairs for x-corr, and 106, 387, 478 units, respectively. (C, D) A shift in the proportion of learning rules towards Hebbian-like (Heb; x-axis) and LTP (y-axis) in the ET group for (C) synchrony and (D) spontaneous activity (SA). (E, F) LTD-LTP index (total magnitude of LTP i.e. green area under curve relative to total magnitude of LTD i.e. blue area above curve of learning rules (E inset) is increased in the ET group for (E) synchrony and (F) spontaneous activity (SA).
Bimodal (but not unimodal) stimulation induces LTD to reduce fusiform-cell synchrony and spontaneous activity
Given that increased synchrony and spontaneous activity correlated with an expansion of the LTP phase of the STDP learning rule, we hypothesized that inducing LTD would reduce synchrony and spontaneous activity. First, we determined the bimodal interval that produced the strongest LTD in the animals with tinnitus by quantifying LTD probability (overall proportion of units showing LTD at a given bimodal interval). We found that more units responded with decreased synchrony and spontaneous activity after bimodal stimulation intervals of −5 and −10 ms. Whereas ± 20 ms intervals showed slight deviation from 0.5, they were not different from chance (Fig. 3A). Suppression of synchrony and spontaneous activity after −5 ms bimodal stimulation was significantly greater (due to less variance) than unimodal auditory or unimodal somatosensory stimulation, neither of which produced long-term effects (one-way ANOVA; F(2)=11.3, P=1.1e-6 for synchrony and F(2)=142, P=5.4e-66 for spontaneous activity) (Fig. 3B,C).
Fig. 3. Targeted bimodal stimulation suppresses synchrony and spontaneous activity in fusiform cells of guinea pigs.
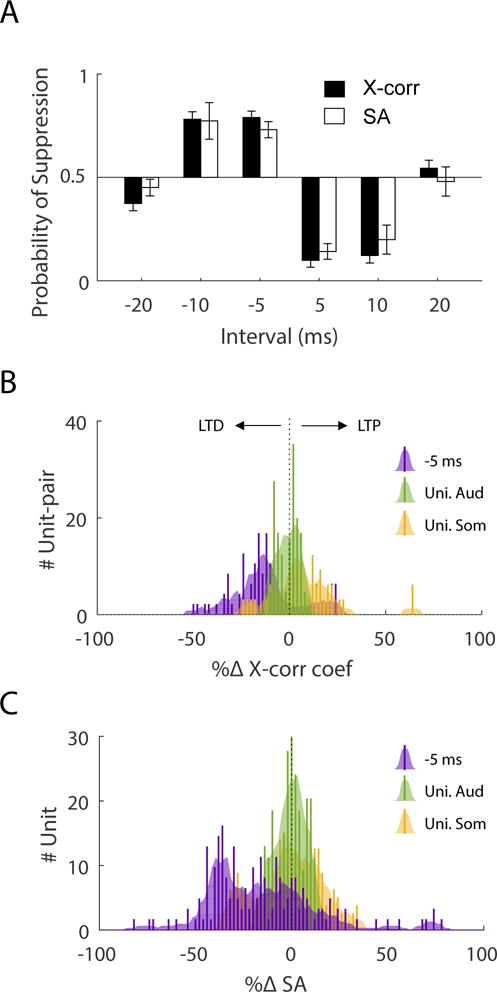
(A) Probability of synchrony (x-corr) or spontaneous activity (SA) suppression as a function of bimodal interval. Probability is computed by proportion of unit-pairs (total n = 159) or units (n = 251) showing decreased x-corr or SA at a given bimodal interval. A probability of 0.5 indicates an equal number of units showing increased or decreased x-corr or SA. The highest probability of suppression occurs for the −10 and −5 ms intervals (error bar = confidence interval for binomial proportion). The −5ms interval was chosen for the treatment. (B) The distributions of suppression vs enhancement of synchrony are compared for the −5 ms bimodal interval, unimodal somatosensory (Uni Som), or unimodal auditory stimulation (Uni Aud). The bimodal stimulus clearly suppressed synchrony whereas unimodal stimuli showed little deviation from zero. (C) Similar to synchrony, the bimodal stimulus suppressed SA, whereas the unimodal stimulus showed little deviation from zero (Bar = 2% bin; shaded curve is fitted by Spline Interpolant).
We next asked whether reducing synchrony in the fusiform-cell circuit would affect the animal’s tinnitus behavior. We hypothesized that repeated bimodal stimulation with an LTD-inducing interval (−5 ms) would reduce synchrony and spontaneous activity as well as behavioral evidence of tinnitus. Unimodal auditory stimulation, on the other hand, should not induce LTD since auditory synapses on the basal dendrites are not plastic; somatosensory input alone has been shown to induce LTP (15, 18). To test this hypothesis, we treated guinea pigs with tinnitus with 20-minute daily sessions of bimodal stimulation consisting of an 8 kHz tone-burst (the frequency at which tinnitus was most prevalent, see Fig. S6) paired with transcutaneous stimulation at the −5 ms interval for 25 days (group ET-treat). Three control groups were used, all expressing tinnitus after noise exposure. A sham group received a sedative but no bimodal or unimodal stimulation (ET-sham); an auditory-only group received the same 8-kHz tone but did not receive transcutaneous somatosensory stimulation (ET-audio); a somatosensory-only group received only electrical stimulation (ET-som). After the 25-day treatment period we quantified tinnitus behavior in the four groups using the tinnitus index (TI), which compared gap startle responses normalized to the pre-exposure baseline before and after noise exposure (Fig. S4). Representative findings presented in Fig 4A (one animal per group) show increased normalized startle responses after noise exposure, indicating tinnitus, which was reduced after treatment only in the animal receiving bimodal stimulation (ET-treat). Group analysis presented in Fig 4B showed that compared to the pre-treatment TI, animals receiving bimodal stimulation (ET-treat) exhibited a significant reduction in the TI at the treated frequency of 8 kHz and not at other tinnitus frequencies, whereas the sham group (ET-sham) and the auditory-only group (ET-audio) showed no changes (two-way ANOVA; F(2,1)=3.70, P=0.0069 for frequency × group). The somatosensory-only group (ET-som) showed a small decrease in TI at one frequency and a large increase in TI in another frequency, but no significant group mean change from control (post-hoc P>0.05). Tinnitus reduction correlated with lower neural synchrony (Fig. 4C) (Pearson’s linear correlation: r(287)=0.15, P=0.010; correction of dependency using linear mixed-effect model: P=0.031) and lower spontaneous activity (Fig. 4D) (r(1125)=0.20, P=6.8e-12; correction of dependency using linear mixed-effect model: P= 0.0011). Together, these results demonstrate that targeted LTD-induction in guinea pigs reduced tinnitus produced by dysregulated STDP, increased neuronal synchrony and spontaneous activity.
Fig. 4. LTD-induction reduces synchrony and spontaneous activity and reduces tinnitus in guinea pigs.
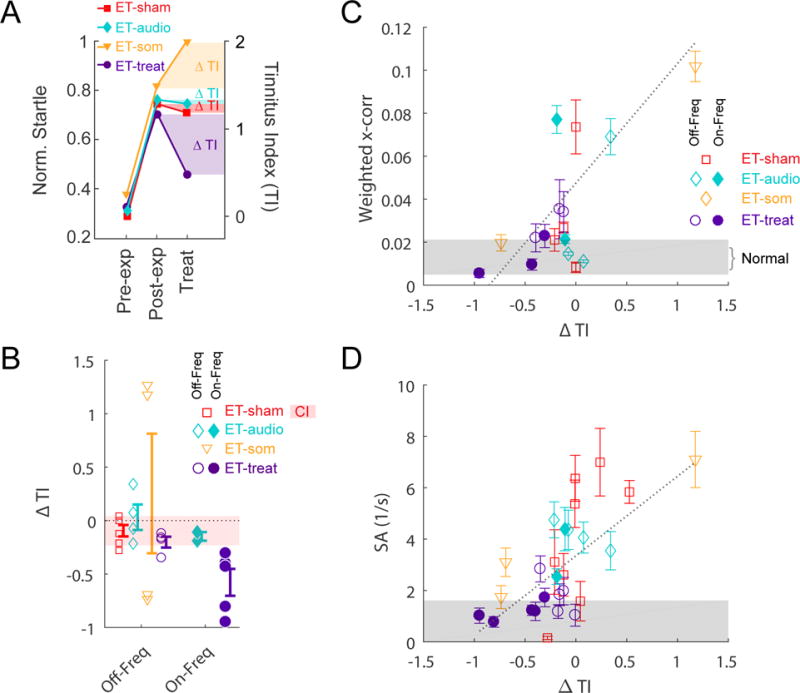
(A) Four representative animals (one from each group) show increased normalized startles after noise-exposure (pre- to post-exposure) indicating tinnitus (left ordinate), quantified as the TI (right ordinate). After LTD-induction by application of a bimodal auditory-somatosensory stimulus to the fusiform cells (ET-treat), there was a reduction in TI in the treated animal (ET-treat). Sham-treated (ET-sham; sedative only), auditory stimulus only (ET-audio), and somatosensory stimulus only (ET-som) animals either showed no reduction in TI or worsened TI. (B) Mean TI was significantly reduced in the ET-treat group at the treated frequency (On-Freq; 8 kHz) but not untreated frequencies (Off-Freq; 12 and 16 kHz). TI was not significantly reduced in the ET-sham, ET-audio, or ET-som groups. Pink horizontal bar indicates the 95% confidence interval (CI) for the ET-sham group. (C) The weighted mean cross-correlation coefficient (x-corr) for fusiform cells (at best frequencies within the TI bandwidth) is plotted as a function of ΔTI (116, 36, 35, 106 unit-pairs for ET-sham, ET-audio, ET-som, and ET-treat groups, respectively). Grey area indicates the range of x-corr for non-exposed animals. Reduction in synchrony significantly correlated with TI reduction. (D) Spontaneous activity (SA) plotted as a function of ΔTI (446, 204, 202, 696 units). Reduction in SA significantly correlated with TI reduction. Data shown are mean ± SEM.
Bimodal (but not unimodal) auditory-somatosensory stimulation reduces tinnitus loudness in humans
The positive animal study outcomes prompted investigating bimodal treatment for humans suffering from tinnitus. A double-blinded, sham-controlled, cross-over study was used to evaluate the effectiveness of bimodal auditory-somatosensory stimulation as a tinnitus treatment. All subjects and investigators were blinded as to whether subjects received an active (bimodal) or sham (unimodal-auditory) treatment for the duration of the study. Upon enrollment, participants were first assigned to either a sham group (n=10; group 1) or an active bimodal treatment group (n=10; group 2) (Fig. 5). Assignment was by a random number list that was precomputed prior to the start of the study. Take-home devices were programmed to deliver the bimodal or unimodal treatment protocols by control software and data encrypted to ensure blinding. The sound stimuli were delivered through calibrated insert earphones and the electrical stimuli using Ag-AgCl cups placed on the skin of the cervical spine or the cheek. Participants used the devices for 30 minutes once a day for two four-week sessions with a four-week washout period following each session. After the washout period, subjects “crossed-over” to receive the other treatment for the 2nd four-week period so that all subjects received both active and sham treatments. Participants returned to the lab weekly for monitoring and tinnitus assessment: loudness was assessed by matching tinnitus loudness to an external sound using TinnTester software, intrusiveness was assessed using the Tinnitus Functional Index (TFI; see Material and Methods).
Fig. 5.
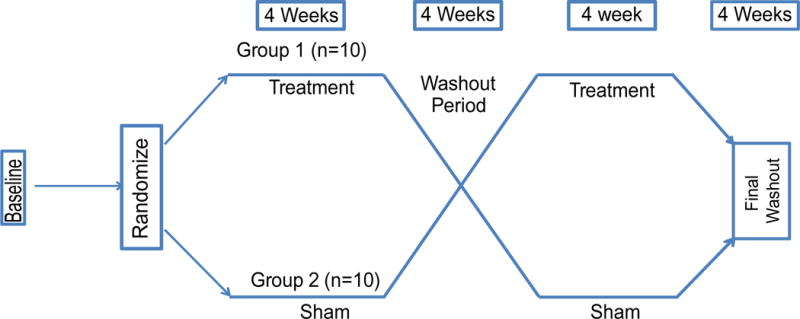
Outline of cross-over design for the human study.
The auditory stimulus (the same for bimodal and sham) was derived from each individual’s tinnitus spectrum and audiogram (see Materials and Methods). Devices provided either bimodal (auditory-electric) stimulation (bimodal active treatment) or unimodal (auditory alone) stimulation (sham treatment) for 30 minutes a day for 28 days. The bimodal interval was the same as that shown to be effective in the guinea pigs (−5 ms). Somatosensory stimulation alone was not provided as the animal study (Figs 3B, 4A, B) indicated that it could exacerbate the tinnitus.
The active bimodal treatment produced a significant (P<0.05) cumulative decrease in tinnitus loudness assessed by TinnTester loudness matching each week of the active treatment (Fig. 6A). The greatest mean change in loudness occurred after the fourth and final week of treatment. In contrast, loudness was stable (unchanged) during sham treatment for both groups. There was no significant difference between groups 1 and 2 (P=0.88), demonstrating that treatment order had no effect. Pooled groups showed a mean decrease of 8.035+/-1.33 dB from a baseline of 54.42+/-13.3 dB in loudness matches during the four weeks of active treatment (two-way ANOVA, F(3,1)=7.768, P=5.5e-5, post-hoc), significantly larger than the changes seen in the other conditions (sham, active washout, sham washout) where changes from baseline were not significant (Fig. 6B). Tinnitus reduction reached an average of 12.2 dB in the fourth week of active treatment. Of the 20 participants tested, two reported complete elimination of their tinnitus towards the end of the active treatment period.
Fig. 6. Bimodal treatment results in reduced tinnitus loudness and reduced TFI scores in human patients.
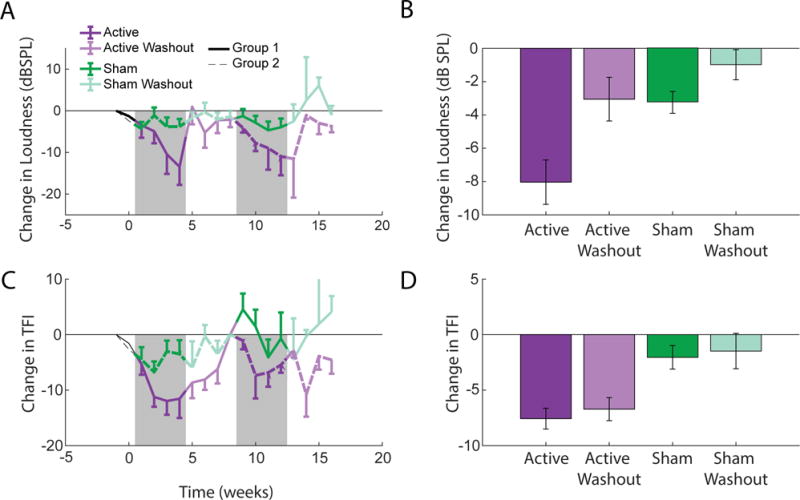
(A) Mean loudness by group. Group 1 (n=10) received the active treatment first; group 2 (n=10) received the sham treatment first. (B) Mean changes (relative to baseline) in loudness matching for each condition. (C) Mean TFI changes (relative to baseline) for groups 1 and 2. (D) Mean changes (relative to baseline) in TFI scores. Error bars are SEM.
Bimodal (but not unimodal) stimulation improves TFI scores
Mean overall TFI scores decreased from baseline of 29.2±2.6 to 22.9±1.8 units during the active treatment, but remained unchanged during sham treatment (Fig. 6C). Improvements in TFI scores were sustained beyond the active treatment and into the washout period, unlike the changes in loudness matching. As treatment order also had no significant effect on TFI scores (GLMM; P=0.819), both groups were pooled for statistical analysis. The mean TFI scores across the different study periods (Fig. 6D) were significantly improved (i.e., reduced relative to baseline) for both active and active washout periods (but not sham periods) (7.33±0.956 TFI units; two-way ANOVA, F(3,1)=7.712, P=6.14e-5) indicating a diminished impact on daily life with mean reductions of 7.51 and 6.71 points, respectively. Eleven participants noted subjective changes in volume, pitch, or quality that resulted in their tinnitus becoming less “harsh” or “piercing” and more “mellow.” Even participants who did not experience a complete elimination of their tinnitus reported anecdotally that their tinnitus was noticeably less obtrusive and easier to ignore.
Ten of the 20 subjects had a clinically significant reduction of at least 13 points in their TFI scores during active treatment, which is considered clinically meaningful for this questionnaire (24). There were no demographic differences across subjects showing significant TFI changes compared to stable subjects (Table S2). Four participants had clinically significant drops during the sham treatment, but two of these also showed significant decreases in TFI during the active treatment. Further, both participants reported that their tinnitus improved more during the active treatment. The two participants who stated that the sham treatment was more effective also had the shortest tinnitus duration (less than 1 year).
Reductions in loudness relative to baseline correlated significantly with reductions in overall TFI scores (Linear mixed-effects model: Beta=0.169±0.058, T=2.94, P=0.0035; Fig. S7). Furthermore, changes in loudness correlated with changes in TFI subscores: sense of control, intrusive, cognitive and sleep (Table S3).
Discussion
Increases in synchrony, spontaneous activity and bursting (14) and altered STDP (15) are established neural correlates of tinnitus. In animal models of tinnitus, increased synchrony has been identified in the DCN (14), inferior colliculus (25) and auditory cortex (26). These studies suggest that the tinnitus percept emerges from increased spontaneous synchrony amongst neurons in cortical and subcortical regions that contribute to perceptual binding (27). Here, we first examined the relationship between synchrony and STDP in normal-hearing guinea pigs, which exhibited Hebbian and anti-Hebbian learning rules as well as rules giving LTP or LTD. We then induced tinnitus in animals using noise-exposure that produced only temporary threshold shifts and observed tinnitus-related increases in neural activity reflecting an overall dominance of LTP. Subsequently, we applied the optimal bimodal interval to induce LTD in sessions of 20 minutes duration for 25 days, which reversed hypersynchrony and behavioral evidence of tinnitus at frequencies corresponding to the treatment frequency. None of the control stimuli (sedative alone, unimodal somatosensory or unimodal auditory stimulation) had any effect on tinnitus behaviors or tinnitus correlates. Based on the outcome of the animal study, we used the same bimodal stimulus protocol to treat tinnitus in humans.
STDP is essential for shaping sensory perception through input-dependent learning. In the visual cortex, STDP modulates tuning of visual neurons to orientation and motion (28). Similar STDP processes shape map plasticity in the somatosensory and auditory cortices for frequency selectivity, pitch encoding and discrimination (29-31). These context-dependent changes in sensory processing can alter connectivity and synchrony of neural ensembles (32, 33). In the fusiform-cell circuit, multi-modal inputs induce context-dependent changes through STDP (34). Auditory-somatosensory integration in DCN constitutes an adaptive filtering process through which perception of behaviorally-relevant sounds is amplified and internally-generated sounds are attenuated (17, 35, 36). Fusiform-cell synchrony regulation by STDP likely contributes to this perceptual task, while dysregulated multimodal STDP gives rise to phantom perception, as we show here.
Synaptic plasticity has been suggested as a foundation for network-level homeostatic adaptation (37). In the fusiform-cell circuit, glutamatergic inputs to the granule cell-parallel fiber circuit are upregulated after hearing loss (38-40), resulting in increases in LTP (41). This homeostatic mechanism in response to altered input is not exclusive to the auditory pathway (42). After light deprivation, visual-cortical neurons exhibit expansion in STDP due to increased NMDA receptor activation (43). Indeed, blocking NMDA receptors in the fusiform-cell circuit reduces neural synchrony (44). Muscarinic acetylcholine receptors, whose expression is upregulated after noise exposure (45), also contribute to STDP (46, 47).
STDP can affect intrinsic membrane excitability by altering ion channel conductance (48, 49). Maladaptive changes to fusiform cell plasticity that decrease inhibition through reduced hyperpolarizing currents could also contribute to increased synchrony and spontaneous activity. Reduced potassium channel activation and reduced glycinergic and GABAergic receptor activation of fusiform cells have been demonstrated in tinnitus models (50, 51). A major source of GABA input and glycinergic input to fusiform cells arises in cartwheel cells (Fig. S1). These DCN interneurons, which receive parallel-fiber synapses that exhibit STDP (17), provide recurrent inhibitory synapses onto fusiform cells. Cartwheel cells therefore may play an essential role in generating fusiform-cell synchrony (19, 20). Another potential player, the golgi cell in the marginal region of the cochlear nucleus, provides feedback modulation of granule-cell output, which may entrain parallel fibers into synchronized firing (52-54). These network components are likely to work together to increase synchrony in fusiform cells, thus potentially playing important roles in tinnitus.
Because the human cochlear nucleus contains all of the cellular elements present in the DCN of rodents (21), we reasoned that the same bimodal protocol might suppress tinnitus in humans. In both the animal and the human studies, bimodal but not unimodal auditory stimulation effectively suppressed tinnitus. The documented failure of unimodal auditory stimulation to produce long-term changes in fusiform-cell firing rates predicted that unimodal auditory stimulation would be inefficient at reducing tinnitus (15, 18, 34, 55). The significant reduction in tinnitus in animals, and tinnitus loudness and distress in humans suggests that the bimodal treatment was successful at inducing frequency-specific LTD, reversing the pathological neural activity responsible for the generation of tinnitus.
Unimodal auditory treatment, in addition to being ineffective at reducing tinnitus during the sham treatment phase, tended to cause an increase in tinnitus loudness and TFI scores at the end of the sham treatment, possibly due to the increased attention paid to the tinnitus during the evaluation periods. Unimodal somatosensory stimulation, on the other hand, shown to cause LTP and not LTD in animal studies (15, 18, 34), predicted that unimodal somatosensory stimulation could exacerbate tinnitus. Unimodal somatosensory stimulation did in fact exacerbate the tinnitus in some animals, preventing us from testing the electrical-only stimulation condition in humans.
Bimodal auditory-somatosensory stimulation in humans had no side-effects, whereas invasive techniques such as deep-brain stimulation and vagal-nerve stimulation can have severe side-effects. Our LTD-induction approach is non-invasive, easy to implement and presents minimal risk. Although reduced tinnitus loudness did not carry over into the washout period, this benefit was persistent enough to accumulate over several days of treatment. Improved adjustment to tinnitus, as reflected in the TFI scores, persisted during the washout period, for up to three weeks. Furthermore, reductions in tinnitus loudness correlated with TFI sub-scores regarding sense of control, intrusiveness, cognition and sleep, suggesting that tinnitus loudness reduction during bimodal treatment conferred psychological benefits that outlasted the treatment.
Other approaches to treat tinnitus, such as the coordinated reset sound therapy or paired sound-vagal nerve stimulation also target putative aberrant neural activity, but have not yet yielded positive results in the clinic. Paired vagal nerve stimulation, although showing promising results in an animal model, requires invasive surgery with accompanying risks and side effects, rendering it only suitable for the most debilitated patients. Sound therapies do not consistently reduce tinnitus loudness (56) perhaps because unimodal auditory stimulation has no effect on modulating long-term plasticity in the DCN (Fig. 3B, C) (15, 18).
There are some limitations to our study. Our study only tested one subgroup of tinnitus patients, those with somatic tinnitus, thus it is unknown whether these results would translate to other subgroups. In addition, ethical considerations prevented us from testing some protocol conditions in the human patients, such as the somatosensory stimulation alone condition, which was observed to exacerbate tinnitus in the guinea-pig study. Nevertheless, the neural de-synchronization strategy presented here offers a new and accessible treatment possibility for tinnitus sufferers.
Materials and Methods
Study Design
All animal procedures were performed per protocols established by the NIH publication No. 80-23 and approved by the University of Michigan’s University Committee on Use and Care of Animals. First, noise-over exposure was used to induce tinnitus in guinea pigs (see Fig. S2). Evidence of tinnitus was provided by a behavioral test (GPIAS) and confirmed with physiological signatures of increased spontaneous rates of firing and synchrony in DCN fusiform cells. 12 guinea pigs were used for physiological assessment after noise exposure and 13 for physiological assessment after treatment. To the latter group (all expressing tinnitus), we applied non-invasive, 30 minute/day auditory-somatosensory stimulation (with three different controls) for 25 days, and assessed behavioral and neurophysiological correlates of tinnitus. Second (Fig. 5), a double-blinded, sham-controlled, cross-over study was performed to evaluate the effectiveness of the auditory-somatosensory stimulation in humans with tinnitus. The study was performed in accordance with the University of Michigan IRBMED. Participants were randomly assigned to either sham (n=10) or active treatment first (n=10) groups. Participants were trained to use a small, customized take-home device that provided the active and sham treatments. Weekly tinnitus spectra-estimation and self-reported questionnaires were obtained on site. All twenty participants who completed the study were included in the analysis.
Tinnitus assessment in guinea pigs
Tinnitus was assessed using GPIAS (14, 15, 41, 57), (Fig. S4A). A normalized startle ratio (NSR) was computed as the ratio of the mean startle amplitude for the gap/pre-pulse trials and the mean of the startle-only trials (Fig. S4B). An animal was defined as having tinnitus in a frequency band if the post-exposure mean NSR value for gap-inhibition was significantly greater than the baseline value. Neural recordings to evaluate spontaneous activity and synchrony were performed after the completion of tinnitus assessments.
Human tinnitus assessment
A computerized procedure (TinnTester) (58) was used for weekly loudness matching in the laboratory throughout the trial. The Tinnitus Functional Index (TFI) questionnaire was used to assess the impact of a subject’s tinnitus on their quality of life (24).
Auditory-somatosensory treatment in guinea pigs and humans
The somatosensory stimulation was provided by transcutaneous active electrodes positioned on the skin overlying either the trigeminal ganglion or the cervical spinal cord in the region of C2 (with the ground electrode adjacent). In humans, electrode location depended on which maneuvers induced the strongest change in tinnitus. In guinea pigs, C2 was used throughout. Auditory stimulation was personalized according to each subject’s tinnitus spectrum. In guinea pigs, 8 kHz (most prevalent tinnitus frequency) was used. For the active treatment, the auditory stimulus preceded the somatosensory stimulus by 5 ms.
Statistics
Two-tail t-test, χ2 contingency tests, Pearson’s linear correlation, one-way and two-way analyses of variance (ANOVAs) were used to determine statistical differences (α=0.05). Post-hoc analyses for ANOVA were performed using the Tukey-Kramer test where indicated. For statistical significance evaluation of guinea pig’s tinnitus behavioral vs neurophysiological results, patients’ loudness vs. TFI, and loudness matching measures, general linear mixed models (GLMM) or linear mixed-effect models were used.
Supplementary Material
Fig. S1. Development of STDP in the fusiform-cell circuit.
Fig. S2: The experimental timeline for the animal study.
Fig. S3. Noise exposure produces only temporary threshold and suprathreshold shifts
Fig. S4. GPIAS behavioral assessment of tinnitus in guinea pigs.
Fig. S5. Human treatment groups and ears had similar hearing thresholds.
Fig. S6. Reduction in tinnitus loudness in humans correlates with reductions in TFI.
Fig. S7. Tinnitus Modulation Maneuver Checklist.
Table S1. Distribution of STDP learning rule type across unit/unit-pairs in guinea pigs.
Table S2. Subject demographics
Table S3. Correlations between changes in loudness and changes in TFI sub-score.
One Sentence Summary.
Non-invasive bimodal auditory stimulation targets the dorsal cochlear nucleus and reduces tinnitus in guinea pigs and humans
Accessible Summary.
The sound of silence
Tinnitus reduces quality of life for millions of tinnitus sufferers worldwide.
Using a guinea pig model of tinnitus after noise trauma, Marks et al. delivered precisely-timed bimodal auditory-somatosensory stimulation designed to induce long-term depression (LTD) in the cochlear nucleus of these animals. Twenty minutes per day of bimodal stimulation to induce LTD in the cochlear nucleus reduced physiological and behavioral evidence of tinnitus in the animals. The same bimodal protocol reduced tinnitus loudness in human subjects in a double-blinded, sham-controlled, crossover clinical study. Unimodal stimulation did not reduce tinnitus in the animals or the humans. LTD-targeting bimodal auditory-somatosensory stimulation may hold promise for suppressing chronic tinnitus in patients.
Acknowledgments
We thank Chris Ellinger, Dwayne Valliencourt, and Dave Thompson for technical assistance, Consulting for Statistics, Computing and Analytics Research (CSCAR) for statistical consultation, and Sandy Bledsoe, Michael Roberts, Amarins Heeringa and Seth Koehler for helpful comments on a previous version of this manuscript.
Funding:
This study was supported by NIH grants R01-DC004825 (SES), T32-DC00011 (DTM and CW), and the Wallace H. Coulter Translational Research Partnership (SES and GJB).
Participation of LER was assisted by funding from the Natural Sciences and Engineering Council of Canada.
Footnotes
Author Contributions: SES conceived and designed the animal and human studies. SES, CW and DTM designed the animal study; SES, KLM, KCSL and LER designed the human study; GJB provided subject medical clearance for study participation; KLM collected the human data; DTM and CW collected the animal data; KLM, DTM and SES analyzed the human data; CW, DTM and SES analyzed the animal data. KLM, DTM, CW, LER, KCSL and SES wrote the manuscript.
Competing Interests: SES and DTM are inventors on US patent # 3A9242067 “Personalized auditory-somatosensory stimulation to treat tinnitus.” All other authors declare no competing interests.
List of Supplementary Materials
Materials and Methods
References
- 1.Axelsson A, Ringdahl A. Tinnitus—a study of its prevalence and characteristics. Br J Audiol. 1989;23:53. doi: 10.3109/03005368909077819. [DOI] [PubMed] [Google Scholar]
- 2.Austin DF. Audiometric thresholds and prevalence of tinnitus among male veterans in the United States: Data from the National Health and Nutrition Examination Survey, 1999-2006. J Rehabil Res Dev. 2011;48:503. doi: 10.1682/jrrd.2010.07.0138. [DOI] [PubMed] [Google Scholar]
- 3.Mazurek B, Haupt H, Olze H, Szczepek AJ. Stress and tinnitus—from bedside to bench and back. Front Syst Neurosci. 2012;6 doi: 10.3389/fnsys.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Ridder D, Vanneste S, Elgoyhen AB, Langguth B, De Nora M. All Treatments in Tinnitus Are Experimental, Controversial, and Futuristic: A Comment on “Experimental, Controversial, and Futuristic Treatments for Chronic Tinnitus” by Folmer et al (2014) J Am Acad Audiol. 2015;26:595. doi: 10.3766/jaaa.14041. [DOI] [PubMed] [Google Scholar]
- 5.Paul BT, Bruce IC, Roberts LE. Evidence that hidden hearing loss underlies amplitude modulation encoding deficits in individuals with and without tinnitus. Hear Res. 2017 Feb;344:170. doi: 10.1016/j.heares.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011 Sep 21;31:13452. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folmer RL, Griest SE. Chronic tinnitus resulting from head or neck injuries. The Laryngoscope. 2003;113:821. doi: 10.1097/00005537-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20:351. doi: 10.1016/s0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- 9.Weisz N, et al. The neural code of auditory phantom perception. J Neurosci. 2007 Feb 7;27:1479. doi: 10.1523/JNEUROSCI.3711-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus–triggers, mechanisms and treatment. Nat Rev Neurol. 2016 Mar;12:150. doi: 10.1038/nrneurol.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006 Mar 1;495:100. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Mizuno N. Single neurons in the spinal trigeminal and dorsal column nuclei project to both the cochlear nucleus and the inferior colliculus by way of axon collaterals: a fluorescent retrograde double-labeling study in the rat. Neurosci Res. 1997;29:135. doi: 10.1016/s0168-0102(97)00082-5. [DOI] [PubMed] [Google Scholar]
- 13.Schofield BR, Coomes DL. Auditory cortical projections to the cochlear nucleus in guinea pigs. Hear Res. 2005 Jan;199:89. doi: 10.1016/j.heares.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Wu C, Martel DT, Shore SE. Increased Synchrony and Bursting of Dorsal Cochlear Nucleus Fusiform Cells Correlate with Tinnitus. J Neurosci. 2016 Feb 10;36:2068. doi: 10.1523/JNEUROSCI.3960-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci. 2013 Dec 11;33:19647. doi: 10.1523/JNEUROSCI.2788-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryugo DK, Haenggeli CA, Doucet JR. Multimodal inputs to the granule cell domain of the cochlear nucleus. Exp Brain Res. 2003 Dec;153:477. doi: 10.1007/s00221-003-1605-3. [DOI] [PubMed] [Google Scholar]
- 17.Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004 Jul;7:719. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- 18.Wu C, Martel DT, Shore SE. Transcutaneous induction of stimulus-timing-dependent plasticity in dorsal cochlear nucleus. Front Syst Neurosci. 2015;9:116. doi: 10.3389/fnsys.2015.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha DB, Ledbetter NM, Barbour DL. Spike-timing computation properties of a feed-forward neural network model. Front Comput Neurosci. 2014;8:5. doi: 10.3389/fncom.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleberg FI, Fukai T, Gilson M. Excitatory and inhibitory STDP jointly tune feedforward neural circuits to selectively propagate correlated spiking activity. Front Comput Neurosci. 2014;8:53. doi: 10.3389/fncom.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagoner JL, Kulesza RJ., Jr Topographical and cellular distribution of perineuronal nets in the human cochlear nucleus. Hear Res. 2009 Aug;254:42. doi: 10.1016/j.heares.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci. 2012;6:42. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner JG, et al. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006 Feb;120:188. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- 24.Meikle MB, et al. The tinnitus functional index: development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012;33:153. doi: 10.1097/AUD.0b013e31822f67c0. [DOI] [PubMed] [Google Scholar]
- 25.Bauer CA, Turner JG, Caspary DM, Myers KS, Brozoski TJ. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J Neurosci Res. 2008 Aug 15;86:2564. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engineer ND, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011 Feb 3;470:101. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999 Sep;24:49. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 28.Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004 Sep 30;44:23. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Dahmen JC, Hartley DE, King AJ. Stimulus-timing-dependent plasticity of cortical frequency representation. J Neurosci. 2008 Dec 10;28:13629. doi: 10.1523/JNEUROSCI.4429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao SW, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nat Neurosci. 2004 Sep;7:974. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- 31.Saeedi NE, Blamey PJ, Burkitt A, Grayden DB. Learning Pitch with STDP: A Computational Model of Place and Temporal Pitch Perception Using Spiking Neural Networks. PLoS Comput Biol. 2016 doi: 10.1371/journal.pcbi.1004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markram H, Gerstner W, Sjostrom PJ. A history of spike-timing-dependent plasticity. Front Synaptic Neurosci. 2011;3:4. doi: 10.3389/fnsyn.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Womelsdorf T, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007 Jun 15;316:1609. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 34.Koehler SD, Shore SE. Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. PLoS One. 2013;8:e59828. doi: 10.1371/journal.pone.0059828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997 May 15;387:278. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- 36.Singla S, Dempsey C, Warren R, Enikolopov AG, Sawtell NB. A cerebellum-like circuit in the auditory system cancels responses to self-generated sounds. Nat Neurosci. 2017 Jul;20:943. doi: 10.1038/nn.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004 Feb;5:97. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- 38.Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and nonauditory inputs to the cochlear nucleus. J Neurosci. 2009 Apr 1;29:4210. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci. 2012 Nov 7;32:15791. doi: 10.1523/JNEUROSCI.2598-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker M, et al. Acoustic overexposure increases the expression of VGLUT-2 mediated projections from the lateral vestibular nucleus to the dorsal cochlear nucleus. PLoS One. 2012;7:e35955. doi: 10.1371/journal.pone.0035955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus–possible basis for tinnitus-related hyperactivity? J Neurosci. 2012 Feb 1;32:1660. doi: 10.1523/JNEUROSCI.4608-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007 Jan;52:156. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 43.Guo Y, et al. Dark exposure extends the integration window for spike-timing-dependent plasticity. J Neurosci. 2012 Oct 24;32:15027. doi: 10.1523/JNEUROSCI.2545-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefanescu RA, Shore SE. NMDA Receptors Mediate Stimulus-Timing-Dependent Plasticity and Neural Synchrony in the Dorsal Cochlear Nucleus. Front Neural Circuits. 2015;9:75. doi: 10.3389/fncir.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin YM, Godfrey DA. Effects of cochlear ablation on muscarinic acetylcholine receptor binding in the rat cochlear nucleus. J Neurosci Res. 2006 Jan;83:157. doi: 10.1002/jnr.20706. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci. 2011 Mar 2;31:3158. doi: 10.1523/JNEUROSCI.5303-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stefanescu RA, Shore SE. Muscarinic acetylcholine receptors control baseline activity and Hebbian stimulus-timing dependent plasticity in fusiform cells of the dorsal cochlear nucleus. J Neurophysiol. 2016 Dec 21; doi: 10.1152/jn.00270.2016. jn 00270 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Debanne D, Poo MM. Spike-timing dependent plasticity beyond synapse - pre- and post-synaptic plasticity of intrinsic neuronal excitability. Front Synaptic Neurosci. 2010;2:21. doi: 10.3389/fnsyn.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansel C, Linden DJ, D’Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci. 2001 May;4:467. doi: 10.1038/87419. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, et al. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009 Dec 1;164:747. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Middleton JW, et al. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci U S A. 2011 May 3;108:7601. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vos BP, Maex R, Volny-Luraghi A, De Schutter E. Parallel fibers synchronize spontaneous activity in cerebellar Golgi cells. J Neurosci. 1999 Jun 1;19:RC6. doi: 10.1523/JNEUROSCI.19-11-j0003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maex R, De Schutter E. Synchronization of golgi and granule cell firing in a detailed network model of the cerebellar granule cell layer. J Neurophysiol. 1998 Nov;80:2521. doi: 10.1152/jn.1998.80.5.2521. [DOI] [PubMed] [Google Scholar]
- 54.Yaeger D, Trussell LO. Auditory Golgi cells are interconnected predominantly by electrical synapses. J Neurophysiol. 2016 Apr 27; doi: 10.1152/jn.01108.2015. jn 01108 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefanescu RA, Koehler SD, Shore SE. Stimulus-timing-dependent modifications of rate-level functions in animals with and without tinnitus. J Neurophysiol. 2015 Feb 1;113:956. doi: 10.1152/jn.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Devesa P, Perera R, Theodoulou M, Waddell A. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst Rev. 2010:CD005233. doi: 10.1002/14651858.CD005233.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berger JI, Coomber B, Shackleton TM, Palmer AR, Wallace MN. A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods. 2013 Mar 15;213:188. doi: 10.1016/j.jneumeth.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts LE, Moffat G, Baumann M, Ward LM, Bosnyak DJ. Residual inhibition functions overlap tinnitus spectra and the region of auditory threshold shift. J Assoc Res Otolaryngol. 2008 Dec;9:417. doi: 10.1007/s10162-008-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yaeger DB, Trussell LO. Auditory Golgi cells are interconnected predominantly by electrical synapses. J Neurophysiol. 2016 Aug 01;116:540. doi: 10.1152/jn.01108.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 1997 Jul;78:248. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- 61.Yaeger DB, Trussell LO. Single granule cells excite Golgi cells and evoke feedback inhibition in the cochlear nucleus. J Neurosci. 2015 Mar 18;35:4741. doi: 10.1523/JNEUROSCI.3665-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaltenbach JA. Tinnitus: Models and mechanisms. Hear Res. 2011 Jun;276:52. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts LE, et al. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010 Nov 10;30:14972. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014 Feb;111:552. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fournier P, Hebert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear Res. 2013 Jan;295:16. doi: 10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Salloum RH, Yurosko C, Santiago L, Sandridge SA, Kaltenbach JA. Induction of enhanced acoustic startle response by noise exposure: dependence on exposure conditions and testing parameters and possible relevance to hyperacusis. PLoS One. 2014;9:e111747. doi: 10.1371/journal.pone.0111747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Buran BN, et al. Onset coding is degraded in auditory nerve fibers from mutant mice lacking synaptic ribbons. J Neurosci. 2010 Jun 2;30:7587. doi: 10.1523/JNEUROSCI.0389-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stabler SE, Palmer AR, Winter IM. Temporal and mean rate discharge patterns of single units in the dorsal cochlear nucleus of the anesthetized guinea pig. J Neurophysiol. 1996 Sep;76:1667. doi: 10.1152/jn.1996.76.3.1667. [DOI] [PubMed] [Google Scholar]
- 69.Voigt HF, Young ED. Cross-correlation analysis of inhibitory interactions in dorsal cochlear nucleus. J Neurophysiol. 1990 Nov;64:1590. doi: 10.1152/jn.1990.64.5.1590. [DOI] [PubMed] [Google Scholar]
- 70.Pinsky PF, Rinzel J. Synchrony measures for biological neural networks. Biol Cybern. 1995 Jul;73:129. doi: 10.1007/BF00204051. [DOI] [PubMed] [Google Scholar]
- 71.Hoare DJ, Kowalkowski VL, Kang S, Hall DA. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope. 2011 Jul;121:1555. doi: 10.1002/lary.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Development of STDP in the fusiform-cell circuit.
Fig. S2: The experimental timeline for the animal study.
Fig. S3. Noise exposure produces only temporary threshold and suprathreshold shifts
Fig. S4. GPIAS behavioral assessment of tinnitus in guinea pigs.
Fig. S5. Human treatment groups and ears had similar hearing thresholds.
Fig. S6. Reduction in tinnitus loudness in humans correlates with reductions in TFI.
Fig. S7. Tinnitus Modulation Maneuver Checklist.
Table S1. Distribution of STDP learning rule type across unit/unit-pairs in guinea pigs.
Table S2. Subject demographics
Table S3. Correlations between changes in loudness and changes in TFI sub-score.


