Abstract
The search for a female autism phenotype is difficult, given the low diagnostic rates in females. Here, we studied potential sex differences in a core feature of autism, difficulty with eye gaze processing, among typically developing individuals who vary in the broad autism phenotype, which includes autistic-like traits that are common, continuously distributed, and similarly heritable in males and females. Participants viewed complex images of an actor in a naturalistic scene looking at one of many possible objects and had to identify the target gazed-at object. Among males, those high in autistic-like traits exhibited worse eye gaze following performance than did those low in these traits. Among females, eye gaze following behavior did not vary with autistic-like traits. These results suggest that deficient eye gaze following behavior is part of the broader autism phenotype for males, but may not be a part of the female autism phenotype.
Keywords: eye gaze, gaze following, face processing, sex differences, autism, phenotype
Introduction
Autism is a developmental disorder that is characterized by difficulties with social communication and social interaction. It is diagnosed four times more often in males than females (Fombonne, 2009, Werling, 2016). As a result, the overwhelming majority of information regarding the symptoms of autism has been acquired from samples that are almost exclusively male, leaving open questions about the nature of autism indicators in females (Werling, 2016). One possibility is that there are sex differences in the development and/or expression of autism behaviors that have made it difficult to diagnose females using the current male-based criteria. This notion has led to an increasing interest in identifying a ‘female autism phenotype’ (Mandy et al., 2012; Bargiela, Steward, & Mandy, 2016; Lai et al., 2016). Compared to males, females diagnosed with autism appear to exhibit more social motivation (Head, McGillivray, & Stokes, 2014, Sedgewick, Hill, Yates, Pickering, & Pellicano, 2016) and nonverbal communication behaviors (Rynkiewicz et al., 2016). However, there is insufficient evidence to provide clinical guidelines regarding sex differences in core symptomology in autism (Bargiela et al., 2016, Rynkiewicz et al., 2016).
Difficulty understanding nonverbal communicative cues expressed on the face, including eye gaze following, is one of the diagnostic symptoms of autism (American Psychiatric Association, 2013). For individuals diagnosed with autism, difficulties understanding the referential nature of eye gaze are present in young children (Bedford et al., 2016; Thorup et al., 2016), adolescents (Riby, Hancock, Jones, & Hanley, 2013) and adults (Vlamings, Stauder, van Son, & Mottron, 2015). However, these findings are predominantly based on research involving male participants and recent findings indicate that impaired gaze following behavior in infancy does not reliably predict future autism symptoms in 3-year-old females like it does in males (Bedford et al., 2016). Thus, although attending to and interpreting eye gaze cues is a persistent difficulty for males diagnosed with autism, it is unclear whether and to what extent impaired eye gaze following is similarly indicated for females.
There are inherent limitations in the study of sex differences in the expression of autism symptoms among individuals with an autism diagnosis. Most research conducted with autism participants has recruited individuals from autism clinics that have excluded females who have been missed by clinical services because their autism may exemplify the female autism phenotype (Van Wijngaarden-Cremers et al., 2014; Lai et al., 2016). Similarly, the diagnosis is based on potentially male-biased criteria, which may lack the sensitivity to the female autism phenotype (Bargiela et al., 2016).
An alternative approach, and one that we take here, is to study potential sex differences in eye gaze following behavior among individuals who vary in the ‘Broader Autism Phenotype’ (BAP). The BAP includes a constellation of social and communication behaviors together with unusual personality features that are typically referred to as ‘autistic traits’ (Baron-Cohen et al., 2001; Constantino & Todd, 2003) or ‘autistic-like traits’ (ALTs; Ronald, Happé, & Plomin, 2008). These traits are milder manifestations of traits characteristic for clinically diagnosed autism (Sucksmith, Roth, & Hoekstra, 2011). They are highly heritable and show substantial variation in the general population (Constantino & Todd, 2003; Posserud et al., 2006). The distributions of ALTs are continuous and largely overlapping for men and women (Ruzich et al., 2015). Critically, these traits are normally distributed throughout the general population and share similar etiology with autism (e.g., Lundsdström et al., 2012), suggesting that individuals diagnosed with autism fall on the extreme end of this distribution.
ALTs are assessed using self-report questionnaires (Baron-Cohen et al., 2001; Hurley, Losh, Parlier, Reznick, & Piven, 2007), which are validated by showing that individuals with autism and their first-degree relatives (mothers, fathers, and male/female siblings) show higher numbers of traits on these measures (Constantino & Todd, 2003; Hurley et al., 2007; Ruzich et al., 2015). Importantly, there is evidence that adults with higher rates of ALTs have difficulty understanding the social referential nature of eye gaze (Swanson & Siller, 2014). Therefore, studying sex differences in eye gaze following behaviors, as a function of differences in ALTs, in a non-clinical sample of adults is a useful strategy for evaluating potentially distinct male and female autism phenotypes.
The current study examines potential sex differences in the relation between autistic traits and eye gaze following behavior. The central goal was to evaluate whether eye gaze following behavior varies as a function of ALTs, regardless of sex, or whether it varies as a function of ALTs and biological sex. If the latter were true, this would provide supportive evidence for the notion that autism-like behaviors manifest differently in males and females. Based on the existing literature, we hypothesized that males with more ALTs would perform worse on the eye gaze following task compared to males with fewer ALTs. However, it was an open question as to whether autistic traits would modulate gaze following behavior among the female participants. If there is a “female autism phenotype,” it could be that atypical gaze following behavior is not related to ALTs in females, which could have implications for reconsidering impaired gaze following as part of the female autism phenotype.
Method
Participants
A total of 120 (60 male) undergraduate students (18 – 23 years; M = 18.8, SD = 1.1) participated in the study. Participants did not have a diagnosis of autism spectrum disorder, anxiety, depression, ADHD, developmental delay, intellectual disability, or seizures. Participants were White (81.6%), Hispanic (5%), Asian, (8.3%), Black (3.3%), and Multiracial (1.6%).
Participants gave written informed consent to participate using procedures approved by the Pennsylvania State University’s (PSU) Internal Review Board. They were recruited from the PSU Department of Psychology Undergraduate Subject Pool and earned one credit hour for completing the experimental procedures.
Autism Trait Groups
We used an extreme subjects design to examine potential sex differences in the relation between autistic-like traits and eye gaze behavior. Potential participants completed the Autism Quotient (Baron-Cohen et al., 2001) as part of a Department of Psychology Subject Pool online screening battery prior to enrollment in the current study. The AQ is a 50-item self-report questionnaire to measure autistic traits using a 4-point Likert scale. We coded the measure based on a 4-point scale so that total scores ranged from 50 – 200 (Hoekstra et al., 2008, Rhodes et al., 2013). Higher scores indicate the presence of more autistic traits. Any individual who failed to answer more than 4 items was excluded from the analysis. We acquired AQ scores from 2257 (643 male and 1614 female) adults to derive a sample mean and standard deviation (M = 109, SD = 12), which is comparable to previous samples (Hoekstra et al., 2008; Rhodes et al., 2013). All individuals who scored 1 SD above (High ≥ 121, 24% of males and 17% of females tested) or below (Low ≤ 97, 14% of males and 17% of females) this mean and who agreed to be contacted for future studies were invited to participate in the study via e-mail. Recruitment continued until the target number of participants tested was reached (i.e., 30/group).
The High trait group had higher AQ total and sub-scores than the Low group and the distribution of scores from the two groups did not overlap (See Table 1). Importantly, the distributions of AQ scores for male and female participants within each AQ group were indistinguishable (see Figure S1 in the Supplemental Material available online).
Table 1.
Demographic information for participants in the high and low autistic-like trait groups depicted as a function of sex. Mean (SD) age in years, race/ethnicity, Autism Quotient (AQ) total scores plus four AQ subscale scores, and Broad Autism Phenotype Questionnaire (BAPQ) total scores are presented.
| High autistic-like traits | Low autistic-like traits | |||||
|---|---|---|---|---|---|---|
| Male | Female | Mean | Male | Female | Mean | |
| N | 30 | 30 | 30 | 30 | ||
| Age | 19.0 (1.3) | 18.6 (1.2) | 18.8 (1.3) | 19.1 (1.0) | 18.4 (0.8) | 18.8 (1.0) |
| Race/Ethnicity | ||||||
| White/Nonhispanic | 20 | 25 | 26 | 30 | ||
| White/Hispanic | 2 | 3 | 1 | 0 | ||
| Black | 2 | 1 | 1 | 0 | ||
| Asian | 6 | 1 | 1 | 0 | ||
| More than 1 race | 0 | 0 | 1 | 0 | ||
| AQ Total | 128.6 (6.9) | 127.8 (5.3) | 128.2 (6.1)* | 92.2 (5.0) | 90.1 (6.2) | 91.2 (5.6) |
| Social Skills | 26.5 (3.8) | 25.6 (3.7) | 26.0 (3.7)* | 14.8 (2.2) | 14.0 (2.3) | 14.4 (2.2) |
| Communication | 25.1 (3.0) | 25.2 (3.1) | 25.1 (3.0)* | 16.0 (2.1) | 15.0 (1.9) | 15.5 (2.0) |
| Attention to Detail | 26.9 (3.2) | 27.3 (3.2) | 27.1 (3.4)* | 22.3 (4.5) | 22.1 (3.6) | 22.2 (4.0) |
| Attention Switching | 28.1 (2.3) | 27.1 (2.5) | 27.6 (2.4)* | 21.6 (3.4) | 21.2 (3.0) | 21.4 (3.1) |
| BAPQ Total | 3.13 (0.4) | 2.95 (0.6) | 3.04 (0.5)* | 2.26 (0.3) | 1.91(0.3)* | 2.08 (0.3) |
Note: There were significant differences between the high and low autistic trait groups on all AQ scores and BAPQ total scores (*p < .001). For the low autistic trait group, males and females differed on BAPQ total scores (*p < .001). The groups were matched on age.
To provide external validity for the grouping procedure, participants also completed the Broad Autism Phenotype Questionnaire (BAPQ; Hurley et al., 2007) in the lab the same day they executed the eye gaze following task. The BAPQ is a 36-item self-report measure of autistic traits that targets aloof and rigid personality and pragmatic language problems. Higher scores indicate more traits. The BAPQ score was missing for 1 male in the low autistic trait group. Consistent with the findings on the AQ scores, the High group exhibited higher BAPQ scores than the Low group for both males and females. In the High group, there were no sex differences. In the Low group, males exhibited more traits on the BAPQ than females (See Table 1).
Stimuli
Stimuli were modeled after those in a previous study of eye gaze following behavior that reported differences between typically developing children and those with autism (Riby et al., 2013) and are shown in Figure S2 (see Supplemental Materials online). Each stimulus included a color digital photograph taken with a Fujifilm Finepix S4200 camera. All images were standardized for size (1440 by 1080 pixels) in Adobe Photoshop 2015.1.2 software. Each image depicted an actor (unfamiliar adult) directing their eye gaze at a target object in a complex scene. There were 8 different actors and 19 different indoor scenes (offices, restaurants, houses). In each image, there was a target object (i.e., the correct gazed-at object), a plausible non-target object (i.e., near by the target object but not gazed-at), and several implausible objects (i.e., farther away from target object and not gazed-at).
The original stimulus set included 218 images. Prior to the study, we identified the most frequent label for each target object by having a separate group of 12 adults look at each image and generate the name of the target gazed-at object. We eliminated 67 images for which there was no consensus (< 50% agreement) on the name of the target object. There was high agreement (M = .78, SD = 0.21) for the names of the target objects in the remaining 151 images. For each of these images, we created 3 additional labels, including one for a plausible non-target object, and two for implausible non-target objects. In addition, we used this same procedure to identify labels for the original stimuli used by Riby et al. (2013) so that these stimuli could be included in the multiple-choice task. As a result, the total number of stimuli in the study included 165 images.
Procedure
Participants completed the eye gaze following task on a Dell Latitude E6540 computer with a 15.6-inch screen using E-Prime 2.0 software. Participants were instructed to view each image and identify the specific object the person was looking at from a list of 4 labels that was presented on a subsequent screen. Participants completed 3 practice trials with feedback prior to beginning the task. Feedback was not provided during the rest of the task. Each stimulus image was presented for 3000 ms and was immediately followed by a response screen that included the 4-alternative-forced-choice answers and the question, “What object was the person looking at?” The response screen remained until participants executed a keyboard response with the number (1, 2, 3, 4) that corresponded with their answer. Following the response, there was a 1000 ms fixation screen before the next trial began.
The possible answers included words that described the target gazed-at object, a plausible non-target object, and two implausible non-target objects. The position of the label for the target object was counterbalanced across trials. In order for participants to identify the target object correctly, they were required to mentally compute the trajectory of the gaze information, which required them to discriminate whether the actor was looking at the target or the nearby plausible non-target object, and then choose the correct label for the target object. This multiple-choice version of the task enabled us to establish a chance level performance at 25%. The task was executed in 3 blocks of randomly ordered trials so that participants could take short breaks.
Data Analysis
Accuracy was the dependent variable. Linear mixed models were used to analyze the fixed effects of sex (male, female) and trait group (Low, High) and the interactions between these factors on performance, while controlling for the random factors of stimulus and participant. Reported means include the estimated marginal means from this analysis. To decompose sex x group interactions, the simple main effects were investigated in separate models starting with the effect of sex within each trait group. In addition, we evaluated the simple main effect of trait group within each sex. Type III tests of fixed effects are reported with Satterthwaite degrees of freedom approximation, along with their parameter estimates (with SE and 95% confidence intervals). One item was removed from the analyses because all the females performed at ceiling on the trial, preventing model convergence. To maintain a familywise error rate on the analyses, we employed a Bonferonni correction corresponding to the 4 follow-up tests utilized to decompose the interaction, p = .05/4 = .013.
In a converging analysis, accuracy was evaluated in a separate model using BAPQ scores as a continuous measure of autistic traits with the fixed effect of sex, and the interaction between sex and BAPQ scores, while controlling for the random factors of stimulus, participant, and participant race/ethnicity. To decompose the interaction, separate models were run for males and females with a familywise error rate of p = .05/2 = .025.
Results
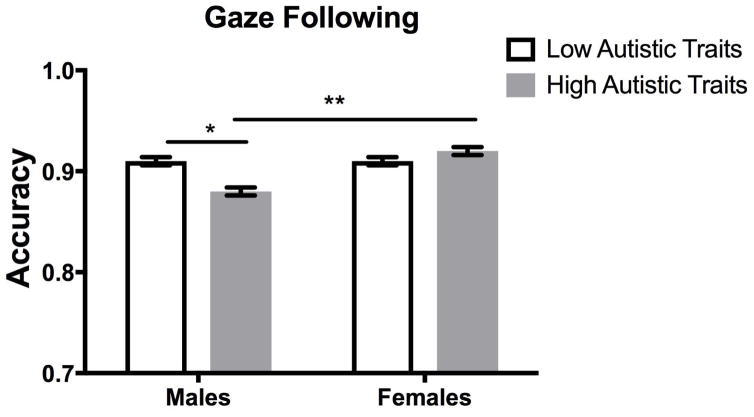
Figure 1 shows the mean accuracy for each autistic trait group plotted as a function of sex. The mixed model containing both trait group and sex failed to reveal a main effect of trait group (p = .09). However, there was a main effect of sex (β = −.03, SE = −.01, CI = −.04/−.02), F(1, 6519.05) = 19.33, p < .001. Males exhibited lower accuracy on the task (M = 0.89, SE = .003) than did females (M = 0.91, SE = .003). Importantly, this effect was qualified by a significant sex x autistic trait group interaction, (β = .03, SE = .01, 95% CI = .01/.04), F(1, 6519.05) = 10.85, p = .001.
Figure 1.
Average group performance on the eye gaze following task. Data are plotted as the mean (± 1 SEM) accuracy for males and females in the high and low autistic-like trait groups. Analyses revealed a significant difference between males in the high and low autistic trait groups (*p < .005) and between males and females in the high autistic trait groups (**p < .001).
To decompose the trait group x sex interaction, we first investigated the potential modulatory effect of group within each sex in separate models. For male participants, there was a significant effect of trait group, (β = .019, SE = .006, 95% CI = .007/.030), F(1, 2354.04) = 9.77, p = .002. Males in the High group performed worse on the eye gaze task (M = 0.88, SE = .004) than did males in the Low group (M = 0.91, SE = .004). In contrast, for female participants, there was no significant effect of trait group (p = .13), indicating that females in the High and Low trait groups were indistinguishable in their performance.
Second, we investigated the modulatory effect of sex on eye gaze following behavior within each trait group. Among the adults with high autistic traits, there was a significant effect of sex, (β = −.028, SE = .005, 95% CI = −.039/−.017), F(1, 1517.88) = 25.45, p < .001. Males high in autistic traits exhibited significantly worse performance in the eye gaze following task (M = 0.88, SE = .004) than did females similarly high in autistic traits (M = .92, SE = .004). In contrast, among the adults low in autistic traits, there was no sex difference (p = .43) indicating that males and females low in autistic traits were indistinguishable in their performance.
The analysis with BAPQ total scores also revealed a significant interaction between sex and BAPQ scores, F(1, 6500.80) = 6.10, p = .014. For male participants, there was a significant effect of BAPQ scores on eye gaze following, F(1, 2352.80) = 5.75, p = .017. For females, there was not a significant effect of BAPQ scores (p = .10).
Discussion
The goal of this study was to evaluate whether eye gaze following behavior varies among a non-clinical sample of adults as a function of autistic-like traits (ALTs) regardless of biological sex, or whether it varies differently as a function of ALTs and biological sex. Participants viewed complex scenes composed of a single actor among multiple objects and determined which object the actor was looking at. Precise identification of the target object required that participants precisely compute the trajectory of the gaze information to discriminate whether the actor was looking at the target, plausible, or implausible objects. We found that males high in ALTs exhibited worse gaze following behavior than did 1) males low in ALTs and 2) females similarly high in ALTs. The presence of ALTs did not modulate performance among the females. In other words, females high in autistic-like traits had indistinguishable gaze following behavior from other females who were low in ALTs. These findings are consistent with previous work suggesting that individuals with higher rates of autistic traits have difficulty processing eye gaze information (Swanson & Siller, 2014). However, our results are importantly different from previous work in that they indicate that disruptions in eye gaze following behavior may only be related to the presence of ALTs in males.
One other study reported a similar sex difference when examining whether face-processing behaviors are related to autistic-like traits in adults (Rhodes et al., 2013). In males, as ALTs increased, adaptive coding of face identity (as measured by face identity aftereffects) and face recognition behavior both degraded, as expected. In contrast, in females, there was an unexpected positive association between ALTs and adaptive coding of face identity and no relation between ALTs and face recognition abilities (Rhodes et al., 2013). There were some limitations in the study design, including unbalanced group sizes, a lack of group matching on AQ scores, and lack of screening for psychiatric diagnoses (e.g., anxiety and depression) that are known to impact face processing. An important advantage of the current study is that it was designed a priori to test hypotheses about sex differences, which is why there were an equal number of males and females within the high and low trait groups. Also, within each trait group, the males and females were matched on AQ scores and age. Therefore, this balanced extreme subjects experimental design had the power to uncover even subtle sex differences in the patterns of eye gaze processing behavior.
It will be important to apply these design considerations in future work employing an individual differences approach that examines how ALTs modulate sensitivity to eye gaze following across a broader continuum of autistic-like traits. This will address concerns regarding how individuals with low and high AQ scores potentially differ (e.g., excel versus have difficulty) from those with average AQ scores in processing eye gaze information. In addition, converging dependent variables, like gaze time and scan path from eye tracking, will be helpful for understanding more strategic and mechanistic factors that influence how autistic traits and sex influence sensitivity to eye gaze following.
In terms of thinking about mechanistic influences that might drive these sex differences in sensitivity to eye gaze following, previous researchers hypothesized that a difference in exposure to prenatal hormones, specifically testosterone, may lead to an increased risk of social communication difficulties for males (Knickmeyer & Baron-Cohen, 2006). This argument critically hinges on predictions that there are sex differences among typically developing individuals in social communication behavior that are related to prenatal hormone exposure and eye gaze behavior specifically. There is some evidence to support this claim. In one study, increased levels of fetal testosterone in boys were negatively related to the number of eye contact episodes in the same children at 12-months of age, an effect that did not exist within girls who overall had lower testosterone exposure, resulting in overall lower eye contact for boys than girls (Luchmaya, Baron-Cohen, & Raggat, 2002). However, the pattern of results from our study does not fit with the predictions of this proposed model, which would have predicted sex differences in sensitivity to eye gaze behavior among the participants in the low autistic traits group, which was not observed in our data. Males and females with low autistic traits were similarly sensitive to eye gaze following.
An alternative mechanistic model focuses on female resilience instead of male risk. The central idea is that there are female-specific factors (i.e., hormones, sexually dimorphic genes) that protect them from developing symptoms of autism (Robinson, Lichtenstein, Anckarsater, Happe, & Ronald, 2013; Werling, 2016; Werling, Parikshak, & Geschwind, 2016). Those females who do present with autism symptoms are expected to have more genetic loading for the disorder and to be more severely impacted by autism than are their male counterparts (Robinson et al., 2013). Indeed, adult females diagnosed with autism have higher self-reported traits on the AQ than do diagnosed males; however, the same females show fewer traits than males on observational measures such as the Autism Diagnostic Observation Schedule (Lai et al. 2011; 2016). Our findings are not consistent with the predictions from this model either, which would expect high trait females to show the biggest deficit in eye gaze behavior. In fact, these females were indistinguishable in their performance on the eye gaze task from the females and males who were low in autistic traits.
A final possibility is that the phenotypic expression of autism is qualitatively different in males and females (Mandy et al. 2012; Lai et al., 2016). There are several existing clinical syndromes, like cardiovascular disease, in which the clinical presentation, pathophysiology, diagnosis, and disease management is sex-specific (Kawamoto et al., 2016). Evidence in support of the notion of sex differences in autism phenotypes includes findings that females with an autism diagnosis have a greater capacity for traditional friendships (Head et al., 2014; Sedgewick et al., 2016), fewer externalizing and more internalizing behaviors (Mandy et al., 2012), less restricted interests (Hiller et al., 2014), and fewer repetitive behaviors (Van Wijngaarden-Cremers et al., 2014) than do males with an autism diagnosis. Our results suggest that disrupted eye gaze processing, which is a core diagnostic feature of autism, is part of the broader autism phenotype for males, but not females. When combined with evidence that eye gaze following is not an effective predictor of autism for high-risk female infants (Bedford et al., 2016), these results lead to the hypothesis that abnormal eye gaze processing is not a reliable diagnostic feature of autism in females. Research evaluating sex differences in the female autism phenotype will need to be extended into high-risk populations (e.g., siblings of individuals with autism) and the autism population to fully evaluate this hypothesis.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by The Pennsylvania State University Center for Online Innovation in Learning; the National Institute of Mental Health (grant number R61MH110624). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We would like to thank the undergraduate and graduate student research assistants who helped create the stimuli.
Footnotes
Author Contributions
E.W. and K.S.S. both conceptualized and wrote the article together. E.W. completed data collection and data analysis under supervision of K.S.S. Both authors approved the final version of the paper for submission.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Bargiela S, Steward R, Mandy W. The experiences of late-diagnosed women with autism spectrum conditions: an investigation of the female autism phenotype. Journal of Autism and Developmental Disorders. 2016;46(10):3281–3294. doi: 10.1007/s10803-016-2872-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31(1):5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Bedford R, Jones EJ, Johnson MH, Pickles A, Charman T, Gliga T. Sex differences in the association between infant markers and later autistic traits. Molecular Autism. 2016;7(1):21. doi: 10.1186/s13229-016-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60(5):524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric Research. 2009;65(6):591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Head AM, McGillivray JA, Stokes MA. Gender differences in emotionality and sociability in children with autism spectrum disorders. Molecular Autism. 2014;5(1):19. doi: 10.1186/2040-2392-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, Weber N. Sex differences in autism spectrum disorder based on DSM-5 criteria: evidence from clinician and teacher reporting. Journal of Abnormal Child Psychology. 2014;42(8):1381–1393. doi: 10.1007/s10802-014-9881-x. [DOI] [PubMed] [Google Scholar]
- Hoekstra RA, Bartels M, Cath DC, Boomsma DI. Factor structure, reliability and criterion validity of the Autism-Spectrum Quotient (AQ): a study in Dutch population and patient groups. Journal of Autism and Developmental Disorders. 2008;38(8):1555–1566. doi: 10.1007/s10803-008-0538-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RS, Losh M, Parlier M, Reznick JS, Piven J. The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders. 2007;37(9):1679–1690. doi: 10.1007/s10803-006-0299-3. [DOI] [PubMed] [Google Scholar]
- Kawamoto KR, Davis MB, Duvernoy CS. Acute Coronary Syndromes: Differences in Men and Women. Current atherosclerosis reports. 2016;18(12):73. doi: 10.1007/s11883-016-0629-7. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Baron-Cohen S. Topical review: fetal testosterone and sex differences in typical social development and in autism. Journal of Child Neurology. 2006;21(10):825–845. doi: 10.1177/08830738060210101601. [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Pasco G, Ruigrok AN, Wheelwright SJ, Sadek SA … MRC AIMS Consortium. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PloS one. 2011;6(6):e20835. doi: 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Ruigrok AN, Chakrabarti B, Auyeung B, Szatmari P … MRC AIMS Consortium. Quantifying and exploring camouflaging in men and women with autism. Autism. 2016 doi: 10.1177/1362361316671012. 1362361316671012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström S, Chang Z, Råstam M, Gillberg C, Larsson H, Anckarsäter H, Lichtenstein P. Autism spectrum disorders and autisticlike traits: similar etiology in the extreme end and the normal variation. Archives of General Psychiatry. 2012;69(1):46–52. doi: 10.1001/archgenpsychiatry.2011.144. [DOI] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P. Foetal testosterone and eye contact in 12-month-old human infants. Infant Behavior and Development. 2002;25(3):327–335. [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. Journal of Autism and Developmental Disorders. 2012;42(7):1304–1313. doi: 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- Posserud Maj-Britt, Lundervold Astri J, Gillberg Christopher. Autistic Features in a Total Population of 7–9-year-old Children Assessed by the ASSQ (Autism Spectrum Screening Questionnaire) Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(2):167–175. doi: 10.1111/j.1469-7610.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Jeffery L, Taylor L, Ewing L. Autistic traits are linked to reduced adaptive coding of face identity and selectively poorer face recognition in men but not women. Neuropsychologia. 2013;51(13):2702–2708. doi: 10.1016/j.neuropsychologia.2013.08.016. [DOI] [PubMed] [Google Scholar]
- Riby DM, Hancock PJ, Jones N, Hanley M. Spontaneous and cued gaze-following in autism and Williams syndrome. Journal of Neurodevelopmental Disorders. 2013;5(1):13. doi: 10.1186/1866-1955-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, Ronald A. Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences. 2013;110(13):5258–5262. doi: 10.1073/pnas.1211070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Happé F, Plomin R. A twin study investigating the genetic and environmental aetiologies of parent, teacher and child ratings of autistic-like traits and their overlap. European Child & Adolescent Psychiatry. 2008;17(8):473–483. doi: 10.1007/s00787-008-0689-5. [DOI] [PubMed] [Google Scholar]
- Ruzich E, Allison C, Smith P, Watson P, Auyeung B, Ring H, Baron-Cohen S. Measuring autistic traits in the general population: a systematic review of the Autism-Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Molecular Autism. 2015;6(1):2. doi: 10.1186/2040-2392-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Lassalle A, Baron-Cohen S. An investigation of the ‘female camouflage effect’in autism using a computerized ADOS-2 and a test of sex/gender differences. Molecular Autism. 2016;7(1):10. doi: 10.1186/s13229-016-0073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgewick F, Hill V, Yates R, Pickering L, Pellicano E. Gender differences in the social motivation and friendship experiences of autistic and non-autistic adolescents. Journal of Autism and Developmental Disorders. 2016;46:1297–1306. doi: 10.1007/s10803-015-2669-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucksmith E, Roth I, Hoekstra RA. Autistic traits below the clinical threshold: reexamining the broader autism phenotype in the 21st century. Neuropsychology Review. 2011;21(4):360–389. doi: 10.1007/s11065-011-9183-9. [DOI] [PubMed] [Google Scholar]
- Swanson MR, Siller M. Brief report: Broad autism phenotype in adults is associated with performance on an eye-tracking measure of joint attention. Journal of Autism and Developmental Disorders. 2014;44(3):694–702. doi: 10.1007/s10803-013-1901-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorup E, Nyström P, Gredebäck G, Bölte S, Falck-Ytter T. Altered gaze following during live interaction in infants at risk for autism: an eye tracking study. Molecular Autism. 2016;7(1):12. doi: 10.1186/s13229-016-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and age differences in the core triad of impairments in autism spectrum disorders: a systematic review and meta-analysis. Journal of Autism and Developmental Disorders. 2014;44(3):627–635. doi: 10.1007/s10803-013-1913-9. [DOI] [PubMed] [Google Scholar]
- Vlamings PH, Stauder JE, van Son IA, Mottron AL. Atypical visual orienting to gaze-and arrow-cues in adults with high functioning autism. Journal of Autism and Developmental Disorders. 2005;35(3):267–277. doi: 10.1007/s10803-005-3289-y. [DOI] [PubMed] [Google Scholar]
- Werling DM. The role of sex-differential biology in risk for autism spectrum disorder. Biology of Sex Differences. 2016;7(1):58. doi: 10.1186/s13293-016-0112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werling DM, Parikshak NN, Geschwind DH. Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders. Nature Communications. 2016:7. doi: 10.1038/ncomms10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.