INTRODUCTION
Tobacco use continues to be the number one cause of preventable morbidity and mortality in the United States (CDC, 2008). Smoking is a major cause of lung and heart disease (CDC, 2008), is now associated with 13 types of cancer (Alberg, Shopland, & Cummings, 2014), and as much as two thirds of smoker will die from a smoking related illness. The population of smokers in the US has changed substantially over recent decades, with smokers today showing greater nicotine dependence, (Cokkinides et al., 2009; Goodwin, Keyes, & Hasin, 2009), higher incidence of psychiatric diagnosis (Annamalai, Singh, & O’Malley, 2015; CDC, 2013), more failed quit attempts (M. Chaiton et al., 2016; Hughes, 2011; Irvin, Hendricks, & Brandon, 2003), and greater resistance to available treatments (Ip et al., 2012; Irvin et al., 2003). Over 50% of smokers attempt to quit smoking each year (CDC, 2011), but under 5% of unassisted quit attempts and only 10% of assisted quit attempts lead to sustained abstinence (Fiore et al., 2008). This growing treatment resistance in the face of profound morbidity and mortality has created a need for more effective therapies (Brandon, 2001; O. M. Chaiton, Cohen, & Frank, 2008). In response to this problem, there has been substantive innovation in tobacco dependence pharmacotherapies (Fiore et al., 2008; Stead, Perera, Bullen, Mant, & Lancaster, 2008), but comparatively little innovation in the development of behavioral therapies with the notable exceptions of Motivational Interviewing (MI) (Lai et al., 2010) and Acceptance and Commitment Therapy (ACT) (Bricker & Tollison, 2011; Gifford & Palm, 2011).
Mindfulness in its simplest form, is cognitive skill that can be taught with only brief instruction (e.g. 5 minutes) and practice (e.g. 30 minutes) with demonstrable reduction of smoking urges and associated brain activity (Westbrook et al., 2013). Mindfulness might be described as mentally disengaging from automatic or reactive behavior to become aware of our experiences, thoughts, or feelings as they occur so that we may respond from a perspective that is engaged, open-minded, and non-judgmental (Kabat-Zinn & Hanh, 2009; Segal, Williams, & Teasdale, 2002). It does not entail changing what we are doing or thinking, but instead bringing intentional awareness and acceptance to ordinary moment-to-moment experience. This acceptance-based approach runs counter to cognitive approaches in which an individual “strives to overcome” a negative emotion or thought (Beck, 1979; Wolpe, 1982). In people who smoke, the act of challenging an urge can paradoxically lead to a prolonged urge (Rogojanski, Vettese, & Antony, 2011; Whitfield, 2006). This finding has led to the exploration of acceptance-based cognitive strategies to overcome tobacco dependence. Studies on mindfulness training have suggested reductions on automatic behavior (Witkiewitz et al., 2014), impulsive reactions (Way et al., 2010), physiologic stress (Goldberg et al., 2014; Grossman et al., 2004), anxiety (Creswell et al., 2007; Hofmann et al., 2010; Koszycki et al., 2007), and depression (Teasdale, Segal, & Williams, 1995).
Mindfulness training was first studied as a treatment for smokers by Dr. James Davis (the author), in a pilot study, which found that time spent practicing mindfulness meditation was associated with smoking abstinence (Davis et al., 2007). Since then, Mindfulness Training for Smokers (MTS) and similar interventions have been evaluated in a number of randomized controlled trials (RCTs) (Brewer et al., 2011; Davis, Goldberg, et al., 2014; Davis et al., 2014; Davis et al., 2013; Vidrine et al., 2016). A recent meta-analysis across multiple RCTs showed that abstinence rates for MTS were almost twice that of matched behavioral controls (RR = 1.88 (95% CI: 1.04–3.40)) (Oikonomou, Arvanitis, & Sokolove, 2016). Studies on MTS and similar interventions have shown a significant association between daily meditation practice time and smoking abstinence (Davis, Goldberg, et al., 2014), as well as significant reductions on measures of stress, negative affect, and urge to smoke compared to controls (Brewer et al., 2011; Davis, Goldberg, et al., 2014; Davis, Manley, et al., 2014). Neuroimaging and cognitive processing studies have led to two compelling models for how mindfulness may function therapeutically within an addiction (Brewer, Elwafi, & Davis, 2013; Elwafi et al., 2013; Garland, Froeliger, & Howard, 2014). In Judson Brewer’s model, mindful attention enhances activity in the pre-frontal cortex, activating top-down regulatory processes with interruption of brain pathways associated with emotion and cue-elicited reward-based behavior. Eric Garland expanded on this model, proposing that mindfulness practice also increases functional connectivity between the pre-frontal cortex and subcortical networks involved in salience of and response to appetitive cues. In this model, greater top-down functional connectivity potentiates greater capacity to respond to drug cues through pre-frontal rather than sub-cortical processes (Garland, 2014).
In an RCT, Davis et al. (2014a) assessed smokers randomized to MTS vs. the Wisconsin Tobacco Quit Line (WTQL); the study showed signifcantly higher abstinence rates in MTS at 4-weeks post-quit (MTS = 45.8% vs. WTQL = 25.4% (p= 0.02)) and 24-weeks post-quit (MTS = 38.7% vs. WTQL = 20.6%; (p= 0.05)). The study utilized design elements commonly employed in RCTs including recruitment through advertisements, use of exclusion criteria, 1:1 randomized group allocation, free behavioral treatment and medications (4 weeks of NRT), participant payment, use of objective and self-report measures, multiple study visits with research staff. Randomized control trials (RCTs) are the gold standard for determining the efficacy of medical treatments (Sibbald & Roland, 1998). A well-designed RCT will commonly use inclusion/exclusion criteria, participant payment, and free treatment (Weisberg, Hayden, & Pontes, 2009) to experimentally isolate specific therapeutic ingredients and reduce bias (Stolberg, Norman, & Trop, 2004). Unfortunately, however, the design elements of RCTs also typically lead to an over-estimation of the effectiveness of a treatment (e.g. stringent exclusion criteria may exclude less motivated and low functioning individuals, free medications help ensure the use of medications, and close oversight by staff increases attendance and outcomes), decreasing the generalizability of results to clinical practice (Moulton, 2004; Weiss, Koepsell, & Psaty, 2008). After RCTs have established a treatment as efficacious, an important next step is to assess the treatment within a clinical environment (Woolf, 2008). In order to bring greater ecological validity to the assessment it is common to conduct an observational study with a naturalistic design. This approach might include for example no recruitment drive, limited or no inclusion/exclusion criteria, therapies with standard costs, no participant payment, no additional research procedures or evaluations, and limited contact with research staff (McLeod, 2015). Testing a new treatment within an observational trial is however often neglected, perhaps because observational studies are vulnerable to a number of problems that together lead to smaller treatment effects: requiring participants to pay for their own treatments can lead to low adherence rates; lack of payment and limited contact with study staff can lead to high attrition (Song & Chung, 2010). Although MTS has performed well within clinical trials, it is currently unknown how it might perform in a clinical setting. We do not know what proportion of clinic patients would choose to enroll in MTS, how many classes they might attend, or whether abstinence rates would be similar to those found in clinical trials.
METHOD
The study was funded through Meriter Foundation Research and Education Grant #477 and was designed to provide a naturalistic observational assessment of MTS as provided through the Meriter Smoking Cessation Program. The study was conducted at Meriter Hospital and Clinics (now UnityPoint), a health system with close ties to the University of Wisconsin Hospital and Clinics in Madison, Wisconsin. The study was approved by the Meriter Hospital Institutional Review Board (IRB) with University of Wisconsin IRB reciprocity. The study itself provided no treatment, no medications, no payment, no free materials, no study visits, no contact with study staff, and no evaluations beyond those routinely provided within the smoking cessation program.
Participants
Study enrollment continued for 12 months, during which all patients seen within the Meriter Smoking Cessation Program were asked if they would provide consent to have their clinic data used for research purposes. All study participants were patients within the Meriter Smoking Cessation Program and the only criteria for participation in the study was participation in the clinical program. The clinical program enrolled adults (18 and over) seeking treatment for tobacco dependence, with no exclusions. All patients in the clinical program were offered an opportunity to sign consent to have their information used for research purposes and all but two signed consent. Their reasons for not signing consent were not recorded.
Procedure
Clinic Visits
All clinic patients (including study participants) completed a brief clinic-based evaluation including questions on demographics and smoking history. For all clinic patients, smoking status was assessed at the initial visit via exhaled breath carbon monoxide (CO) testing and a 7-day smoking history (Brown et al., 1995). Smoking abstinence was defined as self-report of no smoking (not even a single puff) in the past seven days plus a CO test result of under 7 parts per million (ppm) (NICE, 2010). All clinic patients were scheduled for a return clinic visit 2-weeks after their quit day at which they were asked to repeat CO breath testing and 7-day smoking history. At the return visit, clinic patients who choose MTS as their behavioral treatment were asked about the amount of time they spent in daily meditation over the last 7 days. The clinic did not administer standardized tests (e.g. on nicotine dependence, depression, anxiety, stress, or alcohol use) and these tests were not administered to study participants.
Choice of Medications and Behavioral Treatment
All clinic patients (including study participants) chose medications and behavioral treatment through a joint decision making process with the smoking cessation program coordinator. FDA approved smoking cessation medications were recommended to all patients based on the Clinical Practice Guideline Treating Tobacco Use and Dependence (Fiore et al., 2008) with modifications based on medication intolerance, patient preference, insurance coverage, and cost burden. Primary providers were contacted for all patients (when available) to provide prescriptions. Participants were provided with a choice of two behavioral treatments: Mindfulness Training for Smokers or referral to the Wisconsin Tobacco Quit Line (WTQL) with a directly observed initial call or a referral through the Fax to Quit Program (Kobinsky et al., 2010). A description of each intervention was provided which included information about the required payment for MTS with reimbursement for completers (see below). The Program Coordinator explained that all patients in the clinic should receive some form of behavioral treatment, that MTS had been more effective for smoking cessation than the WTQL in a randomized trial, but also that “it is possible that your results will be different from those found in research.” MTS utilized “cost-incentive” pay structure in which patients were required to pay for MTS course enrollment, but would then receive full reimbursement through an insurance provider after completion of the course regardless of smoking status (course costs were on a sliding scale). Clinic patients (including participants) were required to acquire medications on their own (via insurance or self-pay), although NRT was provided through the WTQL for participating patients (see below).
MTS Intervention
The MTS course provided is a group-therapy skills training course comprised of seven weekly 2½-hour classes and a 6½-hour Quit Day Retreat for a total of 24 hours of instruction. The MTS course is led by instructors certified through completion of the 3-day Teacher Training Course. MTS course materials include an instructional DVD, a manual, and audio CDs. The course follows video-based instruction played during class and provides example-based education with language appropriate for 7th grade leaners. The MTS course contains behavioral tools commonly used in smoking cessation interventions such as planning for the quit day, behavioral strategies for management of triggers and urges, but also included training in mindfulness meditation and mindfulness of smoking triggers, urges, emotions, and thoughts. MTS participants were asked to engage in 15–30 minutes of daily meditation with the Guided Meditation Audio-Recording, and to use mindful attention spontaneously throughout the day to manage stress, emotions, triggers, urges, and other relapse challenges. The Quit Day Retreat follows the fifth class and includes multiple instructor-guided mindfulness exercises to facilitate emersion into the practice of more regular use of mindfulness.
Wisconsin Tobacco Quit Line (WTQL) Intervention
The WTQL is operated by Alere Wellbeing (now Optum®) and is the largest telephonic smoking cessation service provider in the US. Individuals who called the WTQL received up to 5 proactive (the coach calls the client) counseling calls from a Quit Coach, access to Web Coach, an interactive web-based program with education and discussion forums, 2-weeks of free nicotine replacement, and printed self-help materials. In addition to proactive calls to smokers, participants were allowed to place an unlimited number of “reactive” calls to the WTQL which provided 24/7 call availability. Whenever possible, the WTQL registration call (to Alere registration specialist) was made under observation at the smoking cessation clinic to ensure enrollment in services. If the patient did not make an observed call from the clinic, a WTQL Fax to Quit Program Referral was placed, initiating registration and proactive calls to the participant.
Measures
No standardized tests were used in this study. Instead only demographic information, smoking history, 7-day smoking calendar and carbon monoxide (CO) breath testing were performed. All of these were performed at baseline but only 7-day smoking calendar and CO testing were performed post treatment.
Data Analyses
Data were analyzed using the R statistical software (R Development Core Team, 2014). Chi-square (χ2) tests were used to compare MTS and WTQL groups on dichotomous variables (e.g., gender, quit status) and odds ratios (OR) were computed using a Fisher’s exact test. Independent t-tests were used to compare groups on continuous variables (e.g., age, number of years smoked). One-sample t-tests were used to compare the number of participants choosing each intervention. Two sample t-tests and Chi-Square tests were used to assess continuous variables including potential association of demographic variables, medication use, and program participation with abstinence. For assessment of smoking status (abstinence vs. relapse) we adhered to the conservative principal that all missing data (e.g. missed return appointment) was coded as “relapsed.” Because the study sample was not randomized, conditions for Data Missing at Random (MAR) were not met to allow for multiple imputation to assess missing data.
RESULTS
Recruitment and Intervention Choice
Over the 12-month study period, 195 patients came to a clinic visit at the Meriter Smoking Cessation Program and all 195 were offered an opportunity join the study. Of these, 92.80% (n = 181) provided consent to have their data used for research purposes and were enrolled in the study. Reasons for not joining the study are provided in the consort diagram (Figure 1). Demographic data on participants is provided in Table 1. Significantly more participants chose to participate in MTS than WTQL (MTS = 65.75% (n = 119), WTQL = 34.25% (n = 62); t [180] = 4.45, p < .001). Based on an interview with the Program Coordinator, the most commonly cited factors leading to the choice of MTS over WTQL was that these participants had already used Quit Line in the past and it was ineffective for them, or that they had tried to quit and failed multiple times and wanted to use the most effective treatment possible (mean number of failed prior quit attempts for the full sample was 5.84).
Fig. 1.
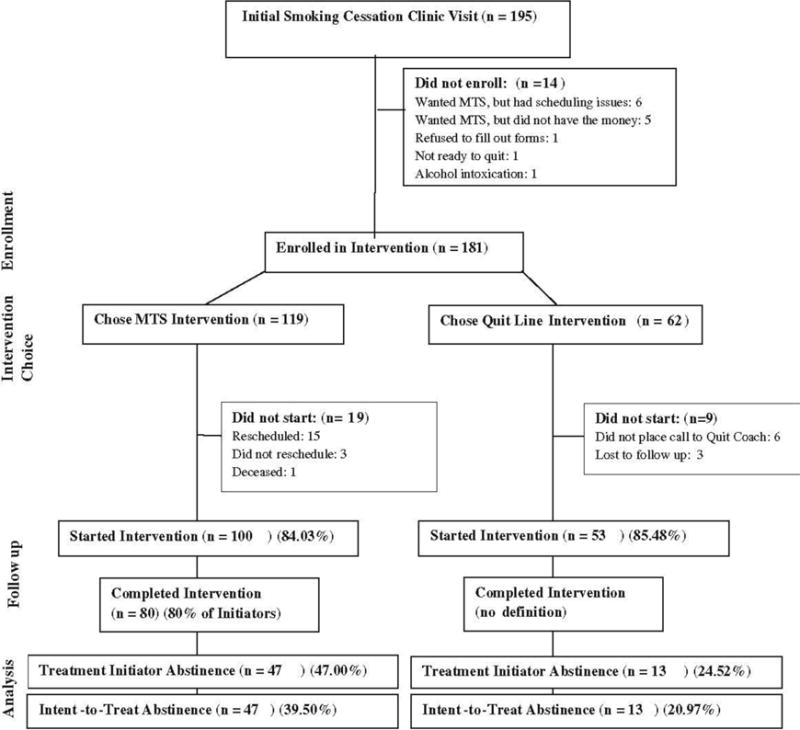
Consort diagram
Table 1.
Sample Demographics
| Variable | Total (n=181) | MTS(n=119) | QL(n=62) | pvalue | |||
|---|---|---|---|---|---|---|---|
| n/mean (SD) | % | n/mean (SD) | % | n/mean (SD) | % | ||
| Age | 51.60 (12.23) | 51.60(12.46) | 51.63(11.80) | 0.987 | |||
| Male | 64 | 35.36 | 38 | 31.93 | 26 | 41.94 | 0.241 |
| Female | 117 | 64.64 | 81 | 68.07 | 36 | 58.06 | 0.241 |
| Race/ethnicity | <.001*** | ||||||
| African-American | 18 | 9.94 | 5 | 4.20 | 13 | 20.97 | |
| American Indian | 2 | 1.10 | 0 | 0.00 | 2 | 3.23 | |
| Asian | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | |
| Caucasian | 157 | 86.74 | 111 | 93.28 | 46 | 74.19 | |
| Latino/Hispanic | 2 | 1.10 | 1 | 0.84 | 1 | 1.61 | |
| Other | 2 | 1.10 | 2 | 1.68 | 0 | 0.00 | |
| Education beyond high school | 78 | 43.09 | 68 | 57.14 | 10 | 16.13 | <.001*** |
| Drinks per week | 1.29 (1.98) | 1.57 (2.27) | 0.76 (1.45) | 0.011* | |||
| Cigs per day | 18.37 (9.94) | 17.74(8.40) | 19.58(12.90) | 0.249 | |||
| Years smoked | 30.68 (14.12 | 30.61(14.08) | 30.82(14.20) | 0.923 | |||
| Quit attempts | 5.84 (7.44) | 5.71 (4.60) | 6.11(12.90) | 0.761 |
p<.05;
p<.01;
p< .001
Baseline Characteristics
MTS and WTQL groups did not differ on age (MTS = 51.60 [SD = 12.46], WTQL = 51.63 [SD = 11.80]), gender (MTS = 31.93% male, WTQL = 41.94% male), or baseline smoking characteristics (cigarettes per day, years smoked, prior quit attempts; all p > .10) (Table 1). MTS participants were more likely to be Caucasian (MTS = 93.28%, WTQL = 74.19%; χ2 [1] = 11.30, p < .001, OR = 4.78), have education beyond high school (MTS = 57.14%, WTQL = 16.13%; χ2 [1] = 26.31, p < .001, OR = 6.86), and consume more alcoholic drinks per week (MTS = 1.57 [SD = 1.57], WTQL = 0.76 [SD = 0.76]; t [179] = 2.56, p = .011).
Intervention Adherence
Enrollment in MTS required payment for the MTS course. Of all MTS enrollees, 84.03% (n = 100) attended at least 1 class (treatment initiators). 19 participants paid for the course, but did not attend any classes during the study period. Based on an interview with Program Coordinator, most of these participants (15 of 19) who paid for MTS but did not attend any classes rescheduled their start date to attend the course after the study period (see Consort Diagram). It is assumed that some portion of the 15 who paid and rescheduled did in fact attend classes. For this reason, intent-to-treat analyses of MTS participants may underestimate the actual percentage of patients who quit smoking and treatment initiator analysis is also provided. Among WTQL participants, 85.48% (n = 53) completed at least one phone call to the quit coach (treatment initiators); the Quit Coach call followed the initial observed call to the Registration Specialist. MTS and WTQL groups did not differ on treatment initiation (χ2 [1] = .002, p = 0.969, OR = 0.89). Among MTS initiators, 72.00% (n = 72) attended the Quit Day Retreat (defined as treatment completers). There was no comparable method for defining “completion” within the WTQL intervention.
Abstinence Rates
An analysis of treatment initiators (n = 153) on biochemically confirmed 7-day point prevalence smoking abstinence at the 2-week post-quit clinic visit showed MTS abstinence rate = 47.00% (n = 47); WTQL abstinence rate = 24.52% (n = 13); χ2 [1] = 6.43, p = .011, OR = 2.71. An intent-to-treat analysis (n = 181) of the same showed MTS abstinence rate = 39.50% (n = 47); WTQL abstinence rate = 20.97% (n = 13); χ2 [1] = 5.51, p = .019, OR = 2.45. Abstinence rates for the MTS course completers was 65.30% (n = 47), with no comparable definition of “completion” for WTQL.
Baseline Variables
Analyses were conducted to determine whether baseline variables were associated with smoking abstinence. There was no difference between abstinent and relapsed participants on number of years smoked (p = .09), number of cigarettes per day (p = .76), gender (p = .29), or race (p = .13). Abstinent vs. relapsed participants did, however, differ on age (abstinent = 54.49 years, relapsed = 50.00 years, t[178] = 2.38, p = .02), and education (beyond high school = 51.89%, high school or less = 21.57%, χ2 [1] = 16.74, p < .001). Binary logistic regression was used to compare Intent-to-Treat smoking abstinence rates between the MTS and WTQL groups while controlling for baseline covariates. In these models differences in abstinence between MTS and WTQL remained significant when controlling for gender, age, race, baseline cigarettes per day, years smoked, and previous quit attempts (ps < .05). The difference between MTS and WTQL was no longer significant, however, when controlling for education beyond high school (p = .304).
Treatment Adherence and Treatment-based Predictors of Abstinence
MTS treatment initiators attended an average of 7.17 out of 8 classes (SD = 1.12), with abstinent smokers attending more classes (7.39) than relapsed smokers (6.79) (p = .02). WTQL treatment initiators completed an average of 1.93 (SD = 1.43) Quit Coach calls. Differences were non-significant between abstinent and relapsed smokers on calls completed (p = .41). Minutes meditated over 7 days assessed in the MTS group at the 2-week post-quit visit showed a mean of 13.82 (SD = 10.08) minutes per day. Abstinent vs. relapsed participants showed a trend (non-significant) toward greater amount of time in daily meditation (abstinent = 15.34 minutes, relapsed = 11.27 minutes, p = .09). At the 2-week post-quit assessment visit, medication use was numerically higher in MTS (MTS = 78% and WTQL = 60%), but failed to reach significance (p = .08). Medication use did not predict abstinence in the full sample (p = .23), or within groups MTS (p = .17), or WTQL (p = .93) (although the WTQL group (who received free patches from the WTQL) was more likely to use smoking cessation medications than the the MTS group (Table 2). When controlling medication use, the difference between MTS and WTQL smoking abstinence remained significant (p < .05) for both the ITT and treatment initiator samples.
Table 2.
Medications
| Medication | MTS patients | WTQL patients | p-value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Patch Alone | 6 | 5.04 | 23 | 37.10 | < .001 |
| Patch + Immediate Release NRT | 1 | 0.84 | 1 | 1.61 | .999 |
| Patch + Other | 0 | 0.00 | 1 | 1.61 | .739 |
| Immediate Release NRT Alone | 2 | 1.68 | 1 | 1.61 | .999 |
| Varenicline Alone | 1 | 0.84 | 1 | 1.61 | .999 |
| Bupropion Alone | 3 | 2.52 | 0 | 0.00 | .517 |
| None | 102 | 85.71 | 33 | 53.23 | < .001 |
| Other | 4 | 3.36 | 2 | 3.23 | .999 |
| Total | 119 | 62 | |||
DISCUSSION
Outcomes
As an observational study, this trial is limited by the fact that participants choose their treatments (no randomization) and paid for medications and treatments. In addition, MTS required payment, whereas WTQL was free and provided free NRT. These differences introduce multiple potential biases related to financial ability, medical literacy, time flexibility, understanding of treatments and make a fair comparison between MTS and WTQL interventions impossible. For a comparison between MTS and various controls (including WTQL), please see randomized trials already conducted on these interventions (Brewer et al., 2011; Davis, Goldberg, et al., 2014; Davis, Manley, et al., 2014; Davis et al., 2013; Vidrine et al., 2016). This trial provides a different perspective through its naturalistic design with more robust ecological validity. The study was designed to provide insight into how these two interventions actually function in a clinical setting including information on intervention enrollment, attendance and smoking abstinence. With this objective in mind, it is noteworthy that MTS and WTQL produced very similar abstinence rates to those found in a recent RCT. Initiator analysis of smoking abstinence in the current study showed abstinence for MTS = 47.0% and WTQL = 24.5% at 2 weeks post quit attempt, whereas initiator analysis within a recent RCT showed abstinence for MTS = 45.8% and WTQL = 25.4% at 4 weeks post quit attempt (Davis, Goldberg, et al., 2014). In a clinical setting, we would expect to see decreased treatment effects overall compared to an RCT due to RCT procedures known to support treatment effects (e.g., exclusion for comorbidities, no payment for medications or treatment, close staff supervision). Baseline variables found to be different between the two studies included age (current study = 51.6 years vs. RCT = 41.2 years), number of Caucasians (current study = 85.0% vs RCT = 77.0%), number of baseline cigarettes per day (current study = 18.4 vs. RCT = 15.7) (ps < .05). Unexpectedly high abstinence rates in the current study might be explained by the fact that this study allowed patients to choose between the two interventions – potentially enhancing motivation, the cost-incentive structure may have increased adherence, or clinic-based participants in the current study may have been more motivated to quit smoking than participants enrolled in a study solely for research purposes. Even with differences in population and design, the similarity of the effects between MTS and WTQL in a clinic and an RCT suggest that the robustness of the MTS intervention itself transcends the impact of participation through clinical or research settings. A large treatment effect of MTS is not surprising given the intensity of MTS (24 hours of total contact time), which easily becomes the most prominent aspect of treatment (as opposed to researcher contact, medication payment etc.).
Recruitment, Attendance and Course Completion
A high percentage (92.8%) of those who came to the smoking cessation clinic also enrolled in the study, such that study results should have relatively good generalizability to clinic population. The study maintained naturalistic design elements with no procedures, study visits, or contact with study staff, such that study outcomes should reliably reflect clinic outcomes. MTS course initiation rate during the study period was lower than expected (84.03% of those who enrolled and paid for MTS actually attended classes), although (15 of 19) who did not attend, rescheduled to take the course after the study period. Because a majority of non-initiators rescheduled to take the course at a later time, treatment initiator analyses may be as accurate as intent-to-treat analyses for estimation of clinical outcomes. WTQL participants showed a similar initiation rate of 85.48%. For the most part, these participants placed an observed call to the Quit Line Registration Specialist, but did not complete a call with a Quit Coach. MTS should have greater barriers for initiating treatment, because it is a high-intensity intervention. These barriers appear to have been ameliorated by other factors however, the most obvious ones being prior payment for the course and perhaps population differences (education, age) between groups.
Choice of MTS vs. Quit Line
This study allowed for a choice between MTS and WTQL with the finding that 65.75% chose to enroll in MTS vs. 34.25% who chose to enroll in the WTQL. It is noteworthy that a majority of participants chose MTS over WTQL. This might be unexpected because MTS was more intensive (took more time) and required payment up front. The finding that a high proportion of participants chose MTS may have been due to the fact that the Program Coordinator informed all patients that MTS had been found to be more effective in randomized trials. The exit interview with the Program Coordinator suggests that some participants did respond to information from prior research on intervention efficacy. This exit interview also indicated that the promise of full reimbursement for the course on completion appeared to make patients more willing to pay for the course. These findings are important to our field since we typically assume that regardless of efficacy, most patients would rather participate in behavioral treatment that is low-intensity and free vs. high-intensity and not free (Rennie et al., 2007). The finding should be understood in the context of the fact that the mean age of participants in this study was 51.6 with mean of 5.84 quit attempts, and it is possible that it is older smokers, or smokers with numerous failed quit attempts, may be most willing to participate in intensive treatment or pay for treatment if they believe will help them quit smoking.
Differences in Baseline Variables
Because the study allowed participants to choose between treatments, it was expected that there would be differences in baseline variables between groups, including variables that might impact abstinence. When baseline variables were included as covariates in models of smoking abstinence, all variables except for education did not affect between group (i.e., MTS vs. WTQL) abstinence differences. The fact that baseline education impacted group differences suggests that the observed differences in MTS and WTQL could have been driven by baseline differences between groups. Population differences between groups is a flaw inherent in naturalistic studies due to lack of randomization. These kinds of methodological challenges are to be expected as researchers embrace calls to test interventions in clinical settings through effectiveness and implementation studies (Dimidjian & Segal, 2015). Future randomized trials on MTS would be helpful to further elucidate the impact of education level on treatment outcomes.
Use of Medications
In this study, neither group was prescribed medications through the study or clinical program, but were advised to obtain medications from their primary provider. Participants using the WTQL, however, received through nicotine patches in the mail after enrollment. This resulted in a greater number of WTQL vs. MTS participants using medications. The lower level use of medications by MTS did not affect differences in abstinence between the two groups in any analysis, but it is concerning. There is consensus among investigators now that medications should be used during smoking cessation attempts whenever possible (Fiore et al., 2008). Because of the low-level of medication use in MTS when relying on primary provider prescriptions, we changed our clinic structure so that all clinic patients now see a prescriber (physician or mid-level provider) trained in tobacco use treatment for prescription of medications. Meriter Hospital and Duke University have both adopted this prescriber-centered model.
Incentive Cost structure
Studies have shown that the use of cost-incentive structures leads to better outcomes because patients are more willing to engage treatment if there is an imminent reward (Volpp et al., 2009). Evidence shows that people are more motivated to avoid losses than they are to seek gains (Kahneman & Tversky, 1979), but are most motivated when both losses and gains (i.e., sticks and carrots) are involved (Halpern et al., 2015). MTS in this study was provided for a fee, but with the promise that a health-system associated insurance provider would reimburse the full cost upon course completion. This presents a “carrot” and a “stick”– if an enrollee does not complete the MTS course they lose their enrollment fee; if they complete the course however, their enrollment fee is refunded. This cost-incentive pay structure was associated with a high rate of course completion in a course with a high rate of abstinence (65.3%) among course completers. From the perspective of the insurance provider, this is an advantageous return on a relatively small payment and provides substantial advantages over models in which the health system pays to make smoking cessation services available to all patients, but only a fraction of patients use these services (An et al., 2010). This may be most advantageous to contemporary medical systems (HMOs and ACOs) in which the provider assumes financial liability for health outcomes of their patients (Madison, Schmidt, & Volpp, 2013). A growing proportion of smokers with high dependence, stress, or other comorbidities will need more intensive treatment to achieve abstinence. Due to the profound impact of smoking on health, it would be wise for health systems, especially those financially tied to health outcomes, to consider the additional use of more intensive behavioral smoking cessation treatments and payment structures that incentivize attendance.
Current Programs Using MTS
Current programs using MTS include Duke University Medical Center, Unity Point in Madison, WI; Mission Hospital in Asheville, North Carolina; and several smaller treatment centers in Wisconsin. All program materials (Instructional DVD, Course Manual, Meditation Recordings) for the course are provided at no cost through the website www.quitresources.com. The MTS Teacher Training is provided through a 3-day course for psychotherapists, addiction treatment specialists, and Mindfulness-Based Stress Reduction (MBSR) instructors. The clinic treatment model surrounding MTS has progressed from the one described in this study to a more comprehensive model integrating the use of a Certified Tobacco Treatment Specialist (Nurse Practitioner or Physician’s Assistant) who prescribes evidence-based smoking cessation medications and offers a menu of behavioral treatments including MTS.
Limitations
Limitations of this study included those found in any naturalistic observational study - self-selection of intervention groups led to population differences between groups, which made isolation of treatment effects impossible. Other problems existed as well - medication use, though not a predictor of outcomes, varied widely across subjects; the follow up period was brief; and the lack of standardized self-report measures undermined any exploration of mechanism. The study, however, was not conducted on its own, but within an iterative series of studies on MTS. The purpose of this study was to provide ecological validity through naturalistic observational design for a treatment that has already been tested and found effective within randomized trials. Within this context, the principal finding of this study was that MTS, when provided in a clinical setting showed outcomes similar to those in randomized trials.
Acknowledgments
Funding: The study was funded through Meriter Foundation Research and Education Grant #477
Footnotes
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Author Contributions:
JD: Secured grant funding for the study, designed and executed the study, oversaw data collection and analysis, and primary manuscript writer.
SG: Conducted statistical analyses, helped write the manuscript.
KA: Conducted day-to-day operations of the study, collected data, helped prepare methods section of manuscript.
RS: Collaborated in manuscript preparation, tables, figures, copy editing, reference checks.
DL: conducted data cleaning, secondary statistical analysis, manuscript preparation and editing.
EK: collaborated in manuscript preparation for background section, constructed tables, figures, copy editing.
References
- Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179(4):403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An LC, Betzner A, Schillo B, Luxenberg MG, Christenson M, Wendling A, Kavanaugh A. The comparative effectiveness of clinic, work-site, phone, and Web-based tobacco treatment programs. Nicotine & Tobacco Research. 2010;12(10):989–996. doi: 10.1093/ntr/ntq133. [DOI] [PubMed] [Google Scholar]
- Annamalai A, Singh N, O’Malley SS. Smoking Use and Cessation Among People with Serious Mental Illness. Yale J Biol Med. 2015;88(3):271–277. [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Brandon TH. Behavioral tobacco cessation treatments: yesterday’s news or tomorrow’s headlines? J Clin Oncol. 2001;19(18 Suppl):64S–68S. [PubMed] [Google Scholar]
- Brewer JA, Elwafi HM, Davis JH. Craving to Quit: psychological models and neurobiological mechanisms of mindfulness training as treatment for addictions. Psychology of addictive behaviors: journal of the Society of Psychologists in Addictive Behaviors. 2013;27(2):366–379. doi: 10.1037/a0028490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, Rounsaville BJ. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug Alcohol Depend. 2011;119(1–2):72–80. doi: 10.1016/j.drugalcdep.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker J, Tollison S. Comparison of motivational interviewing with acceptance and commitment therapy: a conceptual and clinical review. Behav Cogn Psychother. 2011;39(5):541–559. doi: 10.1017/s1352465810000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans MD, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behavior. 1995;12(2):101–112. [Google Scholar]
- CDC. Centers for Disease Control and Prevention : Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226. [PubMed] [Google Scholar]
- CDC. Centers for Disease Control and Prevention: Quitting smoking among adults: United States, 2001-2010. Morbidity and Mortality Weekly Report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- CDC. Vital Signs: Current Cigarette Smoking Among Adults Aged ≥18 Years with Mental Illness — United States, 2009–2011. 2013 [PMC free article] [PubMed] [Google Scholar]
- Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, Schwartz R. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. 2016;6(6):e011045. doi: 10.1136/bmjopen-2016-011045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiton OM, Cohen EJ, Frank J. Population Health and the Hardcore Smoker: Geoffrey Rose Revisited. Journal of Public Health Policy. 2008;29(3):307–318. doi: 10.1057/jphp.2008.14. [DOI] [PubMed] [Google Scholar]
- Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States–recent progress and opportunities. CA Cancer J Clin. 2009;59(6):352–365. doi: 10.3322/caac.20037. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosom Med. 2007;69(6):560–565. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Davis JM, Fleming MF, Bonus KA, Baker TB. A pilot study on mindfulness based stress reduction for smokers. BMC Complement Altern Med. 2007;7:2. doi: 10.1186/1472-6882-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, Baker TB. Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Subst Use Misuse. 2014;49(5):571–585. doi: 10.3109/10826084.2013.770025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Manley AR, Goldberg SB, Smith SS, Jorenby DE. Randomized trial comparing mindfulness training for smokers to a matched control. J Subst Abuse Treat. 2014;47(3):213–221. doi: 10.1016/j.jsat.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Mills DM, Stankevitz KA, Manley AR, Majeskie MR, Smith SS. Pilot randomized trial on mindfulness training for smokers in young adult binge drinkers. BMC Complement Altern Med. 2013;13:215. doi: 10.1186/1472-6882-13-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Segal ZV. Prospects for a clinical science of mindfulness-based intervention. American Psychologist. 2015;70(7):593. doi: 10.1037/a0039589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwafi HM, Witkiewitz K, Mallik S, Thornhill TA, Brewer JA. Mindfulness training for smoking cessation: moderation of the relationship between craving and cigarette use. Drug and alcohol dependence. 2013;130(0):222–229. doi: 10.1016/j.drugalcdep.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Jaen CR, Baker TB. Clinical Practice Guideline: Treating Tobacco Use and Dependence: 2008 Update 2008 [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Front Psychiatry. 2014;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Pierson HM, Piasecki MP, Antonuccio DO, Palm KM. Does acceptance and relationship focused behavior therapy contribute to bupropion outcomes? A randomized controlled trial of functional analytic psychotherapy and acceptance and commitment therapy for smoking cessation. Behavior therapy. 2011;42:700–715. doi: 10.1016/j.beth.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Goldberg SB, Manley AR, Smith SS, Greeson JM, Russell E, Van Uum S, Davis JM. Hair cortisol as a biomarker of stress in mindfulness training for smokers. J Altern Complement Med. 2014;20(8):630–634. doi: 10.1089/acm.2014.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Keyes KM, Hasin DS. Changes in Cigarette Use and Nicotine Dependence in the United States: Evidence From the 2001–2002 Wave of the National Epidemiologic Survey of Alcoholism and Related Conditions. American Journal of Public Health. 2009;99(8):1471–1477. doi: 10.2105/AJPH.2007.127886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, Volpp KG. Randomized Trial of Four Financial-Incentive Programs for Smoking Cessation. New England Journal of Medicine. 2015;372(22):2108–2117. doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78(2):169–183. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. The hardening hypothesis: Is the ability to quit decreasing due to increasing nicotine dependence? A review and commentary. Drug and alcohol dependence. 2011;117(2–3):111–117. doi: 10.1016/j.drugalcdep.2011.02.009. doi: http://dx.doi.org/10.1016/j.drugalcdep.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip DT, Cohen JE, Bondy SJ, Chaiton MO, Selby P, Schwartz R, Ferrence R. Do components of current ‘hardcore smoker’ definitions predict quitting behaviour? Addiction. 2012;107(2):434–440. doi: 10.1111/j.1360-0443.2011.03674.x. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Hendricks PS, Brandon TH. The increasing recalcitrance of smokers in clinical trials II: Pharmacotherapy trials. Nicotine Tob Res. 2003;5(1):27–35. doi: 10.1080/1462220031000070534. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J, Hanh TN. Full catastrophe living: Using the wisdom of your body and mind to face stress, pain, and illness. Delta; 2009. [Google Scholar]
- Kahneman D, Tversky A. Prospect Theory: An Analysis of Decision under Risk. Econometrica. 1979;47(2):263–291. doi: 10.2307/1914185. [DOI] [Google Scholar]
- Kobinsky KH, Redmond LA, Smith SS, Yepassis-Zembrou PL, Fiore MC. The Wisconsin Tobacco Quit Line’s Fax to Quit Program: Participant Satisfaction and Effectiveness. Wisconsin Medical Journal. 2010;109(2):79–84. [PubMed] [Google Scholar]
- Koszycki D, Benger M, Shlik J, Bradwejn J. Randomized trial of a meditation-based stress reduction program and cognitive behavior therapy in generalized social anxiety disorder. Behav Res Ther. 2007;45(10):2518–2526. doi: 10.1016/j.brat.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Lai DT, Cahill K, Qin Y, Tang JL. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2010;(1):CD006936. doi: 10.1002/14651858.CD006936.pub2. [DOI] [PubMed] [Google Scholar]
- Madison K, Schmidt H, Volpp KG. Smoking, obesity, health insurance, and health incentives in the affordable care act. JAMA. 2013;310(2):143–144. doi: 10.1001/jama.2013.7617. [DOI] [PubMed] [Google Scholar]
- McLeod S. S Psychology 2015 [Google Scholar]
- Moulton LH. Covariate-based constrained randomization of group-randomized trials. Clin Trials. 2004;1(3):297–305. doi: 10.1191/1740774504cn024oa. [DOI] [PubMed] [Google Scholar]
- NICE. How to stop smoking in pregnancy and following childbirth. 2010:1–58. [Google Scholar]
- Oikonomou MT, Arvanitis M, Sokolove RL. Mindfulness training for smoking cessation: A meta-analysis of randomized-controlled trials. Journal of Health Psychology. 2016 doi: 10.1177/1359105316637667. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Rennie TW, Bothamley GH, Engova D, Bates IP. Patient choice promotes adherence in preventive treatment for latent tuberculosis. European Respiratory Journal. 2007;30(4):728–735. doi: 10.1183/09031936.00034007. [DOI] [PubMed] [Google Scholar]
- Rogojanski J, Vettese L, Antony M. Coping with Cigarette Cravings: Comparison of Suppression Versus Mindfulness-Based Strategies. Mindfulness. 2011;2(1):14–26. [Google Scholar]
- Segal ZV, Williams MJ, Teasdale JD. Mindfulness-based cognitive therapy for depression. 2nd. New York, NY, US: Guilford Press; 2002. [Google Scholar]
- Sibbald B, Roland M. Understanding controlled trials. Why are randomised controlled trials important? BMJ. 1998;316(7126):201. doi: 10.1136/bmj.316.7126.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. 2010;126(6):2234–2242. doi: 10.1097/PRS.0b013e3181f44abc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;1:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Stolberg HO, Norman G, Trop I. Randomized controlled trials. AJR Am J Roentgenol. 2004;183(6):1539–1544. doi: 10.2214/ajr.183.6.01831539. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Segal Z, Williams JM. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behav Res Ther. 1995;33(1):25–39. doi: 10.1016/0005-7967(94)e0011-7. [DOI] [PubMed] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, Wetter DW. Efficacy of Mindfulness-Based Addiction Treatment (MBAT) for Smoking Cessation and Lapse Recovery: A Randomized Clinical Trial. Journal of Consulting and Clinical Psychology. 2016 doi: 10.1037/ccp0000117. No Pagination Specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpp KG, Troxel AB, Pauly MV, Glick HA, Puig A, Asch DA, Audrain-McGovern J. A randomized, controlled trial of financial incentives for smoking cessation. N Engl J Med. 2009;360(7):699–709. doi: 10.1056/NEJMsa0806819. [DOI] [PubMed] [Google Scholar]
- Way BM, Creswell JD, Eisenberger NI, Lieberman MD. Dispositional mindfulness and depressive symptomatology: correlations with limbic and self-referential neural activity during rest. Emotion. 2010;10(1):12. doi: 10.1037/a0018312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg HI, Hayden VC, Pontes VP. Selection criteria and generalizability within the counterfactual framework: explaining the paradox of antidepressant-induced suicidality? Clin Trials. 2009;6(2):109–118. doi: 10.1177/1740774509102563. [DOI] [PubMed] [Google Scholar]
- Weiss NS, Koepsell TD, Psaty BM. GEneralizability of the results of randomized trials. Archives of Internal Medicine. 2008;168(2):133–135. doi: 10.1001/archinternmed.2007.30. [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, Tindle HA. Mindful attention reduces neural and self-reported cue-induced craving in smokers. Soc Cogn Affect Neurosci. 2013;8(1):73–84. doi: 10.1093/scan/nsr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield HJ. Towards case-specific applications of mindfulness-based cognitive-behavioural therapies: A mindfulness-based rational emotive behaviour therapy. Counselling Psychology Quarterly. 2006;19(2):205–217. doi: 10.1080/09515070600919536. [DOI] [Google Scholar]
- Witkiewitz K, Bowen S, Harrop EN, Douglas H, Enkema M, Sedgwick C. Mindfulness-based treatment to prevent addictive behavior relapse: theoretical models and hypothesized mechanisms of change. Subst Use Misuse. 2014;49(5):513–524. doi: 10.3109/10826084.2014.891845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe J. The practice of behavior therapy. 3rd. New York: Pergamon; 1982. [Google Scholar]
- Woolf SH. THe meaning of translational research and why it matters. JAMA. 2008;299(2):211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]


