Abstract
Engineered nanomaterials (ENM) are utilized in many applications due to their unique physicochemical properties. The increasing use of ENMs in consumer products raises concerns of potential adverse effects in humans and the environment. A common outcome of exposure (intentional, environmental or occupational) to ENMs is altered immune responses including inflammation, hypersensitivity, and immunosuppression. ENMs have been shown to interact with the immune system through key effector cells (i.e. mast cells and antigen presenting cells) or via complement activation leading to consequences to both innate and adaptive immunity. Further, upon introduction into a biological system, ENMs are rapidly coated with proteins, lipids and other macromolecules forming a biocorona which impacts immune cell and complement responses. In this current opinion, we highlight key studies and challenges in understanding cellular mechanisms of ENM-mediated immunomodulation and toxicity.
Keywords: Nanoparticles, nanotoxicology, immunomodulation, immune activation, immune suppression
Graphical abstract
1. INTRODUCTION
Engineered nanomaterials (ENM) are materials with at least one dimension in the range of 1–100 nanometers. Due to their small size, large surface area, and in comparison to their bulk counterparts, ENMs possess unique physicochemical properties making them useful in a wide range of applications [1]. Advancements in physics, chemistry and material sciences has led to promotion of nanotechnology with a theoretically unlimited number of ENMs and applications ranging from electronics and catalysts to biosensors and drug delivery. There are diverse types of ENMs based on their composition including carbon-based, metals, metal oxides, polymers, lipid-, and protein-based. For instance, carbon nanotubes are utilized for their mechanical strength and light weight while silver, gold and iron ENMs are widely utilized for their antimicrobial, optical, and magnetic properties, respectively. The number of consumer products that utilize ENMs is also exponentially increasing [2]. This raises concerns regarding potential human exposure and associated adverse effects of ENMs on human health and the environment. In general, various endpoints of toxicity have been demonstrated following exposure to a wide range of ENMs including formation of reactive oxygen species (ROS), disruption of mitochondrial respiration, induction of apoptosis, lipid peroxidation and DNA damage, however, specific molecular mechanisms are largely lacking [3]. Nonetheless, a common theme arising from many toxicological studies of ENM exposure is immunotoxicity including but not limited to inflammation and immunomodulation.
The immune system is one of the most important systems that can dictate toxicological and pathological consequences following exposure to ENMs (e.g. tissue accumulation vs. clearance). Importantly, the majority of immune responses to ENMs are largely due to their unique physicochemical properties such as size, surface coating/charge and shape. For instance, it has been demonstrated that ENM size influences uptake and activation of antigen presenting cells (APC), such as dendritic cells and macrophages, as well as nanoparticle trafficking to draining lymph nodes and subsequent T-cell responses [4–6]. Surface charge is another key factor which enhances cell membrane interaction, uptake and subsequent immune cell activation [7]. ENM shape has also been shown to influence internalization by cells with high aspect ratio ENMs being associated with higher toxicity due to frustrated phagocytosis in macrophages [7, 8]. Understanding such physicochemical property-mediated changes in immunological responses remains a challenge and is of critical importance for future nanomedicine applications.
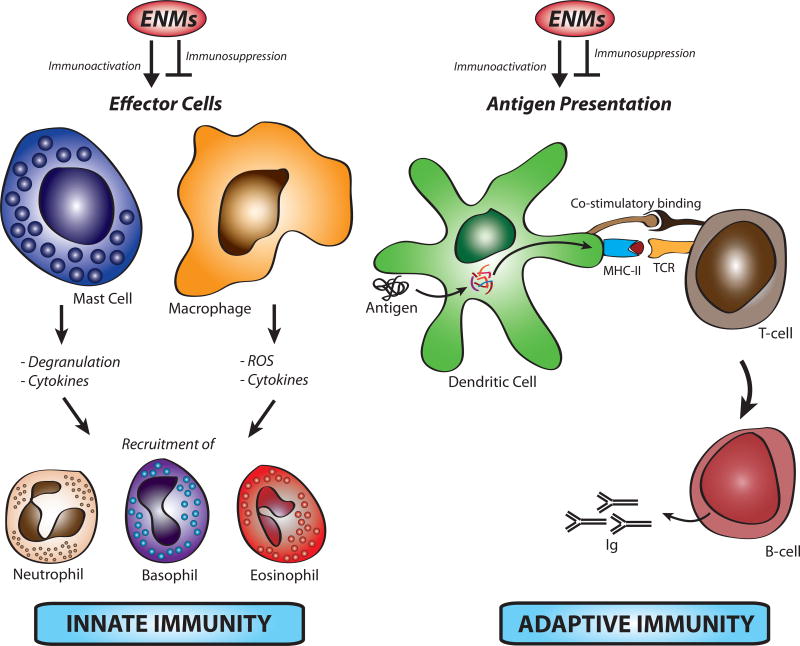
To date, the majority of studies examining ENM immunomodulation have demonstrated immune activation, however, an increasing number of studies are beginning to establish ENM mediated immunosuppression as an outcome. In both cases, exposure could be either intentional (e.g. medical application) or unintentional (e.g. consumer, environmental or occupational) [9]. Further, activation of the immune system by ENMs could be beneficial and is being pursued in areas such as cancer therapy and vaccination or could be detrimental such as in autoimmune disorders and tissue remodeling. In this current opinion, we highlight key findings of cellular and molecular mechanisms of ENM-induced immunostimulatory and immunosuppressive properties (figure 1).
Figure 1. Potential direct mechanisms of engineered nanomaterial-induced immunomodulation.
Engineered nanomaterials (ENM) can activate or suppress immune responses through direct interaction with effector immune cells such as macrophages and mast cells leading to their activation and subsequent recruitment of other effector immune cells such as neutrophils. ENMs can also modulate immune responses through direct interaction with antigen presenting cells such as dendritic cells, which could affect their function toward an adaptive immune activation or suppression.
2. ENM-INDUCED IMMUNE ACTIVATION
The majority of work regarding ENM-induced immunomodulation is focused on the activation of key immune cells such as macrophages, subsequent cellular inflammatory responses and toxicity. In this section, we discuss the role of complement activation, the inflammatory response in ENM-mediated immunomodulation and the impact of ENM immunomodulatory properties on hypersensitivity immune reactions and adaptive immunity.
2.1. Complement activation
The complement system is an important part of the innate immune system in fighting pathogens. The complement system is composed of more than 30 plasma proteins that interact with each other in a cascade manner, leading to opsonization, lysis, release of chemotactic molecules and anaphylatoxins. ENMs have been shown to activate the complement system through the classical, alternative and lectin pathways with the prime consequence being surface opsonization, which subsequently leads to particle clearance by the reticuloendothelial system [10]. Importantly, several clinically approved ENM formulations (mostly liposomes and micelles) have been shown to activate complement resulting in pseudoallergic responses (i.e. release of anaphylatoxins) [11].
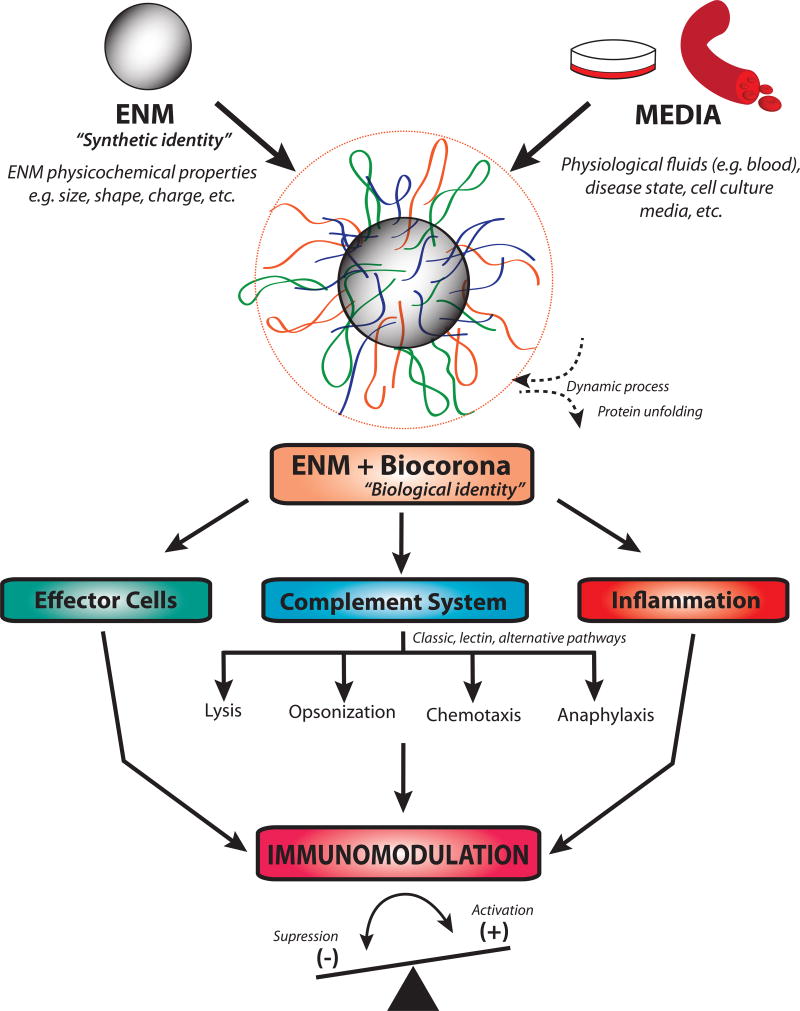
ENM interaction with the complement system is complex and controlled by physicochemical properties [6]. In addition, adsorption of proteins to ENMs and hence the formation of a biomolecular corona (biocorona), which is defined as the adsorption of macromolecules including proteins, lipids, carbohydrates and nucleic acids to the ENM surface, has been shown to play key roles in complement activation [12]. Once ENMs are introduced into biological fluids (e.g. blood) or cell culture media containing serum, they instantly form a biocorona (figure 2) [13]. It remains one of the major challenges in studying ENM interactions with biological systems to understand the influence of the ENM biocorona (ENM biological identity) on cellular responses versus ENM synthetic identity. Importantly, one proposed mechanism of complement activation is through ENM-adsorbed proteins, which undergo conformational changes making them susceptible to C3b attack [14]. This has been demonstrated using superparamagnetic iron oxide nanoparticles (SPION), which activated the alternative complement pathway in human blood via adsorbed proteins [14]. This suggests that ENMs with long circulating half-life may result in sustained inflammatory responses and immunomodulatory consequences. Certainly, further research on the molecular basis of ENM-mediated complement activation including the role of protein adsorption on the surface of ENMs is needed for safer design. In addition, development and implementation of methods for the assessment of ENM-mediated complement activation under realistic conditions (e.g. underlying disease states, changes in serum chemistry, etc.) could represent a key step for future nanosafety research.
Figure 2. The effect of biocorona formation on engineered nanomaterial-induced immunomodulation.
Once ENMs are introduced into biological fluids such as blood, they instantly form a biocorona (resulting in a new biological identity), which could alter interaction with the immune system as compared to their synthetic identity. Formation of the biocorona influences ENM interactions with effector cells, the complement system and induction of inflammatory responses that eventually modulate immune responses.
2.2. The inflammatory response
Innate immune cells express receptors that can recognize certain molecular patterns (e.g. pathogen-associated molecular patterns, PAMP) through pattern recognition receptors (PRR) such as the Toll-like receptors (TLR) and NOD-like receptors (NLR). These receptors can recognize certain molecular patterns of microbial origin such as lipopolysaccharides and peptidoglycan. Innate immune cells also recognize danger- or damage-associated molecular patterns (DAMPs) (also called ‘alarmins’) such as interleukin-1 (IL-1). These are endogenous biomolecules that trigger a sterile inflammatory response. It has been proposed that ENMs may act as danger signals termed nanomaterial-associated molecular patterns (NAMPs), which could provoke cellular stress responses, cell injury and inflammation [15]. Indeed, in an attempt to design a novel vaccine system, Luo et al. have reported that polymeric nanoparticles activated the stimulator of interferon genes (STING) pathway, a recently identified pathway involved in innate immunity in the recognition of cyclic dinucleotides in host cells [16]. Further, several lines of evidence demonstrate a potential production of ‘alarmins’ in response to ENM exposure. For instance, silica micro- and nanoparticles have been shown to induce IL-1α, which was key in acute lung inflammation [17]. Similarly, we have shown that exposing mice to MWCNT induced IL-33 which acted as an alarmin to trigger inflammatory responses in mouse lung [18, 19].
NALP3 (also known as NLRP3 and cryopyrin) is one of the most studied members of the NLRs. It mediates the formation of caspase-1-activating multiprotein complex known as the inflammasome, which processes pro-inflammatory caspases and cytokines [20]. NALP3 senses a wide range of molecules including uric acid crystals, alum, asbestos and bacterial toxins [20]. Of interest, several studies have reported activation of the inflammasome in response to ENM exposure. For instance, Demento et al. have reported that macrophage exposure to LPS-modified antigen loaded poly-lacticco-glycolic acid nanoparticles resulted in IL-1β production, which was diminished in NLRP3-deficient and caspase-1-deficient macrophages [21]. It is worth noting that several molecular mechanisms have been proposed to underlie ENM-induced inflammasome activation. For example, Yazdi et al. have shown that similar to classic NLRP3 agonists, TiO2-induced activation of the inflammasome involves potassium channels and ROS. Silver nanoparticles have been shown to induce activation of the inflammasome through degradation of ATF-6 (activating transcription factor-6), an endoplasmic reticulum stress sensor [22]. These studies provide evidence that ENMs can promote sterile inflammation through PRRs leading to inflammasome activation and subsequent cellular stress, injury and inflammation. Nevertheless, research on the molecular basis of how ENMs are recognized by PRRs is still in its infancy and it is anticipated that unraveling the underlying molecular mechanisms will help in understanding ENM-induced inflammation.
Although it is now well recognized that the biocorona could drastically influence ENM interactions with immune cells including inflammatory responses (figure 2), knowledge regarding the underlying molecular mechanisms is largely lacking. Nevertheless, some efforts have been made to start bridging this gap. For instance, Deng et al. have demonstrated that gold nanoparticles bind to fibrinogen causing its unfolding thereby leading to the activation of the Mac-1 receptor on macrophages and subsequent NFκB signaling [23]. Importantly, fibrinogen binding to integrin receptors is critical for innate immunity and such formation of a biocorona could therefore be of immunomodulatory consequence [24]. We have recently reported that AgNPs with a biocorona from hyperlipidemic serum had unique protein content including higher cholesterol concentrations and resulted in excerbated inflammatory responses indicating a vital role for changes in biocorona constituents that can modulate immune responses [25]. Taken together, it is now evident that the ‘biological identity’ of ENMs is of critical importance from an immunological standpoint as it could largely dictate ENM interaction with membrane and surface receptors of immune cells, and therefore, it should be particularly considered in in vitro experimental design for a better representation of ‘real world’ in vivo settings for improved prediction of ENM-mediated immunotoxicity.
2.3. Hypersensitivity immune reactions and adaptive immunity
The adaptive immune system is critical for eliminating invading pathogens and foreign antigens. However, to develop an adaptive immune response, it takes hours to days and requires a preceding innate immune response. The immune system utilizes different mechanisms for such responses and these are categorized into four types of immune hypersensitivity reactions: type I immune reactions (immediate) are driven by immunoglobulin type E (IgE); type II (cytotoxic) are mediated by IgM and IgG; type III are mediated by antigen-antibody immune complexes; and type VI (delayed) are driven by T helper cells. Because of their unique interaction with the immune system, ENMs could potentially interfere with each of these adaptive immune responses and initiate or exacerbate hypersensitivity reactions.
Type I immune hypersensitivities or allergies develop following exposure to an antigen (e.g. pollen, dust mite, etc.). It requires pre-exposure to an allergen and subsequent production of immunoglobulin type E (IgE) by B-cells. Mast cells and basophils are then primed with IgE bound to the high affinity IgE receptor (FcεRI) and a second exposure to the same allergen leads to crosslinking of IgE bound FcεRI resulting in cell activation and the release of preformed mediators including histamine and various proteases and enzymes. ENM interference at any stage of the process i.e. from first exposure to activation of effector cells could affect (enhance or suppress) allergic immune responses. IgE is a key element in mediating allergic immune responses and several in vivo investigations have shown changes in IgE levels following ENM exposure. For instance, in a mouse model of atopic dermatitis, Hirai et al. have found that co-exposure of amorphous silica nanoparticles and antigen increased IgE levels and augmented T helper 2 (Th2) responses and was associated with aggravated allergic symptoms [26]. Recently, Feraheme®, a SPION formulation for iron replacement medication, was reported to cause severe anaphylactic reactions (79 cases occurred, 18 were fatal) following intravenous administration [27]. This indicates potential ENM-induced adverse effects of type I allergic reactions and severity of such responses undoubtedly underscores the necessity for better understanding and assessment of such interactions. To this end, a number of investigations have attempted to assess ENM interactions with major effector cell types involved in allergy and the underlying mechanisms. Importantly, we have shown that mast cell activation in response to ENMs exacerbates inflammatory responses in murine lung and is detrimental to cardiovascular function [28]. Similarly, we have shown silver nanoparticles (AgNPs) induce a size-dependent mast cell degranulation involving activation of signal transduction pathways and calcium influx, which were independent of IgE or FcεRI crosslinking [29, 30]. It is worth mentioning that ENM-mediated allergy-like reactions could also be induced indirectly through activation of the complement system leading to the release of anaphylatoxins (C4a, C3a and C5a). Together, these findings indicate a potential direct as well as indirect role for ENM-induced modulation of key effector cells involved in allergy. Future studies should examine the severity and consequences of allergic reactions to ENMs, the role of physicochemical properties, and relevance of such reactions to susceptible populations (e.g. patients with preexisting allergies).
Antigen presenting cells play key roles in initiating adaptive immune responses to pathogens and interference in this process by ENM exposure could be of significant consequence. Indeed, a number of investigations have demonstrated immunomodulation following exposure to ENMs in APC and animal models. For example, exposure of dendritic cells to TiO2 and SiO2 nanoparticles resulted in increased expression of CD80, CD86, MHC-II and activation of the inflammasome [31]. It has also been shown that exposure to TiO2 nanoparticles polarized dendritic cells towards a Th1 response whereas cerium dioxide (CeO2) nanoparticles resulted in IL-10 release from APC and induced Th2 responses [32]. Similarly, zinc oxide (ZnO) nanoparticles have been shown to modulate adaptive immune responses through exacerbation of Th2 responses in an ovalbumin model of murine allergic asthma leading to an increase in ovalbumin-specific IgE and IgG1, increased Th2 cytokines and T-cell proliferation [33]. Collectively, these observations along with those discussed earlier suggest a potential capacity for ENMs to activate, exacerbate or alter innate and adaptive immune responses by themselves or during co-exposures and co-morbidities.
3. ENM-INDUCED IMMUNE SUPPRESSION
Most immunotoxicity studies are focused on immune activation and the inflammatory properties of ENMs including cellular stress and injury while the area of ENM-induced immunosuppression is largely under investigated. It is important to note that there are many studies that have employed ENMs as delivery systems (targeted therapy) for immunosuppressive drugs which showed improved pharmacokinetic and pharmacodynamic profiles [34]. This is beyond the scope of this review and in general these studies are typically not concerned with the inherent immunosuppressive properties of ENMs. Nevertheless, evidence is accumulating that demonstrates potential immunosuppressive properties of ENMs. For instance, Chauhan et al. have reported anti-inflammatory properties of poly-amidoamine dendrimers mediated through inhibition of cyclooxygenases and nitric oxide production, which was found to be largely dependent on surface functionalization [35]. Release of cytokines and chemokines by innate immune cells is crucial for producing immunity and IL-1β is key in a variety of acute and chronic inflammatory diseases. It has been demonstrated that exposure to SPION attenuated LPS-induced IL-1β production in primary murine microglial cells by inhibiting cathepsin B, which mediates IL-1β activation [36]. Such findings indicate one of the challenges in understanding ENM-induced biological responses as some ENMs induce IL-1β and the inflammasome while others suppress IL-1β, which may be explained by variations in ENM physicochemical properties. Interference with effector cell activation and signaling is another potential mechanism of ENM-mediated immunosuppression. For example, it has been reported that C60 fullerenes inhibited mediator release from mast cells through inhibition of IgE-mediated activation of the tyrosine kinase Syk and ROS formation [37]. Although ROS formation is recognized as a mechanism of ENM-induced toxicity, some ENMs appear to have the capacity to scavenge reactive species. For instance, CeO2 nanoparticles are reported to scavenge reactive oxygen and nitrogen species and protect against autoimmune-mediated neurodegenerative disease [38].
APCs represent an important interface between innate and adaptive immunity and interference in this process would be detrimental to a robust immune response. Importantly, it has been shown that ENMs can suppress immune responses by interfering with the phagocytic activity and clearance of pathogens as well as antigen processing by APCs and subsequent T-cell activation. For instance, Kodali et al. reported that exposing macrophages to SPION reduced phagocytosis of S. pneumoniae [39]. Similarly, exposure to SPION has been shown to reduce the capacity of antigen processing, activation of CD4+ T-cells, and cytokine release by dendritic cells [40]. These findings suggest compromised adaptive immune responses to pathogens. Indeed, Sanpui et al. have demonstrated that exposure to SWCNT significantly increased the susceptibility to viral infections potentially through inhibition of proinflammatory and anti-viral protein expression as well as modulation of mitochondrial function and viral receptors [41]. Collectively, these studies indicate that various ENMs could aid in or lead to immunosuppression through different mechanisms; however, such studies are barely scratching the surface and undeniably more mechanistic work is needed. It is worth noting that even in the case of utilizing ENMs for their immunosuppressive properties (e.g. fullerenes) and even with a good safety profile, meticulous characterization of such immunomodulatory effects with improved understanding of the underlying mechanisms remains a challenge for safe and sustainable use of nanotechnology in medicine.
4. CONCLUDING REMARKS
As the number of ENMs and their applications increases, health concerns and safety issues increase as well. While large efforts have been made to understand the nature of ENM interactions with biological systems, addressing the safety use of nanotechnology remains a grand challenge. Recent work (highlighted in this review) has demonstrated a critical role of the immune system in dictating toxicological consequences of ENMs. Importantly, the molecular mechanisms of ENM-induced immunological responses are yet to be fully determined. A major issue is lack of rigorous characterization of utilized ENMs, particularly for their biological identity, making comparison between studies nearly impossible. Recommendations to help bridge the gap in knowledge and better understanding of ENM-mediated immunomodulation and immunotoxicity include: i. rigorous experimental design and thorough ENM characterization with specific emphasis on the context of exposure (e.g. route of administration, biological fluid and biocorona, underlying disease states, etc.); ii. test for basic immunological parameters as part of ENM routine characterization – such assays could include RBC hemolysis, complement activation, platelet and granulocyte activation, etc. [9]; iii. focus on delineation of the underlying molecular mechanisms of ENM immunomodulation beyond general endpoints of toxicity (e.g. cytotoxicity); and finally, iv. foster strong interdisciplinary collaborations and dedicate efforts toward consortium type, large studies that utilize thoroughly characterized ENMs across multiple research groups.
Highlights.
-
-
Unique physicochemical properties (size, shape, charge and surface properties) contribute significantly to ENM interactions with the immune system
-
-
ENMs activate the complement system through classical, alternative and lectin pathways potentially via ENM-adsorbed proteins
-
-
ENMs activate the inflammasome through multiple mechanisms
-
-
ENMs mediate immune activation or suppression through direct interaction with effector immune cells and/or interference with antigen presenting cells
-
-
Understanding mechanisms of ENM-mediated immunomodulation represents a grand challenge in development of safe nanotechnologies for use in medicine and consumer products
Acknowledgments
This work was funded by NIEHS grant R01 ES019311 (JMB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
* * of outstanding interest
- 1.Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8(7):543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 2.Vance ME, et al. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769–80. doi: 10.3762/bjnano.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 4.Fifis T, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol. 2004;173(5):3148–54. doi: 10.4049/jimmunol.173.5.3148. [DOI] [PubMed] [Google Scholar]
- 5.Manolova V, et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–13. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 6.Mottram PL, et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol Pharm. 2007;4(1):73–84. doi: 10.1021/mp060096p. [DOI] [PubMed] [Google Scholar]
- 7.Frohlich E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int J Nanomedicine. 2012;7:5577–91. doi: 10.2147/IJN.S36111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8*.Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2(8):469–78. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 10.Moghimi SM, et al. Material properties in complement activation. Adv Drug Deliv Rev. 2011;63(12):1000–7. doi: 10.1016/j.addr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Szebeni J. Complement activation-related pseudoallergy: a new class of drug-induced acute immune toxicity. Toxicology. 2005;216(2–3):106–21. doi: 10.1016/j.tox.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Szebeni J, et al. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Deliv Rev. 2011;63(12):1020–30. doi: 10.1016/j.addr.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Monopoli MP, et al. Biomolecular coronas provide the biological identity of nanosized materials. Nat Nanotechnol. 2012;7(12):779–86. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, et al. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2017;12(4):387–393. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Fadeel B. Clear and present danger? Engineered nanoparticles and the immune system. Swiss Med Wkly. 2012;142:w13609. doi: 10.4414/smw.2012.13609. [DOI] [PubMed] [Google Scholar]
- 16.Luo M, et al. A STING-activating nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2017;12(7):648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabolli V, et al. The alarmin IL-1alpha is a master cytokine in acute lung inflammation induced by silica micro- and nanoparticles. Part Fibre Toxicol. 2014;11:69. doi: 10.1186/s12989-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katwa P, et al. A carbon nanotube toxicity paradigm driven by mast cells and the IL-(3)(3)/ST(2) axis. Small. 2012;8(18):2904–12. doi: 10.1002/smll.201200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, et al. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. Int J Nanomedicine. 2013;8:1733–48. doi: 10.2147/IJN.S44211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–35. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demento SL, et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27(23):3013–21. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simard JC, et al. Silver nanoparticles induce degradation of the endoplasmic reticulum stress sensor activating transcription factor-6 leading to activation of the NLRP-3 inflammasome. J Biol Chem. 2015;290(9):5926–39. doi: 10.1074/jbc.M114.610899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng ZJ, et al. Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation. Nat Nanotechnol. 2011;6(1):39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- 24.Flick MJ, et al. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113(11):1596–606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannahan JH, et al. From the Cover: Disease-Induced Disparities in Formation of the Nanoparticle-Biocorona and the Toxicological Consequences. Toxicol Sci. 2016;152(2):406–16. doi: 10.1093/toxsci/kfw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirai T, et al. Amorphous silica nanoparticles size-dependently aggravate atopic dermatitis-like skin lesions following an intradermal injection. Part Fibre Toxicol. 2012;9:3. doi: 10.1186/1743-8977-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.U.S. Food and Drug Administration. FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). [Drug Safety Communication] 2015 Available from: https://www.fda.gov/Drugs/DrugSafety/ucm440138.htm.
- 28.Wingard CJ, et al. Mast cells contribute to altered vascular reactivity and ischemia-reperfusion injury following cerium oxide nanoparticle instillation. Nanotoxicology. 2011;5(4):531–45. doi: 10.3109/17435390.2010.530004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aldossari AA, et al. Influence of physicochemical properties of silver nanoparticles on mast cell activation and degranulation. Toxicol In Vitro. 2015;29(1):195–203. doi: 10.1016/j.tiv.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alsaleh NB, Persaud I, Brown JM. Silver Nanoparticle-Directed Mast Cell Degranulation Is Mediated through Calcium and PI3K Signaling Independent of the High Affinity IgE Receptor. PLoS One. 2016;11(12):e0167366. doi: 10.1371/journal.pone.0167366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winter M, et al. Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology. 2011;5(3):326–40. doi: 10.3109/17435390.2010.506957. [DOI] [PubMed] [Google Scholar]
- 32.Schanen BC, et al. Immunomodulation and T helper TH(1)/TH(2) response polarization by CeO(2) and TiO(2) nanoparticles. PLoS One. 2013;8(5):e62816. doi: 10.1371/journal.pone.0062816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Roy R, et al. Zinc oxide nanoparticles provide an adjuvant effect to ovalbumin via a Th2 response in Balb/c mice. Int Immunol. 2014;26(3):159–72. doi: 10.1093/intimm/dxt053. [DOI] [PubMed] [Google Scholar]
- 34.Ilinskaya AN, Dobrovolskaia MA. Immunosuppressive and antiinflammatory properties of engineered nanomaterials. Br J Pharmacol. 2014;171(17):3988–4000. doi: 10.1111/bph.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35**.Chauhan AS, et al. Unexpected in vivo anti-inflammatory activity observed for simple, surface functionalized poly(amidoamine) dendrimers. Biomacromolecules. 2009;10(5):1195–202. doi: 10.1021/bm9000298. [DOI] [PubMed] [Google Scholar]
- 36.Wu HY, et al. Iron oxide nanoparticles suppress the production of IL-1beta via the secretory lysosomal pathway in murine microglial cells. Part Fibre Toxicol. 2013;10:46. doi: 10.1186/1743-8977-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan JJ, et al. Fullerene nanomaterials inhibit the allergic response. J Immunol. 2007;179(1):665–72. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- 38.Heckman KL, et al. Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano. 2013;7(12):10582–96. doi: 10.1021/nn403743b. [DOI] [PubMed] [Google Scholar]
- 39**.Kodali V, et al. Dysregulation of macrophage activation profiles by engineered nanoparticles. ACS Nano. 2013;7(8):6997–7010. doi: 10.1021/nn402145t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blank F, et al. Biomedical nanoparticles modulate specific CD4+ T cell stimulation by inhibition of antigen processing in dendritic cells. Nanotoxicology. 2011;5(4):606–21. doi: 10.3109/17435390.2010.541293. [DOI] [PubMed] [Google Scholar]
- 41*.Sanpui P, et al. Single-walled carbon nanotubes increase pandemic influenza A H1N1 virus infectivity of lung epithelial cells. Part Fibre Toxicol. 2014;11:66. doi: 10.1186/s12989-014-0066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]