Abstract
Purpose
Pro-inflammatory cytokines such as Interleukin-17A (IL17A) and Interleukin-32 (IL32), known to enhance natural killer and T cell responses, are also elevated in human malignancies and linked to poor clinical outcomes. To address this paradox, we evaluated relation between IL17A and IL32 expression and other inflammation- and T cell response-associated genes in breast tumors.
Methods
TaqMan-based gene expression analysis was carried out in seventy-eight breast tumors. The association between IL17A and IL32 transcript levels and T cell response genes, ER status as well as lymph node status was also examined in breast tumors from TCGA dataset.
Results
IL17A expression was detected in 32.7% ER-positive and 84.6% ER-negative tumors, with higher expression in the latter group (26.2 vs 7.1-fold, p < 0.01). ER-negative tumors also showed higher expression of IL32 as opposed to ER-positive tumors (8.7 vs 2.5-fold, p < 0.01). Expression of both IL17A and IL32 genes positively correlated with CCL5, GNLY, TBX21, IL21 and IL23 transcript levels (p < 0.01). Amongst ER-positive tumors, higher IL32 expression significantly correlated with lymph node metastases (p < 0.05). Conversely, in ER-negative subtype, high IL17A and IL32 expression was seen in patients with negative lymph node status (p < 0.05). Tumors with high IL32 and IL17A expression showed higher expression of TH1 response genes studied, an observation validated by similar analysis in the TCGA breast tumors (n=1041). Of note, these tumors were characterized by low expression of a potentially immunosuppressive isoform of IL32 (IL32γ).
Conclusion
These results suggest that high expression of both IL17A and IL32 leads to enhancement of T cell responses. Our study, thus, provides basis for the emergence of strong T cell responses in an inflammatory milieu that have been shown to be associated with better prognosis in ER-negative breast cancer.
Keywords: Interleukin-17A, Interleukin-32, Gene expression, Breast cancer, Lymph node status
Introduction
Association of inflammatory responses with the initiation, promotion, and progression of malignancies is well established (Coussens and Werb 2002). This cancer-related inflammation (CRI) is orchestrated by cytokines, chemokines, and growth factors secreted by different cellular constituents of the tumor and the tumor microenvironment. While some of these molecules may represent effector immune responses against the growing neoplasm, their prolonged presence along with pro-inflammatory signals arising from tumor cells heightens the level of inflammation in the tumor milieu (Colotta et al. 2009). This in turn facilitates tumor survival and dissemination via increased tumor vasculature and suppression of effector anti-tumor immune responses (Balkwill et al. 2005; Ben-Baruch 2006; Colotta et al. 2009).
Inflammation has also been linked to poor prognosis in breast cancer patients (Ham and Moon 2013) highlighting the need for better understanding of its influence on tumor escape and invasion. With the presence of distinct biological and molecular subtypes that have varied prognostic implications (Sims et al. 2007), breast cancer provides a natural model to examine such associations. Hence, our study focussed on comparison of expression of primary mediators of inflammation and Th1 response in these breast cancer subtypes. Among them, expression levels of Interleukin 17A (IL17A) and Interleukin 32 (IL32), two dominant pro-inflammatory cytokines in CRI, were highlighted and assessed in clinical context.
Over-expression of IL17A, an inflammatory molecule primarily of lymphoid origin, has been associated with many allergic and autoimmune diseases (Xu and Cao 2010). Despite being extensively studied in various malignancies, influence of IL17A on disease prognosis remains ambiguous (Murugaiyan and Saha 2009; Wilke et al. 2011). Association of IL17A with inflammation, invasiveness, angiogenesis, and recruitment of immunosuppressive myeloid-derived stem cells (MDSCs) or tumorigenic neutrophils at the tumor site has been reported (Numasaki et al. 2003; Zhu et al. 2008; He et al. 2010; Cochaud et al. 2013; Benevides et al. 2015). These observations support correlation between IL17A expression and poor prognosis (Zhang et al. 2009; Chen et al. 2010, 2013; Liu et al. 2011). Conversely, IL17A has also been shown to suppress tumor growth by inducing anti-tumor cytotoxic T- and T-helper (Th1/Th17) cell immune responses (Yamamoto et al. 2009; Martin-Orozco et al. 2009; Phan-Lai et al. 2016).
IL32, on the other hand, is expressed by multiple cell types and is known to potently induce expression of other inflammatory molecules such as TNF alpha (TNFα). But like IL17A, its over-expression has been implicated in the emergence of inflammatory diseases (Joosten et al. 2013). IL32 expression has also been shown to be associated with poor prognosis in various cancers (Sorrentino and Di Carlo 2009; Lee et al. 2012; Guenin et al. 2014). Interestingly, due to its pro-apoptotic influence, IL32 was identified as a tumor suppressor in prostate cancer (Majid et al. 2010) while its role in cervical cancer remains ambiguous (Lee et al. 2011; Punt et al. 2015). Incidentally, some studies also point towards its contribution to anti-mycobacterial and anti-viral responses (Montoya et al. 2014; Zhou and Zhu 2015) supported by its ability to enhance function of various immune response-associated cell types (Jung et al. 2011; Park et al. 2012; Yun et al. 2013).
IL17A and IL32 also share signaling intermediates (Turner-Brannen et al. 2011) which may have a bearing upon their ability to contribute to effector immunity in a variety of settings. Expression of immune response-associated genes has been shown to enhance prognostic power of clinical markers independent of proliferative gene signatures (Haibe-Kains et al. 2010). These considerations provide strong rationale for analyzing gene expression profiles of these two cytokines with respect to T-cell response markers. Association between IL17A and IL32 expression levels with hormone receptor and regional lymph node status of breast cancer patients was also studied to explore possible relevance of these mediators of inflammation to disease prognosis. Finally, data from TCGA database were analyzed to further examine trends of expression of T-cell response-associated genes in the context of intra-tumoral IL17A and IL32 expression patterns.
Materials and methods
Sample collection
One hundred and sixty breast tumor specimens were collected from tumor repositories at TMH and ACTREC from 2009 to 2012 with the approval of the ethics committee at TMH. The inclusion criteria were, (1) confirmed diagnosis of primary breast cancer (Invasive Ductal Carcinoma), and (2) tumor size ≤5 cm. The exclusion criteria were (1) excision biopsy, (2) therapy prior to surgery, and (3) strong history of autoimmune disorders. Tumors collected were either stored in liquid nitrogen or in RNAlater™ (Ambion, Inc., Austin, TX, USA). Of these, 81 specimens were found to have tumor cell content greater than 60% and were processed further for RNA extraction. Gene expression data for all markers studied were available for 78 tumors. Clinical information of the patients including age at diagnosis, lymph nodes status, and the hormone receptor expression profiles of the tumors was collected from the medical records of individual patients (Table 1).
Table 1.
Summary of clinicopathological features of breast cancer patients
| Tumor variable | All | ER positive | ER negative |
|---|---|---|---|
| n | 78 (100%) | 52 (67%) | 26 (33%) |
| ERBB2 | |||
| Positive | 22 (28%) | 15 (29%) | 7 (27%) |
| Negative | 55 (71%) | 37 (71%) | 18 (69%) |
| Unknown | 1 (1%) | 0 | 1 (4%) |
| Age | |||
| <45 | 33 (42%) | 20 (38%) | 13 (50%) |
| 46–55 | 17 (22%) | 13 (25%) | 4 (15%) |
| >56 | 28 (36%) | 19 (37%) | 9 (35%) |
| Median age | 48.0 | 48.0 | 47.5 |
| Nodal status | |||
| Positive | 42 (54%) | 33 (63%) | 9 (37%) |
| Negative | 36 (46%) | 19 (37%) | 17 (63%) |
ER estrogen receptor, ERBB2 human epidermal growth factor receptor 2
RNA extraction, cDNA synthesis, and real-time PCR
Total RNA was extracted from the selected tissues either by TRIzol (TRI Reagent, Ambion Inc.)-based method or using RNA extraction columns from mirVana™ miRNA Isolation kit (Ambion Inc.) as per the recommended protocol. RNA was quantified using NanoDrop ND 2000 spectrophotometer (NanoDrop Inc., Wilmington, DE, USA) and the quality was assessed on agarose gels. First-strand synthesis was performed using high-capacity cDNA synthesis kit [Applied Biosystems (ABI), Foster City, CA, USA]. Transcript levels of IL17A, IL32, and related markers (Supplementary Table S1) were analyzed using TaqMan assays (ABI). Expression levels were normalized using PUM1 as the endogenous control/housekeeping gene (McNeill et al. 2007). Real-time PCR was performed on the ABI Prism 7900 platform. Due to inconsistent RNA yields from normal breast tissues, we used commercially available normal breast-derived RNA preparation (Human Breast Total RNA, Ambion Inc.) as a calibrator to compute fold change (2−ΔΔCt method).
Analysis of TCGA expression dataset
mRNA expression data and corresponding clinical information of breast cancer samples were obtained (April 2015) from The Cancer Genome Atlas (TCGA) data repository. Level 3 expression data of IL17A and IL32 were extracted for further analysis. The cohort (n = 1041) was divided into three classes on the basis of IL32 expression data; IL32_hi (n = 260), IL32_moderate (n = 521), and IL32_lo (n = 260)) using the 25th–75th percentile criteria for sample division. For IL17A, tumor samples were divided into positive (n = 131) and negative (n = 910) groups on the basis of presence or absence of the transcript for the cytokine. Available ER/PR and lymph node status was correlated with gene expression patterns.
Statistical analysis
Differential expression of quantitative variables and their statistical analysis was performed using non-parametric Mann–Whitney U test. Each marker was classified into high and low producers on the basis of median fold value for that marker, in all tumors or specific subgroups. Difference in distribution of high and low producers for each marker across ER subtypes and lymph node status was tested for significance using Chi-square analysis. Correlations between expressions of all genes were estimated using Pearson’s correlation. Differences with p values ≤0.05 were considered to be statistically significant. Statistical analysis was performed using SPSS (SPSS for Windows v16.0; SPSS Inc., Chicago, IL, USA) and graphs were generated using GraphPad Prism Software (Version 5.01, GraphPad, San Diego, CA).
Results
Clinicopathological features of patients with primary breast cancer
With the exception of two tumors, all the collected specimens represented infiltrating ductal carcinomas (IDCs) with majority (>75%) belonging to grade III disease. In the randomly selected and eligible 78 breast tumor specimens, 52 (67%) were ER positive (Table 1). ERBB2 expression was seen at equal frequency (~30%) within the tumors from both ER subtypes. Age distribution was also comparable in patients with ER-positive and ER-negative tumors. Sixty-three percent of ER-positive tumor-bearing patients were lymph node positive while this was true for 37% of ER-negative patients.
IL17A transcript expression is highly variable in human breast tumors
IL17A transcripts were undetected in the commercially available normal breast-derived RNA preparation (see “Materials and methods”). ER-negative subgroup contained a relatively higher fraction of IL17A-positive tumors as compared to ER-positive subgroup (84 vs. 32%, p < 0.05, Table 3). Additionally, IL17A mRNA levels detected in ER-negative tumors were significantly higher compared to those in ER-positive tumors (median fold, 26 and 7, respectively, p < 0.01; Table 2).
Table 3.
Analysis of IL17A and IL32 gene expression patterns in all tumors and individual ER subtypes
| IL17AposIL32hi n (%) |
IL17AposIL32lo n (%) |
IL17AnegIL32hi n (%) |
IL17AnegIL32lo n (%) |
Total n (%) |
|
|---|---|---|---|---|---|
| All tumors | |||||
| Total | 23 (30%) | 16 (20%) | 16 (20%) | 23 (30%) | 78 |
| Lymph node | |||||
| Negative | 14 (61%) | 8 (50%) | 4 (25%) | 10 (43%) | 36 |
| Positive | 9 (39%) | 8 (50%) | 12 (75%) | 13 (57%) | 42 |
| Hormone receptor-positive tumors | |||||
| Total | 9 (17%) | 8 (15%) | 17 (33%) | 18 (35%) | 52 |
| Lymph node | |||||
| Negative | 2 (29%) | 4 (50%) | 5 (29%) | 8 (44%) | 19 |
| Positive | 7 (71%) | 4 (50%) | 12 (71%) | 10 (56%) | 33 |
| Hormone receptor-negative tumors | |||||
| Total | 11 (42%) | 11 (42%) | 2 (8%) | 2 (8%) | 26 |
| Lymph node | |||||
| Negative | 9 (82%) | 7 (64%) | 1 (50%) | 0 (0%) | 17 |
| Positive | 2 (18%) | 4 (36%) | 1 (50%) | 2 (100%) | 9 |
IL32 hi/lo groups were categorized on the basis of median value
Table 2.
Higher expression of Th1 response- and inflammation-associated genes in negative tumors
| Marker | All tumors (n = 78) |
ER positive (n = 52) |
ER negative (n = 26) |
Individual | Corrected | |||
|---|---|---|---|---|---|---|---|---|
| Folda | Range | Fold | Range | Fold | Range | p | p | |
| CCL2 | 1.9 | (0.2–20.9) | 1.5 | (0.2–20.9) | 2.7 | (0.5–17.2) | 0.01 | NS |
| CCL3 | 3.5 | (0.2–50.1) | 3.4 | (0.3–50.1) | 4.1 | (0.2–12.1) | 0.70 | NS |
| CCL4 | 4.3 | (0.1–29.7) | 3.9 | (0.1–21.0) | 6.8 | (0.1–29.7) | 0.01 | NS |
| CCL5 | 2.7 | (0.2–35.5) | 1.7 | (0.2–23.4) | 4.6 | (0.5–35.5) | 0.00 | 0.01 |
| CCL20 | 3.2 | (0.1–199.8) | 2.3 | (0.1–39.0) | 6.4 | (0.7–199.8) | 0.01 | NS |
| GNLY | 1.2 | (0.1–28.0) | 0.7 | (0.1–4.8) | 4.6 | (0.9–28.0) | 0.00 | 0.00 |
| IFNγ | 1.2 | (0.1–19.3) | 0.8 | (0.1–19.3) | 1.8 | (0.1–11.9) | 0.02 | NS |
| IL1B | 9.9 | (0.3–71.8) | 9.9 | (0.3–71.8) | 9.6 | (1.5–58.2) | 0.89 | NS |
| IL6 | 0.4 | (0.0–13.6) | 0.3 | (0.0–8.4) | 0.8 | (0.1–13.6) | 0.02 | NS |
| IL8 | 0.7 | (0.0–13.4) | 0.5 | (0.0–12.6) | 1.8 | (0.1–13.4) | 0.00 | 0.04 |
| IL12 | 1.1 | (0.1–12.7) | 0.8 | (0.1–10.5) | 2.4 | (0.5–12.7) | 0.00 | 0.00 |
| IL17Ab | 12.4 | (1.5–1214.9) | 7.1 | (2.8–634.3) | 26.2 | (1.5–1214.9) | 0.00 | 0.00 |
| IL21 | 23.5 | (0.1–1007.0) | 15.0 | (0.1–305.2) | 64.4 | (1.7–1007.0) | 0.00 | 0.00 |
| IL23 | 2.9 | (0.1–191.1) | 2.1 | (0.1–10.6) | 8.1 | (1.0–191.1) | 0.00 | 0.00 |
| IL32 | 3.2 | (0.2–35.8) | 2.5 | (0.2–10.5) | 8.7 | (1.3–35.8) | 0.00 | 0.00 |
| TNFα | 6.1 | (0.4–53.9) | 5.5 | (0.4–42.0) | 7.4 | (1.0–53.9) | 0.48 | NS |
| TBX21 | 0.8 | (0.0–7.9) | 0.6 | (0.0–7.9) | 1.4 | (0.2–6.1) | 0.00 | 0.00 |
NS not significant, IL interleukin, CCL chemokine (C–C motif) ligand, GNLY granulysin, IFNγ interferon gamma, TNFα tumor necrosis factor alpha, TBX21 T-box 21
Represent median fold values (with range in brackets). Differences across the ER subtypes were analyzed using Mann–Whitney U test
IL17A was detected in 39 tumors(17 ER positive, 22 ER negative)
IL32 transcript is expressed at elevated levels in majority of breast tumors
IL32 transcripts were ubiquitously expressed in all breast tumors studied. Sixty-eight percent tumors (53/78) displayed two-fold or higher IL32 expression compared to normal tissue (data not shown). As in case of IL17A, IL32 transcript levels were higher in ER-negative tumors. Accordingly, the median fold value for IL32 in ER-negative tumors was over three times higher compared to values seen in ER-positive tumors (8.7 vs. 2.5 fold, p < 0.01, Table 2).
ER-negative tumors are characterized by higher expression of genes representing mediators of inflammation
ER-negative tumors also showed significantly higher levels of expression of genes associated with inflammation and Th1 responses such as CCL5, GNLY, IL8, IL12, IL21, IL23, and TBX21 as compared to their ER-positive counterparts (p < 0.05, Table 2). Of these, GNLY, IL8, IL12, IL21, and IL23 along with IL17A and IL32 showed at least three-fold difference in expression across the ER-receptor subtypes.
IL17A and IL32 levels have common associations with expression of IFNγ, CCL5, GNLY, TBX21, IL21, and IL23
Expression of CCL2, CCL5, IL21, IL23, IFNγ, and IL12 moderately correlated with IL17A (Pearson’s correlation, r > 0.4, p ≤ 0.01). Similar trends were seen for correlation between IL32 expression and IFNγ, IL12, IL21, IL23, TNF, CCL2, and CCL5 levels (Supplementary Tables S2a and S2b).
These correlations were further assessed by comparing expression levels of different markers in tumors grouped on the basis of high and low transcript levels of IL17A or IL32 (Mann–Whitney U test). In case of IL17A, the classification of tumors was made on the basis of presence or absence of IL17A transcripts in these tumors. This analysis showed that TBX21, CCL5, GNLY, IL8, IL12, IL21, IL23, and IL32 were significantly over-expressed in IL17A-positive tumors as compared to IL17A-negative tumors (p < 0.05, Fig. 1a). Similarly, CCL2, CCL4, CCL5, GNLY, IFNγ, IL21, IL23, and TBX21 transcript levels were significantly higher in high IL32 expressing tumors in comparison to low IL32-expressing tumors (p < 0.05, Fig. 1b). Thus, IL17A and IL32 have common expression correlation with CCL5, TBX21, GNLY, IL21, and IL23.
Fig. 1.
Markers associated with IL17A and IL32 expression. a, b Markers associated with expression of IL17A and IL32, respectively. Tumors were classified into high and low producers for IL17A and IL32 using median fold values for these cytokines as classifier. The other markers studied were then compared across the IL17A and IL32 subgroups and tested for statistical significance using Mann–Whitney U test. *Significance at p < 0.05 levels while **significance at p < 0.01 level
Influence of high IL17A and IL32 expression levels on lymph node status may depend on ER subtype
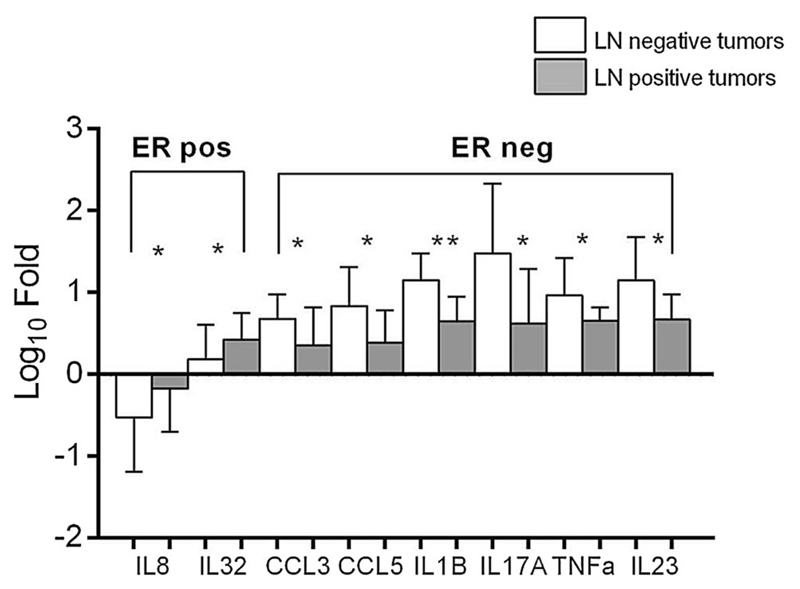
Expression levels of all markers were compared in the context of lymph node status of the patients (Mann–Whitney U test). Of 52 ER-positive tumor-bearing patients, 33 (63%) were lymph node positive. In this subtype, higher levels of IL8 and IL32 were significantly associated with lymph node-positive status. On the other hand, of the 26 patients with ER-negative tumors, 17 (63%) had lymph node-negative status. In this subtype, higher expression levels of CCL3, CCL5, IL1B, IL17A, and TNFα were associated with lymph node-negative status (p < 0.05, Fig. 2).
Fig. 2.
Markers differentially expressed across lymph node-positive and lymph node-negative patients in both ER subtypes of breast cancer. Mann–Whitney U test was used to compute statistical significance. *Significance at p < 0.05 levels while **significance at p < 0.01 level
ER-negative tumors expressing high levels of IL17A and IL32 are predominantly associated with lymph node-negative status
For combined expression of both these cytokines in relation to lymph node status, four different expression profiles were generated (Table 3). In ER-negative subset, tumors positive for IL17A expression were predominantly from lymph node-negative patients (16/22) when compared to the IL17A-deficient tumors (1/4) (72 vs. 25%, p < 0.05). IL17A-positive tumors with high IL32 expression had the highest frequency of lymph node-negative patients (9/11, 82%) suggesting strong association of combined expression of both these cytokines with lymph node-negative status in ER-negative subset.
This observed synergistic effect of high IL17A and IL32 expression on lymph node-negative status of patients was also seen when such analysis was extended to IL17A-positive-only tumors (on the basis of their IL17A median fold value, tumors were classified as IL17Ahi and IL17Alo). Herein, ER-negative tumors with IL17AhiIL32hi profile were found to belong to lymph node-negative patients (6/6, Supplementary Table S3).
However, such synergistic effect was not observed in ER-positive subset, wherein tumors with high IL32 expression belonged predominantly to lymph node-positive patients (19/26) as opposed to the low IL32 expressing tumors (14/26), although this trend was not statistically significant (73 vs. 53%, p = 0.12). In this group of tumors, frequencies of lymph node-positive and lymph node-negative patients were comparable in IL17AposIL32hi and IL17AnegIL32hi subgroups (Table 3).
Increased IL17A and IL32 levels are associated with high expression of T-cell response genes
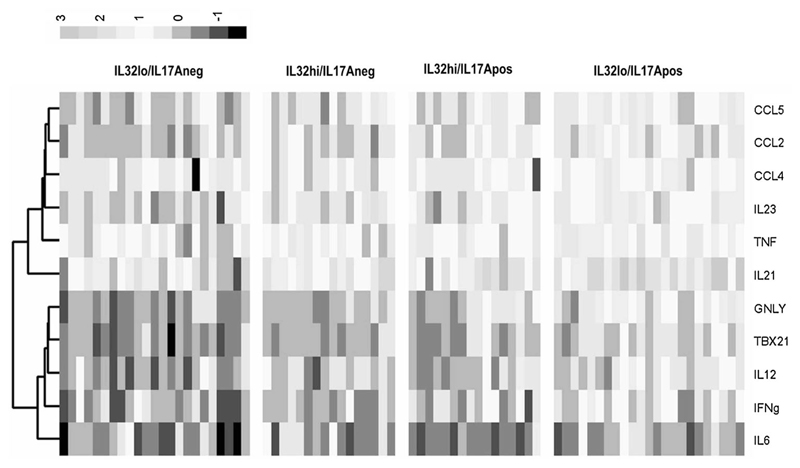
Earlier data analysis indicated that high IL32 expression may increase risk for lymph node metastasis in patients with ER-positive tumors. Interestingly, tumors with higher expression of IL17A or IL32 were also characterized by higher levels of GNLY and TBX21 (Fig. 1) further justifying the assessment of expression patterns of the selected markers in the context of different combinations of IL17A and IL32 expression levels. Distinctly higher levels and frequency of T-cell response-associated genes such as IL12, TBX21, and GNLY expression were noted in ‘IL17A-positive, IL32-high’ group of tumors (Fig. 3). These tumors were also characterized by strikingly higher expression levels of IL23, CCL4, CCL5, and CCL2.
Fig. 3.
Heat Map depicting comparison of expression of inflammation and T-cell response-associated genes in patients grouped on the basis of IL17A and IL32 expression levels
Analysis of TCGA data supports the association between IL17A and IL32 expression patterns and expression of T-cell response-associated genes
Our real-time data also revealed much higher ΔCt values for IL17A as compared to those of IL32 and other immune markers tested (data not shown) indicating very low expression of IL17A transcripts in tumors. Data from the TCGA repository clearly supported this finding as IL17A transcript levels were found to be present at ten-fold lower levels compared to those of the IL32 in agreement with our semi-quantitative expression analysis (Supplementary Figure S1). Further, IL17A expression was seen in 131 out of 1041 breast samples (12.5%). ER expression status was available for ~75% of the tumors (101/131). Accordingly, of the 101 IL17A-positive tumors, 45% belonged to ER-negative category (Table 4). Within the ER-negative tumors, ~25% tumors were IL17A positive (46/179) as against ~9% of ER-positive tumors (55/600) which showed detectable levels of IL17A transcript.
Table 4.
Distribution of breast cancer subtypes in the TCGA database w.r.t. IL17A and IL32 expression pattern
| IL32_Hi |
IL32_Moderate |
IL32_Lo |
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
N = 260 |
N = 521 |
N = 260 |
|||||||
| ER Neg | ER Pos | Not known | ER Neg | ER Pos | Not known | ER Neg | ER Pos | Not known | |
| IL17A_negative | N = 193 | N = 469 | N = 248 | ||||||
| (n = 910) | 59 | 69 | 65 | 53 | 293 | 123 | 21 | 183 | 44 |
| IL17A_positive | N = 67 | N = 52 | N = 12 | ||||||
| (n = 131) | 30 | 20 | 17 | 15 | 27 | 10 | 1 | 8 | 3 |
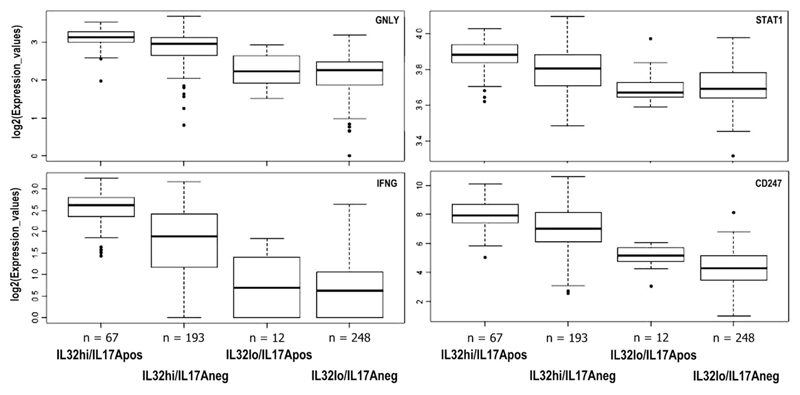
Expression levels of T-cell response-associated genes, GNLY, CD247 (CD3zeta chain), STAT1, and IFNγ observed in the context of levels of IL17A and IL32 transcripts could be summarized as IL17AposIL32hi > IL17negIL32hi > IL17AposIL32lo/IL17AnegIL32lo, clearly highlighting association between high levels of these two inflammatory cytokines and T-cell responses. In each case, the differences in the expression levels of the markers analyzed were statistically significant when compared with the first group (p < 0.001; Fig. 4).
Fig. 4.
Analysis of expression of T-cell response-associated genes in breast tumors from the TCGA database grouped on the basis of combinations of expression levels of IL17A and IL32 transcripts
Discussion
With the link between inflammation and cancer established, next challenge lies in deducing contributions of different cytokines and associated pathways to cancer-related inflammation and anti-cancer immunity. In this study, using naturally defined subsets of hormone receptor-positive and hormone receptor-negative breast cancers, we examined expression profiles of a group of cytokines and chemokines that are considered to be major players in cancer-related inflammation. In view of recent interests in the role of IL17A and IL32 in cancer, we focussed on their expression patterns in breast cancer and their association with expression of genes that mediate inflammation and T-cell response. IL17A and IL32 levels were also correlated with lymph node status of patients, used as a surrogate marker for prognosis (Carter et al. 1989; Jatoi et al. 1999).
The strikingly higher expression of genes associated with inflammation observed in ER-negative tumors is in agreement with previous data reported by Chavey et al. (2007). Contrasting frequencies and levels of IL17A expression in different ER subtypes found in our study are compatible with data from studies employing immunohistochemistry (Droeser et al. 2012; Chen et al. 2013). Furthermore, similar trends were also seen in breast tumors from the TCGA database with nearly three-fold higher frequency of IL17A expressing tumors in ER-negative tumors compared to the ER-positive ones (25 vs. 9%, p < 0.05). Higher IL32 expression observed in ER-negative tumors than ER-positive tumors is in concurrence with high levels of this cytokine reported in basal-like breast cancers (Player et al. 2014).
Strong association of IL17A levels with IL21 and IL23 expression is in accordance with the role of these two cytokines in IL17A induction and maintenance of IL17A-producing cell populations (Chen et al. 2010). IL21 and IL23 expression also correlated significantly with IL32 levels which may represent a common signaling cascade shared by this cytokine with IL17A (Turner-Brannen et al. 2011). Furthermore, high IL17A and IL32 expression positively correlated with IFNγ, CCL5, GNLY, and TBX21 levels, which collectively represent Th1/CTL response genes, an observation primarily seen in ER-negative subgroup (Supplementary Table 2a, b). Thus, ER-negative tumors expressing high IL17A and IL32 levels were characterized by strong combined Th1/CTL and Th17 signatures, an effect that may suggest the role of IL17A in augmenting effector anti-tumor T-cell-mediated immune responses (Benchetrit et al. 2002; Ankathatti Munegowda et al. 2011; Nunez et al. 2013). This is further highlighted by higher frequency of lymph node-negative patients in ER-negative tumors expressing high levels of both cytokines (Supplementary Table 3).
Expression of high IL32 levels in ER-positive tumors, on the other hand, was associated with positive lymph node status, independent of IL17A expression. This effect can be attributed to relatively lower frequency and levels of IL17A expression in this tumor subset and ensuing lack of synergism between IL17A and IL32 in mounting effector anti-tumor immune responses. Additionally in ER-positive tumors, IL32 and IL8 were significantly over-expressed in patients with positive lymph node status as compared to their lymph node-negative counterparts. IL32 is known to be an inducer of TNFα (Kim et al. 2005), a phenomenon that is also reflected in our analysis (Supplementary Tables S2b). Potent pro-inflammatory influence of this cytokine could promote angiogenesis and invasiveness via IL8 and TNFα (Shoda et al. 2006; Nold-Petry et al. 2014) and could contribute to the observed positive lymph node status in patients with ER-positive breast tumors. Association between high levels of IL32 with lymph node positivity has also been reported in other malignancies (Sorrentino and Di Carlo 2009).
The proposed synergism between IL17A and IL32 towards enhancing Th1 responses was further validated in breast tumors from the TCGA cohort. In agreement with our observations, similar trends were seen in the TCGA samples wherein transcript levels of Th1 response genes were the highest in tumors with the IL32hiIL17Apos profile (IL17AposIL32hi > IL17negIL32hi > IL17AposIL32lo/IL17AnegIL32lo). This trend was seen in both ER subsets of tumors (Supplementary Figure S2). Furthermore, marginally higher frequency of lymph node negativity in IL17AhiIL32hi compared to that in IL17AnegIL32hi in ER-negative subgroup (58 vs. 55%) was seen in breast cancer patients from the TCGA cohort. Such a trend was not seen in case of ER-positive tumors (Supplementary Table 4) implying a probable subtype-specific effect of synergy between the two cytokines on the disease prognosis. However, it must be noted that the hormone receptor status was not known for nearly 25% of these patients. Concurrently, our data are compatible with the reported association between T-cell response signatures and favorable prognosis in breast cancer patients with ER-negative tumors (Reyal et al. 2008; Rody et al. 2009; Teschendorff et al. 2010). The possibility that high levels of IL17A and IL32 in ER-negative tumors from lymph node-negative patients seen in this study would have an impact on the disease prognosis is supported by observation documenting significantly better overall survival (80% at 5 years) in such patients compared to their lymph node-positive counterparts (65% or less; p < 0.001) (Hernandez-Aya et al. 2011).
Thus, briefly, our analysis highlighted several important aspects of gene expression patterns in breast cancers such as, (a) significant differences between expression levels of inflammation-associated genes, especially, IL17A and IL32 in the two subtypes studied, (b) association between high IL17A and IL32 expression and expression of T-cell response-associated genes, and (c) association of high IL17A levels and lymph node-negative status in ER-negative tumors. In addition, the reported associations between IL23 expression with both IL17A and IL32, between IL32 and TNFα, as well as association of high IL32 levels with lymph node metastasis in ER-positive tumors were also reflected in our results.
In our immunohistochemical analysis of paraffin-embedded tissues from representative ER-negative and ER-positive tumors (data not shown), we were able to detect only sporadically IL17A-positive cells (Chen et al. 2010; Zhu et al. 2008). Ubiquitous expression of IL32 in normal as well as malignant breast tissues was seen with low to moderate staining in tumor cells as well as in lymphoid compartment. Staining patterns in hormone receptor-positive and hormone receptor-negative tumors were comparable. Immunohistochemical detection of cytokines and interleukins is known to be a daunting task since a majority of them are secreted. In addition, the choice of primary antibodies and presence of different isoforms of the target molecules may contribute to the variations in staining patterns. Hence, profiling of gene expression patterns remains a sensitive and efficient method to study the interplay between multiple immunological parameters in tumor tissues (Rody et al. 2009; Reyal et al. 2008; Teschendorff et al. 2010), which is also emphasized by recent studies in cervical (Omrane and Benammar-Elgaaied 2015) and colorectal cancers (Punt et al. 2015).
In view of various roles assigned to different isoforms of IL 32 (Choi et al. 2009; Heinhuis et al. 2012; Kang et al. 2012; Park et al. 2015) and the suggested influence of combinations of IL17A and IL32 expression on the lymph node status in patients studied herein, we examined expression of four IL32 isoforms (IL32-β, -γ, -δ, -ε) in breast cancer subtypes in the context of IL17A expression. Significantly lower levels of IL32γ transcripts (p < 0.05; Mann–Whitney U test) were seen in ER-positive tumors that also expressed IL17A transcripts (Supplementary Figure S3A). Lower expression of IL32γ also seen in ER-positive tumors with high IL32 transcripts levels (Supplementary Figure S3A) may be due to the higher frequency of IL17A-positive tumors in this group compared to tumors with lower IL32 expression. Interestingly, in contrast to IL32γ isoform, levels of other three isoforms were significantly higher in former group of tumors (p < 0.05). The possibility of inverse association between IL17A and IL32γ expression was further supported by similar observation in ER-negative tumors wherein tumors with higher IL17A levels compared to those with lower IL17A transcripts were characterized by reduced expression of IL32γ (Supplementary Figure S3B).
Incidentally, IL32γ has been described as the most potent inflammatory isoform of the cytokine (Choi et al. 2009; Heinhuis et al. 2012) and was found to induce several-fold increase in IDO expression by various subtypes of leukocytes (Smith et al. 2011). This mechanism was proposed to contribute to immune-suppression in HIV patients (El-Far et al. 2016). Similar mechanism may operate in tumors with high expression of IL32 and IL17A as these tumors also displayed higher levels of T-cell response-associated genes and negative lymph node status.
Skewed frequencies of lymph node-positive and lymph node-negative patients in both ER subsets represents a limitation of the present study that arose in the course of random selection of eligible specimens. An excellent correlation between gene expression patterns of IL17A, IL32, and their related markers as well as overall compatibility of our data with those reported by other studies and from the TCGA cohort rules out the effect of skewed distribution of lymph node metastases on the inferences drawn. The role of other IL17 family members (IL17B–F) in the observed phenomenon remains unexplored. Same may be true of IL32 isoforms (Kang et al. 2014), especially in view of lack of direct measurement of levels IL32α isoform which has been shown to modulate NFkB expression in hepatocellular carcinoma cells (Kang et al. 2012) and a variety of interactions between the IL32 isoforms and effects of IL32 heter-odimers (Kang et al. 2013).
It is interesting to note that despite highly inflammatory microenvironment in ER-negative tumors several studies have shown the existence of strong TH1/Tc responses in these tumors with their positive impact on the disease outcome (Reyal et al. 2008; Rody et al. 2009; Teschendorff et al. 2010). Data obtained in the present study are also in accordance with reports that highlight the potential of these two cytokines to enhance T-cell responses and point towards a synergistic effect of these pro-inflammatory entities in generating anti-tumor T-cell response. Thus, the findings provide possible basis for association between T-cell response gene signature and disease prognosis in ER-negative breast tumors. Effects of pleiotropic cytokines can vary depending upon the site of the inflammation owing to the microenvironment (Joshi et al. 2014), due to synergistic, antagonistic, or balancing effects which play an important part in influencing disease outcome. The suggested synergy between IL17A and IL32 highlights one such phenomenon and provides basis to the reported association between strong immune response signatures and better prognosis in hormone receptor-negative breast cancers. As revealed by the analysis of IL32 isoforms, the observed effect may operate via suppression of inflammatory isoform of IL32 (IL32γ) contributing to an enhanced T-cell metagene signature and subsequent lower disease spread.
To summarize, our results indicate that IL17A and IL32 together boost T-cell metagene signature in both ER-positive and ER-negative tumors. High expression of the two cytokines was associated with lymph node negativity in ER-negative subgroup but not in the ER-positive subgroup. This disparity may arise from the differences in the levels of expression of these two cytokines in ER-negative as against ER-positive tumors, implying a ‘threshold’ effect.
Supplementary Material
Acknowledgements
This work was supported by Grant from the Department of Biotechnology, Ministry of Science and Technology, Government of India. The authors would like to thank Mrs. Manisha Kulkarni and Mr. Anand Deshpande at ICMR National Tumor Tissue Repository at TMH and Dr. Kishore Amin and Mr. Madan Ludbe at tumor tissue repository at ACTREC. Help provided by Dr. Sridhar, Consultant Pathologist, ACTREC, and Mrs. Pallavi Rane, ECTU, CRC, ACTREC is gratefully acknowledged.
Funding This study was funded by Department of Biotechnology, Government of India (Grant Number BT/PR10199/Med/30/68/2007).
Abbreviations
- IL
Interleukin
- ER
Estrogen receptor
- LN
Lymph node
- CRI
Cancer-related inflammation
- CTLs
Cytotoxic T lymphocytes
- Th
T helper
- Tc
T cytotoxic
- PCR
Polymerase chain reaction
Footnotes
Compliance with ethical standards
Conflict of interest All authors declares that they have no conflict of interest.
Animal study This article does not contain any studies with animals performed by any of the authors.
Ethical approval for studies in patients All the procedures involving human participants were performed in accordance with the ethical standards of the institutional and national research committees (guidelines) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated CD8(+) T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother. 2011;60(10):1473–1484. doi: 10.1007/s00262-011-1054-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16(1):38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99(6):2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- Benevides L, da Fonseca DM, Donate PB, Tiezzi DG, De Carvalho DD, de Andrade JM, Martins GA, Silva JS. IL17 promotes mammary tumor progression by changing the behavior of tumor cells and eliciting tumorigenic neutrophils recruitment. Cancer Res. 2015;75(18):3788–3799. doi: 10.1158/0008-5472.CAN-15-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63(1):181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9(1):R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69(3):348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63(2):225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- Choi JD, Bae SY, Hong JW, Azam T, Dinarello CA, Her E, Choi WS, Kim BK, Lee CK, Yoon DY, Kim SJ, et al. Identification of the most active interleukin-32 isoform. Immunology. 2009;126(4):535–542. doi: 10.1111/j.1365-2567.2008.02917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, Cure H, Mascaux C, Alberici G, Bonnefoy N, Eliaou JF, et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci Rep. 2013;3:3456. doi: 10.1038/srep03456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droeser R, Zlobec I, Kilic E, Guth U, Heberer M, Spagnoli G, Oertli D, Tapia C. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer. 2012;12:134. doi: 10.1186/1471-2407-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Far M, Kouassi P, Sylla M, Zhang Y, Fouda A, Fabre T, Goulet JP, van Grevenynghe J, Lee T, Singer J, Harris M, et al. Proinflammatory isoforms of IL-32 as novel and robust biomarkers for control failure in HIV-infected slow progressors. Sci Rep. 2016;15(6):22902. doi: 10.1038/srep22902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenin S, Mouallif M, Hubert P, Jacobs N, Krusy N, Duray A, Ennaji MM, Saussez S, Delvenne P. Interleukin-32 expression is associated with a poorer prognosis in head and neck squamous cell carcinoma. Mol Carcinog. 2014;53(8):667–673. doi: 10.1002/mc.21996. [DOI] [PubMed] [Google Scholar]
- Haibe-Kains B, Desmedt C, Rothe F, Piccart M, Sotiriou C, Bontempi G. A fuzzy gene expression-based computational approach improves breast cancer prognostication. Genome Biol. 2010;11(2):R18. doi: 10.1186/gb-2010-11-2-r18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham M, Moon A. Inflammatory and microenvironmental factors involved in breast cancer progression. Arch Pharm Res. 2013;36(12):1419–1431. doi: 10.1007/s12272-013-0271-7. [DOI] [PubMed] [Google Scholar]
- He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184(5):2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA. Interleukin-32: a predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine. 2012;60(2):321–327. doi: 10.1016/j.cyto.2012.07.010. [DOI] [PubMed] [Google Scholar]
- Hernandez-Aya LF, Chavez-Macgregor M, Lei X, Meric-Bernstam F, Buchholz TA, Hsu L, Sahin AA, Do KA, Valero V, Horto-bagyi GN, Gonzalez-Angulo AM. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29(19):2628–2634. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatoi I, Hilsenbeck SG, Clark GM, Osborne CK. Significance of axillary lymph node metastasis in primary breast cancer. J Clin Oncol. 1999;17(8):2334–2340. doi: 10.1200/JCO.1999.17.8.2334. [DOI] [PubMed] [Google Scholar]
- Joosten LA, Heinhuis B, Netea MG, Dinarello CA. Novel insights into the biology of interleukin-32. Cell Mol Life Sci. 2013;70(20):3883–3892. doi: 10.1007/s00018-013-1301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi N, Kannan S, Kotian N, Bhat S, Kale M, Hake S. Interleukin 6–174G>C polymorphism and cancer risk: meta-analysis reveals a site dependent differential influence in Ancestral North Indians. Hum Immunol. 2014;75(8):901–908. doi: 10.1016/j.humimm.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Jung MY, Son MH, Kim SH, Cho D, Kim TS. IL-32gamma induces the maturation of dendritic cells with Th1- and Th17-polarizing ability through enhanced IL-12 and IL-6 production. J Immunol. 2011;186(12):6848–6859. doi: 10.4049/jimmunol.1003996. [DOI] [PubMed] [Google Scholar]
- Kang YH, Park MY, Yoon DY, Han SR, Lee CI, Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, Jang YJ, et al. Dysregulation of overexpressed IL-32α in hepatocellular carcinoma suppresses cell growth and induces apoptosis through inactivation of NF-κB and Bcl-2. Cancer Lett. 2012;318(2):226–233. doi: 10.1016/j.canlet.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Park SH, Ham SY, Yang Y, Hong JT, Yoon DY. Interleukin-32delta interacts with IL-32beta and inhibits IL-32beta-mediated IL-10 production. FEBS Lett. 2013 doi: 10.1016/j.febslet.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Kang JW, Park YS, Lee DH, Kim MS, Bak Y, Ham SY, Park SH, Kim H, Ahn JH, Hong JT, Yoon DY. Interaction network mapping among IL-32 isoforms. Biochimie. 2014;101:248–251. doi: 10.1016/j.biochi.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22(1):131–142. doi: 10.1016/j.immuni.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim JH, Kim H, Kang JW, Kim SH, Yang Y, Kim J, Park J, Park S, Hong J, Yoon DY. Activation of the interleukin-32 pro-inflammatory pathway in response to human papillomavirus infection and over-expression of interleukin-32 controls the expression of the human papillomavirus oncogene. Immunology. 2011;132(3):410–420. doi: 10.1111/j.1365-2567.2010.03377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Liang ZL, Huang SM, Lim JS, Yoon DY, Kim JM. Overexpression of IL-32 is a novel prognostic factor in patients with localized clear cell renal cell carcinoma. Oncol Lett. 2012;3(2):490–496. doi: 10.3892/ol.2011.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407(2):348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Majid S, Dar AA, Saini S, Yamamura S, Hirata H, Tanaka Y, Deng G, Dahiya R. MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer. 2010;116(24):5637–5649. doi: 10.1002/cncr.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill RE, Miller N, Kerin MJ. Evaluation and validation of candidate endogenous control genes for real-time quantitative PCR studies of breast cancer. BMC Mol Biol. 2007;8:107. doi: 10.1186/1471-2199-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya D, Inkeles MS, Liu PT, Realegeno S, Teles RM, Vaidya P, Munoz MA, Schenk M, Swindell WR, Chun R, Zavala K, et al. IL-32 is a molecular marker of a host defense network in human tuberculosis. Sci Transl Med. 2014;6(250):250ra114. doi: 10.1126/scitranslmed.3009546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, Saha B. Protumor vs. antitumor functions of IL-17. J Immunol. 2009;183(7):4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- Nold-Petry CA, Rudloff I, Baumer Y, Ruvo M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, Dinkel H, et al. IL-32 promotes angiogenesis. J Immunol. 2014;192(2):589–602. doi: 10.4049/jimmunol.1202802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Nunez S, Saez JJ, Fernandez D, Flores-Santibanez F, Alvarez K, Tejon G, Ruiz P, Maldonado P, Hidalgo Y, Manriquez V, Bono MR, et al. T helper type 17 cells contribute to anti-tumour immunity and promote the recruitment of T helper type 1 cells to the tumour. Immunology. 2013;139(1):61–71. doi: 10.1111/imm.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrane I, Benammar-Elgaaied A. The immune microenvironment of the colorectal tumor: involvement of immunity genes and microRNAs belonging to the TH17 pathway. Biochem Biophys Acta. 2015;1856(1):28–38. doi: 10.1016/j.bbcan.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Park MH, Song MJ, Cho MC, Moon DC, Yoon do Y, Han SB, Hong JT. Interleukin-32 enhances cytotoxic effect of natural killer cells to cancer cells via activation of death receptor 3. Immunology. 2012;135(1):63–72. doi: 10.1111/j.1365-2567.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Lee S, Jeong AL, Han S, Ka HI, Lim JS, Lee MS, Yoon DY, Lee JH, Yang Y. Hypoxia-induced IL-32β increases glycolysis in breast cancer cells. Cancer Lett. 2015;356(2 Pt B):800–808. doi: 10.1016/j.canlet.2014.10.030. [DOI] [PubMed] [Google Scholar]
- Phan-Lai V, Dang Y, Gad E, Childs J, Disis ML. The antitumor efficacy of IL2/IL21-cultured polyfunctional Neu-specific T cells is TNFα/IL17 dependent. Clin Cancer Res. 2016;22(9):2207–2216. doi: 10.1158/1078-0432.CCR-15-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Player A, Oguamanam T, Okanmelu J, Burrell K, Hollomon M. Preliminary characterization of IL32 in basal-like/triple negative compared to other types of breast cell lines and tissues. BMC Res Notes. 2014;7:501. doi: 10.1186/1756-0500-7-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt S, Houwing-Duistermaat JJ, Schulkens IA, Thijssen VL, Osse EM, de Kroon CD, Griffioen AW, Fleuren GJ, Gorter A, Jordanova ES. Correlations between immune response and vascularization qRT-PCR gene expression clusters in squamous cervical cancer. Mol Cancer. 2015;14:71. doi: 10.1186/s12943-015-0350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyal F, van Vliet MH, Armstrong NJ, Horlings HM, de Visser KE, Kok M, Teschendorff AE, Mook S, van’t Veer L, Caldas C, Salmon RJ, et al. A comprehensive analysis of prognostic signatures reveals the high predictive capacity of the proliferation, immune response and RNA splicing modules in breast cancer. Breast Cancer Res. 2008;10(6):R93. doi: 10.1186/bcr2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rody A, Holtrich U, Pusztai L, Liedtke C, Gaetje R, Ruckhaeberle E, Solbach C, Hanker L, Ahr A, Metzler D, Engels K, et al. T-cell metagene predicts a favorable prognosis in estrogen receptor-negative and HER2-positive breast cancers. Breast Cancer Res. 2009;11(2):R15. doi: 10.1186/bcr2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoda H, Fujio K, Yamaguchi Y, Okamoto A, Sawada T, Kochi Y, Yamamoto K. Interactions between IL-32 and tumor necrosis factor alpha contribute to the exacerbation of immune-inflammatory diseases. Arthritis Res Ther. 2006;8(6):R166. doi: 10.1186/ar2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims AH, Howell A, Howell SJ, Clarke RB. Origins of breast cancer subtypes and therapeutic implications. Nat Clin Pract Oncol. 2007;4(9):516–525. doi: 10.1038/ncponc0908. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Toledo CM, Wietgrefe SW, Duan L, Schacker TW, Reilly CS, Haase AT. The immunosuppressive role of IL-32 in lymphatic tissue during HIV-1 infection. J Immunol. 2011;186(11):6576–6584. doi: 10.4049/jimmunol.1100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino C, Di Carlo E. Expression of IL-32 in human lung cancer is related to the histotype and metastatic phenotype. Am J Respir Crit Care Med. 2009;180(8):769–779. doi: 10.1164/rccm.200903-0400OC. [DOI] [PubMed] [Google Scholar]
- Teschendorff AE, Gomez S, Arenas A, El-Ashry D, Schmidt M, Gehrmann M, Caldas C. Improved prognostic classification of breast cancer defined by antagonistic activation patterns of immune response pathway modules. BMC Cancer. 2010;10:604. doi: 10.1186/1471-2407-10-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Brannen E, Choi KY, Arsenault R, El-Gabalawy H, Napper S, Mookherjee N. Inflammatory cytokines IL-32 and IL-17 have common signaling intermediates despite differential dependence on TNF-receptor 1. J Immunol. 2011;186(12):7127–7135. doi: 10.4049/jimmunol.1002306. [DOI] [PubMed] [Google Scholar]
- Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32(5):643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7(3):164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kamigaki T, Yamashita K, Hori Y, Hasegawa H, Kuroda D, Moriyama H, Nagata M, Ku Y, Kuroda Y. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer. Oncol Rep. 2009;22(2):337–343. [PubMed] [Google Scholar]
- Yun HM, Oh JH, Shim JH, Ban JO, Park KR, Kim JH, Lee DH, Kang JW, Park YH, Yu D, Kim Y, et al. Antitumor activity of IL-32beta through the activation of lymphocytes, and the inactivation of NF-kappaB and STAT3 signals. Cell Death Dis. 2013;4:e640. doi: 10.1038/cddis.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50(5):980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zhu Y. Important role of the IL-32 inflammatory network in the host response against viral infections. Viruses. 2015;7:3116–3299. doi: 10.3390/v7062762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10(6):R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.