Abstract
Background
Paraquat (PQ) poisoning can cause multiple organ failure, in which the lung is the primary target organ. There is currently no treatment for PQ poisoning. Mesenchymal stem cells (MSCs), which differentiate into multiple cell types, have generated much enthusiasm regarding their use for the treatment of several diseases. The aim of this study was to systematically review and analyze published preclinical studies describing MSC administration for the treatment of PQ poisoning in animal models to provide a basis for cell therapy.
Methods
The electronic databases PubMed and CBMdisc were searched in this systematic review and meta-analysis. The MSC treatment characteristics of animal models of PQ poisoning were summarized. After quality assessment was performed, the effects of MSC transplantation were evaluated based on the survival rate, lung wet/dry weight, fibrosis scores, oxidative stress response, and inflammatory response. Publication bias was assessed.
Results
Eleven controlled preclinical studies involving MSC transplantation in animal models of PQ poisoning were included in this review. MSC therapy improved the survival rate and reduced the lung wet/dry weight and histopathological fibrosis changes in most studies. MSCs decreased serum or plasma malondialdehyde levels in the acute phase after 7 and 14 d and increased serum or plasma superoxide dismutase and glutathione levels at the same time points. IL-1β, TNF-α and TGF-β1 levels in blood or lung tissues were decreased to different degrees by MSCs. Lung hydroxyproline was decreased by MSCs after 14 d. No obvious evidence of publication bias was found.
Conclusion
MSCs showed anti-fibrosis therapeutic effects in animal models of lung injury caused by PQ poisoning, which may be related to reduced oxidative stress and inflammatory cytokine levels. Our review indicates a potential therapeutic role for MSC therapy to treat PQ poisoning and serves to augment the rationale for clinical studies.
Introduction
Paraquat (1,1-dichloro-4,4-bipyridine, PQ) is a quaternary nitrogen herbicide used worldwide that exerts strong toxic effects on humans and animals [1]. Due to its low price and easy access, PQ has become a familiar cause of death by pesticide poisoning in developing countries. The lethal dose of PQ is 5–15 ml of water solution containing 20% PQ (20–40 mg/kg) in adults. The fatality rate of PQ poisoning is as high as 50–70% [2]. PQ absorbed through the digestive tract, skin and respiratory tract can cause multiple organ damage, in which the lung is the primary target organ [3–5]. It is thought that the structural similarity of PQ to lung diamines and polyamines such as putrescine, spermine, and spermidine induces the lung to accumulate PQ competitively [6]. The pathologic manifestations of PQ poisoning are pulmonary acute inflammatory infiltration and the rapid development of fibrosis. In clinical practice, there is no specific treatment for PQ poisoning other than minimizing its absorption and attempting to prevent organ injury.
Mesenchymal stem cells (MSCs) are a class of multipotent adult stem cells characterized by both self-proliferation and highly plasticity, unlike immortalized cell lines, which are not stem cells [7]. MSCs differentiate into mesodermal-derived tissues, such as adipocytes, osteoblasts, and chondrocytes, in vitro and in vivo [8] and secrete a variety of cytokines. MSCs are derived from many sources, including bone marrow, umbilical cord blood, dental pulp, adipose tissue and adult organs [9]. These cells exhibit fibroblastic morphology, are generally isolated via adherence to a plastic surface and share a common immuno-phenotype consisting of CD105, CD73 and CD90 expression; they are also negative for CD45, CD34, CD14, CD19 and HLA-DR expression [10]. MSCs showing low immunogenicity are used for xenogenous transplantation to achieve immunomodulation and improve tissue repair. They have also been evaluated for their regenerative potential to treat conditions such as myocardial infarction [11], certain neurodegenerative disorders [12] and chronic lung diseases [13]. Studies have shown that bone marrow and umbilical cord blood-derived MSCs selectively home to sites of lung injury through the stromal derived factor-1/chemokine receptor CXCR4 signaling pathway, differentiate into cells expressing lung epithelial markers and exert paracrine functions [14,15]. MSCs exhibit the potential to improve lung function in bleomycin and lipopolysaccharide pulmonary disease models and are being exploited in the clinic for their therapeutic potential in the treatment of pulmonary fibrosis and pulmonary hypertension [13,16].
The aim of this systematic review and meta-analysis was to investigate the effects of MSCs derived from different sources for the treatment of lung injury induced by PQ. The results obtained from the present review may serve as a reference for the assessment of MSC therapy translation in clinical trials.
Materials and methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) criteria [17], which are briefly described here (S1 Table). Additionally, we deposited our laboratory protocols at protocols.io; the identifier is dx.doi.org/10.17504/protocols.io.mwqc7dw.
Literature search
The PubMed and CBMdisc databases were searched with a date range starting on December 12, 1961; the searches were last updated on September 26, 2017. Medical subject headings (MeSH) combined with individual words were used to select the search terms. Terms used in the search included “Mesenchymal Stem Cell”, “Paraquat” and “Pulmonary” (refer to S4 Table). We also searched the reference lists of the retrieved articles to identify any additional studies that were missed.
Study selection
All experiments evaluated animals with PQ poisoning treated with normal MSCs alone and a PQ damage group as a control. Reviews and repeated studies were excluded.
Data extraction and screening
All studies were read, and all data were extracted independently by two reviewers. Disagreements were resolved by a third reviewer. The following study characteristics were extracted from each article: the first author’s name; the year of publication; the sample size of the PQ control group and MSC experimental group; the source, dose, time and delivery route of MSCs; the dose and delivery route of PQ; the species of the recipient animal; and time at outcome assessment. According to the literature [7,8,10], MSC characterization criteria and general methodology in the included studies were also collected and compared. Parameters including changes in survival rate, the lung wet/dry weight ratio, fibrosis scores, and malondialdehyde (MDA), superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GSH-PX), interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and transforming growth factor beta (TGF-β1) levels were reviewed via systematic description. In addition, MDA, SOD, and GSH levels in serum or plasma (insufficient statistical data were excluded) were chosen as functional indices to evaluate the therapeutic effects of MSCs through meta-analysis.
Risk of bias
Two reviewers (FH and ATZ) assessed the risk of bias in each experiment using SYRCLE's Risk of Bias tool [18], which is based on the Cochrane Risk of Bias tool for animal intervention studies. In cases with discrepancies, a third investigator (SF) was requested to discuss the different opinions. Ten assessment items related to selection, performance, detection, attrition, reporting and other biases in the SYRCLE tool were assessed and scored as low, high, or unclear risk of bias. The questions in the tool had responses of “yes”, “no” or “do not explicitly state”, corresponding to “low risk of bias”, “high risk of bias” or “unclear”, respectively.
Statistical analysis
Meta-analysis was conducted using Review Manager Version 5.3 (Cochrane collaboration) to generate forest plots and funnel plots. The following data items were used for data entry: (1) mean, standard deviation, and number of animals in the PQ or MSC administration group; and (2) the P value between the two groups. The data were pooled to estimate the mean difference and corresponding 95% confidence intervals. Heterogeneity between studies was assessed with a Chi-squared-based Q test and I2. P<0.1 or I2>50% denotes significant heterogeneity. Because of the large heterogeneity of the total data set, subgroup analysis of comparisons was performed. Outcomes measured 3, 7, 14 and 21 d after transplantation were extracted separately and used for stratified comparisons. The significance of pooled estimates was assessed with a Z test, in which P<0.05 was considered significant. The presence of publication bias and small-study effects were evaluated and explored using funnel plots. Data unsuitable for quantitative analysis were evaluated by statistical qualitative analysis.
Results
Included studies and their characteristics
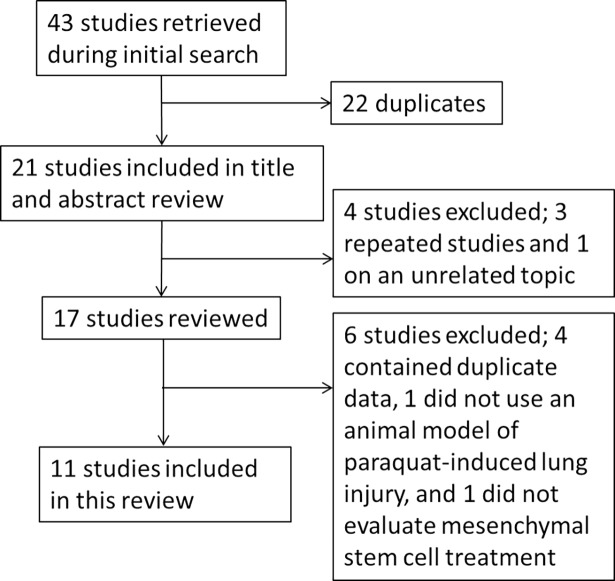
We identified 43 articles in our search, and only 11 articles met the selection criteria for inclusion in this review (Fig 1). The first authors of the included studies are all from China, and the articles were published from 2011 to 2017. All articles included a PQ poisoning group and an MSC treatment group. Sprague-Dawley rats, Balb/c mice and Wistar rats were used in the studies. Different doses of PQ were used to induce lung injury by intraperitoneal injection or intragastric administration. MSCs were obtained from the bone marrow, umbilical cord and adipose tissue. The dose given varied from 0.1×106 to 10×106 cells, although one study did not indicate the specific dose used. The MSCs were mostly administered intravenously, but two studies used retrobulbar injection. All results in these studies were observed within 30 d (Table 1).
Fig 1. Included and excluded studies.
Table 1. Characteristics of the included studies.
| First author | Year | Recipient animals (MSC/PQ) | MSC source | MSC dose (1×106)/time (after PQ)/delivery method | PQ dose (delivery method) | Time at outcome measurement |
|---|---|---|---|---|---|---|
| Xiong Jianfei | 2014 | SD rats (24/24) | SD rat bone marrow | 1/6 h/i.v. | 20% PQ for 15 mg/kg (i.p.) | 3, 7, 14 d after MSC transplantation |
| Gao Jing | 2011 | Wistar rats (9/9) | Wistar rat bone marrow | 0.1/?/i.v. | 20% PQ for 18 mg/kg (i.p.) | 72 h after MSC transplantation |
| Chen Min | 2016 | SD rats (18/18) | Bone marrow | 1/6 h/i.v. | 20 mg/ml PQ for 120 mg/kg (i.g.) | 7, 14, 28 d after PQ administration |
| Huang Yang | 2012 | SD rats (15/15) | SD rat bone marrow | 10/6 h/i.v. | 20% PQ for 5 mg/kg (i.p.) | 1, 3, 7 d after MSC transplantation |
| Huang Yang | 2013 | SD rats (24/24) | Rat bone marrow | 10/6 h /i.v. | 200 g/L PQ for 20 mg/kg (i.p.) | 1, 3, 7, 14 d after PQ administration |
| Zhang Yanmin | 2011 | Wistar rats (20/20) | Wistar rat bone marrow | 1/4 h/i.v. | 20% PQ for 18 mg/kg (i.p.) | 28 d after PQ administration |
| Wu You | 2016 | Balb/c mice (24/24) | Balb/c mouse bone marrow | 1/6 h/retrobulbar injection | 20% PQ for 25 mg/kg (i.p.) | 3, 7, 14, 21 d after PQ administration |
| Lu Shuanghong | 2014 | Balb/c mice (29/30) | Human umbilical cord | ?/24 h/i.v. | 40 mg/kg (i.p.) | 7, 21 d after MSC transplantation |
| Liu Hong | 2016 | Balb/c mice (20/20) | Bone marrow | 1/4 h/retrobulbar injection | 20% PQ for 25 mg/kg (i.p.) | 3, 7, 14, 21 d after MSC transplantation |
| Wu Lin | 2017 | C57BL/6 mice (15/15) | Mouse adipose tissue | 1/6 h/i.v. | 20% PQ for 0.02 L/kg (i.p.) | 12, 24, 48 h after MSC transplantation |
| Hsin-Lin Tsai | 2013 | SD rats (7/7) | Human bone marrow | 5/6 h/i.v. | 24 mg/kg (i.p.) | 30 d after PQ administration |
PQ: paraquat; MSC: mesenchymal stem cell; i.g: intragastric administration; i.v: intravenous administration; i.p: intraperitoneal injection
Risk of bias
Only one of the studies was judged as having a high risk of bias for all data entries assessed (S2 Table). The MSC and PQ groups were similar at baseline in all studies. Allocation of subjects to MSC and PQ groups in all studies was random, and two studies expressly described the method used for random sequence generation. None of the studies described the method used to conceal allocation, whether animals were randomly housed, whether caregivers and examiners were blinded and the method for outcome assessment by random selection. Blinding of the outcome assessor was described in nine studies and was not described in one study. Only one study was judged as having a high risk for outcome blinding. All studies were assessed as having a low risk of attrition and reporting bias. None of the studies had other problems that might lead to a high risk of bias.
MSC characteristics
The criteria and general methodology for MSCs used in the studies are summarized in S3 Table. Eight of the studies reported that the MSCs were separated in the lab. The plastic adherence of MSCs was reported in all studies except for one. Positive and negative markers specific to MSCs were detected in seven studies and were not reported in four studies. The mesodermal-derived tissue differentiation capability of MSCs was tested in four studies. Dulbecco’s modified Eagle’s medium ± fetal bovine serum were conventionally used in cell expansion media, with the exception of one study that did not report the medium used. Two studies reported the use of antibiotic solution in the culture medium. The MSC passage number was reported in eight studies; passage numbers ranged between 3 and 13.
The overall effects of MSCs on PQ-induced lung injury in animals
Survival rates were mentioned in three studies [19–21]. MSCs improved the mean survival time or final survival rate of PQ-poisoned rats. The lung wet/dry weight ratio was measured in 3 studies [22–24]. MSCs reduced the lung wet/dry weight ratio at 72 h and 7 d after transplantation, but no significant differences were observed at 1, 3 and 14 d. Pulmonary fibrosis was assessed in 3 studies [20,21,25]. The Szapiel method and Ashcroft method were used in the studies by Chen et al. [12] and Lü et al. [20], while the study by Tsai et al. [21] did not describe the details of this assessment (Table 2).
Table 2. Summary of the major experimental results.
| First author | Year | Survival rate | Lung wet/dry weight | Lung fibrosis score | MDA | SOD | GSH | GSH-PX | IL-1β | TNF-α | TGF-β1 | HYP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xiong Jianfei | 2014 | ↑ | Plasma NS (3, 14 d), ↓ (7 d) | Plasma ↓ (3 d), ↑ (7 d), NS (14 d) | Plasma NS (3, 14 d), ↓ (7 d) | Plasma NS (3, 14 d), ↓ (7 d) | ||||||
| Gao Jing | 2011 | ↓ (72 h) | ↓ a(72 h) | ↑ a(72 h) | ↑ a(72 h) | |||||||
| Chen Min | 2016 | ↓ c(7, 14, 28 d) | ↓ b(7, 14, 28 d) | Lung NS (7 d), ↓ (14, 28 d) | ||||||||
| Huang Yang | 2012 | NS (1, 3 d), ↓ (7 d) | Plasma NS (1 d), ↓ (3, 7 d) | Plasma NS (1 d), ↓ (3, 7 d) | ||||||||
| Huang Yang | 2013 | NS (1, 3, 14 d), ↓ (7 d) | Plasma NS (1, 14 d), ↓ (3, 7 d) | Plasma NS (1, 14 d), ↓ (3, 7 d) | Plasma ↓ (1, 3, 7 d), NS (14 d) | Plasma ↓ (1, 3, 7 d), NS (14 d) | ||||||
| Zhang Yanmin | 2011 | Serum ↓ (28 d) | Lung ↓ (28 d) | |||||||||
| Wu You | 2016 | Serum ↓ (3, 7, 14 d), NS (21 d) | Serum ↑ (3, 7, 14, 21 d) | Serum ↑ (3, 7, 14, 21 d) | Serum ↓ (3, 7, 14, 21 d) | Serum ↓ (3, 7, 14, 21 d) | ||||||
| Lu Shuanghong | 2014 | ↑ | ↓ d(21 d) | |||||||||
| Liu Hong | 2016 | Serum ↓ (3, 7, 14, 21 d) | Lung protein ↑ (3, 21 d); serum ↑ (3, 7, 14 d), NS (21 d) | Serum ↑ (3, 7, 21 d), NS (14 d) | Serum NS (3 d), ↓ (7, 14, 21 d) | Serum ↓ (3, 7, 14 d), NS (21 d) | ||||||
| Wu Lin | 2017 | Plasma NS (12, 24 h), ↓ (48 h) | Plasma NS (12, 24 h), ↓ (48 h) | |||||||||
| Hsin-Lin Tsai | 2013 | ↑ | ↓ e(30 d) |
↑: increased compared with the PQ group (<0.05)
↓: decreased compared with the PQ group (<0.05); NS: not significant; MDA: malondialdehyde; SOD: superoxide dismutase; GSH: glutathione; GSH-PX: glutathione peroxidase; HYP: hydroxyproline
a Lung tissue homogenate
b Lung immunohistochemical semiquantitative analysis
c Szapiel method
d Ashcroft method
e Did not indicate the method
The effects of MSCs on the oxidative stress response
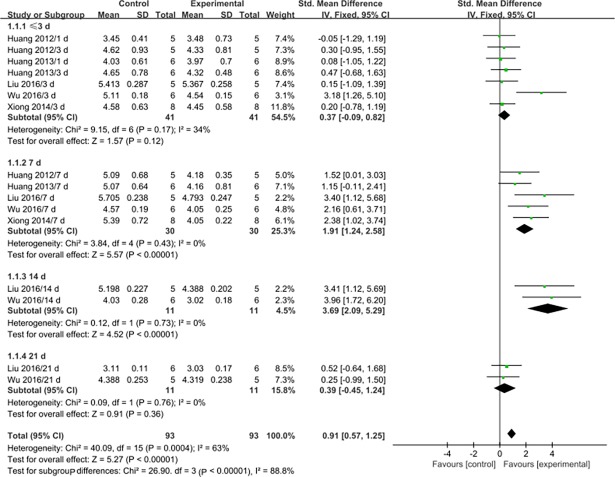
Serum or plasma MDA levels were assessed at 3 d after MSC transplantation or earlier in seven studies [19,23,24,26,27], at 7 d after transplantation in five studies [19,23,24,26,27], at 14 d after transplantation in two studies [26,27], and at 21 d after transplantation in two studies [26,27] (Table 2). Generally, MSC therapy was associated with significantly decreased MDA levels at 7 d (SMD: 1.91, 95% CI: 1.24 to 2.58, P<0.00001) and 14 d after transplantation (SMD: 3.69, 95% CI: 2.09 to 5.29, P<0.00001), but not at 3 d or earlier (SMD: 0.37, 95% CI: -0.09 to 0.82, P = 0.12) or 21 d after transplantation (SMD: 0.39, 95% CI: - 0.45 to 1.24, P = 0.36). No significant heterogeneity was observed in the groups (Fig 2).
Fig 2. Forest plots showing the effects of MSCs on MDA levels.
Control: PQ group; Experimental: MSC treatment group; CI: confidence interval; IV: independent variable; SD: standard deviation.
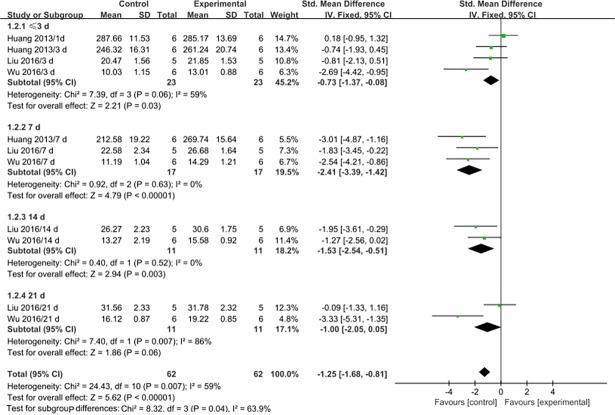
Serum or plasma SOD levels were assessed at 3 d after MSC transplantation or earlier in four studies [24,26,27], at 7 d after transplantation in three studies [24,26,27], at 14 d after transplantation in two studies [26,27], and at 21 d after transplantation in two studies [26,27] (Table 2). Generally, MSC therapy was associated with significantly increased SOD levels at 3 d or earlier (SMD: -0.73, 95% CI: -1.37 to -0.08, P = 0.03), 7 d (SMD: -2.41, 95% CI: -3.39 to -1.42, P<0.00001) and 14 d after transplantation (SMD: -1.53, 95% CI: -2.54 to 0.51, P = 0.003). However, no difference was observed at 21 d after transplantation (SMD: -1.00, 95% CI: -2.05 to 0.05, P = 0.06). Significant heterogeneity was observed at 3 d or earlier and 21 d after transplantation (I2 = 59% and 86%). The heterogeneity was mainly associated with the time point (Fig 3).
Fig 3. Forest plots showing the effects of MSCs on SOD levels.
Control: PQ group; Experimental: MSC treatment group; CI: confidence interval; IV: independent variable; SD: standard deviation.
Serum or plasma GSH levels were assessed at 3 d after MSC transplantation or earlier in two studies [26,27], at 7 d after transplantation in two studies [26,27], at 14 d after transplantation in two studies [26,27], and at 21 d after transplantation in two studies [26,27] (Table 2). Generally, MSC therapy was associated with significantly increased GSH levels at 3 d (SMD: -1.15, 95% CI: -2.10 to -0.21, P = 0.02) 7 d (SMD: -1.09, 95% CI: -2.02 to -0.15, P = 0.02), 14 d (SMD: -1.07, 95% CI: -2.00 to -0.14, P = 0.02) and 21 d after transplantation (SMD: -1.06, 95% CI: - 1.98 to -0.13, P = 0.03). No significant heterogeneity was observed in the groups, and the findings were highly stable (Fig 4).
Fig 4. Forest plots showing the effects of MSCs on GSH levels.
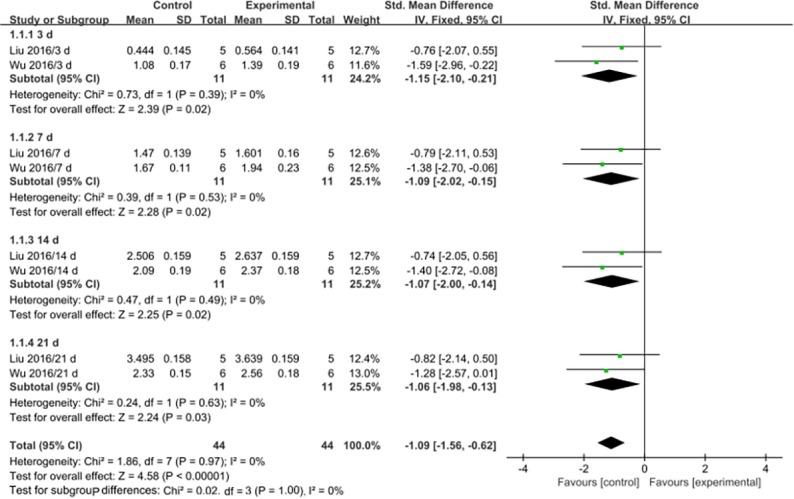
Control: PQ group; Experimental: MSC treatment group; CI: confidence interval; IV: independent variable; SD: standard deviation.
GSH-PX was measured in two studies (Table 2) [19,22]. The study by Xiong et al. [19] showed that plasma GSH-PX was decreased at 3 d after transplantation but increased at 7 d after transplantation, and no significant difference was observed at 14 d after transplantation. The study by Xiong et al. [19] showed that GSH-PX in lung tissue homogenates was increased at 72 h after transplantation.
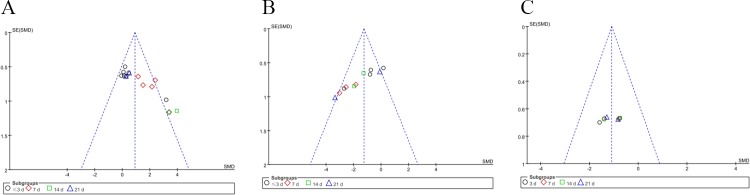
A funnel plot of the MDA, SOD and GSH data showed that the values were distributed around the overall estimate, with no obvious publication bias (Fig 5). In addition, we present the MDA, SOD and GSH data in clear histogram format (S1 File).
Fig 5.
Funnel Plot of MDA (A), SOD (B) and GSH (C) Data. SE: standard error; SMD: standard mean difference.
The effects of MSCs on the inflammatory response
Blood serum or plasma IL-1β was measured in four studies (Table 2). IL-1β levels were significantly decreased by MSC therapy at 7 d after transplantation [19,24,27]. Some studies showed no significant effect on IL-1β expression at 12 h [28], 24 h [28], 3 d [19], and 14 d [19,24] after transplantation, while others showed that IL-1β decreased at 48 h [28], 1 day [24], 3 d [24], 14 d [27], and 21 d [27] after transplantation.
Blood serum or plasma TNF-α was measured in six studies (Table 2). TNF-α levels were significantly decreased by MSC therapy at 7 d after transplantation [19,23,24,26,27]. Some studies showed no significant effect on TNF-α at 12 h [28], 24 h [28], 1 day [23], 3 d [19,26], and 14 d [19,24] after transplantation, while others showed that TNF-α decreased at 48 h [28], 1 day [24], 3 d [23,24,27], 14 d [26,27], and 21 d [26,27] after transplantation.
Blood serum or plasma TGF-β1 levels were measured in two studies (Table 2). No significant effect on TGF-β1 was observed at 21 d after transplantation [26], but its expression decreased at 3 d [26], 7 d [26], 14 d [26], and 28 d after transplantation [29]. Lung TGF-β1 expression decreased at 7 d, 14 d, and 28 d after transplantation [25].
Lung hydroxyproline was assessed in two studies (Table 2) [25,29]. Lung hydroxyproline levels were decreased by MSCs at 14 and 28 d after transplantation, but no significant difference was observed at 7 d after transplantation.
Discussion
Hemodialysis, or blood filtration, to remove toxins is the primary method of treating PQ poisoning in the clinic [30]. In addition, drugs such as adiponectin [31], sodium ferulate combined with oxymatrine [32] and pirfenidone plus prednisolone [33] are being studied to treat lung injury, but the therapeutic effects are not ideal. This study summarized preclinical data describing the use of MSCs for the treatment of PQ poisoning in animal models of lung injury. MSCs derived from bone marrow, fat and umbilical cord were used to treat PQ poisoning in animal models within 24 h of PQ administration. The oxidative stress response, inflammatory response, survival status and histopathology of experimental animals were improved by MSCs.
The shortcomings of experimental reports in the included studies were determined by using the SYRCLE Risk of Bias tool. None of the 11 studies included was assessed as having a low risk of bias based on all of the reporting data entries included in the tool. Among all studies, a low risk of bias was assigned only if detailed, specific methodological descriptions were included. In other words, the appropriate methodology may be applied in these studies, but there are no explicit methodological descriptions in the articles. Our review suggests that it is imperative to improve description of the experimental methods, especially in preclinical studies of MSC therapy for PQ poisoning. The SYRCLE Risk of Bias tool may be a good reference tool to allow preclinical studies to reduce bias. In addition, according to the summary of MSC study characteristics (S3 Table), four of the studies did not report the markers and differentiation capability of the MSCs used. We also suggest that the criteria and general methodology for MSCs described in future studies must be more elaborate; greater article stringency will help to optimize conversion studies.
Large amounts of oxygen free radicals and MDA are produced during PQ-induced lung damage, resulting in the oxidation of membrane lipids, damage to pulmonary vascular endothelial cells, and the destruction of cellular barriers. SOD and GSH are important free radical scavengers. Quantitative analysis showed that MSCs significantly reduce MDA content in the serum or plasma of PQ-poisoned animals at 7 and 14 d after transplantation, but no significant differences are observed 3 d after transplantation or earlier or 21 d after transplantation. By contrast, serum or plasma SOD and GSH levels increase significantly after MSC transplantation at 3 d or earlier, 7 d or 14 d. This finding suggests that MSC administration reduces MDA levels by promoting serum or plasma SOD and GSH levels within 14 d, reducing oxidative stress and protecting lung tissues. In addition, blood MDA levels in the MSC treatment groups do not significantly different from those in the PQ group at or before 3 d after MSC transplantation, potentially because the transplanted MSCs have not yet arrived at their “niche” location to exert anti-oxidative effects. Moreover, PQ poisoning results in the development of fibrotic lesions in the target organ by 21 d after poisoning, and MDA levels primarily reflect oxidative stress in the acute phase; this may explain why there is no obvious difference in MDA content between the PQ and MSC treatment groups at 21 d.
Pro-inflammatory and pro-fibrogenic cytokines are closely associated with the initial and developmental stages of PQ-induced pulmonary fibrosis [34]. PQ activates the NF-κB signaling pathway, resulting in the release of TNF-α, IL-1β, and IL-6 and leading to acute lung injury [35]. IL-1β inhibits fluid transport across the distal lung epithelium, resulting in surfactant abnormalities and increased protein permeability across the alveolar-capillary barrier, playing a key role in the development of acute PQ-induced lung injury [36]. TNF-α is produced by alveolar macrophages during the early stage of lung injury and the later fibrosis stage, triggering the release of various pro-inflammatory cytokines and leading to fibroblast proliferation [37]. In addition, TNF-α causes cell death and alveolar epithelial dysfunction [38]. TGF-β promotes collagen deposition and the differentiation of fibroblasts into myofibroblasts and inhibits fibroblast autophagy [39,40]. Our review found that IL-1β, TNF-α and TGF-β1 levels in blood or lung tissues are decreased to differing degrees by MSCs within 28 d, suggesting that transplanted MSCs modulate inflammatory cytokines in the blood or local lung tissues via paracrine secretion functions and thus play anti-inflammatory and anti-fibrosis roles in the lung. In addition, hydroxyproline content in lung tissues is decreased by MSCs at 14 d and 28 d after transplantation, indicating that MSC administration decreases the collagen content in lung tissue. This result also confirms that MSCs exert an anti-fibrosis effect.
Interestingly, a single clinical study named “Human umbilical cord derived mesenchymal stem cell therapy in PQ poisoning induced lung injury” is shown on clinicaltrials.gov. The status of this study is unknown, and the notes for this study state: “Study has passed its completion date and status has not been verified in more than two years”. According to the clinical situation during PQ poisoning, the status quo of sponsors and collaborators, and the progression of Chinese stem cell research, we infer that there are three main reasons for the study status given above. First, PQ poisoning demonstrates acute progression in clinical practice, with respiratory failure usually occurring within 2 or 3 weeks, and large doses of PQ (> 40 mg/kg) cause multiple organ failure within days. Meanwhile, MSC preparation also requires adequate time. Patients usually receive emergency treatment to block the absorption of toxic substances in the short term. Therefore, there are certain difficulties associated with patient recruitment for this study. Second, the sponsors and collaborators shown in the study details are “Affiliated Hospital to Academy of Military Medical Sciences” and “Ivy institute of stem cells Co. Ltd”. After searching on the Internet (https://xin.baidu.com/detail/compinfo?pid=llHczwJVAmS4YZhuGUSXktjSo77kpo*crgkh&from=ps&tab=changeRecord), we found that the business scope and legal representation of this company have changed since May 2015, the initial start date of the study. Therefore, changes to the company's operating policies may constitute a commercial factor affecting enrollment. Third, the implementation of stem cell translational research policy is very important to standardize clinical research involving stem cells in China. An official document designated “Notification of the management of stem cell clinical research (trial)” was published in August 2015, and relevant policies are currently being updated; all stem cell clinical research must follow these scientific standards. Therefore, patient recruitment, commercial sponsorship, and policy support should be carefully considered when performing clinical cell therapy transformation research for PQ poisoning.
This study has some limitations. First, there is a high incidence of PQ poisoning in China, and most of the preclinical studies in this review come from the Chinese literature. Meanwhile, it is necessary to improve detailed descriptions of experimental methods to reduce the risk of bias. Second, we quantitatively meta-analyzed the oxidative stress indices MDA, SOD and GSH and qualitatively analyzed factors such as inflammatory cytokines and fibrosis, but these factors do not completely simulate the response to PQ poisoning in humans. Third, sub-groups in the SOD quantitative analysis demonstrated an I2>50%, which may affect the credibility of the results. In addition, the MDA funnel chart shows that four studies fall outside of the 95% confidence interval and that the overall I2 value of MDA is 63%, suggesting that the moderate heterogeneity may be caused by these four studies, although the heterogeneity of each subgroup was acceptable. Fourth, the maximum time point we statistically analyzed in this study was 28 d after PQ poisoning. Whether MSCs directly differentiate into alveolar epithelial cells to prevent PQ-induced fibrosis and exert long-term therapeutic effects on lung regeneration is worthy of further exploration.
Conclusion
Preclinical data regarding MSC treatment of PQ-induced lung injury showed that MSCs exerted therapeutic effects on animal models of lung injury induced by PQ poisoning; these effects may be associated with the reduction of oxidative stress and inflammatory cytokines. MSCs may be a new and effective biological agent for the treatment of clinical PQ poisoning, and our review serves to augment the rationale for clinical studies.
Supporting information
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Professor Limei Yu for help with the methodology.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no funding for this work.
References
- 1.Neves FF, Sousa RB, Pazin-Filho A, Cupo P, Junior JE, Nogueira-Barbosa MH. Severe paraquat poisoning: clinical and radiological findings in a survivor. J Bras Pneumol. 2010;36: 513–516. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Chen Y, Mao L, Zhao G, Hong G, Li M, et al. Effects of hemoperfusion and continuous renal replacement therapy on patient survival following paraquat poisoning. PLoS One. 2017;12: e0181207 doi: 10.1371/journal.pone.0181207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng CH, Chen HH, Hu CC, Huang WH, Hsu CW, Fu JF, et al. Predictors of acute kidney injury after paraquat intoxication. Oncotarget. 2017;8: 51345–51354. doi: 10.18632/oncotarget.17975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller TE, Nunes ME, Menezes CC, Marins AT, Leitemperger J, Gressler AC, et al. Sodium selenite prevents paraquat-induced neurotoxicity in zebrafish. Mol Neurobiol. 2017. doi: 10.1007/s12035-12017-10441-12036 [DOI] [PubMed] [Google Scholar]
- 5.Yang C, Song HW, Liu W, Dong XS, Liu Z. Protective effects of chymostatin on paraquat-induced acute lung injury in mice. Inflammation. 2017. doi: 10.1007/s10753-10017-10670-x [DOI] [PubMed] [Google Scholar]
- 6.Guo F, Sun YB, Su L, Li S, Liu ZF, Li J, et al. Losartan attenuates paraquat-induced pulmonary fibrosis in rats. Hum Exp Toxicol. 2015;34: 497–505. doi: 10.1177/0960327114543840 [DOI] [PubMed] [Google Scholar]
- 7.Tirino V, Paino F, d'Aquino R, Desiderio V, De Rosa A, Papaccio G. Methods for the identification, characterization and banking of human DPSCs: current strategies and perspectives. Stem Cell Rev. 2011;7: 608–15. doi: 10.1007/s12015-011-9235-9 [DOI] [PubMed] [Google Scholar]
- 8.Tirino V, Paino F, De Rosa A, Papaccio G. Identification, isolation, characterization, and banking of human dental pulp stem cells. Methods Mol Biol. 2012;879: 443–63. doi: 10.1007/978-1-61779-815-3_26 [DOI] [PubMed] [Google Scholar]
- 9.Papaccio F, Paino F, Regad T, Papaccio G, Desiderio V, Trino V. Concise review: cancer cells, cancer stem cells, and mesenchymal stem cells: influence in cancer development. Stem Cells Transl Med. 2017;6: 2115–25. doi: 10.1002/sctm.17-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8: 315–17. doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 11.Giacomelli E, Mummery CL, Bellin M. Human heart disease: lessons from human pluripotent stem cell-derived cardiomyocytes. Cell Mol Life Sci. 2017;74: 3711–3739. doi: 10.1007/s00018-017-2546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Wang S, Cao W. Mesenchymal stem cell-mediated immunomodulation in cell therapy of neurodegenerative diseases. Cell Immunol. 2017. doi: 10.1016/j.cellimm.2017.1006.1006 [DOI] [PubMed] [Google Scholar]
- 13.Conese M, Piro D, Carbone A, Castellani S, Di Giola S. Hematopoietic and mesenchymal stem cells for the treatment of chronic respiratory diseases: role of plasticity and heterogeneity. Scientific World Journal. 2014;2014: 859817 doi: 10.1155/2014/859817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, et al. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24: 1254–64. doi: 10.1634/stemcells.2005-0271 [DOI] [PubMed] [Google Scholar]
- 15.Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, et al. Derivation of lung epithelium from human cord blood–derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177: 701–11. doi: 10.1164/rccm.200706-859OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol. 2009;175: 303–313. doi: 10.2353/ajpath.2009.080629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10): 1006–12. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 18.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14: 43 doi: 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong J, Zhu C, Zhang K, Lv L. [Bone marrow mesenchymal stem cells reduce acute paraquat poisoning]. Hebei Medical Journal. 2014;36: 3295–3298. doi: 10.3969/j.issn.1002-7386.2014.21.037 [Google Scholar]
- 20.Lü S, Wang H, Du L, Song Y, Wu D, Liu B, et al. [Therapeutic effect of human umbilical cord mesenchymal stem cells on paraquat-induced lung injury in mouse]. Letters in Biotechnology. 2014;25: 809–812. doi: 10.3969/j.issn.1009-0002.2014.06.013 [Google Scholar]
- 21.Tsai HL, Chang JW, Yang HW, Chen CW, Yang CC, Yang AH, et al. Amelioration of paraquat-induced pulmonary injury by mesenchymal stem cells. Cell Transplant. 2013;22: 1667–1681. doi: 10.3727/096368912X657765 [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Cai X, Liu H, Bai H, Han X. [Effect of transplantation of bone marrow mesenchymal stem cells on treatment of acute lung injury induced by paraquat]. Int J Respir. 2011;31: 270–274. doi: 10.3760/cma.j.issn.1673-436X.2011.004.008 [Google Scholar]
- 23.Huang Y, Yin W, Zhang X-M, Wang Y-T, Yu H-Y, Hao L, et al. [Optimal conditions of bone marrow mesenchymal stem cells on paraquat-induced acute lung injury in rats]. Chinese Journal of Industrial Hygiene and Occupational Diseases. 2012;30: 645–649. [PubMed] [Google Scholar]
- 24.Huang Y, Wen Y, Hou-You Y, Yu-Tong W, Chuan-Ming L, Jian X, et al. Combined treatment with bone marrow mesenchymal stem cells and methylprednisolone in paraquat-induced acute lung injury. BMC Emerg Med. 2013;13: S5 doi: 10.1186/1471-227X-13-S1-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen M, Chen Y, Lin Q, Zhu R, Ke J, Chen F. [The protective effect of exogenous bone marrow mesenchymal stem cells on pulmonary fibrosis induced by paraquat]. Chin J Emerg Med. 2016;25: 610–616. doi: 10.3760/cma.j.issn.1671-0282.2016.05.014 [Google Scholar]
- 26.Liu H, Ding Y, Hou Y, Zhao G, Lu Y, Chen X, et al. [The protective effect of bone marrow mesenchymal stem cells carrying antioxidant gene superoxide dismutase on paraquat lung injury in mice]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2016;34: 1–7. doi: 10.3760/cma.j.issn.1001-9391.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Liu H, Ding Y, Lu Y, Chen X, Cai Q, et al. [The protective effect of anti-oxidant gene nuclear factor erythroid 2-related factor 2 on paraquat induced lung injury in mice]. Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care. 2016;23: 36–41. doi: 10.3969/j.issn.1008-9691.2016.01.010 [Google Scholar]
- 28.Wu L, Li J, Zhang S, Zhao W, Yin W. [Therapeutic effect of adipose tissue-derived stromal cells on paraquat poisoning-induced acute lung injury in mice]. Journal of Clinical Emergency (China). 2017;18: 90–97. [Google Scholar]
- 29.Zhang Y, Qiu Z, Liu G. [Effect of mesenchymal stem cell used on prevention and treatment for acute pulmonary injury induced by paraquat poisoning in rats]. Chin J Emerg Med. 2011;20: 39–43. doi: 10.3760/cma.j.issn.1671-0282.2011.01.010 [Google Scholar]
- 30.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72: 745–757. doi: 10.1111/j.1365-2125.2011.04026.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao R, Zhou Y, He Y, Jiang Y, Liu P, Ye L, et al. Adiponectin protects against paraquat-induced lung injury by attenuating oxidative/nitrative stress. Exp Ther Med. 2015;9: 131–136. doi: 10.3892/etm.2014.2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Pei X, Xu M, Sun S, Zhang C, Mu K, et al. The protective effect of sodium ferulate and oxymatrine combination on paraquat-induced lung injury. Iran J Pharm Res. 2015;14: 573–583. [PMC free article] [PubMed] [Google Scholar]
- 33.Rasooli R, Pourgholamhosein F, Kamali Y, Nabipour F, Mandegary A. Combination therapy with pirfenidone plus prednisolone ameliorates paraquat-induced pulmonary fibrosis. Inflammation. 2017. doi: 10.1007/s10753-10017-10671-10759 [DOI] [PubMed] [Google Scholar]
- 34.Toygar M, Aydin I, Agilli M, Aydin FN, Oztosun M, Gul H, et al. The relation between oxidative stress, inflammation, and neopterin in the paraquat-induced lung toxicity. Hum Exp Toxicol. 2015;34: 198–204. doi: 10.1177/0960327114533808 [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Luo F, Zhao H. Paraquat-induced reactive oxygen species inhibit neutrophil apoptosis via a p38 MAPK/NF-κB–IL-6/TNF-α positive-feedback circuit. PLoS One. 2014;9: e93837 doi: 10.1371/journal.pone.0093837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, et al. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem. 2005;280: 18579–18589. doi: 10.1074/jbc.M410561200 [DOI] [PubMed] [Google Scholar]
- 37.Lee IT, Lin CC, Wu YC, Yang CM. TNF-alpha induces matrix metalloproteinase-9 expression in A549 cells: role of TNFR1/TRAF2/PKCalpha-dependent signaling pathways. J Cell Physiol. 2010;224: 454–464. doi: 10.1002/jcp.22142 [DOI] [PubMed] [Google Scholar]
- 38.Patel BV, Wilson MR, O'Dea KP, Takata M. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol. 2013;190: 4274–4282. doi: 10.4049/jimmunol.1202437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee S, Kolb MR, Duan F, Janssen LJ. Transforming growth factor-beta evokes Ca2+ waves and enhances gene expression in human pulmonary fibroblasts. Am J Respir Cell Mol Biol. 2012;46: 757–764. doi: 10.1165/rcmb.2011-0223OC [DOI] [PubMed] [Google Scholar]
- 40.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, et al. Autophagy in idiopathic pulmonary fibrosis. PLoS One. 2012;7: e41394 doi: 10.1371/journal.pone.0041394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.