Abstract
Objectives
To compare clinical outcomes of concurrent chemoradiotherapy (CCRT) with those of radiotherapy alone for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy (IMRT) era.
Materials and methods
We comprehensively searched PubMed, Embase, and the Cochrane Library to identify eligible studies. Overall survival (OS), progression-free survival (PFS), distant metastasis-free survival (DMFS), locoregional recurrence-free survival (LRRFS) with hazard ratios (HRs), and toxicities with odd ratios (ORs) were analyzed.
Results
A total of seven studies met the criteria, with 1302 patients who were treated with IMRT alone or IMRT plus concurrent chemotherapy. No significant survival benefit was shown by CCRT regardless of OS (HR = 1.17, 95% CI 0.73–1.89, P = 0.508), PFS (HR = 0.76, 95% CI 0.38–1.50, P = 0.430), DMFS (HR = 0.89, 95% CI 0.33–2.41, P = 0.816), or LRRFS (HR = 1.03, 95% CI 0.95–1.12, P = 0.498). Additionally, CCRT notably increased the risk of acute grade 3–4 leukopenia (OR = 4.432, 95% CI 2.195–8.952, P < 0.001), compared to IMRT alone.
Conclusion
Adding concurrent chemotherapy to IMRT led to no survival benefit and increased acute toxicity reactions for stage II nasopharyngeal carcinoma.
Introduction
Nasopharyngeal carcinoma (NPC) is quite rare in Europe and the United States but relatively endemic in Southeast Asia, Southern China, the Arctic, and North Africa, especially in Southern China[1, 2]. Radiotherapy is the primary and only curative treatment modality. Additionally, sequential and/or concurrent chemotherapy is widely applied for the treatment of NPC for its chemosensitivity. As is well known, concurrent chemoradiotherapy (CCRT) with/without adjuvant chemotherapy is recommended for locoregionally advanced NPC cases, and radiotherapy alone is suggested for stage I NPC patients. With regard to stage II NPC, CCRT is more acceptable[3, 4]. A phase III randomized trial[5] by Chen et al. demonstrated that adding concurrent chemotherapy to two-dimensional radiotherapy (2D-RT) significantly improved overall survival (OS) in stage II (the Chinese 1992 staging system) NPC, predominantly through a decrease in distant failures. In the context of conventional radiotherapy, many other trials[6, 7] have also determined that CCRT can improve survival for stage II NPC compared with radiotherapy alone. Recently, intensity-modulated radiotherapy (IMRT), an advanced form of conventional radiotherapy and a great stride in the management of NPC, has been widely used clinically. Compared to conventional 2D-RT, this technique offers a more satisfactory balance between target dose coverage and the sparing of adjacent organs at risk. As a number of studies have confirmed, IMRT is superior to conventional radiotherapy in local control, progression-free survival (PFS), and even OS[8–11]. Thus, a crucial question is whether stage II NPC patients can still obtain a significant survival benefit from concurrent chemotherapy in the IMRT era. Additionally, some studies have reported satisfactory therapeutic effects in stage II NPC patients treated with IMRT alone[12, 13].
A recent meta-analysis[14] explored the value of chemoradiotherapy in stage II NPC compared to radiotherapy alone. However, patients treated with various neoadjuvant chemotherapy or adjuvant chemotherapy combined with CCRT were included. The real role of adding concurrent chemotherapy to IMRT for NPC patients remains unclear. Hence, we performed this study to compare the clinical treatment outcomes and toxicities of pure CCRT with those of IMRT alone for stage II NPC patients, with the hope of providing valuable evidence for treatment guidelines and suggestions for future trials.
Material and methods
Literature search strategy
This systematic review and meta-analysis was conducted according to Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA)[15]. The electronic databases Embase, PubMed, and the Cochrane Library were comprehensively searched for all relevant studies without restrictions to language or region before June 13, 2017. The following search terms and their combinations were used: (nasopharyngeal OR nasopharynx) AND (carcinoma OR cancer OR neoplasm OR tumor OR malignant OR malignancy) AND (radiotherapy) AND (chemotherapy). To ensure a comprehensive review, we also screened the citation lists of all included articles.
Selection criteria
All eligible trials had to meet the following predefined criteria: (1) studies that compared IMRT plus concurrent chemotherapy versus IMRT alone in stage II NPC patients; (2) included patients were previously untreated with histologically proven NPC; (3) patients who received neoadjuvant chemotherapy or adjuvant chemotherapy were excluded; (4) at least one of the following terms could be acquired from the paper directly or indirectly using Tierney’s Methods[16]: time-to-event data including OS, PFS, distant metastasis-free survival (DMFS), and locoregional recurrence-free survival (LRRFS), and instances of grade 3–4 adverse events; and (5) commentaries, editorials, reviews, case reports, and letters to editors were excluded.
Data extraction
Two investigators independently extracted relevant characteristics from all included studies using a standard extraction form. For each individual study, we summarized the data including first author, publication year, study design, inclusion period, region where research was conducted, number of patients, histologic type (WHO criteria), staging system and detailed stage data, follow-up duration, treatment protocols, time-to-event data (OS, PFS, DMFS, LRRFS), and instances of acute grade 3–4 adverse events. Any discrepancies were resolved by consulting with a third researcher. If necessary, the study authors were contacted via e-mail.
The primary outcomes were OS, PFS, DMFS, and LRRFS. OS was defined as the time from diagnosis until death or the latest day known to be alive. The duration of time to distant metastasis or recurrence was counted from the initiation of treatment to treatment failure. The secondary end points were the rates of acute grade 3–4 toxicity reactions including hematological events (anemia, leukopenia, neutropenia, thrombocytopenia) and non-hematological events (mucositis and gastrointestinal).
Quality assessment and data analysis
The Cochrane risk of bias tool[17] and the modified Newcastle-Ottawa scale[18] were used to appraise the methodological quality of included randomized controlled trials and retrospective studies, respectively. The quality of each retrospective study was scored ranging from 0 to 9, and studies with scores ≥ 6 were considered high-quality. Furthermore, according to the criteria published by Oxford Centre for Evidence-Based Medicine[19], the levels of evidence for each included studies were evaluated.
Statistical analyses were performed using STATA 14 (STATA Corporation, College Station, TX, USA). All time-to-event data (OS, PFS, DMFS, LRRFS) were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). Odd ratios (ORs) with 95% CIs were used as summary statistics for toxicities. If the 95% CI did not include the value 1 with P < 0.05, the estimate of the outcome was considered statistically significant. An observed HR or OR <1 signified that patients treated with CCRT had survival benefits or sustained less toxicities. Statistical heterogeneity across studies was evaluated using the Cochrane Q test and the I2 statistic[20–22]. Heterogeneity was defined as when the P value of the Cochrane Q test was < 0.10 or the I2 value was > 50%. If P > 0.10 and I2 < 50%, a fixed-effects model was applied for analysis. If not, a random-effects model was used.
For sensitivity analysis, we excluded several trials each time according to different criteria and analyzed the remaining trials to assess the stability of the results. Publication bias was evaluated using Begg’s and Egger’s tests, P > 0.1 was considered no potential publication bias[23, 24].
Results
Search results and characteristics of studies
A total of 1595 studies were identified from the databases and references. After excluding 328 duplicate publications, 1220 non-relevant studies were discarded by screening their titles and/or abstracts. Of the 47 full-text articles assessed for eligibility, 11 were abandoned for no matched comparison, 15 for using non-IMRT technique, 10 for including patients with locoregionally advanced NPC, and 4 for lack of time-to-event data (OS, PFS, DMFS, LRRFS) or instances of adverse events, as we predefined. Consequently, 7 trials[25–31] fulfilled the inclusion criteria, and the flow diagram is presented in Fig 1.
Fig 1. Flow chart showing inclusion and exclusion of trials.
Of 1302 total patients included in this study, 716 received CCRT and 586 received IMRT alone. All seven studies were performed in China. Apart from a single randomized controlled trial[30], six of the seven trials were retrospective studies. All studies recruited stage II NPC patients except for two studies that also included a small fraction of stage III patients (T3N0M0, 18.0%)[27] and stage I patients (T1N0M0, 23.2%)[31]. The general quality of the seven studies was evaluated, and four were classified as high-quality. Moreover, four studies reached evidence level 2b. The baseline characteristics of the included studies are summarized in Table 1.
Table 1. Main characteristics of all the included studies.
| Study | Inclusion Period | Study design | Patients (treatment/control) | Female% (treatment/control) | Median age (treatment/control) | Histology (WHO classification) | Clinical stage | Median follow-up time(range),mo. | Concurrent chemotherapy | IMRT | High quality | Level of evidence[19] | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | ||||||||||||
| Ng/2015 | 2004–2012 | R | 210(124/86) | NA | NA(48.0/52.2) | NA | AJCC 7th edition II | 49.2(NA) | 57: cisplatin 40 mg/m2, qw; 59: cisplatin 100 mg/ m2, q3w; 8: carboplatin | 70Gy/33f | No | 4 | ||
| Chen/2016 | 2007–2014 | R | 122(80/42) | 32.0(28.8/38.1) | NA | 14 | 108 | AJCC 7th edition II | 56.0(9.0–100.0) | 2–3 × q3w cisplatin 80–100 mg/m2 | 68-70Gy/30-31f for PGTVnx and PGTVnd, 60-66Gy/30-31f for PCTV1, 50-56Gy/30-31f for PCTV2 | Yes | 2b | |
| Zhang/2015 | 2003–2013 | R | 482(241/241) | 25.5(25.3/25.7) | NA(47/46) | 16 | 466 | AJCC 7th edition II +T3N0M0(18.0%) | 47.6(10.0–138.0)/50.7(10.9–138.0) | 2–3 × q3w cisplatin- or nedaplatin-based regimen 80–100 mg/m2;or cisplatin- or nedaplatin-based regimen 30–40 mg/m2, qw; or docetaxel-based regimen 20–30 mg/m2, qw | 68Gy/30f for PGTVnx, 60-66Gy/30f for PGTVnd, 60Gy/30f for PCTV1, 54Gy/30f for PCTV2 | Yes | 2b | |
| Su/2016 | 2005–2010 | R | 249(143/106) | 28.5(30.8/25.5) | NA | 13 | 236 | AJCC 7th edition II | 59.4(4.0–115.7) | 123: platinum single-agent (qw or q3w),20: paclitaxel, PF, or TP | 66-70Gy/25-30f for PGTVnx, 60-64Gy/25-30f for PGTVnd, 55-62Gy/25-30f for PCTV1, 42-54Gy/25-30f for PCTV2 | Yes | 4 | |
| Xu/2015 | 2009–2011 | R | 86(43/43) | 26.7(25.6/27.9) | 50(50/51) | NA | AJCC 6th edition II | 37.4(4.8–66.2) | cisplatin 40 mg/m2, qw | 66Gy/30f for PGTVnx and PGTVnd, 60Gy/30f for PCTV1, 54Gy/30f for PCTV2 | Yes | 2b | ||
| Yi/2015 | 2010–2012 | RCT | 84(41/43) | NA | NA | NA | AJCC 7th edition II | 38.0(NA) | cisplatin 40 mg/m2, qw | NA | No | 2b | ||
| Luo/2014 | 2006–2010 | R | 69(44/25) | 44.9(NA) | 42(NA) | 49 | 20 | AJCC 6th edition II + T1N0M0(23.2%) | 34.0(12.0–64.0) | cisplatin 80–100 mg/m2, q3w | 68-72Gy/30-33f for PGTVnx, 66-70Gy/30-33f for PGTVnd, 60-63Gy/30-33f for PCTV1, 50.4-56Gy/28f for PCTV2 | No | 4 | |
Abbreviations: R, retrospective; RCT, randomized controlled trial; AJCC, American Joint Committee on Cancer; WHO, World Health Organization (WHO classification: type I, squamous cell carcinoma; type II, nonkeratinizing carcinoma; type III, undifferentiated carcinoma); NA, not available; mo., months; IMRT, intensity-modulated radiotherapy; qw, weekly; q3w, every 3 weeks; PF, cisplatin combined with 5-fluorouracil; TP, docetaxel combined with cisplatin; f, fraction; GTV, gross tumor volume; CTV, clinical target volume.
Survival outcome
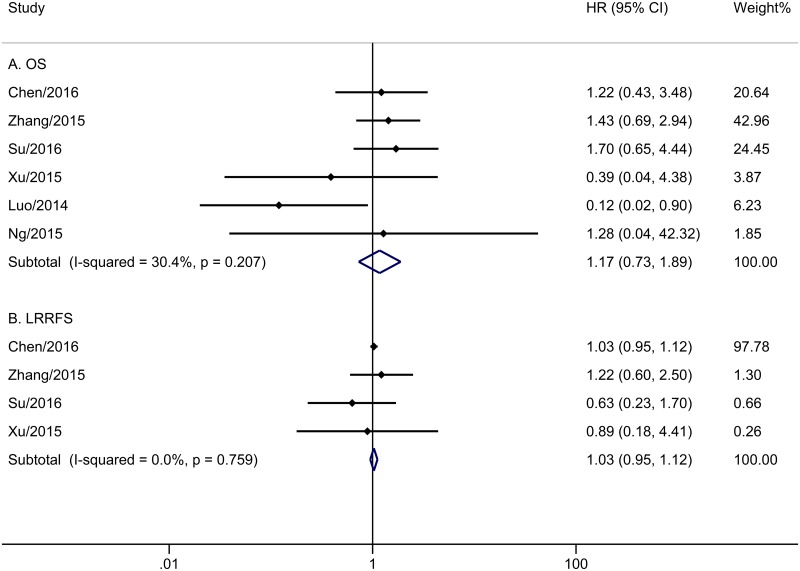
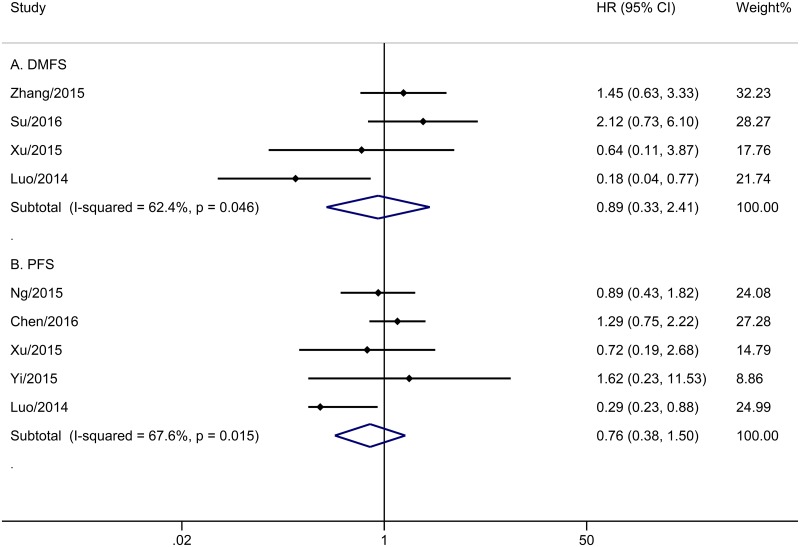
The meta-analysis of OS was based on six trials with 1218 patients. No obvious heterogeneity was observed among these trials (P = 0.207, I2 = 30.4%). Analysis by a fixed-effects model showed that the CCRT group did not have improved OS compared with the IMRT alone group (HR = 1.17, 95% CI 0.73–1.89, P = 0.508; Fig 2A). Five trials with 571 patients reported PFS (Fig 3B). The merge HR was 0.76 (95% CI 0.38–1.50, P = 0.430; heterogeneity: P = 0.015, I2 = 67.6%), indicating that there was no significant difference in PFS between the two groups. Four studies with 886 cases reported DMFS. Fig 3A shows the pooled results. Unfortunately, stage II NPC patients who underwent IMRT did not appear to benefit from concurrent chemotherapy (HR = 0.89, 95% CI 0.33–2.41, P = 0.816; heterogeneity: P = 0.046, I2 = 62.4%). Data regarding LRRFS were reported in four trials with 939 patients. The addition of concurrent chemotherapy led to no benefit for patients who received IMRT (P = 0.498), with HR of 1.03 (95% CI 0.95–1.12) based on a fixed-effects model, since there was no obvious evidence of heterogeneity (P = 0.759, I2 = 0.0%) among the included papers (Fig 2B).
Fig 2. Forest plot and meta-analysis of OS (A) and LRRFS (B).
Fig 3. Forest plot and meta-analysis of DMFS (A) and PFS (B).
A sensitivity analysis was performed to identify whether the survival results were sharply influenced by certain trials. As showed in Table 2, the survival outcomes remained stable after separately excluding three trials that recruited less than 100 patients[29–31], three low-quality studies[25, 30, 31], one trial with the median follow-up time less than 36 months[31], and two trials that enrolled a small number of stage I or III NPC patients[27, 31]. Considering that the weight of one study[26] was over 97% for the pooled result of LRRFS, we excluded this study and analyzed the residual trials. The merge HR was 0.966 (95% CI 0.559–1.667, P = 0.90; heterogeneity: P = 0.570, I2 = 0.0%), drawing a similar conclusion as the primary (HR = 1.03, 95% CI 0.95–1.12, P = 0.498). Generally speaking, the survival results of CCRT versus IMRT alone were of high stability.
Table 2. Sensitivity analysis for the comparison of CCRT with IMRT alone.
| Outcome | Patients | Effect | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| CCRT | IMRT alone | HR(95% CI) | P-value | X2 | df | I2(%) | P-value | |
| Sample size > 100 patients | ||||||||
| OS | 588 | 475 | 1.442(0.874, 2.380) | 0.152 | 0.22 | 3 | 0 | 0.975 |
| PFS | 204 | 128 | 1.128(0.731, 1.740) | 0.586 | 0.65 | 1 | 0 | 0.42 |
| DMFS | 384 | 347 | 1.676(0.870, 3.226) | 0.122 | 0.3 | 1 | 0 | 0.581 |
| LRRFS | 464 | 389 | 1.029(0.948, 1.116) | 0.493 | 1.14 | 2 | 0 | 0.564 |
| High-quality studies | ||||||||
| OS | 507 | 432 | 1.368(0.833, 2.245) | 0.215 | 1.3 | 3 | 0 | 0.729 |
| PFS | 123 | 85 | 1.185(0.718, 1.957) | 0.507 | 0.65 | 1 | 0 | 0.421 |
| DMFS | 427 | 390 | 1.498(0.809, 2.771) | 0.198 | 1.26 | 2 | 0 | 0.531 |
| LRRFS | 507 | 432 | 1.029(0.948, 1.116) | 0.498 | 1.18 | 3 | 0 | 0.759 |
| Median follow-up time > 36 months | ||||||||
| OS | 631 | 518 | 1.366(0.836, 2.231) | 0.213 | 1.3 | 4 | 0 | 0.861 |
| PFS | 288 | 214 | 1.098(0.734, 1.643) | 0.649 | 1.21 | 3 | 0 | 0.75 |
| DMFS | 427 | 390 | 1.498(0.809, 2.771) | 0.198 | 1.26 | 2 | 0 | 0.531 |
| LRRFS | 507 | 432 | 1.029(0.948, 1.116) | 0.498 | 1.18 | 3 | 0 | 0.759 |
| Studies enrolling absolutely stage II NPC patients | ||||||||
| OS | 390 | 277 | 1.314(0.675, 2.559) | 0.442 | 1.27 | 3 | 0 | 0.736 |
| PFS | 288 | 214 | 1.098(0.734, 1.643) | 0.649 | 1.21 | 3 | 0 | 0.75 |
| DMFS | 186 | 149 | 1.557(0.625, 3.882) | 0.342 | 1.25 | 1 | 20.1 | 0.263 |
| LRRFS | 266 | 191 | 1.026(0.945, 1.114) | 0.536 | 0.95 | 2 | 0 | 0.621 |
Abbreviations: CCRT, concurrent chemoradiotherapy; IMRT, intensity-modulated radiotherapy; HR, hazard ratio; CI, confidence interval; df, degrees of freedom; OS, overall survival; PFS, progression-free survival; DMFS, distant metastasis-free survival; LRRFS, locoregional recurrence-free survival.
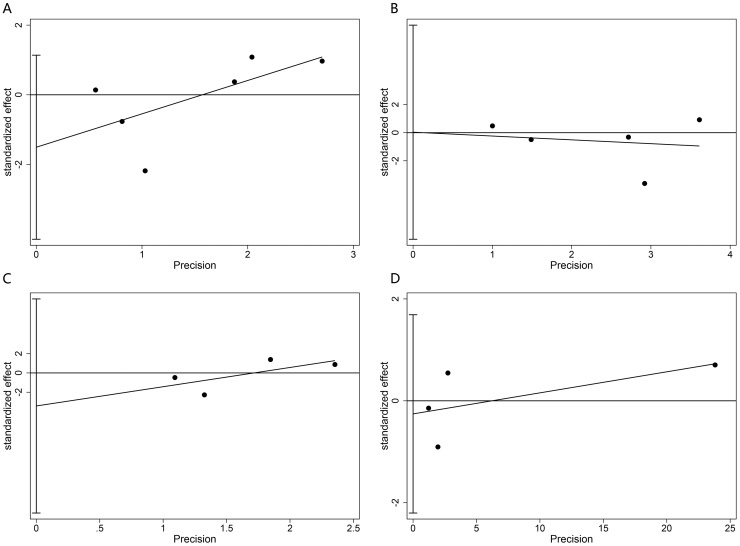
Both Begg’s and Egger’s tests (Fig 4) were performed, and no obvious publication bias was observed in OS, PFS, DMFS, or LRRFS (Begg’s tests, P = 0.260, 1.000, 0.734, 0.734, respectively; Egger’s tests, P = 0.189, 0.989, 0.316, 0.627, respectively).
Fig 4. Egger’s tests for possible publication bias of OS (A), PFS (B), DMFS (C), LRRFS (D).
Treatment-related adverse events
The grade 3–4 acute adverse events that were available for pooled analysis are presented in Table 3. Compared with IMRT alone, concurrent chemoradiotherapy notably increased the risk of acute grade 3–4 leukopenia (OR = 4.432, 95% CI 2.195–8.952, P < 0.001). No significant difference was observed between the two arms with regard to the incidence of anemia (OR = 1.378, 95% CI 0.418–4.538, P = 0.598), neutropenia (OR = 1.905, 95% CI 0.801–4.529, P = 0.145), thrombocytopenia (OR = 1.981, 95% CI 0.794–4.944, P = 0.143), gastrointestinal complications (OR = 7.038, 95% CI 0.890–55.640, P = 0.064), or mucositis (OR = 1.578, 95% CI 0.949–2.623, P = 0.079).
Table 3. Grade 3–4 acute adverse events of CCRT versus IMRT alone for stage II nasopharyngeal carcinoma.
| Grade 3–4 acute adverse events | Availability | Effect | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| CCRT (events/total) | IMRT alone (events/total) | OR (95% CI) | P-value | X2 | df | I2(%) | P-value | |
| Anemia | 7/464 | 4/389 | 1.378(0.418, 4.538) | 0.598 | 0.38 | 2 | 0 | 0.827 |
| Leukopenia | 49/507 | 10/432 | 4.432(2.195, 8.952) | <0.001 | 1.91 | 3 | 0 | 0.591 |
| Neutropenia | 17/321 | 8/283 | 1.905(0.801, 4.529) | 0.145 | 0.14 | 1 | 0 | 0.713 |
| Thrombocytopenia | 15/507 | 6/432 | 1.981(0.794, 4.944) | 0.143 | 3.11 | 3 | 3.6 | 0.375 |
| Gastrointestinal | 10/223 | 0/148 | 7.038(0.890, 55.640) | 0.064 | 0.05 | 1 | 0 | 0.815 |
| Mucositis | 58/186 | 36/149 | 1.578(0.949, 2.623) | 0.079 | 1.71 | 1 | 41.4 | 0.192 |
Abbreviations: CCRT, concurrent chemoradiotherapy; IMRT, intensity modulated radiotherapy; OR, odd ratio; CI, confidence interval; df, degrees of freedom.
Discussion
At present, the most acceptable treatment modality for stage II NPC patients is CCRT, and the majority of evidence for this is based on conventional radiotherapy. In this systematic review and meta-analysis, we explored the real role of concurrent chemotherapy for early stage NPC patients in the IMRT era.
The results showed that the addition of concurrent chemotherapy led to comparable survival conditions for stage II NPC patients. There are several possible explanations for the non-significant survival difference. First and foremost, IMRT is obviously superior to 2D-RT in local tumor control, especially for early T-stage cases[8–11]. A retrospective study by Lai et al.[10] reported significantly improved 5-year local relapse-free survival (LRFS) (92.7% vs. 86.8%) for NPC patients treated with IMRT compared to 2D-RT, and the improvement was even greater for stage T1 patients (100% vs. 94.4%; P = 0.016). Peng et al. conducted a prospective randomized study[32] to compare clinical outcomes of IMRT versus 2D-RT for the treatment of NPC. With a median follow-up time of 42 months, the 5-year OS and local control rates were 79.6% and 90.5% for the IMRT group, and 67.1% and 84.7% for the 2D-RT group, respectively. In addition, in the study by Zhang et al.[27], NPC patients who received IMRT alone had similar survival rates with patients who received concurrent chemotherapy and 2D-RT in the study by Chen et al.[5] (4-year OS, 97.4% vs. 97.4%; 4-year DMFS, 96.5% vs. 97.3%; 4-year LRFS, 93.8% vs. 95.7%, respectively). We wonder that stage II NPC patients might not have a survival benefit from concurrent chemotherapy because IMRT is able to improve significantly the local control rate. Next, the single prospective study[5] to date that demonstrated the value of concurrent chemotherapy in the 2D-RT era enrolled stage II NPC patients evaluated using the Chinese 1992 staging system. According to the 2010 UICC/AJCC staging system, 31 of the included patients were restaged as N2 and stage III. The OS results might be falsely affected by the survival benefit from concurrent chemotherapy in these stage N2 patients. Lastly, clinical stage II NPC consisted of three subgroups-T2N0M0, T1N1M0, and T2N1M0-with different prognoses: T2b classification has a relatively greater risk of local recurrence, and T2N1 NPC might have a greater risk of distant metastasis and poorer survival[5, 13, 31, 33, 34]. Hence, we considered whether T2N1 NPC patients treated with IMRT could benefit from concurrent chemotherapy. Due to a lack of detailed data of individual patients, a subgroup assessment of stage II NPC with precise population stratification was not performed.
The pooled analysis showed that the incidence of acute grade 3–4 toxicity reactions in the CCRT group was higher than in the IMRT alone group. In particular the incidence of leukopenia reached statistical significance (OR = 4.432, 95% CI 2.195–8.952, P < 0.001). A meta-analysis by Zhang et al.[35] analyzed the overall risk of treatment-related mortality with additional chemotherapy in NPC. Compared to radiotherapy alone, chemoradiotherapy significantly increased the risk of treatment-related mortality (0.8% vs. 1.7%). Considering the increased risk of adverse events and treatment-related mortality, the management of stage II NPC should be considered with caution. At present, several phase II-III trials are ongoing to evaluate the role of CCRT for stage II NPC patients treated with IMRT (e.g., NCT02610010, NCT02116231), and we are looking forward to the eventual conclusions.
This systematic review and meta-analysis had several limitations. First, most of the included trials were retrospective, which made biases inevitable. Second, only three trials[27, 29, 31] reported survival data as HRs and 95% CIs directly. We acquired these values for the remaining trials using Tierney’s methods, which could cause potential biases and errors. Third, all of the included studies were performed in China, which might be attributed to the epidemiological characteristics of NPC. Undeniably, the generalization of the conclusions has to be carefully considered. Additionally, not all of the included studies provided sufficient data for analysis, and there was insufficient evidence of late adverse reactions to perform a pooled analysis. Despite these drawbacks, this meta-analysis may provide some significant guidance and reference to identify the optimal treatment strategy for stage II NPC patients.
Conclusions
In brief, the present study suggested that the addition of concurrent chemotherapy led to no survival benefit and increased acute toxicity reactions for stage II NPC who received IMRT. Because patients with T2N1 have a relatively greater risk of distant metastasis, the role of adding concurrent chemotherapy to IMRT for these cases requires further research. Prospective, randomized controlled clinical trials with large sample sizes are needed.
Supporting information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was sponsored by the Major programs of Sichuan Science and Technology Department (No. 2017SZ0015). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Cao S-M, Simons MJ, Qian C-N. The prevalence and prevention of nasopharyngeal carcinoma in China. Chinese Journal of Cancer. 2011;30(2):114–9. doi: 10.5732/cjc.010.10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang ET, Adami HO. The Enigmatic Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiology Biomarkers & Prevention. 2006;15(10):1765–77. doi: 10.1158/1055-9965.EPI-06-0353 [DOI] [PubMed] [Google Scholar]
- 3.Chan AT, Gregoire V, Lefebvre JL, Licitra L, Hui EP, Leung SF, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii83–5. Epub 2012/11/20. doi: 10.1093/annonc/mds266 . [DOI] [PubMed] [Google Scholar]
- 4.NCCN Guidelines Version 2.2017 Head and Neck Cancers. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 5.Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent Chemoradiotherapy vs Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma: Phase III Randomized Trial. Journal of the National Cancer Institute. 2011;103(23):1761–70. doi: 10.1093/jnci/djr432 [DOI] [PubMed] [Google Scholar]
- 6.Cheng S, Tsai S, Yen K, Jian J, Chu N, Chan K, et al. Concomitant radiotherapy and chemotherapy for early-stage nasopharyngeal carcinoma. J Clin Oncol. 2000;18(10):2040–5. doi: 10.1200/JCO.2000.18.10.2040 [DOI] [PubMed] [Google Scholar]
- 7.Xu T, Hu C, Wang X, Shen C. Role of chemoradiotherapy in intermediate prognosis nasopharyngeal carcinoma. Oral Oncology. 2011;47(5):408–13. doi: 10.1016/j.oraloncology.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 8.Fang FM, Chien CY, Tsai WL, Chen HC, Hsu HC, Lui CC, et al. Quality of Life and Survival Outcome for Patients With Nasopharyngeal Carcinoma Receiving Three-Dimensional Conformal Radiotherapy vs. Intensity-Modulated Radiotherapy—A Longitudinal Study. International Journal of Radiation Oncology Biology Physics. 2008;72(2):356. [DOI] [PubMed] [Google Scholar]
- 9.Ng WT, Lee MCH, Hung WM, Choi CW, Lee KC, Chan OSH, et al. Clinical Outcomes and Patterns of Failure After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma. International Journal of Radiation Oncology*Biology*Physics. 2011;79(2):420–8. doi: 10.1016/j.ijrobp.2009.11.024 . [DOI] [PubMed] [Google Scholar]
- 10.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? International Journal of Radiation Oncology*Biology*Physics. 2011;80(3):661–8. [DOI] [PubMed] [Google Scholar]
- 11.Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-Modulated Radiation Therapy With or Without Chemotherapy for Nasopharyngeal Carcinoma: Radiation Therapy Oncology Group Phase II Trial 0225. Journal of clinical oncology. 2009;27(22):3684–90. doi: 10.1200/JCO.2008.19.9109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tham IW-K, Lin S, Pan J, Han L, Lu JJ, Wee J. Intensity-Modulated Radiation Therapy Without Concurrent Chemotherapy for Stage IIB Nasopharyngeal Cancer. American journal of clinical oncology. 2010:1 [DOI] [PubMed] [Google Scholar]
- 13.Su S-F, Han F, Zhao C, Chen C-Y, Xiao W-W, Li J-X, et al. Long-Term Outcomes of Early-Stage Nasopharyngeal Carcinoma Patients Treated With Intensity-Modulated Radiotherapy Alone. International Journal of Radiation Oncology*Biology*Physics. 2012;82(1):327–33. doi: 10.1016/j.ijrobp.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Zhang L-H, Chen Y-P, Liu X, Zhou G-Q, Lin A-H, et al. Chemoradiotherapy Versus Radiotherapy Alone in Stage II Nasopharyngeal Carcinoma: A Systemic Review and Meta-analysis of 2138 Patients. Journal of Cancer. 2017;8(2):287–97. doi: 10.7150/jca.17317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.L A, A DG, T J, M C, G PC, I JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700 Epidemiology Biostatistics & Public Health. 2009;6(4):e1-e34. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8(1):2815 doi: 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins Julian PT. Cochrane handbook for systematic reviews of interventions. 2008:102–8. [Google Scholar]
- 18.Wells GA, Shea BJ, O'Connell D, Peterson J, Welch V, Losos M et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Applied Engineering in Agriculture. 2014;18(6):págs. 727–34. [Google Scholar]
- 19.Phillips B BC, Sackett D. Levels of Evidence (March 2009). Accessed 30 June 2016. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009.
- 20.Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539 doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 22.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Jnci Journal of the National Cancer Institute. 1959;22(4):719–48. [PubMed] [Google Scholar]
- 23.Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50(4):1088 doi: 10.2307/2533446 [PubMed] [Google Scholar]
- 24.Schnee S, Enoch M, Noriega-Crespo A, Sayers J, Terebey S, Caselli P, et al. Bias in meta-analysis detected by a simple, graphical test. Bmj British Medical Journal. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng AWY, Tung SY, Cheung AKW, Chan PC. No Role of Using Chemoradiation in T2N0 and T1N1 With Small Lymph Node Size Stage II Nasopharyngeal Carcinoma. International Journal of Radiation Oncology*Biology*Physics. 2015;93(3):E306–E7. doi: 10.1016/j.ijrobp.2015.07.1329 [Google Scholar]
- 26.Chen KH, Zhu XD, Li L, Qu S, Liang ZQ, Liang X, et al. Comparison of the efficacy between concurrent chemoradiotherapy with or without adjuvant chemotherapy and intensity-modulated radiotherapy alone for stage II nasopharyngeal carcinoma:. Oncotarget. 2016;7(42):69041–50. doi: 10.18632/oncotarget.11978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang LN, Gao YH, Lan XW, Jie T, Zhen S, Ma J, et al. Propensity score matching analysis of cisplatin-based concurrent chemotherapy in low risk nasopharyngeal carcinoma in the intensity-modulated radiotherapy era. Oncotarget. 2015;6(41):44019–29. doi: 10.18632/oncotarget.5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.S Z, M YP, T J, L XW, O PY, X FY. Long-term outcomes of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma treated with IMRT: a retrospective study. Tumour Biology the Journal of the International Society for Oncodevelopmental Biology & Medicine. 2016;37(4):4429. [DOI] [PubMed] [Google Scholar]
- 29.Xu T, Shen C, Zhu G, Hu C. Omission of Chemotherapy in Early Stage Nasopharyngeal Carcinoma Treated with IMRT: A Paired Cohort Study. Medicine. 2015;94(39):E293–E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi J, Zhao C, Chen X, Huang X, Gao L, Luo JW, et al. The Value of Adding Chemotherapy to Intensity Modulated Radiation Therapy for Stage II Nasopharyngeal Carcinoma: A Multicenter Phase 2 Study. International journal of radiation oncology, biology, physics. 2015;93(3):S128–S. [Google Scholar]
- 31.Luo S, Zhao L, Wang J, Xu M, Li J, Zhou B, et al. Clinical outcomes for early-stage nasopharyngeal carcinoma with predominantly WHO II histology treated by intensity-modulated radiation therapy with or without chemotherapy in nonendemic region of China. Head & Neck. 2014;36(6):841–7. [DOI] [PubMed] [Google Scholar]
- 32.Peng G, Wang T, Yang KY, Zhang S, Zhang T, Li Q, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2012;104(3):286–93. [DOI] [PubMed] [Google Scholar]
- 33.Xiao WW, Han F, Lu TX, Chen CY, Huang Y, Zhao C. Treatment outcomes after radiotherapy alone for patients with early-stage nasopharyngeal carcinoma. International Journal of Radiation Oncology*Biology*Physics. 2009;74(4):1070–6. [DOI] [PubMed] [Google Scholar]
- 34.Guo Q, Lu T, Lin S, Zong J, Chen Z, Cui X, et al. Long-term survival of nasopharyngeal carcinoma patients with Stage II in intensity-modulated radiation therapy era. Japanese journal of clinical oncology. 2016;46(3):241–7. doi: 10.1093/jjco/hyv192 [DOI] [PubMed] [Google Scholar]
- 35.Zhang A-M, Fan Y, Wang X-X, Xie Q-C, Sun J-G, Chen Z-T, et al. Increased treatment-related mortality with additional cisplatin-based chemotherapy in patients with nasopharyngeal carcinoma treated with standard radiotherapy. Radiotherapy and Oncology. 2012;104(3):279–85. doi: 10.1016/j.radonc.2012.08.022 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.