Abstract
Oral sensitivity to fats varies in individuals influencing nutritional status and health. Variations in oleic acid perception are associated with CD36 and odorant binding protein (OBPIIa) polymorphisms, and 6-n-propylthiouracil (PROP) sensitivity, which is mediated by TAS2R38 receptor. L-Arginine (L-Arg) supplementation was shown to modify the perception of the five taste qualities. Here we analyzed the effect of three concentrations (5, 10, 15 mmol/L) of L-Arg on oral perception of oleic acid in forty-six subjects classified for PROP taster status and genotyped for TAS2R38, CD36 and OBPIIa polymorphisms. L-Arg supplementation was effective in increasing the perceived intensity of oleic acid in most subjects. The lowest concentration was the most effective, especially in PROP non-tasters or medium tasters, and in subjects with at least an allele A in CD36 and OBPIIa loci. Density Functional Theory (DFT) calculations were exploited to characterize the chemical interaction between L-Arg and oleic acid, showing that a stable 1:1 oleate·ArgH+ adduct can be formed, stabilized by a pair of hydrogen bonds. Results indicate that L-Arg, acting as a ‘carrier’ of fatty acids in saliva, can selectively modify taste response, and suggest that it may to be used in personalized dietetic strategies to optimize eating behaviors and health.
Introduction
Dietary fatty acids play an important role in the regulation of energy and lipid metabolism, and many are their effects on health and illness outcomes of individuals [1]. Consequently, the ability to discriminate dietary fatty acids, selectively and quantitatively, may have crucial implications for nutritional status and human health. Oral sensitivity to dietary fatty acid greatly varies among individuals [2–4]. Therefore, understanding the range of fatty acid oral sensitivity and how it is influenced by genetic and environmental factors may lead to significant insights on the role of taste in fat-rich food intake regulation and metabolism.
Fat taste in humans has been suggested as a sixth primary taste quality by Mattes 2010 [5] and confirmed by others [6–8], but the confirmation will require additional data. The following receptors for taste perception of fatty acids have been shown in rodents and humans: the delayed-rectifying potassium (DRK) channel Kv1.5 [9], the G protein-coupled receptor family (GPR120 and GPR40) [10,11], and the multifunctional CD36 scavenger receptor [8,12], which is expressed on taste bud cells of the circumvallate papillae where it has been shown to initiate the cephalic phase activated by FA perception [12–14]. CD36 expression in taste bud has been found to be reduced in high-fat diet-induced obese rats [15], suggesting that a decreased sensitivity to fat, resulting from a diminished expression of CD36, could lead to an increase of fatty food intake as a compensatory mechanism. In addition, in humans the role of CD36 in orosensory perception and preference of dietary lipids has been well proved [8,16]. Several studies showed that variations in fat perception and obesity are associated with common variants in CD36 gene [8,16,17]. In particular, several data showed that the polymorphism rs176166 (A/G), whose allele A is characterized by a decreased protein expression, could explain individual variations in fat orosensory perception [18–20], as well as the different metabolic pattern found between lean and obese subjects [21].
Variations in sensitivity to oleic acid have been related to changes in general taste sensitivity as indicated by differences in the expression of salivary proteins, such as carbonic anhydrase 6 [22], which have been associated with sensitivity to the prototypical taste stimulus, 6-n-propylthiouracil (PROP) [23–26]. The importance gained by this stimulus in the fields of nutrition and taste is based on results showing that subjects who perceive PROP as more bitter (PROP super-tasters), compared with those who perceive PROP only at high concentrations or not at all (non-tasters), also present a higher sensitivity to a wide range of oral stimuli of all taste qualities [27–38], which could be explained by a higher density of fungiform papillae on their tongue with respect to other PROP taster groups [24,34,39–42]. The relationships between PROP tasting and perception and preferences for fats have been extensively investigated. PROP non-tasters show a lower ability to discriminate fat and creaminess in fatty foods [43–48]. They also exhibit a higher level of preference for dietary fat [44,47,49–52] and consume more fats and high-energy foods than do tasters [50,53]. However, other authors report inconsistent results [54,55].
Recently, individual differences in oleic acid perception has been reported to be associated with variability in a human odorant-binding protein gene (OBPIIa) [56]. Subjects who were homozygous for the A-allele in rs2590498 polymorphism reported to perceive bitterness when tasting a milkshake containing dilute concentrations of oleic acid. Bitter perception was dramatically reduced after eliminating retronasally perceived odorants. Variants of OBPIIa could also explain differences in PROP bitterness perception independently from the genotype of the gene codifying for the specific bitter receptor TAS2R38, which explains most of the PROP phenotypic differences with its allelic diversity [57,58].
Individual differences in taste sensitivity have also been attributed to many other factors including morphology and density of taste papillae [24–26,39–41], physical properties of saliva [59], and its chemical constituents [60–63]. Among them, some proteins belonging to the basic proline-rich protein family (Ps-1 and II-2 proteins) and specific amino acids of their sequence, such as L-Arginine (L-Arg), enhance PROP bitterness perception, depending on their concentration in saliva [61–63]. Besides, recently supplementation with L-Arg has been shown to specifically modify the perception of the five taste qualities, thus suggesting this mechanism as altering taste response related to foods [38].
Based on these considerations and given the nutritional value of dietary lipids, it would be of great interest to find a mechanism to modify the taste response related to fats, which might influence lipids intake, also as a function of the factors involved in individual differences of fat perception. To this aim we analyzed the effect of L-Arg supplementation on the oral perception of oleic acid, as a function of the common variants in CD36 (rs1761667) and OBPIIa (rs2590498) genes, and PROP-tasting genotype and phenotype of subjects. In order to evaluate possible variations of the multimodal oral perception of oleic acid due to L-Arg administration, subjects were tested by delivering the fat stimulus to the oral cavity in absence of nose clips, as it happens when food is ingested. The effect of three concentration of L-Arg (5, 10, 15 mmol/L), which have already been shown to be effective in modifying taste perception of the five taste qualities, was analyzed for the purpose of identifying the concentration with the greatest effectiveness.
Materials and methods
Ethics statement
Subjects were informed about the procedure and the aim of the study and signed an informed consent form. The Ethical Committee of the University Hospital of Cagliari approved the study procedures, which were performed in accordance with the latest revision of the Declaration of Helsinki.
Subjects
Forty-six non-smoking healthy young Caucasian volunteers (8 men and 38 women, age 26.6 ± 0.79 years) were recruited through public advertisements at the University of Cagliari (Italy). All were originally from Sardinia, Italy. They were normal weight with a body mass index (BMI) ranging from 18.6 to 25.3 kg/m2 and experienced no change in body weight larger than 5 kg over the previous 3 months. None were dieting or taking medications that might interfere with oral sensory perception. None had food allergies, or scored high on eating behavior scales (assessed by using the Three-Factor Eating Questionnaire) [64]. Normogeusia for four basic tastes (sweet, sour, salty, and bitter) was verified in all participants by a taste strip test (Burghart Messtechnik, Wedel, Germany). This trial was registered at ClinicalTrials.gov (identifier number: UNICADBSITB-1).
Experimental protocol
Subjects were tested in three sessions. In the first two (on two consecutive days) subjects were classified for PROP-taster status. In the third session, 1-month later, their oral perception for oleic acid, and its changes due to L-Arg administration were assessed. Subjects were requested to refrain from eating, drinking (except water) and using oral care products for at least 2 hours prior to testing. In women, the assessments were done on the sixth/seventh day of their menstrual cycle to avoid changes of oral sensitivity due to the estrogen phase [65–68]. The testing room was kept virtually free from odors, was lit with standard solar lighting (15,000 lux) and noise level was kept at a minimum. Subjects had to be in the testing room 15 min before the beginning of trials in order to adapt to the environmental conditions (23–24°C, 40–50% relative humidity), which were kept constant during the experimental session. Solutions were prepared in spring water the day before each session and stored in the refrigerator until 1 hour before testing.
A sample (2-mL) of whole mixed saliva was collected from each subject into an acid-washed polypropylene test tube. Samples of saliva were stored at –80°C until molecular analyses were completed, as described below.
PROP-taster status
In order to classify each subject for PROP-taster status (as PROP super-taster, medium taster, or non-taster), taste intensity ratings were collected, in 2 successive days, by using two different psychophysical procedures: the three-solution test [69], and the impregnated paper screening test [70], which have been validated in several studies [23,25,26,62]. Both procedures are highly reliable as they strongly correlate with the degree of activation of peripheral taste function [71,72]. In the three-solution test, the taste-intensity ratings were collected for three suprathreshold solutions (in 10-mL samples) of PROP (0.032, 0.32, and 3.2 mmol/L) (Sigma-Aldrich, Milan, Italy) and NaCl (0.01, 0.1, 1.0 mol/L) (Sigma-Aldrich, Milan, Italy). Instead, the impregnated paper screening test is based on the ratings of 2 paper disks, one impregnated with PROP solution (50 mmol/L) and the other with NaCl (1.0 mol/L). Stimuli were presented at room temperature. In both tests, taste-intensity ratings for PROP and sodium chloride (NaCl) were collected from each subject by using the Labeled Magnitude Scale (LMS) [73]. LMS gives subjects the freedom to rate the perceived taste intensity of PROP and NaCl, in relation to the ‘strongest imaginable’ oral stimulus they had ever experienced in their life. Each subject was trained in the use of the LMS before testing. In both procedures, PROP and NaCl were presented in a blind and counterbalanced order. Subjects who gave intensity ratings higher to PROP solutions than to NaCl, or rated the PROP disk higher than 67 mm on the LMS, were classified as PROP super-tasters, while those who gave ratings to PROP solutions lower than to NaCl solutions, or rated the PROP disk lower than 13 mm on the LMS were classified as non-tasters. Finally, those who gave comparable ratings to the two stimuli, or rated PROP disk with intermediate ratings, were classified as medium-tasters. Only subjects likewise classified by the two procedures were included in the study. Ten subjects were classified as PROP super-tasters (21.74%); 19 as medium-tasters (41.30%) and 17 as non-tasters (36.96%). Three-way ANOVA was used to validate the presence of the three taster groups (see S1 Table).
Molecular analysis
DNA was extracted from saliva samples using the QIAamp® DNA Mini Kit (QIAGEN S.r.l., Milan, Italy) according to the manufacturer’s instructions. Purified DNA concentration was estimated by measuring the optical density at 260 nm with an Agilent Cary 60 UV-Vis Spectrophotometer.
Subjects were genotyped for three single nucleotide polymorphisms (SNPs) rs713598, rs1726866, and rs10246939, respectively at base pairs 145 (C/G), 785 (C/T), and 886 (G/A) of the TAS2R38 locus, that consist of three amino acid substitutions (Pro49Ala, Ala262Val, and Val296Ile), which give rise to two major haplotypes, PAV (the dominant taster variant) and AVI (the non-taster recessive one) and three rare haplotypes (AAI, AAV, and PVI). A polymerase chain reaction (PCR) was employed to amplify the short region of the TAS2R38 locus, including the first polymorphism of interest (rs713598), followed by analysis with restriction enzyme (HaeIII) of the fragments obtained according to our previous work [24]. The rs1726866 and rs10246939 SNPs were determined by using the TaqMan® SNP Genotyping Assay (C_9506827_10 for the rs1726866 assay and C_9506826_10 for the rs10246939 assay; Applied Biosystems by Life-Technologies Italia, Europe BV) [74–76] according to the manufacturer’s specifications. Replicates and positive and negative controls were included in all reactions.
Subjects were also genotyped for the single nucleotide polymorphism (SNP), rs1761667 (G/A), of CD36, located at the −31118 promoter region of exon 1A. To genotype the CD36 rs1761667 polymorphism molecular analyses were carried out by PCR followed by analysis with restriction enzyme (HhaI) of the fragments obtained according to Banerjee et al. 2010 [77]. All digestion products were separated by electrophoresis on a 2% agarose gel and the DNA bands were visualized by ethidium bromide staining and ultraviolet light to score the deletion. PCR 50 bp Low Ladder DNA was used as a molecular mass marker (Gene Ruler™ -Thermo Scientific).
Finally, subjects were genotyped for the OBPIIa gene polymorphism rs2590498 (A/G) by using custom TaqMan® SNP Genotyping Assay (Applied Biosystems by Life-Technologies Italia, Europe BV) according to Tomassini et al. 2017 [56], and as briefly described below. The following primers sets were used: the forward GCCAGGCAGGGACAGA and the reverse CTACACCTGAGACCCCACAAG and two TaqMan probes were designed according to the OBPII gene (bold and underlined), probe/reporter 1: VIC-TCGGTGACATGAACC and probe/reporter 2: FAM–TCGGTGACGTGAACC. Replicates as well as positive and negative controls were included in all reactions.
Molecular analysis at the three SNPs of the TAS2R38 locus identified 8 subjects who were PAV homozygous, 20 were heterozygous, and 15 were AVI homozygous. Three subjects with rare haplotype were excluded. Molecular analysis at the SNP (rs1761667) of the CD36 identified: 15 subjects who were homozygous GG for CD36 locus, 23 who were heterozygous, and 8 who were homozygous AA. While, the observed genotype distribution for OBPIIa locus identified 9 subjects who were homozygous AA, 14 who were heterozygous, and 23 who were homozygous GG.
Quantum-mechanical calculations
Theoretical calculations were carried out at the Density Functional Theory (DFT) [78] level with the aim of investigating the relative stabilities of oleic acid in its neutral protonated and anionic deprotonated form, L-Arg, L-argininium (L-ArgH+) and the neutral molecular adduct resulting from the interaction of L-Arg and oleic acid (oleic acid·Arg). In DFT methods, the relationship between the electron density and the energy of the molecular system is described by a “functional”, i.e. a functions of another function. Based on the results of our previous calculations on Arg, and a variety of different molecular systems, also including the evaluation of intermolecular interactions, we adopted an hybrid functional (mPW1PW) [79]. Electron shells were mathematically represented by split-valence basis sets, including polarization functions [80–82]. Atomic charges, bond orders, and interaction strengths were calculated at Natural Bond Orbital level (NBO) [83]. Calculations were carried out on isolated molecules (gas phase) and in the presence of solvents, namely water and n-pentadecane to mimic the differently polar environment within and outside oleic acid vesicles. More details on the adopted QM methods are available as S1 File.
Effect of L-Arg supplementation on oleic acid oral perception
The effect of supplementation with of L-Arg on oleic acid multimodal oral perception was assessed in each subject in the third session. In order to evaluate possible variations in oleic acid perception due to L-Arg administration, an amount (1 μL) of oleic acid just above threshold [17] was also presented supplemented with three concentrations of L-Arg (5, 10 and 15 mmol/L), which had previously been shown to be effective in modulating taste perception of five taste qualities [38,62,63].
Stimuli were presented to each subject, in the absence of nose clips, by means of filter paper disks (1.5 cm diameter) according to Melis et al [17]. After rinsing mouth with spring water, each subject was presented, in a random order and a double blinded way, with 5 filter paper disks impregnated with 15 μL of each stimulus. One contained only mineral oil (10 μL) supplemented with spring water (5 μL); one contained a mixture of oleic acid (1 μL) and mineral oil (9 μL) supplemented with spring water (5 μL); three contained a mixture of oleic acid (1 μL) and mineral oil (9 μL) supplemented with L-Arg (5 μL) at different concentrations. Subjects were instructed to place the paper disk on the center of their tongue, keep it in the mouth to savor it for 10 s in order to facilitate the release of the stimulus, then spit it out. Each stimulation was followed by oral rinsing with spring water. The interstimulus interval was set at 5 min. After 1 h, each subject was presented with three more paper disks as controls which contained mineral oil (10 μL) supplemented with L-Arg (5 μL) at different concentrations. Perceived intensity ratings for each stimulation were collected by having the subject place a mark on the LMS corresponding to his/her stimulus perception.
Statistical analyses
Fisher’s exact test was used to compare the percentages of subjects who showed an increase of perceived intensity ratings when the oleic acid was supplemented with the three concentrations of L-Arg (5, 10 and 15 mmol/L), also as function of PROP-taster status, or polymorphisms of TAS2R38, CD36 (rs1761667) and OBPIIa (rs2590498) genes. One-way ANOVA was used to compare the perceived intensity ratings according to PROP taster status, or polymorphisms of TAS2R38, CD36 (rs1761667) and OBPIIa (rs2590498) genes. Repeated-measures ANOVA was used to analyze the percentage increase of perceived intensity ratings by subjects in which the three concentration of L-Arg (5, 10 and 15 mmol/L) were effective, also as function of PROP-taster status, or polymorphisms of TAS2R38, CD36 (rs1761667) and OBPIIa (rs2590498) genes. Post hoc comparisons were conducted with the Fisher’s least significant difference (LSD) test, unless the assumption of homogeneity of variance was violated, in which case the Duncan’s test was used. Statistical analyses were conducted using STATISTICA for WINDOWS (version 7; StatSoft Inc, Tulsa, OK, USA) with 95% confidence interval. P values < 0.05 were considered significant.
Results
Theoretical calculation: Oleic acid/L-Arg interaction
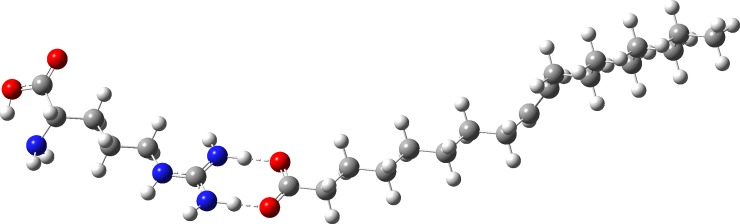
Quantum-mechanical (QM) calculations at the DFT level [78] allowed us to optimize the geometry of oleic acid, the oleate anion, L-Arg, and the protonated form of L-Arg (L-ArgH+). The interaction of oleic acid and L-Arg, or oleate and L-ArgH+, was hypothesized to lead to the hypothetical adduct oleic acid·L-Arg. The formation of this adduct was evaluated in the gas phase, in water, and in an organic solvent (n-pentadecane) featuring the same very low polarity as oleic acid (εr = 2.033 [84] and 2.03, respectively, at 298 K). In all cases, the adduct features a double hydrogen-bonded system involving the carboxylate group of deprotonated oleic acid and the two terminal NH2 groups of L-ArgH+ (Fig 1) featuring two O⋯H–N linear systems (average distances, gas phase: C-O, 1.256; N-H, 1.105; O⋯H, 1.461 Å; O–H–N, 178.85°; water: C-O, 1.257; N-H, 1.060; O⋯H, 1.619 Å; O–H–N, 179.05°; n-pentadecane: C-O, 1.257; N-H, 1.084; O⋯H, 1.523 Å; O–H–N, 178.97°). The optimized bond distances and the total electronic energies (E) calculated for the free synthons and the adduct in the gas phase, in water, and in the organic phase, show that it is very strong in the limit conditions explored by the calculations (ΔEadd = -31.81, -34.88, and -32.83 kcal mol-1 in vacuo, in water and in n-pentadecane). An NBO analysis was carried out to verify the effect of the solvent variation on the strength of the formed hydrogen bonds, showing that, as expected, the two H-bonds are energetically equivalent (< 5%) and derive from the charge-transfer (CT) interaction of the lone pairs of electrons (LP) on the negatively-charged carboxylate oxygen atoms to the antibonding NBOs localized on the N–H bonds (the average CT varies between 42.86 and 72.99 in water and in the gas phase, respectively).
Fig 1. DFT optimized structure of H-bonded adduct between oleic acid (right) and L-ArgH+ (left) calculated in water.
Oxygen atoms are depicted in red, nitrogen atoms in blue, carbon atoms in grey and hydrogen atoms in white.
Effect of L-Arg supplementation on oleic acid perception
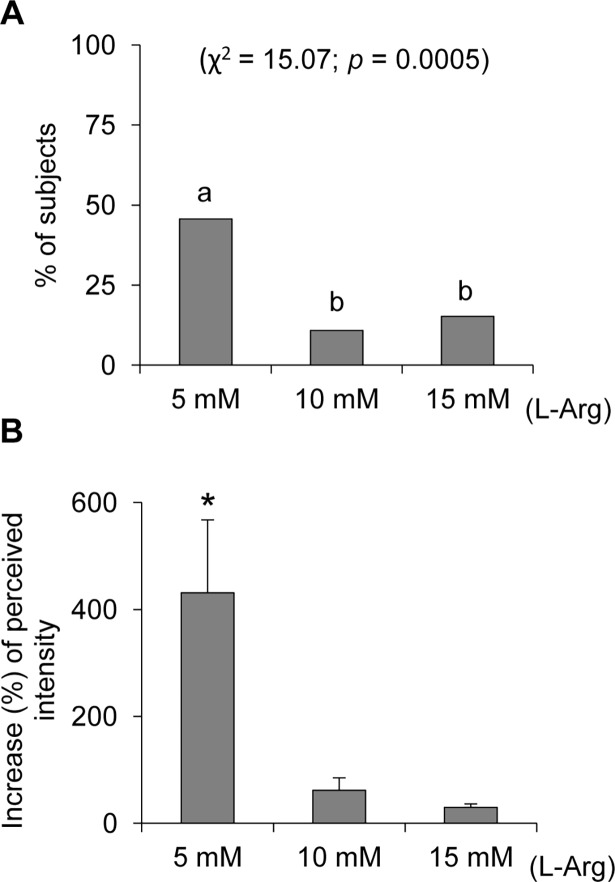
The paper disks containing only mineral oil supplemented with spring water did not evoke any taste perception in all subjects. Differently, most subjects (89.1%) described a perceived intensity between weak and moderate on the LMS (12.0 ± 1.54 mm) when they tested the paper disk impregnated with the mixture of oleic acid and mineral oil supplemented with spring water, while 10.9% (n = 5) could not perceive this stimulus. The supplementation with L-Arg to the mixture of oleic acid and mineral oil was effective in increasing perceived intensity in 72% of subjects, and the individuals who perceived no oleic acid mixture with no L-Arg added, experienced perception for the first time. Fisher’s exact test showed that the percentage of subjects who showed an increase of responsiveness when the oleic acid was supplemented with L-Arg was different at the three concentrations of L-Arg (5, 10 and 15 mmol/L) (χ2 = 15.07, p = 0.0005) (Fig 2A). Specifically, the lowest concentration (5 mmol/L) was effective in 46% of subjects, while the supplementation with 10 or 15 mmol/L determined only a small increase in the number of subjects who became responsive (11% or 15%, respectively). These later values did not differ statistically from each other (χ2 = 0.390, p = 0.553).
Fig 2. Effect of L-Arginine (L-Arg) supplementation on oleic acid responsiveness.
(A) Percentage of subjects who showed an effective increase of perceived intensity ratings when the oleic acid was supplemented with three concentrations of L-Arg (5, 10 and 15 mmol/L). (B) Percentage increase of perceived intensity ratings by the subjects in which the three concentrations of L-Arg (5, 10 and 15 mmol/L) were effective. n = 46. Different letters on top of bars (a or b) indicate significant differences (p ≤ 0.0044; Fisher’s exact test). * = Significant difference with respect to the corresponding value assessed before supplementation (F(1,20) = 8.1696, p = 0.0097; Repeated-measures ANOVA).
The percentage increase in the perceived intensity ratings by subjects in which the three concentrations of L-Arg (5, 10 and 15 mmol/L) were effective is shown in Fig 2B. The supplementation with the lowest concentration (5 mmol/L) determined a significant increase in perceived intensity with respect to values determined in response to stimulation with oleic acid with no L-Arg added (F(1,20) = 8.169, p = 0.0097; Repeated-measures ANOVA), while no significant changes were found after supplementation with 10 and 15 mmol/L of L-Arg in those subjects in which these concentrations were effective (Fig 2B).
The perceived intensity of oleic acid with no L-Arg added, according to PROP taster status of subjects, did not show significant differences (p > 0.05), and was as follows: PROP super-tasters showed an intensity of 13.60 ± 3.14 mm in the LMS, medium tasters of 11.70 ± 2.41 mm and non-tasters of 8.53 ± 2.28 mm. The perceived intensity for oleic acid without added L-Arg by subjects genotyped for CD36 gene did not show significant differences (p > 0.05), and was as follows: subjects with genotype GG showed an intensity of 13.83 ± 2.06 mm, heterozygous subjects of 8.00 ± 2.55 mm and genotypes AA of 8.33 ± 4.02 mm. The perceived intensity for oleic acid without added L-Arg by subjects genotyped for OBPIIa gene was as follows: subjects with genotype AA showed an intensity of 17.44 ± 3.19 mm, heterozygous subjects of 8.43 ± 2.56 mm and genotypes GG of 9.65 ± 1.99 mm. In this case, the perceived intensity by genotypes AA was higher than that tested by genotypes AG and GG (p < 0.041; Duncan test), which gave no different ratings (p > 0.05).
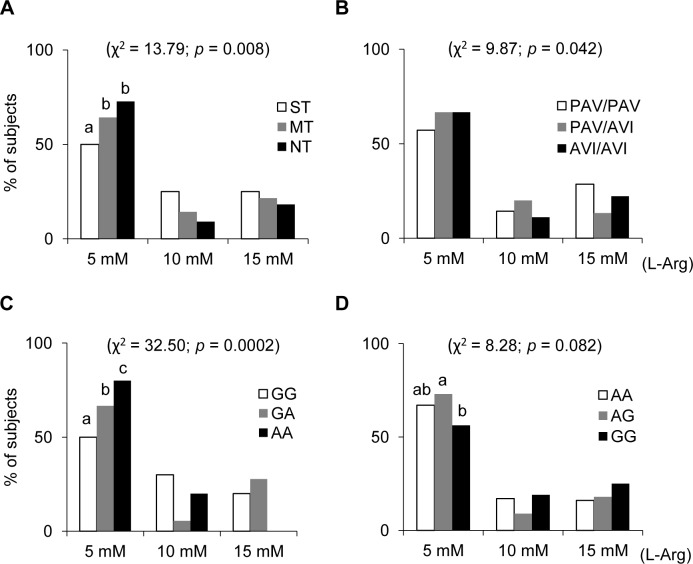
The percentage of subjects who showed an increase of responsiveness when the oleic acid was supplemented with three concentrations of L-Arg (5, 10 and 15 mmol/L), according to PROP taster status or polymorphisms of TAS2R38, CD36 (rs1761667) and OBPIIa (rs2590498) genes is shown in Fig 3. Fisher’s exact test showed that the percentage of subjects responsive to L-Arg classified as non-tasters, medium tasters or PROP super-tasters was different at the three concentrations (χ2 = 13.79, p = 0.008) (Fig 3A). Specifically, the percentage of subjects responsive to the lowest concentrations (5 mmol/L) who were classified as non-tasters (73%) or medium tasters (64%) was higher than that of PROP super-tasters (50%) (χ2 > 3.99, p < 0.045; Fisher’s exact test). No significant changes were found between non-tasters and medium tasters, or in subjects responsive to 10 and 15 mmol/L of L-Arg (p > 0.05). Consistent data, although not significant, were found in subjects genotyped for TAS2R38 gene (Fig 3B). Fisher’s exact test also showed that the percentage of subjects responsive to L-Arg with genotyped GG, GA and AA in CA36 gene was different at the three concentrations (χ2 = 34.497, p = 0.00002) (Fig 3C). In particular, the percentage of subjects responsive to the lowest concentrations of L-Arg who had a pair of alleles A (80%) was higher than that of subjects with only one allele A (67%) (χ2 = 4.77, p = 0.029; Fisher’s exact test), which in turn was higher than that of subjects with homozygous GG genotype (50%) (χ2 = 5.95, p = 0.0147; Fisher’s exact test). No significant changes related to CD36 gene were found in subjects responsive to 10 and 15 mmol/L of L-Arg (p > 0.05). The percentage of subjects responsive to L_Arg (5 mmol/L) with genotype AA in OBPIIa gene (67%) was not different from that of heterozygous (73%), which was higher than that of subjects with homozygous GG genotype (56%) (χ2 = 6.31, p = 0.012; Fisher’s exact test). No significant changes related to OBPIIa genotypes were found in subjects responsive to 10 and 15 mmol/L of L-Arg (p > 0.05).
Fig 3. Percentage of subjects who showed an effective increase of responsiveness when oleic acid was supplemented with three concentration of L-Arg (5, 10 and 15 mmol/L).
(A) Data shown for each PROP taster group. (B) Data shown for each genotype of TAS2R3 gene. (C) Data shown for each genotype of CD36 gene. (D) Data shown for each genotype of OBPIIa gene. n = 46. Different letters on top of bars (a, b or c) indicate significant differences (p ≤ 0.045; Fisher’s exact test).
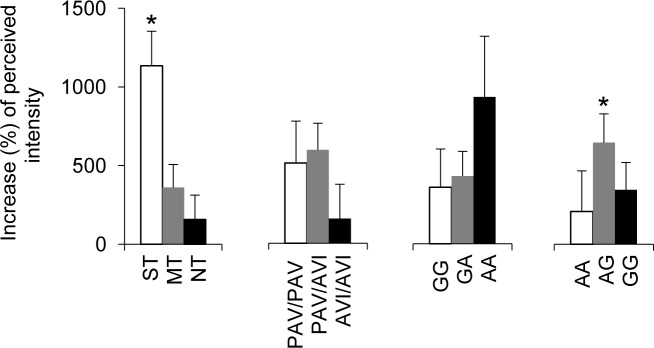
Fig 4 shows the percentage increase of perceived intensity ratings by the subjects in which the lowest concentration of L-Arg (5 mmol/L) were effective according to PROP taster status or polymorphisms of TAS2R38, CD36 (rs1761667) and OBPIIa (rs2590498) genes. Responsive subjects classified as PROP super-tasters showed a significant increase of perceived intensity, with respect to that perceived in response to oleic acid alone (p = 0.0018; Fisher’s LSD test subsequent to repeated-measures ANOVA). No significant changes of perceived intensity were found in non-tasters or medium tasters (p > 0.05). No significant changes were also found when data were analyzed according to polymorphisms in TAS2R38 or CD36 gene, although subjects who carried genotype AA in CD36 gene showed an increase of perceived intensity higher than GG and GA genotypes. In addition, responsive subjects who were heterozygous for OBPIIa gene showed a significant change of perceived intensity, with respect to that perceived in response to oleic acid alone (p = 0.019; Fisher’s LSD test subsequent to repeated-measures ANOVA), while no significant changes were found in subjects with AA or GG genotype.
Fig 4. Percentage increase of perceived intensity ratings by the subjects in which L-Arg (5 mmol/L) was effective according to PROP taster status or polymorphisms of TAS2R38, CD36 (rs1761667) and OBPIIa (rs2590498) genes.
n = 33. * = Significant difference with respect to the corresponding value assessed before supplementation (p = 0.019; Fisher’s LSD test subsequent to repeated-measures ANOVA).
L-Arg did not evoke significant changes in oral perception of oleic acid in 13 subjects (28%) who were not characterized by a particular CD36 or OBPIIa genotype or PROP taster status.
The paper disks containing mineral oil supplemented with l-Arg (5 mmol/L and 10 mmol/L) did not evoke taste perception in all subjects. Differently, the paper disks containing mineral oil supplemented with l-Arg (15 mmol/L) were described as weakly bitter in LMS (4.74 ± 1.01 mm) in 70% of subjects, while the remainder (30%) were not able to identify any of the tastes.
No harms or unintended effects were observed.
Discussion
There is some evidence that a link exists between accumulation of adiposity and reduced chemosensory functions, such as decreased oral fat sensation [85–91], sweet taste [92], salty and bitter taste [93], umami taste [94] and general taste and smell capacity [95]. However, other authors report inconsistent data [96,97], which could be due to the presence of confounding variables [98–100]. In any case, an optimal body composition, favourable to fulfil physiological needs, depends on the balance between energy expenditure and food intake, which is in turn regulated by an adequate food choice governed by individual taste sensitivity [101–103]. Given the nutritional value of dietary fats [1] and the ample individual variations shown in perception, preference for, and consumption of lipids [16,104], the present work provides new insights toward the identification of a mechanism capable of modifying orosensory responses related to fats, also as function of factors implied in individual differences in fat perception.
Our results show that oral supplementation with L-Arg was effective in increasing the perception of oleic acid. The role of L-Arg in taste function and its versatility in modifying taste responses has been extensively underscored. It has been described as a salivary component that contributes to individual differences in PROP bitterness perception, depending on its concentration in saliva [61–63]. Besides, its supplementation has been shown to enhance the responsiveness to bitter compounds, such as PROP or caffeine [38,62,63], the perception of which is mediated by different bitter receptors [58,105]. This suggests that the facilitating action of L-Arg is due to an increase in the availability of molecules at receptor sites, rather than to the binding of molecules with the specific receptor [63]. L-Arg is also known to suppress the bitter taste of quinine by specifically blocking the T2R4 receptor [106–108] or to determine profound modification of perception of other taste qualities [38]. Our results extend the knowledge to the fat taste, by demonstrating the L-Arg supplementation increases perception of oleic acid by affecting both the number of subjects who can perceive an amount of oleic acid which previously resulted to be just above threshold [17] and the intensity of sensation perceived. The results also show that L-Arg was more effective when presented at the lowest test concentration used, thus suggesting that a small surplus of this amino acid in saliva is sufficient to determine a significant increase in the perception of oleic acid, while further amounts seem be in excess. However, our data do not allow to judge which concentration range may actually have the strongest effect. The fact that the stimulation with the most effective concentration of L-Arg (used as a control) was tasteless, rules out the possibility that L-Arg contributes to the perception enhancement of oleic acid.
In addition, our results show that the modifications of perception of oleic acid induced by L-Arg administration are related to the PROP taster status of subjects and common variants in CD36 (rs1761667) and OBPIIa (rs2590498) genes. In particular, supplementation of L-Arg at the lowest concentration determined an increase in the number of subjects who could perceive oleic acid, mostly in PROP non-tasters and medium tasters. These results, which cannot be explained by variations found in relation to TAS2R38 gene, are in accordance with previous data showing that L-Arg enhanced PROP bitterness perception in PROP non-tasters and medium tasters who had low levels of this amino acid in their saliva, as compared to PROP super-tasters [63]. However, the fact that PROP super-tasters showed a greater increase of perceived intensity ratings is not surprising given the fact that these individuals have a higher density of fungiform papillae on their tongue, as compared with other PROP taster groups [24,34,39–42]. Therefore, by considering that the facilitating action of L-Arg is due to an increase in the availability of molecules at receptor sites as previously shown [63], in PROP super-tasters who have a higher density of papillae, L-Arg can facilitate the activation of a higher number of taste receptors.
Interestingly, the analysis of data according to polymorphism, rs1761667 in CD36 indicate that the effectiveness of L-Arg supplementation in increasing the perception of oleic acid is directly related to the presence of allele A in rs1761667 polymorphism of CD36 gene, which has been associated with a lower expression of CD36 scavenger receptor, with respect to that of allele G [109,110]. In fact, L-Arg was more effective in increasing the number of subjects who could perceive oleic acid, when they had an allele A in CD36 locus, with respect to subjects carrying the allele G; besides, having a pair of alleles A further increased the number of responding subjects and their degree of responsiveness, although the latter not significantly.
Surprisingly, our data showed that the presence of a single taster variant in the human odorant-binding protein gene (OBPIIa) resulted to be the most favorable condition for having the strongest effect of L-Arg in increasing oleic acid perception. In fact, the percentage of responsive subjects with genotype homozygous AA, which has been previously associated to an increased oleic acid perception [56], was not different from that of heterozygous, which was higher than that of subjects with homozygous genotype for the non-taster variant (GG). In addition, responsive heterozygous gave higher perceived intensity ratings than genotypes AA or GG. These results seem to suggest that the effect of L-Arg on the olfactory component of perception of oleic acid is greater in the presence of a single taster variant of the odorant-binding protein, while the supplementation of L-Arg in subjects with a pair of alleles AA did not give further advantages.
Our results also confirm previous evidence [17] showing that the paper screening test is an effective and quick method for assessing the multimodal oral perception of dietary fats when they are presented in a mixture with mineral oil which does not evoke taste perception.
Quantum-mechanical DFT calculations were carried out to get an insight on the nature of the interaction between L-Arg and oleic acid. L-Arg is a basic amino acid (pKr = 12.48) that remains protonated at the guanidine residue, both in the free form, in proteins [111,112], and lipid membranes [113], across the entire physiological pH range. Oleic acid, as a long chain fatty acid, spontaneously forms vesicles at a pH close or lower to its pKa value [114]. The pKa value of carboxylic acids is generally close to 4.8 [115], but self-association processes reduce the apparent value of the acidity constants [116]. In the case of oleic acid, an apparent value of pKa (pKapp) of 9.85 was reported [117]. The acidity of oleic acid and the basicity of L-Arg suggest that at physiological pH they are in largely present as oleate and L-argininium, respectively. Since L-ArgH+ was proved to form a H-bonded adduct with PROP, we have turned to study the nature of the interaction between the oleate anion and L-ArgH+ cation or, which stoichiometrically is the same, between L-Arg and oleic acid (oleic acid ·L-Arg). The optimization of the geometry of oleic acid ·L-Arg carried out at DFT level shows that the adduct is stabilized by ΔEadd ≈ 30 kcal mol–1 with respect to the free synthons, due to the formation of two strong H-bond O⋯H–N interactions. This type of interaction is well-known in the literature, and more than 50 structures have been deposited at the Cambridge Structural Database showing L-ArgH+ interacting with carboxylic acids by means of the same–CO2⋯(H2N)2C< 8-membered ring motif, such as the complex of L-Arg and pimelic acid [118]. Since the carbonyl groups of oleic acid are exposed to water in the vesicles, calculations were extended to two limit solvation conditions. Implicit solvation calculations were carried out in water and n-pentadecane, a solvent featuring the same polarity as that reported for oleic acid at 298 K (εr = 2.033) [119], therefore mimicking the L-Arg/oleic acid interaction at the interface and between the vesicles, respectively. Interestingly, notwithstanding a large variation of the H-bonds strengths evaluated by a SOPT analysis, in both solvents the results of the gas phase were confirmed, with stabilization energies of about -35 and -33 kcal mol-1. Since the carboxyl group is important for the taste cues of fatty acids [120,121], it is conceivable that the stability of the adduct is possibly responsible for the transport of free oleic acid molecules from polydispersed vesicles to saliva in the oral cavity. In this sense, the role of L-Arg in increasing the taste sensitivity to oleic acid could be analogous to that previously hypothesized in increasing the bitter-sensitivity to PROP [63]. Remarkably, since the interaction with L-Arg only involves the carboxylate–COO–group of the acid, the same mechanism in principle could be extended to different long-chain fatty acid and L-Arg could induce an enhancement in their perception.
Conclusions
The present results further elucidate the role of L-Arg supplementation in modifying taste responses related to foods highlighting its facilitating effects on oral perception of oleic acid, the major fatty acid in human diet [122], also as a function of the factors implied in individual taste differences, and hence food preferences. Therefore, the use of L-Arg administration may be an helpful tool in designing efficient personalized nutritional strategies aimed at optimizing feeding behaviors and health.
Supporting information
Values are means ± SEM. n = 46. Three-way ANOVA was used to compare PROP bitterness intensity ratings with NaCl saltiness intensity ratings across groups (F(4,258) = 5.199; p = 0.00048). * Significant difference between PROP and the corresponding NaCl concentration (p < 0.0015; Newman Keuls test).
(PDF)
(PDF)
(PDF)
Results of the TaqMan® SNP Genotyping Assay.
(XLS)
Acknowledgments
The Authors thank the volunteers, without whose contribution this study would not have been possible. We also thank, Dr. Caterina Chillotti for running clinical trials.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a grant from the University of Cagliari (Progetti di Ricerca di Interesse Dipartimentale, PRID 2015).
References
- 1.Smit LA, Mozaffarian D, Willett W Review of fat and fatty acid requirements and criteria for developing dietary guidelines. Ann Nutr Metab. 2009; 55: 44–55. doi: 10.1159/000228995 [DOI] [PubMed] [Google Scholar]
- 2.Chalé-Rush A, Burgess JR, Mattes RD Multiple routes of chemosensitivity to free fatty acids in humans. Am J Physiol Gastrointest Liver Physiol. 2007; 292: G1206–1212. doi: 10.1152/ajpgi.00471.2006 [DOI] [PubMed] [Google Scholar]
- 3.Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, et al. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010; 104: 145–152. doi: 10.1017/S0007114510000267 [DOI] [PubMed] [Google Scholar]
- 4.Mattes RD Oral detection of short-, medium-, and long-chain free fatty acids in humans. Chem Senses. 2009; 34: 145–150. doi: 10.1093/chemse/bjn072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattes RD Fat Taste in Humans: Is It a Primary? In: Montmayeur JP, le Coutre J, editors. Fat Detection: Taste, Texture, and Post Ingestive Effects. Boca Raton (FL) 2010. [Google Scholar]
- 6.Ebba S, Abarintos RA, Kim DG, Tiyouh M, Stull JC, et al. The examination of fatty acid taste with edible strips. Physiol Behav. 2012; 106: 579–586. doi: 10.1016/j.physbeh.2012.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mattes RD Oral fatty acid signaling and intestinal lipid processing: support and supposition. Physiol Behav. 2011; 105: 27–35. doi: 10.1016/j.physbeh.2011.02.016 [DOI] [PubMed] [Google Scholar]
- 8.Pepino MY, Love-Gregory L, Klein S, Abumrad NA The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 2012; 53: 561–566. doi: 10.1194/jlr.M021873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbertson TA, Fontenot DT, Liu L, Zhang H, Monroe WT Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997; 272: C1203–1210. doi: 10.1152/ajpcell.1997.272.4.C1203 [DOI] [PubMed] [Google Scholar]
- 10.Matsumura S, Eguchi A, Mizushige T, Kitabayashi N, Tsuzuki S, et al. Colocalization of GPR120 with phospholipase-Cbeta2 and alpha-gustducin in the taste bud cells in mice. Neurosci Lett. 2009; 450: 186–190. doi: 10.1016/j.neulet.2008.11.056 [DOI] [PubMed] [Google Scholar]
- 11.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, et al. Taste Preference for Fatty Acids Is Mediated by GPR40 and GPR120. J Neurosci. 2010; 30: 8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin C, Chevrot M, Poirier H, Passilly-Degrace P, Niot I, et al. CD36 as a lipid sensor. Physiol Behav. 2011; 105: 36–42. doi: 10.1016/j.physbeh.2011.02.029 [DOI] [PubMed] [Google Scholar]
- 13.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005; 115: 3177–3184. doi: 10.1172/JCI25299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sclafani A, Ackroff K, Abumrad NA CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007; 293: R1823–1832. doi: 10.1152/ajpregu.00211.2007 [DOI] [PubMed] [Google Scholar]
- 15.Zhang XJ, Zhou LH, Ban X, Liu DX, Jiang W, et al. Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem. 2011; 113: 663–667. doi: 10.1016/j.acthis.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 16.Keller KL, Liang LC, Sakimura J, May D, van Belle C, et al. Common variants in the CD36 gene are associated with oral fat perception, fat preferences, and obesity in African Americans. Obesity (Silver Spring). 2012; 20: 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melis M, Sollai G, Muroni P, Crnjar R, Barbarossa IT Associations between orosensory perception of oleic acid, the common single nucleotide polymorphisms (rs1761667 and rs1527483) in the CD36 gene, and 6-n-propylthiouracil (PROP) tasting. Nutrients. 2015; 7: 2068–2084. doi: 10.3390/nu7032068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, et al. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 2011; 6: e24014 doi: 10.1371/journal.pone.0024014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X, Bacci S, Mlynarski W, Gottardo L, Soccio T, et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum Mol Genet. 2004; 13: 2197–2205. doi: 10.1093/hmg/ddh233 [DOI] [PubMed] [Google Scholar]
- 20.Madden J, Carrero JJ, Brunner A, Dastur N, Shearman CP, et al. Polymorphisms in the CD36 gene modulate the ability of fish oil supplements to lower fasting plasma triacyl glycerol and raise HDL cholesterol concentrations in healthy middle-aged men. Prostaglandins Leukot Essent Fatty Acids. 2008; 78: 327–335. doi: 10.1016/j.plefa.2008.04.003 [DOI] [PubMed] [Google Scholar]
- 21.Melis M, Carta G, Pintus S, Pintus P, Piras CA, et al. Polymorphism rs1761667 in the CD36 Gene Is Associated to Changes in Fatty Acid Metabolism and Circulating Endocannabinoid Levels Distinctively in Normal Weight and Obese Subjects. Front Physiol. 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mounayar R, Morzel M, Brignot H, Tremblay-Franco M, Canlet C, et al. Salivary markers of taste sensitivity to oleic acid: a combined proteomics and metabolomics approach. Metabolomics. 2014; 10: 688–696. [Google Scholar]
- 23.Calò C, Padiglia A, Zonza A, Corrias L, Contu P, et al. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol Behav. 2011; 104: 1065–1071. doi: 10.1016/j.physbeh.2011.06.013 [DOI] [PubMed] [Google Scholar]
- 24.Melis M, Atzori E, Cabras S, Zonza A, Calò C, et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PLoS One. 2013; 8: e74151 doi: 10.1371/journal.pone.0074151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Padiglia A, Zonza A, Atzori E, Chillotti C, Calò C, et al. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am J Clin Nutr. 2010; 92: 539–545. doi: 10.3945/ajcn.2010.29418 [DOI] [PubMed] [Google Scholar]
- 26.Barbarossa IT, Melis M, Mattes MZ, Calò C, Muroni P, et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), is associated with fungiform papilla density, whereas PROP bitterness is mostly due to TAS2R38 in an ethnically-mixed population. Physiol Behav. 2015; 138: 6–12. doi: 10.1016/j.physbeh.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 27.Bartoshuk LM The biological basis of food perception and acceptance. Food Qual Prefer. 1993; 4: 21–32. [Google Scholar]
- 28.Gent J, Bartoshuk L Sweetness of sucrose, neohesperidin dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil. Chem Senses. 1983; 7: 265–272. [Google Scholar]
- 29.Bartoshuk L, Fast K, Karrer T, Marino S, Price R, et al. PROP supertasters and the perception of sweetness and bitterness. Chem Senses. 1992; 17: 594. [Google Scholar]
- 30.Bartoshuk LM Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science. 1979; 205: 934–935. [DOI] [PubMed] [Google Scholar]
- 31.Bartoshuk LM, Rifkin B, Marks LE, Bars P Taste and aging. J Gerontol. 1986; 41: 51–57. [DOI] [PubMed] [Google Scholar]
- 32.Bartoshuk LM, Rifkin B, Marks LE, Hooper JE Bitterness of KCl and benzoate: related to genetic status for sensitivity to PTC/PROP. Chem Senses. 1988; 13: 517–528. [Google Scholar]
- 33.Bartoshuk LM, Duffy VB, Lucchina LA, Prutkin J, Fast K PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Ann N Y Acad Sci. 1998; 855: 793–796. [DOI] [PubMed] [Google Scholar]
- 34.Yeomans MR, Tepper BJ, Rietzschel J, Prescott J Human hedonic responses to sweetness: role of taste genetics and anatomy. Physiol Behav. 2007; 91: 264–273. doi: 10.1016/j.physbeh.2007.03.011 [DOI] [PubMed] [Google Scholar]
- 35.Prescott J, Soo J, Campbell H, Roberts C Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiol Behav. 2004; 82: 459–469. doi: 10.1016/j.physbeh.2004.04.009 [DOI] [PubMed] [Google Scholar]
- 36.Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, et al. Bitter Receptor Gene (TAS2R38), 6-n-Propylthiouracil (PROP) Bitterness and Alcohol Intake. Alcohol Clin Exp Res. 2004; 28: 1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prescott J, Swain-Campbell N Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem Senses. 2000; 25: 239–246. [DOI] [PubMed] [Google Scholar]
- 38.Melis M, Tomassini Barbarossa I Taste Perception of Sweet, Sour, Salty, Bitter, and Umami and Changes Due to l-Arginine Supplementation, as a Function of Genetic Ability to Taste 6-n-Propylthiouracil. Nutrients. 2017; 9: 541–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartoshuk LM, Duffy VB, Miller IJ PTC/PROP tasting: anatomy, psychophysics, and sex effects. Physiol Behav. 1994; 56: 1165–1171. [DOI] [PubMed] [Google Scholar]
- 40.Essick G, Chopra A, Guest S, McGlone F Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol Behav. 2003; 80: 289–302. [DOI] [PubMed] [Google Scholar]
- 41.Shahbake M, Hutchinson I, Laing DG, Jinks AL Rapid quantitative assessment of fungiform papillae density in the human tongue. Brain Res. 2005; 1052: 196–201. doi: 10.1016/j.brainres.2005.06.031 [DOI] [PubMed] [Google Scholar]
- 42.Bajec MR, Pickering GJ Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol Behav 2008; 95: 581–590. doi: 10.1016/j.physbeh.2008.08.009 [DOI] [PubMed] [Google Scholar]
- 43.Duffy V, Lucchina L, Bartoshuk L Genetic variation in taste: potential biomarker for cardiovascular disease risk? In: Prescott J TB, editor. Genetic variations in taste sensitivity: measurement, significance and implications. Dekker; New York: 2004. pp. 195–228. [Google Scholar]
- 44.Hayes JE, Duffy VB Revisiting sugar-fat mixtures: sweetness and creaminess vary with phenotypic markers of oral sensation. Chem Senses. 2007; 32: 225–236. doi: 10.1093/chemse/bjl050 [DOI] [PubMed] [Google Scholar]
- 45.Prescott J, Bartoshuk LM, Prutkin J 6-n-Propylthiouracil tasting and the perception of nontaste oral sensations In: Prescott J, Tepper BJ, editors. Genetic Variation in Taste Sensitivity. New York: Marcel Dekker; 2004. pp. 89–104. [Google Scholar]
- 46.Tepper BJ, Nurse RJ Fat perception is related to PROP taster status. Physiol Behav. 1997; 61: 949–954. [DOI] [PubMed] [Google Scholar]
- 47.Hayes JE, Duffy VB Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol Behav. 2008; 95: 77–87. doi: 10.1016/j.physbeh.2008.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kirkmeyer SV, Tepper BJ Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem Senses. 2003; 28: 527–536. [DOI] [PubMed] [Google Scholar]
- 49.Tepper BJ, Nurse RJ PROP taster status is related to fat perception and preference. Ann N Y Acad Sci. 1998; 855: 802–804. [DOI] [PubMed] [Google Scholar]
- 50.Keller KL, Steinmann L, Nurse RJ, Tepper BJ Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002; 38: 3–12. doi: 10.1006/appe.2001.0441 [DOI] [PubMed] [Google Scholar]
- 51.Duffy VB, Bartoshuk LM Food acceptance and genetic variation in taste. J Am Diet Assoc. 2000; 100: 647–655. doi: 10.1016/S0002-8223(00)00191-7 [DOI] [PubMed] [Google Scholar]
- 52.Forrai G, Bánkövi G Taste perception for phenylthiocarbamide and food choice—a Hungarian twin study. Acta Physiol Hung. 1984; 64: 33–40. [PubMed] [Google Scholar]
- 53.Tepper BJ, Neilland M, Ullrich NV, Koelliker Y, Belzer LM Greater energy intake from a buffet meal in lean, young women is associated with the 6-n-propylthiouracil (PROP) non-taster phenotype. Appetite. 2011; 56: 104–110. doi: 10.1016/j.appet.2010.11.144 [DOI] [PubMed] [Google Scholar]
- 54.Drewnowski A, Henderson SA, Barratt-Fornell A Genetic sensitivity to 6-n-propylthiouracil and sensory responses to sugar and fat mixtures. Physiol Behav. 1998; 63: 771–777. [DOI] [PubMed] [Google Scholar]
- 55.Drewnowski A, Henderson SA, Cockroft JE Genetic sensitivity to 6-n-propylthiouracil has no influence on dietary patterns, body mass indexes, or plasma lipid profiles of women. J Am Diet Assoc. 2007; 107: 1340–1348. doi: 10.1016/j.jada.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 56.Tomassini Barbarossa I, Ozdener MH, Melis M, Love-Gregory L, Mitreva M, et al. Variant in a common odorant-binding protein gene is associated with bitter sensitivity in people. Behav Brain Res. 2017; 329: 200–204. doi: 10.1016/j.bbr.2017.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, et al. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003; 299: 1221–1225. doi: 10.1126/science.1080190 [DOI] [PubMed] [Google Scholar]
- 58.Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005; 15: 322–327. doi: 10.1016/j.cub.2005.01.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuo R Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000; 11: 216–229. [DOI] [PubMed] [Google Scholar]
- 60.Fox AL The relationship between chemical constitution and taste. Proc Natl Acad Sci USA. 1932; 18: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cabras T, Melis M, Castagnola M, Padiglia A, Tepper BJ, et al. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS One. 2012; 7: e30962 doi: 10.1371/journal.pone.0030962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Melis M, Aragoni MC, Arca M, Cabras T, Caltagirone C, et al. Marked increase in PROP taste responsiveness following oral supplementation with selected salivary proteins or their related free amino acids. PLoS One. 2013; 8: e59810 doi: 10.1371/journal.pone.0059810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Melis M, Arca M, Aragoni MC, Cabras T, Caltagirone C, et al. Dose-Dependent Effects of L-Arginine on PROP Bitterness Intensity and Latency and Characteristics of the Chemical Interaction between PROP and L-Arginine. PLoS One. 2015; 10: e0131104 doi: 10.1371/journal.pone.0131104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stunkard AJ, Messick S The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985; 29: 71–83. [DOI] [PubMed] [Google Scholar]
- 65.Than TT, Delay ER, Maier ME Sucrose threshold variation during the menstrual cycle. Physiol Behav. 1994; 56: 237–239. [DOI] [PubMed] [Google Scholar]
- 66.Alberti-Fidanza A, Fruttini D, Servili M Gustatory and food habit changes during the menstrual cycle. Int J Vitam Nutr Res. 1998; 68: 149–153. [PubMed] [Google Scholar]
- 67.Glanville EV, Kaplan AR Taste Perception and the Menstrual Cycle. Nature. 1965; 205: 930–931. [DOI] [PubMed] [Google Scholar]
- 68.Pal T, Bhattacharyya AK Cyclic changes in salivary lactate dehydrogenase, peroxidase and leucine aminopeptidase during menstrual cycle. Indian J Exp Biol. 1989; 27: 695–698. [PubMed] [Google Scholar]
- 69.Tepper BJ, Christensen CM, Cao J Development of brief methods to classify individuals by PROP taster status. Physiol Behav. 2001; 73: 571–577. [DOI] [PubMed] [Google Scholar]
- 70.Zhao L, Kirkmeyer SV, Tepper BJ A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol Behav. 2003; 78: 625–633. [DOI] [PubMed] [Google Scholar]
- 71.Sollai G, Melis M, Pani D, Cosseddu P, Usai I, et al. First objective evaluation of taste sensitivity to 6-n-propylthiouracil (PROP), a paradigm gustatory stimulus in humans. Sci Rep. 2017; 7: 40353 doi: 10.1038/srep40353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pani D, Usai I, Cosseddu P, Melis M, Sollai G, et al. An automated system for the objective evaluation of human gustatory sensitivity using tongue biopotential recordings. PLoS ONE. 2017; 12: e0177246 doi: 10.1371/journal.pone.0177246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Green BG, Shaffer GS, Gilmore MM Derivation and evaluation of a semantic scale of oral sensation magnitude with apparent ratio properties. Chem Senses. 1993; 18: 683–702. [Google Scholar]
- 74.Smutzer G, Desai H, Coldwell SE, Griffith JW Validation of edible taste strips for assessing PROP taste perception. Chem Senses. 2013; 38: 529–539. doi: 10.1093/chemse/bjt023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem Senses. 2008; 33: 255–265. doi: 10.1093/chemse/bjm084 [DOI] [PubMed] [Google Scholar]
- 76.Sandell M, Hoppu U, Mikkilä V, Mononen N, Kähönen M, et al. Genetic variation in the hTAS2R38 taste receptor and food consumption among Finnish adults. Genes Nutr. 2014; 9: 433 doi: 10.1007/s12263-014-0433-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banerjee M, Gautam S, Saxena M, Bid HK, Agrawal CG Association of CD36 gene variants rs1761667 (G > A) and rs1527483 (C > T) with Type 2 diabetes in North Indian population. Int J Diab Mellitus. 2010; 2: 179–183. [Google Scholar]
- 78.Koch W, Holthausen MC A Chemist’s Guide to Density Functional Theory; ed n, editor. Wiley-VCH, Weinheim, Germany: 2002. [Google Scholar]
- 79.Adamo C, Barone V Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J Chem Phys. 1998; 108: 664–675. [Google Scholar]
- 80.Weigend F Accurate Coulomb-fitting basis sets for H to Rn. Phys Chem Chem Phys. 2006; 8: 1057–1065. doi: 10.1039/b515623h [DOI] [PubMed] [Google Scholar]
- 81.Weigend F, Ahlrichs R Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys. 2005; 7: 3297–3305. doi: 10.1039/b508541a [DOI] [PubMed] [Google Scholar]
- 82.Schäfer A, Horn H, Ahlrichs R Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J Chem Phys. 1992; 97: 2571–2577. [Google Scholar]
- 83.Reed AE, Weinstock RB, Weinhold F Natural population analysis. J Chem Phys. 1985; 83: 735–746. [Google Scholar]
- 84.Francisco Ferreira de Sousa SGCM, dos Santos da Silva Shirsley J., Jordan Del Nero aPA Jr Dielectric Properties of Oleic Acid in Liquid Phase. J Bionanosci. 2010; 3: 1–4. [Google Scholar]
- 85.Chevrot M, Passilly-Degrace P, Ancel D, Bernard A, Enderli G, et al. Obesity interferes with the orosensory detection of long-chain fatty acids in humans. Am J Clin Nutr. 2014; 99: 975–983. doi: 10.3945/ajcn.113.077198 [DOI] [PubMed] [Google Scholar]
- 86.Sayed A, Sery O, Plesnik J, Daoudi H, Rouabah A, et al. CD36 AA genotype is associated with decreased lipid taste perception in young obese, but not lean, children. Int J Obes (Lond). 2015; 39: 920–924. [DOI] [PubMed] [Google Scholar]
- 87.Stewart JE, Newman LP, Keast RS Oral sensitivity to oleic acid is associated with fat intake and body mass index. Clin Nutr. 2011; 30: 838–844. doi: 10.1016/j.clnu.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 88.Drewnowski A Taste preferences and food intake. Annu Rev Nutr. 1997; 17: 237–253. doi: 10.1146/annurev.nutr.17.1.237 [DOI] [PubMed] [Google Scholar]
- 89.Simchen U, Koebnick C, Hoyer S, Issanchou S, Zunft HJ Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur J Clin Nutr. 2006; 60: 698–705. doi: 10.1038/sj.ejcn.1602371 [DOI] [PubMed] [Google Scholar]
- 90.Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring). 2010; 18: 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sartor F, Donaldson LF, Markland DA, Loveday H, Jackson MJ, et al. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite. 2011; 57: 237–246. doi: 10.1016/j.appet.2011.05.107 [DOI] [PubMed] [Google Scholar]
- 92.Feeney EL, O'Brien SA, Scannell AG, Markey A, Gibney ER Suprathreshold measures of taste perception in children—Association with dietary quality and body weight. Appetite. 2017; 113: 116–123. doi: 10.1016/j.appet.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 93.Rawal S, Huedo-Medina TB, Hoffman HJ, Swede H, Duffy VB Structural equation modeling of associations among taste-related risk factors, taste functioning, and adiposity. Obesity (Silver Spring). 2017; 25: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Donaldson LF, Bennett L, Baic S, Melichar JK Taste and weight: is there a link? Am J Clin Nutr. 2009; 90: 800S–803S. doi: 10.3945/ajcn.2009.27462Q [DOI] [PubMed] [Google Scholar]
- 95.Fernandez-Garcia JC, Alcaide J, Santiago-Fernandez C, Roca-Rodriguez MM, Aguera Z, et al. An increase in visceral fat is associated with a decrease in the taste and olfactory capacity. PLoS One. 2017; 12: e0171204 doi: 10.1371/journal.pone.0171204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tucker RM, Kaiser KA, Parman MA, George BJ, Allison DB, et al. Comparisons of Fatty Acid Taste Detection Thresholds in People Who Are Lean vs. Overweight or Obese: A Systematic Review and Meta-Analysis. PLoS One. 2017; 12: e0169583 doi: 10.1371/journal.pone.0169583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tucker RM, Nuessle TM, Garneau NL, Smutzer G, Mattes RD No Difference in Perceived Intensity of Linoleic Acid in the Oral Cavity between Obese and Nonobese Individuals. Chem Senses. 2015; 40: 557–563. doi: 10.1093/chemse/bjv040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barbarossa IT, Carta G, Murru E, Melis M, Zonza A, et al. Taste sensitivity to 6-n-propylthiouracil is associated with endocannabinoid plasma levels in normal-weight individuals. Nutrition. 2013; 29: 531–536. doi: 10.1016/j.nut.2012.09.018 [DOI] [PubMed] [Google Scholar]
- 99.Carta G, Melis M, Pintus S, Pintus P, Piras CA, et al. Participants with Normal Weight or with Obesity Show Different Relationships of 6-n-Propylthiouracil (PROP) Taster Status with BMI and Plasma Endocannabinoids. Sci Rep. 2017; 7: 1361 doi: 10.1038/s41598-017-01562-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tepper BJ, Melis M, Koelliker Y, Gasparini P, Ahijevych KL, et al. Factors Influencing the Phenotypic Characterization of the Oral Marker, PROP. Nutrients. 2017; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tepper BJ Nutritional implications of genetic taste variation: the role of PROP sensitivity and other taste phenotypes. Annu Rev Nutr. 2008; 28: 367–388. doi: 10.1146/annurev.nutr.28.061807.155458 [DOI] [PubMed] [Google Scholar]
- 102.Tepper BJ, White EA, Koelliker Y, Lanzara C, d'Adamo P, et al. Genetic variation in taste sensitivity to 6-n-propylthiouracil and its relationship to taste perception and food selection. Ann N Y Acad Sci. 2009; 1170: 126–139. doi: 10.1111/j.1749-6632.2009.03916.x [DOI] [PubMed] [Google Scholar]
- 103.Tepper BJ, Banni S, Melis M, Crnjar R, Tomassini Barbarossa I Genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) and its association with physiological mechanisms controlling body mass index (BMI). Nutrients. 2014; 6: 3363–3381. doi: 10.3390/nu6093363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keller KL, Olsen A, Cravener TL, Bloom R, Chung WK, et al. Bitter taste phenotype and body weight predict children's selection of sweet and savory foods at a palatable test-meal. Appetite. 2014; 77: 113–121. doi: 10.1016/j.appet.2014.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spielman AI Interaction of saliva and taste. J Dent Res. 1990; 69: 838–843. doi: 10.1177/00220345900690030101 [DOI] [PubMed] [Google Scholar]
- 106.Ahijevych K, Tepper BJ, Graham MC, Holloman C, Matcham WA Relationships of PROP Taste Phenotype, Taste Receptor Genotype, and Oral Nicotine Replacement Use. Nicotine Tob Res. 2015; 17: 1149–1155. doi: 10.1093/ntr/ntu281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leksrisompong P, Gerard P, Lopetcharat K, Drake M Bitter taste inhibiting agents for whey protein hydrolysate and whey protein hydrolysate beverages. J Food Sci. 2012; 77: S282–287. doi: 10.1111/j.1750-3841.2012.02800.x [DOI] [PubMed] [Google Scholar]
- 108.Ogawa T, Hoshina K, Haginaka J, Honda C, Tanimoto T, et al. Screening of bitterness-suppressing agents for quinine: the use of molecularly imprinted polymers. J Pharm Sci. 2005; 94: 353–362. doi: 10.1002/jps.20248 [DOI] [PubMed] [Google Scholar]
- 109.Love-Gregory L, Sherva R, Schappe T, Qi JS, McCrea J, et al. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum Mol Genet. 2011; 20: 193–201. doi: 10.1093/hmg/ddq449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ghosh A, Murugesan G, Chen K, Zhang L, Wang Q, et al. Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. 2011; 117: 6355–6366. doi: 10.1182/blood-2011-02-338582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Strittmatter EF, Williams ER Structures of Protonated Arginine Dimer and Bradykinin Investigated by Density Functional Theory: Further Support for Stable Gas-Phase Salt Bridges. J Phys Chem A. 2000; 104: 6069–6076. doi: 10.1021/jp000038y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.André I, Linse S, Mulder FAA Residue-Specific pKa Determination of Lysine and Arginine Side Chains by Indirect 15N and 13C NMR Spectroscopy: Application to apo Calmodulin. J Am Chem Soc 2007; 129: 15805–15813. doi: 10.1021/ja0721824 [DOI] [PubMed] [Google Scholar]
- 113.Yoo J, Cui Q Does arginine remain protonated in the lipid membrane? Insights from microscopic pK(a) calculations. Biophys J. 2008; 94: L61–L63. doi: 10.1529/biophysj.107.122945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Salentinig S, Sagalowicz L, Glatter O Self-assembled structures and pKa value of oleic acid in systems of biological relevance. Langmuir. 2010; 26: 11670–11679. doi: 10.1021/la101012a [DOI] [PubMed] [Google Scholar]
- 115.Cistola DP, Small DM, Hamilton JA Carbon 13 NMR studies of saturated fatty acids bound to bovine serum albumin. I. The filling of individual fatty acid binding sites. J Biol Chem. 1987; 262: 10971–10979. [PubMed] [Google Scholar]
- 116.Cistola DP, Hamilton JA, Jackson D, Small DM Ionization and phase behavior of fatty acids in water: application of the Gibbs phase rule. Biochemistry. 1988; 27: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 117.Kanicky JR, Shah DO Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J Colloid Interface Sci. 2002; 256: 201–207. [DOI] [PubMed] [Google Scholar]
- 118.Saraswathi NT, Roy S, Vijayan M X-ray studies on crystalline complexes involving amino acids and peptides. XLI. Commonalities in aggregation and conformation revealed by the crystal structures of the pimelic acid complexes of L-arginine and DL-lysine. Acta Crystallogr B. 2003; 59: 641–646. [DOI] [PubMed] [Google Scholar]
- 119.Dennington R, Keith T, Millam J Semichem Inc., Shawnee Mission KS, GaussView, Version 5. 2009.
- 120.Galindo MM, Voigt N, Stein J, van Lengerich J, Raguse JD, et al. G protein-coupled receptors in human fat taste perception. Chem Senses. 2012; 37: 123–139. doi: 10.1093/chemse/bjr069 [DOI] [PubMed] [Google Scholar]
- 121.Tsuruta M, Kawada T, Fukuwatari T, Fushiki T The orosensory recognition of long-chain fatty acids in rats. Physiol Behav. 1999; 66: 285–288. [DOI] [PubMed] [Google Scholar]
- 122.Sette S, Le Donne C, Piccinelli R, Arcella D, Turrini A, et al. The third Italian National Food Consumption Survey, INRAN-SCAI 2005-06—part 1: nutrient intakes in Italy. Nutr Metab Cardiovasc Dis. 2011; 21: 922–932. doi: 10.1016/j.numecd.2010.03.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values are means ± SEM. n = 46. Three-way ANOVA was used to compare PROP bitterness intensity ratings with NaCl saltiness intensity ratings across groups (F(4,258) = 5.199; p = 0.00048). * Significant difference between PROP and the corresponding NaCl concentration (p < 0.0015; Newman Keuls test).
(PDF)
(PDF)
(PDF)
Results of the TaqMan® SNP Genotyping Assay.
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.