Abstract
Zinc is an essential trace element that is required for the function of a large number of proteins. As these zinc-binding proteins are found within the cytosol and organelles, all eukaryotes require mechanisms to ensure that zinc is delivered to organelles, even under conditions of zinc deficiency. Although many zinc transporters belonging to the Cation Diffusion Facilitator (CDF) families have well characterized roles in transporting zinc into the lumens of intracellular compartments, relatively little is known about the mechanisms that maintain organelle zinc homeostasis. The fission yeast Schizosaccharomyces pombe is a useful model system to study organelle zinc homeostasis as it expresses three CDF family members that transport zinc out of the cytosol into intracellular compartments: Zhf1, Cis4, and Zrg17. Zhf1 transports zinc into the endoplasmic reticulum, and Cis4 and Zrg17 form a heterodimeric complex that transports zinc into the cis-Golgi. Here we have used the high and low affinity ZapCY zinc-responsive FRET sensors to examine cytosolic zinc levels in yeast mutants that lack each of these CDF proteins. We find that deletion of cis4 or zrg17 leads to higher levels of zinc accumulating in the cytosol under conditions of zinc deficiency, whereas deletion of zhf1 results in zinc accumulating in the cytosol when zinc is not limiting. We also show that the expression of cis4, zrg17, and zhf1 is independent of cellular zinc status. Taken together our results suggest that the Cis4/Zrg17 complex is necessary for zinc transport out of the cytosol under conditions of zinc-deficiency, while Zhf1 plays the dominant role in removing zinc from the cytosol when labile zinc is present. We propose that the properties and/or activities of individual CDF family members are fine-tuned to enable cells to control the flux of zinc out of the cytosol over a broad range of environmental zinc stress.
Author summary
All organisms require homeostasis mechanisms to maintain sufficient levels of zinc for normal cell metabolism and to avoid toxicity. As zinc-binding proteins are located in the cytosol and within intracellular compartments, all cells have to balance intracellular zinc ion distribution so that there are sufficient, but non toxic levels of zinc in the cytosol as well as organelles. Although much is known about the mechanisms that control cytosolic zinc levels, relatively little is known about the mechanisms that maintain organelle zinc homeostasis. As proteins belonging to the CDF family transport zinc into organelles, here we used a fission yeast model system to determine if the expression or function of zinc transporters belonging to this family was regulated by zinc. We find that two CDF family members, Cis4 and Zrg17, facilitate the transport of zinc out of the cytosol of zinc-deficient cells, whereas the CDF family member Zhf1 preferentially transports zinc out of the cytosol when zinc is not limiting. As the expression of the genes encoding these transport proteins is not regulated by zinc, the results suggest that different CDF family members have complementary roles in transporting zinc out of the cytosol that are independent of changes in transcription. These results provide new insights into the regulatory mechanisms that control cytosolic and organelle zinc homeostasis.
Introduction
Zinc is an essential trace metal that is required for the structure and activity of a large number of proteins. In eukaryotes these proteins include transcription factors containing structural domains stabilized by zinc ions, such as the C2H2-type and C4-type zinc fingers [1]. Zinc is also a cofactor for many enzymes that are located in the cytosol (e.g. alcohol dehydrogenase 1), and in subcellular compartments such as the nucleus (e.g. RNA polymerases), mitochondria (e.g. cytochrome c oxidase), and endoplasmic reticulum (e.g. calreticulin) [2–4]. Due to the essential nature of some of these proteins, all organisms are challenged with obtaining sufficient levels of zinc for incorporation into newly synthesized proteins. A further complicating factor is that excessive levels of zinc are toxic to cells. As a consequence, zinc acquisition, compartmentalization, storage, and efflux need to be tightly regulated to maintain zinc at a level that is sufficient, but not toxic to cell metabolism.
In many organisms zinc-responsive transcription factors maintain zinc homeostasis by controlling the expression of genes that are required for the transport of zinc into and out of the cytosol. In eukaryotes these zinc transport proteins commonly belong to either the Zrt- Irt- like protein family (ZIP) or CDF family. Members of the ZIP family typically facilitate zinc uptake or the release of zinc from intracellular stores, whereas the CDF family members usually transport zinc into the lumens of intracellular compartments or out of a cell [5]. As zinc transport by a ZIP family member typically results in an increase in cytosol zinc levels, the expression of genes encoding ZIP family members is often up-regulated when zinc is limiting [6]. As an example, in Saccharomyces cerevisiae the transcriptional activator Zap1 controls the expression of genes encoding ZIP family members required for zinc uptake (Zrt1 and Zrt2) and release of zinc from the vacuolar stores (Zrt3) [7]. As Zap1 is active in zinc-limited cells and is inactive when zinc is in excess, the expression of ZRT1-3 increases when cells need zinc. Importantly, as zinc transport into the cytosol by the ZIP proteins inactivates Zap1, a negative feedback loop is created that prevents zinc from reaching toxic levels.
Negative feedback circuits also control the expression of CDF family members. In humans, the metal-responsive transcription factor 1 (MTF-1) regulates the expression of ZnT1, an essential CDF family member that is required for zinc efflux from cells [8]. MTF-1 is activated by excess zinc in the cytosol, which in turn transcriptionally induces ZnT1 expression when zinc is high. Similarly, when dietary zinc levels are high in the nematode Caenorhabditis elegans, the high zinc-responsive factor 1 (HIZR-1) induces the expression of CDF family members required for the excretion of zinc from intestinal cells (ttm-1b) and storage of zinc in intestinal gut granules (cdf-2) [9]. As the end result of these transcriptional changes is a reduction in cytosolic zinc levels, thereby inactivating MTF-1 and HIZR-1, a negative feedback loop is created that prevents the cytosol from being depleted of zinc.
Although a number of genes encoding transporters required for zinc uptake, storage, and efflux are subject to negative feedback control, the expression of some CDF family members increases in zinc-limited cells. For example, Zrg17 and Msc2 are two CDF family members from S. cerevisiae that form a heterodimeric complex that transports zinc into the endoplasmic reticulum [10]. Although this complex transports zinc out of the cytosol, ZRG17 is a Zap1-target gene that is expressed at higher levels in zinc-deficient cells [11]. While this regulation at first seems counterintuitive, as it would further deplete zinc from the cytosol, the induction of ZRG17 by Zap1 is critical for preventing the unfolding of proteins in the endoplasmic reticulum under this condition [11]. As zinc transport by the Zrg17/Msc2 complex would also further increase Zap1 activity, the zinc-dependent regulation of ZRG17 presumably results in a positive feedback circuit to supply zinc to compartmentalized proteins when the cytosol is limited for zinc.
The regulation of ZRG17 by Zap1 illustrates a mechanism of how zinc can be supplied to an intracellular compartment in a zinc-limited environment. As few other studies have examined the regulatory circuits that maintain zinc levels in organelles during periods of zinc starvation, the goal of this work was to determine if related mechanisms were present in the distantly related yeast S. pombe. We chose to use S. pombe because multiple aspects of zinc homeostasis differ between fission and budding yeast. These differences include the transcription factor used to control zinc homeostasis (Loz1 vs. Zap1), the primary site for the storage of excess zinc (endoplasmic reticulum vs. vacuole), and the presence of metallothioneins that preferentially bind divalent metal ions such as zinc (Zym1 from S. pombe) or monovalent ions such as copper (Cup1 from S. cerevisiae) [12–16]. Another difference between fission and budding yeasts is the subcellular localization of zinc transporters within the secretory pathway. In S. cerevisiae two CDF family members, Zrc1 and Cot1, transport zinc into the vacuole [17]. In S. pombe, the homolog of Zrc1, named Zhf1, transports zinc into the endoplasmic reticulum, and the homologs of Msc2 and Zrg17 (named Cis4 and Zrg17 respectively) form a complex that localizes to the cis-Golgi [18, 19]. The biological significance of these differences in subcellular localization of the CDF family members between budding and fission yeasts is currently unclear.
To gain a better understanding of the mechanisms that control the supply of zinc to organelles, we used multiple genetic approaches to determine the extent to which three CDF family members from S. pombe (Zhf1, Zrg17, and Cis4) facilitate zinc transport out of the cytosol under conditions of zinc deficiency and zinc excess. We found that deletion of zhf1 results in a strong growth defect when zinc is in excess and that deletion of zrg17 or cis4 leads to a mild growth defect in the presence of the zinc chelator EDTA. These latter results suggest that the Cis4/Zrg17 complex may play an important role under zinc deficiency conditions. To further investigate whether transport via Cis4 and Zrg17 is affected by cellular zinc status we developed methods to monitor changes in cytosolic zinc availability in fission yeast. These analyses revealed that that cis4Δ and zrg17Δ cells accumulate higher levels of zinc in the cytosol under conditions of zinc deficiency, while zhf1Δ cells accumulate higher levels of zinc in the cytosol when zinc is not limiting. We also show that the transcription of zhf1, cis4, and zrg17 genes is not dependent upon zinc. These results reveal that different CDF family members have complementary roles in transporting zinc out of the cytosol. They also suggest that either the activities or the properties of different CDF family members are fine-tuned to transport zinc out of the cytosol under different environmental zinc stresses.
Results
Zinc-dependent phenotypes of zinc homeostasis mutants
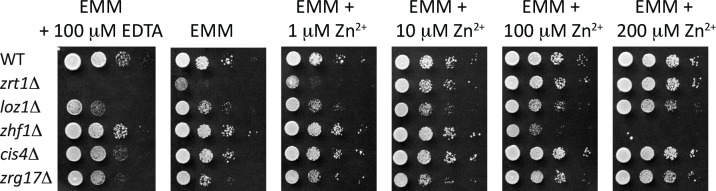
Three members of the CDF family transport zinc into the secretory pathway in fission yeast: Zhf1, Cis4, and Zrg17 [13, 15, 18, 19]. Although previous studies have shown that Zhf1 is required for growth in the presence of high zinc, relatively little was known about the roles of Cis4 and Zrg17 in zinc homeostasis. To determine if Cis4 and Zrg17 were necessary for growth under low or high zinc conditions, serial dilutions of cis4Δ and zrg17Δ cells were plated onto zinc-limiting (EMM + 100 μM EDTA) or zinc-replete medium (EMM + 0–200 μM zinc) (Fig 1). Cells lacking the Zrt1 or Zhf1, which are required for survival during zinc deficiency or zinc toxicity respectively [18], were also plated as controls. In the presence of 100 μM EDTA, cis4Δ cells exhibited a slight growth defect relative to the wild-type. zrg17Δ cells had a modest growth defect under all conditions, but grew more slowly in the presence of EDTA relative to cis4Δ. As Cis4 and Zrg17 form a heteromeric complex, these results are consistent with Cis4 and Zrg17 playing an important role in supplying zinc to the secretory pathway when zinc is limiting. The slower growth of zrg17Δ relative to cis4Δ also suggests that Zrg17 may have additional functions that are independent of Cis4 and zinc.
Fig 1. Growth phenotypes of yeast mutants with impaired zinc homeostasis.
10-fold serial dilutions of the indicated strains were plated onto Edinburgh Minimal Medium (EMM) supplemented with 100 μM EDTA or the indicated level of Zn2+. Plates were incubated for 3–5 days at 31°C before photography.
The ZapCY1 and ZapCY2 zinc-responsive FRET sensors detect dynamic changes in the labile intracellular zinc pool
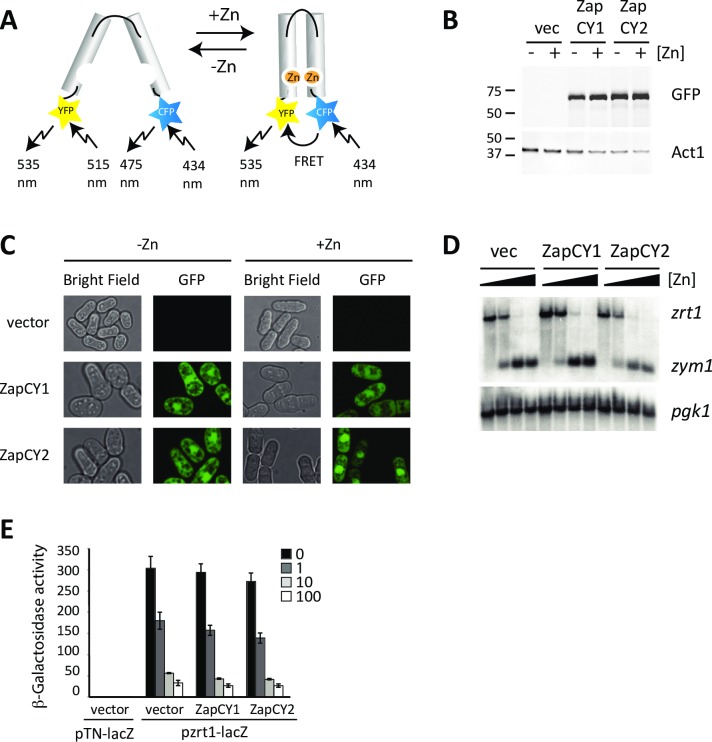
To determine if Cis4 and Zrg17 were required for zinc transport out of the cytosol of zinc-limited cells, we developed constructs to express the genetically encoded ZapCY1 and ZapCY2 zinc-responsive FRET sensors in the cytosol of fission yeast. The ZapCY1/2 sensors have been widely used to monitor dynamic changes in intracellular zinc levels [20]. Both sensors contain the regulatory zinc fingers 1 and 2 from the transcription factor Zap1, flanked by citrine YFP, hereafter referred to as YFP, and eCFP (Fig 2A). In zinc-limited environments, the Zap1 zinc finger domain 1 and 2 are largely unstructured. However, in the presence of zinc, the zinc finger domains fold together to form a single structural unit [21]. As this closed conformation brings together YFP and eCFP increasing FRET, the FRET signal in cells expressing ZapCY1 and ZapCY2 is directly coupled to intracellular zinc availability. Previous studies have shown that ZapCY1 binds zinc in vitro with an apparent dissociation constant of ~ 2.5 pM, while ZapCY2 contains substitutions within the zinc finger domains, which result in it binding zinc with an ~ 300 fold lower affinity [20].
Fig 2. Expression of the ZapCY1 and ZapCY2 FRET reporters in fission yeast.
(A) Schematic diagram of the ZapCY1 FRET sensor. Zinc fingers are shown as gray cylinders, and YFP and CFP are shown as yellow and blue colored stars. (B) Wild-type cells expressing ZapCY1, ZapCY2, or the empty vector were grown overnight in ZL-EMM without (-Zn) or with a 100 μM Zn2+ supplement (+Zn). Cells were collected and crude protein extracts prepped for immunoblot analysis. Immunoblots were probed with antibodies raised against GFP and the loading control Act1 (Actin). A protein ladder with sizes in kDa is shown on the left (C) Wild-type cells expressing the indicated reporters or the vector were grown in ZL-EMM (-Zn) or ZL-EMM + 100 μM Zn2+ (+Zn) and were analyzed by fluorescence microscopy (GFP). Bright field images are also shown. (D) Wild-type cells expressing ZapCY1, ZapCY2, or an empty vector (vec) were grown in ZL-EMM supplemented with 0, 1, 10, or 100 μM Zn2+ and total RNA purified for RNA blot analysis. RNA blots were probed for the zinc-regulated zrt1 and zym1 transcripts, and loading control pgk1. (E) β-galactosidase activity was measured in wild-type cells co-expressing a zrt1-lacZ reporter with the empty vector, ZapCY1, or ZapCY2. Cells were grown as described in panel D. Each bar shows the average value from three independent experiments and error bars show the standard deviations.
To determine if the ZapCY1 and ZapCY2 FRET reporters were stably produced in S. pombe, strains expressing ZapCY1 and ZapCY2 from the constitutive pgk1 promoter were grown overnight in ZL-EMM, and the levels of each sensor examined by immunoblotting. Both reporters accumulated to similar levels in zinc-limited and zinc-replete cells indicating that their stability was not affected by zinc (Fig 2B). We also assessed the subcellular localization of each reporter in response to cellular zinc status using fluorescent microscopy. As shown in Fig 2C, there was strong fluorescence in cells expressing ZapCY1 or ZapCY2, which was absent from cells transformed with the empty vector. The ZapCY1 and ZapCY2 proteins were both localized to the cytosol and nucleus, and were excluded from the vacuole. For unknown reasons, higher levels of the ZapCY2 reporter accumulated in the nucleus of zinc-replete cells.
A potential concern with using ZapCY1 and ZapCY2 to assess alterations in the labile pools of zinc in yeast is that both sensors bind zinc ions, which in turn might reduce the levels of zinc that are normally available for cellular metabolism. In previous studies we have shown that the expression of the Loz1 target genes zrt1 and zym1 is dependent upon intracellular zinc levels [22]. Specifically, zrt1 is expressed in zinc-limited cells and zym1 is expressed in zinc-replete cells. We therefore predicted that zrt1 and zym1 expression would be altered if the ZapCY1/2 FRET sensors interfere with zinc homeostasis. As shown in Fig 2D, the introduction of the ZapCY1/2 FRET sensors had no major effect on zym1 and zrt1 mRNA levels. In addition, when the FRET reporters were co-expressed with a zrt1-lacZ reporter, there were no differences in β-galactosidase activity when compared to cells expressing the vector (Fig 2E). Taken together the above results show that the ZapCY1/2 FRET sensors accumulate within the cytosol and nucleus of cells without any substantial effect on zinc homeostasis.
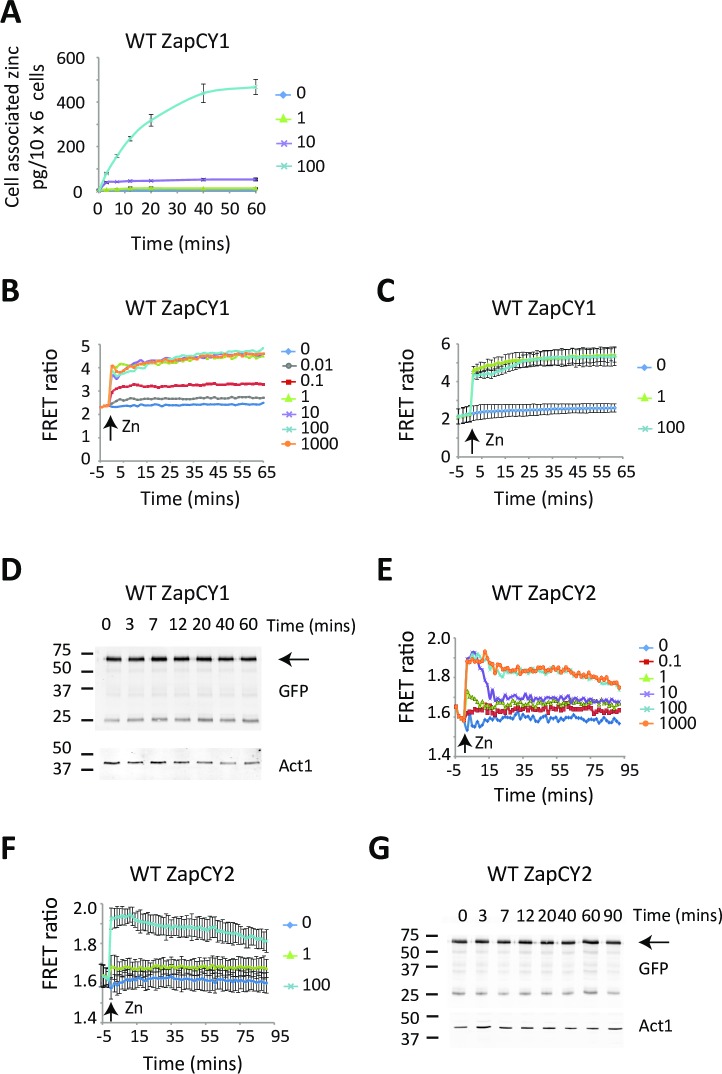
To determine if the ZapCY1 and ZapCY2 FRET sensors are able to detect dynamic changes in the labile pool of zinc in fission yeast, we measured the activity of each reporter in vivo following a ‘zinc shock’. In a zinc shock experiment cells are initially depleted of zinc by growing overnight in ZL-EMM, which leads to increased expression of zrt1 and high levels of Zrt1 on the plasma membrane. As zinc-limited cells are primed and ready to uptake zinc, zinc rapidly enters cells in a dose-dependent manner when it is added to the growth medium (Fig 3A). To examine the response of the high affinity FRET sensor to zinc shock, wild-type cells expressing ZapCY1 were grown overnight in ZL-EMM before being transferred to temperature-controlled microplate wells. Cells were excited at 434 nm and a FRET ratio calculated by dividing the intensity of the emission at 535 nm by the emission at 475 nm. The growth overnight in ZL-EMM resulted in a FRET ratio of 2.29 +/- 0.20 (Fig 3B and 3C, t = -5 min). This ratio remained constant until the addition of zinc, which led to a rapid increase in FRET in a dose-dependent manner (Fig 3B, 0.01–1 μM zinc). These changes in FRET occurred without affecting the stability of ZapCY1 (Fig 3D), consistent with the ZapCY1 sensor binding zinc and forming the closed conformation which brings YFP and CFP closer together. Zinc shocks with higher levels of zinc (10–1000 μM Zn2+) also led to a rapid increase in the FRET ratio (Fig 3B and 3C). However, the magnitude of the response was similar to that seen following a zinc shock with 1 μM zinc. We conclude from these results that a zinc shock with 1 μM Zn2+ results in sufficient levels of zinc accumulating within the cytosol and nucleus of cells to saturate the high affinity ZapCY1 sensor.
Fig 3. FRET responses of wild-type cells expressing ZapCY1 or ZapCY2 during zinc shock.
(A) Wild-type cells expressing ZapCY1 were grown overnight in ZL-EMM. At t = 0 cells were exposed to 0, 1, 10, or 100 μM. Cells were harvested at the indicated time points and total cellular zinc measured using Atomic Absorption Spectroscopy. The final concentration of zinc/cell was calculated by comparing values to a standard curve. (B and C) Wild-type cells expressing ZapCY1 were grown overnight in ZL-EMM. Cells were transferred to temperature-controlled cuvettes and were assayed for FRET by spectrofluorometry. At t = 0 cells were shocked with 0–1000 μM Zn2+ and the changes in FRET monitored over time. The FRET ratio was determined by dividing the FRET emission at 535 nm by the eCFP emission at 475 nm following excitation of samples at 434 nm. Panel B shows a representative experiment and panel C shows the average values from 3 independent experiments with error bars representing standard deviations. (D) Wild-type cells expressing ZapCY1 were grown overnight in ZL-EMM. At t = 0 cells were shocked with 100 μM Zn2+. At the indicated time point cells were harvested and crude protein extracts prepped for immunoblot analysis. Immunoblots were incubated with antibodies to GFP and loading control Act1. (E and F) Wild-type cells expressing ZapCY2 were grown overnight in ZL-EMM. At t = 0 cells were shocked with 0–1000 μM Zn2+ and the FRET response measured as described in panel B. Panel E shows a representative experiment and panel F shows the average values from 3 independent experiments with error bars representing standard deviations. (G) Wild-type cells expressing ZapCY2 were grown overnight in ZL-EMM. At t = 0 cells were shocked with 10 μM Zn2+. Crude protein extracts were then prepped for immunoblot analysis as described in panel D.
To assess the effects of a zinc shock on the low affinity reporter, similar experiments were performed with wild-type cells expressing ZapCY2. In these cells, growth overnight in ZL-EMM resulted in an initial FRET ratio of 1.57 +/- 0.05 (Fig 3E and 3F, t = 0 min). As ZapCY2 binds zinc with a lower affinity than ZapCY1, we predicted that higher levels of zinc would be needed to saturate ZapCY2. Consistent with this hypothesis, no significant increase in FRET was observed following a zinc shock with 0.1 μM Zn2+ and a modest response was seen with 1 μM Zn2+. A rapid increase in the FRET ratio to 1.92 +/- 0.03 was detected following a zinc shock with 10 μM Zn2+ (Fig 3E, t = 2 min). However, this FRET signal subsequently decreased until it reached a more constant ratio of ~ 1.75 after 15 minutes (Fig 3E, t = 15–90 min). As the zinc shock did not affect the stability of ZapCY2 (Fig 3G), these results are consistent with ZapCY2 rapidly binding zinc, and then zinc being lost to higher affinity zinc binding sites in the surrounding environment. Zinc shocks with higher levels of zinc (Fig 3E and 3F, 100 μM zinc) were sufficient to saturate the FRET sensor, but in contrast to ZapCY1, the FRET ratio slowly decreased with time. Thus, the ZapCY1 and ZapCY2 sensors are both able to detect changes in cytosolic zinc levels. However, higher levels of zinc are necessary to saturate ZapCY2 and the zinc bound to ZapCY2 is more readily lost to the surrounding environment.
The FRET signal in cells expressing ZapCY1 and ZapCY2 is dependent upon zrt1 expression
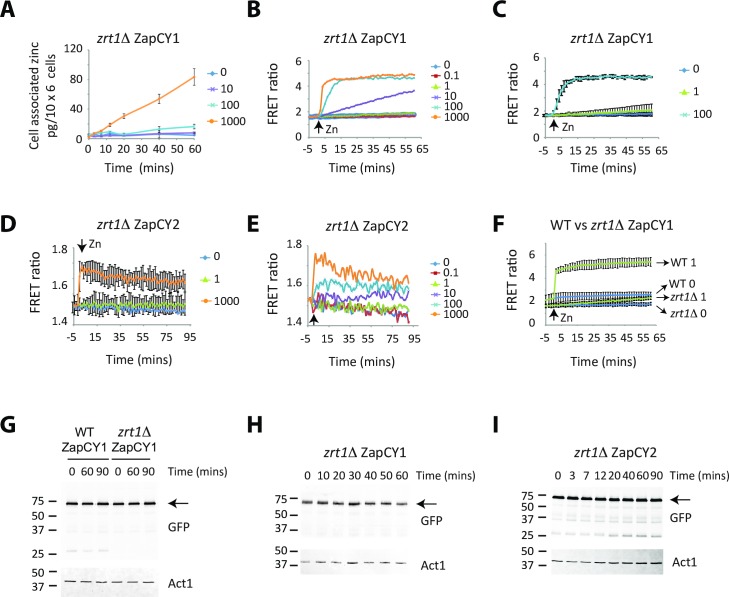
To gain further evidence that the sensors were measuring changes in cytosolic zinc levels, ZapCY1 and ZapCY2 were introduced into cells lacking zrt1. In the absence of Zrt1, higher levels of zinc are needed during a zinc shock experiment to see an increase in total cellular zinc because cells rely on low affinity systems for zinc uptake (compare Fig 3A to Fig 4A). We therefore predicted that higher levels of zinc would be necessary to saturate ZapCY1 and ZapCY2 in zrt1Δ. Consistent with this prediction, higher levels of zinc were required to obtain a maximal FRET ratio change in zrt1Δ cells compared to the wild-type (Fig 4B–4E). As an example, a zinc shock with 1 μM zinc was sufficient to saturate ZapCY1 in the wild-type, but did not affect the activity of the ZapCY1 reporter in zrt1Δ (Fig 4F). The differences in FRET response were a result of loss of zrt1, as both reporters were expressed at similar levels to the wild-type, and a zinc shock had no effect on the stability of either reporter (Fig 4G–4I). Together, these results indicate that the FRET signal in cells is dependent upon the expression of zrt1 and also is consistent with the activity of both reporters being directly regulated by cytosolic zinc levels.
Fig 4. zrt1Δ cells accumulate lower levels of zinc in the cytosol.
(A) zrt1Δ cells expressing ZapCY1 were grown overnight in ZL-EMM. At t = 0 cells were exposed to 0–1000 μM. Total cellular zinc was then determined by AAS as described for Fig 3A. (B-F) zrt1Δ cells expressing ZapCY1 or ZapCY2 were grown overnight in ZL-EMM. Cells were transferred to temperature-controlled cuvettes and at t = 0 shocked with 0–1000 μM Zn2+. Changes in the FRET ratio were measured as described in Fig 3B. Panels B and D show representative experiments and panels C and E show the average values from 3 independent experiments with error bars representing standard deviations. Panel F shows a comparison of a 1 μM zinc shock in wild-type and zrt1Δ cells expressing ZapCY1 (G-I) Wild-type and zrt1Δ cells expressing ZapCY1 or ZapCY2 were grown overnight in ZL-EMM. At t = 0 cells were shocked with 100 μM Zn2+. Cells were harvested at the indicated time points and crude protein extracts prepped for immunoblot analysis. Immunoblots were incubated with antibodies to GFP and loading control Act1.
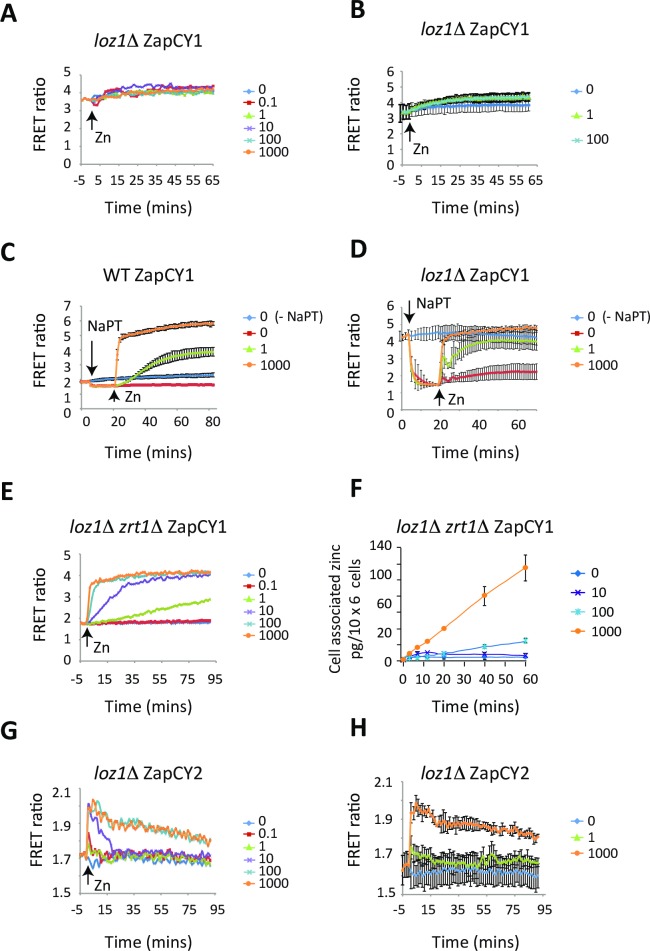
In our previous studies we found that genetic mutations that disrupt Loz1 function result in the constitutive de-repression of zrt1 transcription, leading to increased expression levels. Therefore, to assess the effects of overexpression of zrt1 on cytosolic zinc levels, ZapCY1 and ZapCY2 were introduced in loz1Δ cells and the FRET response was measured during a zinc shock experiment. Following the growth of loz1Δ ZapCY1 cells overnight in ZL-EMM, an initial FRET ratio of 3.3 +/- 0.5 was detected (Fig 5A and 5B), which is higher than the initial FRET ratio in cells expressing Loz1. Further, only a minor increase in FRET was seen following a zinc shock with 0.1–1000 μM zinc. As loz1Δ cells constitutively express zrt1, one explanation for the high initial FRET ratio in this mutant is that they have higher levels of zinc uptake leading to the saturation of ZapCY1. It was also possible that the ZapCY1 reporter was unable to respond to zinc in this genetic background. To distinguish between these possibilities, we used sodium pyrithione (NaPT) to artificially lower cytosolic zinc levels. Pyrithione is a membrane permeable ionophore that readily forms complex with zinc [23]. When 50 μM NaPT was added to wild-type ZapCY1 cells grown overnight in ZL-EMM, a small decrease in the FRET ratio consistent with this molecule binding or releasing accessible zinc within the cytosol (Fig 5C). Importantly, a rapid increase in FRET was seen when zinc was added to the NaPT treated cells. When a similar experiment was performed with loz1Δ cells, a large decrease in the FRET ratio was seen upon the addition of NaPT, which could be reversed by the addition of zinc (Fig 5D). These results indicate that the ZapCY1 reporter is functional in loz1Δ cells, and suggest that in the absence of strong chelators and ionophores it is saturated with zinc under all conditions.
Fig 5. loz1Δ cells accumulate higher levels of zinc in the cytosol under zinc-limiting conditions.
(A and B) loz1Δ cells expressing ZapCY1 were grown overnight in ZL-EMM and cells were transferred to temperature-controlled cuvettes. At t = 0 cells were shocked with 0–1000 μM Zn2+ and changes in the FRET ratio measured as described in Fig 3B. Panel A shows a representative experiment and panel B shows the average values from 3 independent experiments with error bars representing standard deviations. (C and D) Wild-type and loz1Δ cells expressing ZapCY1 were grown overnight in ZL-EMM. Cells were transferred to temperature-controlled cuvettes and at the indicated times were exposed to +/- 50 μM NaPT or 0, 1, or 1000 μM Zn2+. Changes in the FRET ratio were determined as described in Fig 3B. Results show the average values from 3 independent experiments with error bars representing standard deviations. (E) loz1Δ zrt1Δ cells expressing ZapCY1 were grown and subject to zinc shock as described in Fig 3B. A representative experiment is shown. (F) loz1Δ zrt1Δ cells were grown overnight in ZL-EMM. At t = 0 cells were exposed to 0–1000 μM. Total cellular zinc was then determined by AAS as described for Fig 3A. (G and H) loz1Δ cells expressing ZapCY2 were grown overnight in ZL-EMM. Cells were shocked with 0–1000 μM Zn2+ and changes in the FRET ratio measured as described in Fig 3B. Panel G shows a representative experiment and panel H shows the average values from 3 independent experiments with error bars representing standard deviations.
To test whether the saturation of the ZapCY1 reporter in loz1Δ cells was a result of high zrt1 expression, we examined the activity of the ZapCY1 reporter in double mutants lacking loz1 and zrt1. In these cells a low FRET ratio of 1.8 +/- 0.2 was detected following growth overnight in ZL-EMM (Fig 5E). These results suggest that the high expression of zrt1 significantly contributes to the saturation of ZapCY1 in loz1Δ cells. We also noted that higher levels of total zinc accumulated in loz1Δ zrt1Δ when compared to zrt1Δ following a zinc shock (Fig 5F). These results suggest that Loz1 controls the expression of a second lower affinity zinc uptake system and/or regulates the expression of other genes that affect cytosolic zinc availability. Consistent with this hypothesis, a zinc shock with 1 μM zinc did not result in an increased FRET ratio in zrt1Δ, but did lead to a slow increase in FRET in loz1Δ zrt1Δ (compare Figs 4B and 5E). Similarly, the ZapCY1 reporter was close to saturation after a 30 min zinc shock with 10 μM Zn in loz1Δ zrt1Δ cells, and yet in zrt1Δ, a zinc shock with 10 μM Zn zinc only led to a slow gradual increase in FRET over 60 min.
As loz1Δ cells accumulate higher levels of zinc within the cytosol, we also assessed the effects of this allele on the response of the low affinity ZapCY2 reporter. In contrast to the response of ZapCY1, the basal FRET ratio in zinc-limited loz1Δ ZapCY2 cells was similar to the wild-type (compare Figs 3F to 5H). The responses of the ZapCY2 reporter to zinc also resembled those of the wild-type (Fig 5G and 5H). Thus, under conditions of zinc deficiency, loz1Δ cells accumulate higher levels of zinc in the cytosol/nucleus relative to the wild-type. However, when zinc is not limiting in these cells it is effectively buffered and/or transported out of the cytosol.
Cis4 and Zrg17 play a central role in the transport of zinc out of the cytosol during zinc deficiency
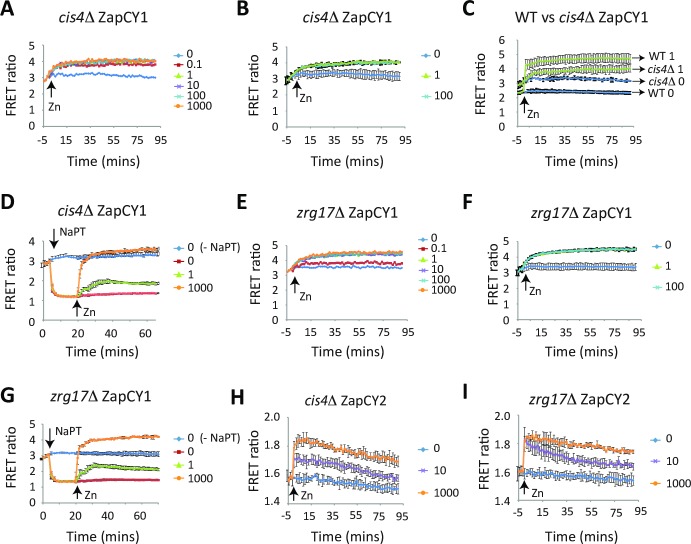
The above results show that the ZapCY1 and ZapCY2 sensors can be used in S. pombe to measure dynamic changes in the levels of labile zinc in the cytosol and nucleus. As deletion of cis4 or zrg17 resulted in a growth defect on low zinc medium, we used these sensors to test whether Cis4 and Zrg17 were necessary for zinc transport out of the cell under this condition. When a zinc shock experiment was performed with cis4Δ ZapCY1 cells, the starting FRET ratio was higher than that observed in the wild-type. Additionally, only a small increase in the FRET ratio was seen with 0.1 μM zinc (from 2.8 +/- 0.23 to 4.0 +/-0.2) and also when cells were shocked with higher levels of zinc (1–1000 μM) (Fig 6A–6C). In contrast, the addition of NaPT resulted in a large decrease in the FRET ratio, which could be reversed by the addition of zinc (Fig 6D). For the most part, similar trends were seen with zrg17Δ ZapCY1 and cis4Δ zrg17Δ ZapCY1. However, for zrg17Δ cells the maximum FRET ratio was slightly higher than that observed with cis4Δ cells; and a zinc shock with 0.1 μM zinc was not sufficient to totally saturate ZapCY1 (Fig 6E–6G and S1 Fig). As ZapCY1 was largely saturated in cis4Δ and zrg17Δ cells following growth overnight in ZL-EMM, these results are consistent with Cis4 and Zrg17 being required for the transport of zinc out of the cytosol under conditions of zinc deficiency.
Fig 6. cis4Δ and zrg17Δ cells accumulate higher levels of zinc in the cytosol under zinc-limiting conditions.
(A, B, and C) cis4Δ cells expressing ZapCY1 were grown and subject to zinc shock as described in Fig 3B. Panel A shows a representative experiment and panel B shows the average values from 3 independent experiments with error bars representing standard deviations. Panel C shows a comparison of a 1 μM zinc shock in wild-type and cis4Δ cells expressing ZapCY1. (D) cis4Δ ZapCY1cells were grown as described in Fig 3B. Cells were transferred to temperature-controlled cuvettes and at the indicated times were exposed to +/- 50 μM NaPT or 0, 1, or 1000 μM Zn2+. Changes in the FRET ratio were determined as described in Fig 3B. Results show the average values from 3 independent experiments with error bars representing standard deviations. (E-G) The FRET ratio was measured in zrg17Δ cells expressing ZapCY1 as described in panel C. (H and I) cis4Δ and zrg17Δ cells expressing ZapCY2 were grown and subject to zinc shock as described in Fig 3B. Each panel shows the average values from 3 independent experiments with error bars representing standard deviations.
To determine if the higher levels of zinc that accumulated in cis4Δ and zrg17Δ also affected zinc binding to low affinity sites, similar experiments were performed with cells expressing ZapCY2. A zinc shock with 10 or 1000 μM zinc resulted in smaller increase in FRET compared to the wild-type (Fig 6H and 6I and S1 Fig). The signal also decreased over time. While it is possible that Cis4 and Zrg17 transport zinc out of the cytosol following a zinc shock, the decrease in FRET response in these mutants suggests that other mechanisms that are independent of Cis4 and Zrg17 protect the cytosol from accumulating high levels of labile zinc.
Zhf1 transports labile zinc from the cytosol
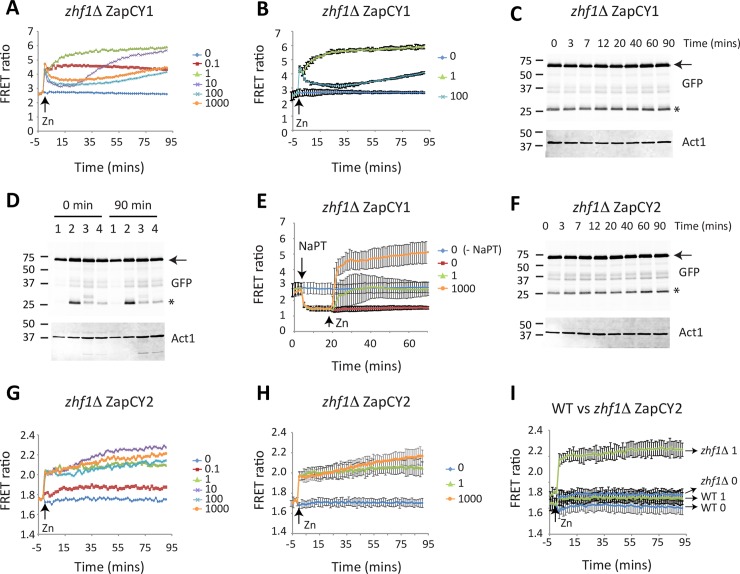
As Zhf1 is predicted to play the primary role in protecting cells from zinc toxicity, we next examined the activity of ZapCY1 and ZapCY2 in strains lacking zhf1. In zhf1Δ ZapCY1 cells grown overnight in ZL-EMM, the basal FRET ratio and response of this sensor to zinc shocks with 0.1 and 1 μM zinc were similar to those seen in wild-type cells (Fig 7A and 7B). In contrast, a zinc shock with 10 μM zinc led to a rapid increase in the FRET ratio, followed by an immediate decrease. After these rapid changes the FRET ratio slowly increased for the remainder of the experiment. A similar response was seen with zinc shocks with higher levels of zinc, with the exception that it took longer (~30 min) to see the increase in FRET ratio. To test whether this atypical response was a result of the instability of the ZapCY1 reporter in zhf1Δ cells, immunoblot analysis was used to examine the stability of ZapCY1 during a zinc shock with 100 μM Zn2+. As shown in Fig 7C and 7D, elevated levels of a lower molecular weight band accumulated in this strain (see asterisk), suggesting that ZapCY1 was more prone to degradation in this strain relative to others. Despite this higher level of degradation, there were no changes in the levels of the full-length reporter and experiments using the zinc chelator NaPT resulted in FRET profiles that resembled those observed in the wild-type (Fig 7E). Although we do yet understand the zinc-dependent changes in the FRET response in zhf1Δ cells, the observation that they are not observed in the presence of NaPT suggests that they result from zinc accumulating in the cytosol of this strain.
Fig 7. zhf1Δ cells accumulate higher levels of zinc in the cytosol following a zinc shock.
(A and B) zhf1Δ cells expressing ZapCY1 were grown and subject to zinc shock as described in Fig 3B. Panel A shows a representative experiment and panel B shows the average values from 3 independent experiments with error bars representing standard deviations. (C) zhf1x expressing ZapCY1 were grown overnight in ZL-EMM. At t = 0 cells were shocked with 100μM Zn2+. Cells were harvested at the indicated time points and crude protein extracts prepped for immunoblot analysis. Immunoblots were incubated with antibodies to GFP and loading control Act1. (D) Wild-type (1), zhf1Δ (2), cis4Δ (3), and zrg17Δ (4) cells expressing ZapCY1 were grown overnight in ZL-EMM. Cells were harvested for immunoblot analysis following the overnight growth (t = 0) or after a zinc shock with 100 μM Zn2+ for 90 min (t = 90). Immunoblots were performed as described in panel C. (E) zhf1Δ ZapCY1cells were grown overnight in ZL-EMM. Cells were transferred to temperature-controlled cuvettes and were treated with +/- 50 μM NaPT for 20 min followed by the addition of 0–1000 μM Zn2+. Changes in the FRET ratio were determined as described in Fig 3B. (F) zhf1Δ cells expressing ZapCY2 were grown overnight in ZL-EMM. Cells were shocked with 100 μM Zn2+ and cell harvested for immunoblot analysis at the indicated time points. Immunoblots were performed as described in panel C. Results show the average values from 3 independent experiments with error bars representing standard deviations. (G-I) zhf1Δ cells expressing ZapCY2 were grown and subject to zinc shock as described in Fig 3B. Panel F shows a representative experiment and panel G shows the average values from 3 independent experiments with error bars representing standard deviations. Panel I shows a comparison of a 1 μM zinc shock in wild-type and zhf1Δ cells expressing ZapCY2.
To gain further evidence that Zhf1 protects the cytosol from excess zinc, similar experiments were performed with zhf1Δ ZapCY2. In these cells, zinc had no effect on the stability of the full length reporter and a zinc shock with 1 μM zinc was sufficient to saturate ZapCY2 (Fig 7F–7I). The FRET ratio after a zinc shock with 1 μM zinc also remained high for the duration of the experiment. These results reveal that higher levels of zinc accumulate in the cytosol of zhf1Δ following a zinc shock and indicate that Zhf1 has a central role in removing labile zinc from the cytosol.
The expression of cis4, zrg17, and zhf1 is not dependent upon zinc
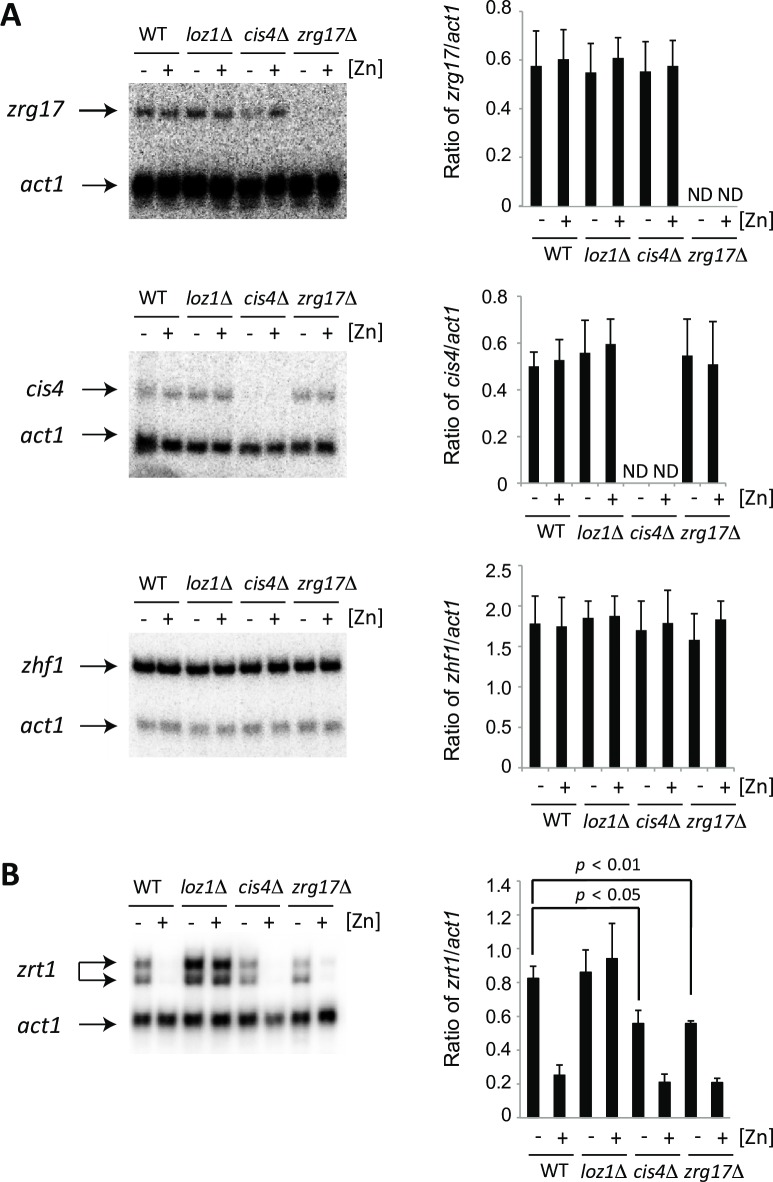
The above results suggest that the Cis4/Zrg17 complex plays a primary role in the transport of zinc out of a zinc-limited cytosol, while Zhf1 has the dominant role in transporting labile zinc from the cytosol. In S. cerevisiae the expression ZRG17 increases under conditions of zinc deficiency and this increase is critical for normal endoplasmic reticulum function under this condition [11]. To determine if the expression of cis4 and zrg17 was dependent on zinc in fission yeast we used S1 nuclease analysis to examine mRNA levels in wild-type and loz1Δ cells grown under zinc-limiting and zinc-replete conditions. As shown in Fig 8A, cis4 and zrg17 transcripts accumulated under all conditions indicating that their expression is not affected by zinc or Loz1. The levels of zhf1 mRNAs were also not regulated by zinc and Loz1, consistent with previous studies that have demonstrated experimentally that the expression of zhf1 is not affected by cellular zinc status [13, 24].
Fig 8. The expression of cis4, zrg17, and zhf1 is not dependent upon zinc.
(A) Total RNA was isolated from wild-type and the indicated mutants grown overnight in ZL-EMM supplemented with 0 or 100 μM Zn2+. The levels of cis4, zrg17, and zhf1 transcripts were compared to the loading control act1 using S1 nuclease analysis. A representative experiment is shown in the left panel and the average values from three independent experiments are shown on the right, with error bars representing standard deviations. ND = Not determined. (B). S1 nuclease analysis was performed as described above with the exception that mRNA was hybridized to probes complementary to zrt1 and act1. Radiolabelled act1 probes were diluted by 10-fold with unlabeled probe for experiments with cis4, zrg17, and zhf1. When cells were grown under zinc-limiting conditions, lower levels of zrt1 transcript accumulated in the cis4Δ and zrg17Δ mutants compared to the wild-type. p values were determined using a student's t-test.
As deletion of cis4 or zrg17 resulted in increased saturation of the high affinity ZapCY1 reporter, we also used S1 nuclease analysis to test whether these mutants accumulated sufficient levels of zinc in the cytosol to trigger increased Loz1-mediated gene repression. The rationale for these experiments is that Loz1 represses target gene expression when zinc levels are high. As a consequence, if higher levels of zinc accumulate in the cytosol of cis4Δ and zrg17Δ, this could result in increased repression of Loz1 target genes. When cells were grown under zinc-limiting conditions, lower levels of zrt1 transcripts accumulated in cis4Δ and zrg17Δ cells relative to the wild-type control (Fig 8B). These results are consistent with the Cis4/Zrg17 complex transporting zinc out of cytosol of zinc-limited cells.
Discussion
Yeast are useful model systems to study zinc homeostasis, as they are able to survive in low zinc environments and rapidly adapt to conditions of zinc excess. In this work we took advantage of these properties by examining the activity of the zinc-responsive ZapCY FRET reporters following overnight growth in a zinc-limited medium and during a zinc shock. We show that ZapCY1 and ZapCY2 are both able to measure dynamic changes in cytosolic zinc levels in fission yeast and that higher levels of zinc are necessary to saturate ZapCY2. We also show that there is a transient increase in FRET following a zinc shock in wild-type cells expressing ZapCY2, suggesting that zinc bound to this sensor exchanges with other ligands within the cytosol that can bind or buffer zinc. As ZapCY1 is able to detect zinc ions binding to high affinity sites within proteins, and ZapCY2 detects binding to low affinity sites, these sensors create useful tools for monitoring the factors that influence cytosolic zinc ion availability and zinc ion binding within the cytosol.
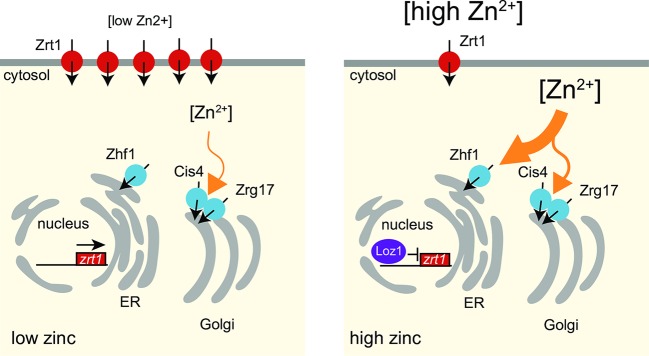
To identify additional factors that affect the levels and availability of zinc within the cytosol, we used ZapCY1/2 to test whether Cis4, Zrg17, and Zhf1 have redundant or complementary roles in zinc transport out of the cytosol. We found that deletion of cis4 or zrg17 resulted in higher levels of saturation of ZapCY1 under conditions of zinc deficiency, whereas deletion of zhf1 had little effect on the saturation of ZapCY1 under this condition. In contrast, significantly lower levels of zinc were necessary to saturate ZapCY2 in zhf1Δ cells compared to cis4Δ or zrg17Δ following a zinc shock. We propose that the Cis4/Zrg17 heterodimer preferentially transports zinc out of the cytosol into the secretory pathway under zinc-limiting conditions, whereas Zhf1 has the dominant role in transporting labile zinc out of the cytosol when zinc is not limiting (Fig 9). In this model, the transport activity of the Cis4/Zrg17 heterodimer ensures that zinc is supplied to the secretory pathway under zinc-limiting conditions. As a reduction in cytosolic zinc levels also triggers the inactivation of Loz1 and increased expression of the zrt1 zinc uptake system, cells are able to balance the levels of zinc uptake with zinc flux out of the cytosol. Cells face a different challenge when zinc is in excess, as too much zinc is toxic to cell metabolism. Under these conditions, the dominant role of Zhf1 results in excess zinc being directed to intracellular stores, protecting the cytosol and other organelles from the toxic effects of too much zinc.
Fig 9. The Cis4/Zrg17 heterodimer and Zhf1 have complementary roles in transporting zinc out of the cytosol into the secretory pathway.
When intracellular zinc levels are low, the Cis4/Zrg17 heterodimer transports zinc out of the cell into the secretory pathway (left panel). When zinc is not limiting, Zhf1 plays the primary role in transporting zinc out of the cytosol into cellular zinc stores (right panel). See text for further details.
A key question that our studies raise is what is the mechanism by which individual CDF family members preferentially transport zinc under zinc-limiting or zinc-replete conditions? Studies with S. cerevisiae have revealed much of what we know about the ability of CDF proteins to transport zinc under varying conditions of zinc stress. In this yeast, the Msc2/Zrg17 complex facilitates the transport of zinc into the endoplasmic reticulum, whereas Zrc1 and Cot transport zinc into the vacuole [10, 14]. One factor that affects zinc transport via the Msc2/Zrg17 complex is ZRG17 expression. ZRG17 is a Zap1 target gene that is expressed at higher levels in zinc-deficient cells [7, 11]. Importantly, in the absence of the Zap1-dependent induction of ZRG17, zinc-deficient cells experience greater levels of ER stress [11]. These results suggest that the levels of Zrg17 protein limit zinc transport by the Msc2/Zrg17 complex and that the increase in ZRG17 expression is critical for normal ER function under conditions of zinc-deficiency. Recent studies have also revealed that higher levels of MSC2 mRNAs accumulate in zinc-deficient cells [25]. The levels of Msc2 may also be an important factor that limits zinc transport by the Msc2/Zrg17 complex. While changes in gene expression are an integral part of zinc transport into the ER under zinc-deficient conditions, it is also important to note that ZRC1 is a Zap1 target gene, and yet overexpression of ZRC1 does not affect cytosolic zinc availability in zinc-deficient cells [26]. These results suggest that Zrc1 does not play a significant role in transporting zinc out of the cytosol under this condition. They also reveal that increased expression of a zinc transport gene does not necessarily result in more zinc being transported out of the cytosol, and that other factors likely affect zinc transporter function.
As the expression of cis4, zrg17, and zhf1 is not dependent upon zinc, what other factors could affect their ability to transport zinc out of the cytosol? One possibility is that there are intrinsic differences in the ability of Zhf1 and Cis4/Zrg17 to transport zinc. For example, if the affinities of the zinc binding sites on the cytosolic face of Zhf1 were weaker than those of the Cis4/Zrg17 heterodimer, this latter complex may only be able to acquire zinc when the cytosolic zinc pools are less saturated. An alternative possibility is that the labile pool of zinc that is accessible to Zhf1 under zinc-limiting conditions is dependent on the presence of an active Cis4/Zrg17 heterodimer. Potential mechanisms that could lead to a pool of labile zinc that is inaccessible to Zhf1 include an increase in the total number of buffering components in the cytosol (i.e. the total amount of zinc remains the same and the buffering capacity increases) and/or tighter buffering of cytosolic zinc (preventing zinc from being available to the weaker binding sites of Zhf1). While future experiments are necessary to determine the precise nature of the buffering components of the cytosol, and the affinity of different CDF transporters for zinc, it is noteworthy that Cis4/Zrg17 and Msc2/Zrg17 complexes both appear to have a primary role in transporting zinc out of the cytosol into the secretory pathway under zinc-limiting conditions. In addition, Zhf1 and Zrc1 both facilitate the transport of zinc from the cytosol when zinc availability is not limited. These similar functions suggest that at least some features of these transporters are conserved in fission and budding yeast.
In addition to the potential differences above, multiple other factors could affect the function of the Cis4/Zrg17 heterodimer and Zhf1. For example, in yeast and humans, zinc transporters from the ZIP family are targeted for degradation in response to high zinc [27, 28]. Although it is currently not known if the stability or activity of each of the S. pombe CDF proteins are regulated at a post-translational level in response to cellular zinc levels, proteomic studies that compared the copy numbers of proteins in fission yeast during vegetative growth in minimal medium revealed the presence of ~12,000–14,000 Zhf1 molecules/cell, ~3000–6000 Zrg17 molecules/cell, and ~ 1300–5500 Cis4 molecules/cell [29, 30]. The higher levels of Zhf1 relative to Zrg17 and Cis4 may therefore be one factor that contributes to Zhf1 having the principal role in transporting zinc out of the cytosol in a zinc-replete environment. In addition to factors that directly affect the function of zinc transport proteins, it is unclear if the subcellular localization of zinc transporters or their local environment affects their function. Moreover, relatively little is known about the molecules that buffer zinc within organelles and the cytosol, and whether the buffering capacity of an organelle for zinc, and/or the number and affinity of zinc-binding proteins within a compartment influences zinc transport. Thus, future studies with CDF proteins from S. pombe and other organisms are warranted to identify additional factors that alter zinc transport function.
We also examined the effects of the loz1Δ allele on cytosol zinc availability. We had previously found that loz1Δ cells constitutively express zrt1 and hyperaccumulate zinc when excess zinc is present in the growth medium [22]. Although loz1Δ cells hyperaccumulate zinc, paradoxically they have a more severe growth defect under zinc-deficient conditions compared to zinc replete (Fig 1). Here we find that the loz1Δ allele results in the saturation of the high affinity ZapCY1 sensor following growth overnight in ZL-EMM, indicating that this mutant accumulates higher levels of zinc in the cytosol relative to the wild-type. We also find that the response of the ZapCY2 reporter was similar to that of the wild-type, revealing that labile zinc entering loz1Δ cells is rapidly removed into stores and/or is effectively buffered. These latter results reveal that other mechanisms that are independent of Loz1 help S. pombe to maintain zinc homeostasis. They also provide an explanation for the viability of the loz1 mutant in high zinc. Another observation that we made was that ZapCY1 was not saturated in double mutants lacking zrt1 and loz1, and that this double mutant accumulated higher levels of zinc in the cytosol relative to zrt1. These results indicate that the constitutive derepression of zrt1 is the primary reason for the saturation of ZapCY1 in loz1Δ cells. They also suggest that Loz1 regulates other genes that affect cytosolic zinc availability. Known Loz1 target genes include zrt1, as well as adh4 (alcohol dehydrogenase 4), gcd1 (glucose dehydrogenase 1), and SPBC1348.06c, which encodes a small fungal protein of unknown function [22, 31]. Loz1 also represses the expression of non-protein coding RNAs that interfere with the expression of the adh1 (alcohol dehydrogenase 1) and zym1 (zinc metallothionein 1) genes [22, 32]. The expression of adh1 and zym1 is therefore inverse to that of other Loz1 targets, in that they are repressed under conditions of zinc deficiency. Although no known Loz1 target gene other than zrt1 has a role in transporting zinc, altered expression of some of its targets could affect intracellular zinc availability. For example, as the loz1Δ allele results in the constitutive repression of adh1, which encodes the abundant zinc binding protein Adh1, the lower levels of this protein could result in higher levels of zinc being available for other proteins. Thus, future studies to identify new Loz1 target genes and to examine the roles of existing target genes in controlling intracellular zinc availability may provide additional insight into factors affecting zinc homeostasis.
The ability of some CDF proteins to transport zinc is a manner that is dependent upon the levels of ‘labile’ or ‘readily available’ zinc in the cytosol could be of particular importance in organisms that express large numbers of CDF family members. For example, humans express 10 CDF family members (named ZnT1-10), while Arabidopsis thaliana and C. elegans each express 14 family members [33]. Potential reasons for why these organisms have so many CDF proteins include that they have unique subcellular localizations, different metal ion specificities, and/or that they have more specialized roles in supplying zinc to smaller subsets of proteins [5, 34–38]. Some genes encoding CDF proteins also show tissue- or developmental- specific expression patterns, while others are regulated by zinc and/or by hormonal or stress-responsive signaling pathways [5, 39, 40]. Although it is currently unclear in other organisms if the activity of specific CDF proteins is dependent upon cellular zinc status, the conserved role of this family in supplying zinc to organelles and storage compartments raises the possibility that the activity of other CDF proteins may also be fined tuned according to cytosolic zinc ion availability.
Materials and methods
Yeast strains and growth conditions
To generate the strains used for the FRET analysis, the plasmids pZapCY1 and pZapCY2 were linearized with NruI and were integrated into the leu1-32 locus of the wild-type strain JW81 (h- ade6-M210 leu1-32 ura4-D18) [41]. All other strains expressing ZapCY1 or ZapCY2 were generated from genetic crosses with the wild-type ZapCY1 (ABY795) or WT ZapCY2 (ABY797) with the respective mutant. The strains co-expressing the ZapCY1 and ZapCY2 with the zrt1-lacZ reporter were generated from genetic crosses with JW81 containing the integrated reporter TN-zrt1-lacZ [24]. To generate zinc-deficient and zinc-replete cells, yeast strains were initially grown to exponential phase in the nutrient rich YES medium. Cells were then spun down and washed twice in ZL-EMM, a derivative of Edinburgh minimal medium that lacks zinc (ZL-EMM). Washed cells were then diluted to 0.02 OD600 with fresh ZL-EMM and were grown for 16 hrs at 31°C in ZL-EMM or in this medium supplemented with 1, 10, or 100 μM ZnCl2. For all zinc shock experiments, cells were grown as described above in ZL-EMM without zinc. The indicated amount of zinc (0.01–1000 μM ZnCl2) was then added to induce the zinc shock.
Plasmid construction
The plasmids pZapCY1 and pZapCY2 were generated by PCR amplifying the coding regions for ZapCY1 and ZapCY2 from the vectors pcDNA3.1-ZapCY1 and pcDNA3.1-ZapCY2 respectively, with primers containing EcoRI and BamHI restriction sites. The ZapCY1/2 PCR products were then digested with EcoRI and BamHI and cloned into similar sites into the vector JK-pgk1-adh4T. The vector JK-pgk1-adh4T is a derivative of JK148 that contains 840 bp of the pgk1 promoter inclusive of its 5’UTR and 726 bp of the adh4 terminator. It was generated by initially PCR amplifying the pgk1 promoter with primers containing KpnI and EcoRI restriction sites. KpnI- and EcoRI- digested PCR products were then cloned into the vector JK148 to generate JK-pgk1. The adh4 terminator was cloned using a similar approach with the exception that primers were designed to introduce the adh4 PCR product into the BamHI/SacI sites of JK-pgk1.
β-Galactosidase assays and Atomic Absorption Spectroscopy (AAS)
β-Galactosidase assays were performed as described previously [42]. Activity units were calculated as follows: (ΔA420 x 1000)/(min x ml of culture x culture absorbance at 600 nm). For AAS 10 ml of cells were grown in ZL-EMM as described above. After the OD600 was measured, the indicate amount of zinc was added at t = 0 min. Cells were then grown at 31°C with shaking and 1.5 ml aliquots removed at the indicated time point. To remove extracellular zinc, cells were washed twice with 0.5 M EDTA and twice with ddH2O. Cell pellets were then digested by boiling in 150 μl of metal free nitric acid for 45 min and the zinc content measured using a SpectrAA 220FS Atomic Absorption Spectrometer. The final zinc concentration/cell was calculated by comparing the readings to a standard curve generated using a zinc standard (Sigma 18827). All values are the average from three independent experiments and error bars represent standard deviations.
FRET Measurements
For FRET experiments, cells were grown for 16 hrs in ZL-EMM as described above. ~2.5 x 106 cells were directly transferred to temperature controlled 96 well plates and the FRET emission intensities measured using spectrofluorometry using the following excitation and emission wavelengths: eCFP excitation 434 nm / emission 474 nm, and FRET excitation 434 nm / emission 535 nm. The FRET ratio was calculated by dividing the FRET emission intensity by the eCFP emission intensity. All average values show the mean FRET ratio from three independent experiments that were performed on independent days. Error bars show standard deviations.
Immunoblotting, RNA blotting, S1 nuclease assays and microscopy
For immunoblotting, total protein extracts were prepared by a trichloroacetic acid precipitation. Proteins were separated by SDS/PAGE analysis using a 10% resolving gel before transfer to nitrocellulose membranes. Proteins were detected using anti-GFP (Sigma G1544) or anti-Actin (Abcam ab3280), and secondary antibodies IR-Dye800CW conjugated anti-mouse IgG (LICOR) and IRDye680 conjugated anti-rabbit IgG (LICOR). Signal intensities were measured using an Odyssey infrared imaging system. For RNA analysis, total RNA was purified using hot acidic phenol method. RNA blots and S1 nuclease analyses were performed as described previously [32, 43]. Probes for the RNA blot analyses were generated using the MAXISCRIPT T7 kit (Ambion) according to manufacturers instructions, whereas probes for the S1 nuclease analyses were generated by 5’ end labeling the following oligonucleotides: zrg17 5’-GATCACTAATAGTTACAGAGACATTATTATTTATAGGGTTTTGAATCTGAATAGCAGTCGGGATG- 3’, cis4 5’- CGAACGCAGAAGAATTAACATTCATTTTTGTCGTCAGGAACACCCAAAAGCTGTGGTTGAC-3’, zhf1 5’-GTTGCCAGCCATATGTGTATTTTGGTTCGTGAGATGTTGAATGTGCTAGACGAGTAGCCCA-3’, zrt1 5’- CCATATTCGTTGAATTCATTGGCATCACCTCCACAAGTCACAGTAGCAGAGCTATCATCGTC-3’, and act1 5’-GTCCCATACCTACCATAATACCATGGTGACGGGGTCTACCGAC-3’. Act1 probes were diluted with unlabeled probe where indicated. Fluorescent microscopy of live cells was performed with an Olympus FV 1000 Filter Confocal system, using filter sets for GFP.
Statistical analysis
Data are presented as the mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, La Jolla, CA, USA). Where appropriate, data were analyzed by a Student unpaired t-test. A p value of <0.05 was considered statistically significant.
Supporting information
cis4Δ zrg17Δ cells expressing ZapCY1 (A) or ZapCY2 (B) were grown overnight in ZL-EMM. Cells were transferred to temperature-controlled cuvettes and were assayed for FRET by spectrofluorometry. At t = 0 cells were shocked with the indicated amount of Zn2+ and the changes in FRET monitored over time. The FRET ratio was determined by dividing the FRET emission at 535 nm by the eCFP emission at 475 nm following excitation of samples at 434 nm. Results represent the average values from 3 independent experiments with error bars representing S.D.
(TIF)
Acknowledgments
We thank members of the Bird lab and Dr. R. Michael Townsend for critical reading of the manuscript and Dr. Kurt W. Runge for assistance with strain generation.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research reported in this publication was supported by the National Institutes of General Medical Sciences awards GM105695 to A.J.B and GM084027 to A.E.P., and from the Office of the Director, National Institutes of Health of the National Institutes of Health under Award Number S10OD023582. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Andreini C, Banci L, Bertini I, Rosato A. Counting the zinc-proteins encoded in the human genome. J Proteome Res. 2006;5(1):196–201. doi: 10.1021/pr050361j . [DOI] [PubMed] [Google Scholar]
- 2.Coleman JE. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341 . [DOI] [PubMed] [Google Scholar]
- 3.Coyne HJ 3rd, Ciofi-Baffoni S, Banci L, Bertini I, Zhang L, George GN, et al. The characterization and role of zinc binding in yeast Cox4. J Biol Chem. 2007;282(12):8926–34. doi: 10.1074/jbc.M610303200 . [DOI] [PubMed] [Google Scholar]
- 4.Baksh S, Spamer C, Heilmann C, Michalak M. Identification of the Zn2+ binding region in calreticulin. FEBS Lett. 1995;376(1–2):53–7. . [DOI] [PubMed] [Google Scholar]
- 5.Kambe T, Tsuji T, Hashimoto A, Itsumura N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol Rev. 2015;95(3):749–84. doi: 10.1152/physrev.00035.2014 . [DOI] [PubMed] [Google Scholar]
- 6.Choi S, Bird AJ. Zinc'ing sensibly: controlling zinc homeostasis at the transcriptional level. Metallomics. 2014;6(7):1198–215. doi: 10.1039/c4mt00064a . [DOI] [PubMed] [Google Scholar]
- 7.Wu CY, Bird AJ, Chung LM, Newton MA, Winge DR, Eide DJ. Differential control of Zap1-regulated genes in response to zinc deficiency in Saccharomyces cerevisiae. BMC Genomics. 2008;9:370 doi: 10.1186/1471-2164-9-370 ; PubMed Central PMCID: PMCPMC2535606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunther V, Lindert U, Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim Biophys Acta. 2012;1823(9):1416–25. doi: 10.1016/j.bbamcr.2012.01.005 . [DOI] [PubMed] [Google Scholar]
- 9.Warnhoff K, Roh HC, Kocsisova Z, Tan CH, Morrison A, Croswell D, et al. The Nuclear Receptor HIZR-1 Uses Zinc as a Ligand to Mediate Homeostasis in Response to High Zinc. PLoS Biol. 2017;15(1):e2000094 doi: 10.1371/journal.pbio.2000094 ; PubMed Central PMCID: PMCPMC5240932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis CD, Macdiarmid CW, Eide DJ. Heteromeric protein complexes mediate zinc transport into the secretory pathway of eukaryotic cells. J Biol Chem. 2005;280(31):28811–8. doi: 10.1074/jbc.M505500200 . [DOI] [PubMed] [Google Scholar]
- 11.Wu YH, Frey AG, Eide DJ. Transcriptional regulation of the Zrg17 zinc transporter of the yeast secretory pathway. Biochem J. 2011;435(1):259–66. doi: 10.1042/BJ20102003 ; PubMed Central PMCID: PMCPMC4804349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson S, Bird AJ. Zinc sensing and regulation in yeast model systems. Arch Biochem Biophys. 2016;611:30–6. doi: 10.1016/j.abb.2016.02.031 ; PubMed Central PMCID: PMCPMC5010796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens S, Bloss T, Vess C, Neumann D, Nies DH, Zur Nieden U. A transporter in the endoplasmic reticulum of Schizosaccharomyces pombe cells mediates zinc storage and differentially affects transition metal tolerance. J Biol Chem. 2002;277(20):18215–21. doi: 10.1074/jbc.M201031200 . [DOI] [PubMed] [Google Scholar]
- 14.Simm C, Lahner B, Salt D, LeFurgey A, Ingram P, Yandell B, et al. Saccharomyces cerevisiae vacuole in zinc storage and intracellular zinc distribution. Eukaryot Cell. 2007;6(7):1166–77. doi: 10.1128/EC.00077-07 ; PubMed Central PMCID: PMCPMC1951117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrelly GP, Harrison MD, Robinson AK, Cox SG, Robinson NJ, Whitehall SK. Surplus zinc is handled by Zym1 metallothionein and Zhf endoplasmic reticulum transporter in Schizosaccharomyces pombe. J Biol Chem. 2002;277(33):30394–400. doi: 10.1074/jbc.M203145200 . [DOI] [PubMed] [Google Scholar]
- 16.Jensen LT, Howard WR, Strain JJ, Winge DR, Culotta VC. Enhanced effectiveness of copper ion buffering by CUP1 metallothionein compared with CRS5 metallothionein in Saccharomyces cerevisiae. J Biol Chem. 1996;271(31):18514–9. . [DOI] [PubMed] [Google Scholar]
- 17.Eide DJ. Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J Biol Chem. 2009;284(28):18565–9. doi: 10.1074/jbc.R900014200 ; PubMed Central PMCID: PMCPMC2707215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boch A, Trampczynska A, Simm C, Taudte N, Kramer U, Clemens S. Loss of Zhf and the tightly regulated zinc-uptake system SpZrt1 in Schizosaccharomyces pombe reveals the delicacy of cellular zinc balance. FEMS Yeast Res. 2008;8(6):883–96. doi: 10.1111/j.1567-1364.2008.00414.x . [DOI] [PubMed] [Google Scholar]
- 19.Fang Y, Sugiura R, Ma Y, Yada-Matsushima T, Umeno H, Kuno T. Cation diffusion facilitator Cis4 is implicated in Golgi membrane trafficking via regulating zinc homeostasis in fission yeast. Mol Biol Cell. 2008;19(4):1295–303. doi: 10.1091/mbc.E07-08-0805 ; PubMed Central PMCID: PMCPMC2291430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin Y, Dittmer PJ, Park JG, Jansen KB, Palmer AE. Measuring steady-state and dynamic endoplasmic reticulum and Golgi Zn2+ with genetically encoded sensors. Proc Natl Acad Sci U S A. 2011;108(18):7351–6. doi: 10.1073/pnas.1015686108 ; PubMed Central PMCID: PMCPMC3088641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao W, Mooney M, Bird AJ, Winge DR, Eide DJ. Zinc binding to a regulatory zinc-sensing domain monitored in vivo by using FRET. Proc Natl Acad Sci U S A. 2006;103(23):8674–9. doi: 10.1073/pnas.0600928103 ; PubMed Central PMCID: PMCPMC1482638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corkins ME, May M, Ehrensberger KM, Hu YM, Liu YH, Bloor SD, et al. Zinc finger protein Loz1 is required for zinc-responsive regulation of gene expression in fission yeast. Proc Natl Acad Sci U S A. 2013;110(38):15371–6. doi: 10.1073/pnas.1300853110 ; PubMed Central PMCID: PMCPMC3780850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krezel A, Maret W. The biological inorganic chemistry of zinc ions. Arch Biochem Biophys. 2016;611:3–19. doi: 10.1016/j.abb.2016.04.010 ; PubMed Central PMCID: PMCPMC5120989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehrensberger KM, Corkins ME, Choi S, Bird AJ. The double zinc finger domain and adjacent accessory domain from the transcription factor loss of zinc sensing 1 (loz1) are necessary for DNA binding and zinc sensing. J Biol Chem. 2014;289(26):18087–96. doi: 10.1074/jbc.M114.551333 ; PubMed Central PMCID: PMCPMC4140304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu YH, Taggart J, Song PX, MacDiarmid C, Eide DJ. An MSC2 Promoter-lacZ Fusion Gene Reveals Zinc-Responsive Changes in Sites of Transcription Initiation That Occur across the Yeast Genome. PLoS One. 2016;11(9):e0163256 doi: 10.1371/journal.pone.0163256 ; PubMed Central PMCID: PMCPMC5033525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacDiarmid CW, Milanick MA, Eide DJ. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J Biol Chem. 2003;278(17):15065–72. doi: 10.1074/jbc.M300568200 . [DOI] [PubMed] [Google Scholar]
- 27.Gitan RS, Eide DJ. Zinc-regulated ubiquitin conjugation signals endocytosis of the yeast ZRT1 zinc transporter. Biochem J. 2000;346 Pt 2:329–36. ; PubMed Central PMCID: PMCPMC1220857. [PMC free article] [PubMed] [Google Scholar]
- 28.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem. 2007;282(10):6992–7000. doi: 10.1074/jbc.M610552200 . [DOI] [PubMed] [Google Scholar]
- 29.Marguerat S, Schmidt A, Codlin S, Chen W, Aebersold R, Bahler J. Quantitative analysis of fission yeast transcriptomes and proteomes in proliferating and quiescent cells. Cell. 2012;151(3):671–83. doi: 10.1016/j.cell.2012.09.019 ; PubMed Central PMCID: PMCPMC3482660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carpy A, Krug K, Graf S, Koch A, Popic S, Hauf S, et al. Absolute proteome and phosphoproteome dynamics during the cell cycle of Schizosaccharomyces pombe (Fission Yeast). Mol Cell Proteomics. 2014;13(8):1925–36. doi: 10.1074/mcp.M113.035824 ; PubMed Central PMCID: PMCPMC4125727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corkins ME, Wilson S, Cocuron JC, Alonso AP, Bird AJ. The gluconate shunt is an alternative route for directing glucose into the pentose phosphate pathway in fission yeast. J Biol Chem. 2017;292(33):13823–32. doi: 10.1074/jbc.M117.798488 ; PubMed Central PMCID: PMCPMC5566534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehrensberger KM, Mason C, Corkins ME, Anderson C, Dutrow N, Cairns BR, et al. Zinc-dependent regulation of the Adh1 antisense transcript in fission yeast. J Biol Chem. 2013;288(2):759–69. doi: 10.1074/jbc.M112.406165 ; PubMed Central PMCID: PMCPMC3543025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roh HC, Collier S, Deshmukh K, Guthrie J, Robertson JD, Kornfeld K. ttm-1 encodes CDF transporters that excrete zinc from intestinal cells of C. elegans and act in a parallel negative feedback circuit that promotes homeostasis. PLoS Genet. 2013;9(5):e1003522 doi: 10.1371/journal.pgen.1003522 ; PubMed Central PMCID: PMCPMC3662639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, et al. Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet. 2012;90(3):467–77. doi: 10.1016/j.ajhg.2012.01.017 ; PubMed Central PMCID: PMCPMC3309204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tuschl K, Clayton PT, Gospe SM Jr., Gulab S, Ibrahim S, Singhi P, et al. Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet. 2012;90(3):457–66. doi: 10.1016/j.ajhg.2012.01.018 ; PubMed Central PMCID: PMCPMC3309187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolaj-Robin O, Russell D, Hayes KA, Pembroke JT, Soulimane T. Cation Diffusion Facilitator family: Structure and function. FEBS Lett. 2015;589(12):1283–95. doi: 10.1016/j.febslet.2015.04.007 . [DOI] [PubMed] [Google Scholar]
- 37.Fujimoto S, Tsuji T, Fujiwara T, Takeda TA, Merriman C, Fukunaka A, et al. The PP-motif in luminal loop 2 of ZnT transporters plays a pivotal role in TNAP activation. Biochem J. 2016;473(17):2611–21. doi: 10.1042/BCJ20160324 ; PubMed Central PMCID: PMCPMC5557410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustin JL, Zanis MJ, Salt DE. Structure and evolution of the plant cation diffusion facilitator family of ion transporters. BMC Evol Biol. 2011;11:76 doi: 10.1186/1471-2148-11-76 ; PubMed Central PMCID: PMCPMC3073911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishihara K, Yamazaki T, Ishida Y, Suzuki T, Oda K, Nagao M, et al. Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J Biol Chem. 2006;281(26):17743–50. doi: 10.1074/jbc.M602470200 . [DOI] [PubMed] [Google Scholar]
- 40.Liuzzi JP, Bobo JA, Cui L, McMahon RJ, Cousins RJ. Zinc transporters 1, 2 and 4 are differentially expressed and localized in rats during pregnancy and lactation. J Nutr. 2003;133(2):342–51. . [DOI] [PubMed] [Google Scholar]
- 41.Wu JQ, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell. 2003;5(5):723–34. . [DOI] [PubMed] [Google Scholar]
- 42.Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–91. . [DOI] [PubMed] [Google Scholar]
- 43.Dohrmann PR, Butler G, Tamai K, Dorland S, Greene JR, Thiele DJ, et al. Parallel pathways of gene regulation: homologous regulators SWI5 and ACE2 differentially control transcription of HO and chitinase. Genes Dev. 1992;6(1):93–104. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
cis4Δ zrg17Δ cells expressing ZapCY1 (A) or ZapCY2 (B) were grown overnight in ZL-EMM. Cells were transferred to temperature-controlled cuvettes and were assayed for FRET by spectrofluorometry. At t = 0 cells were shocked with the indicated amount of Zn2+ and the changes in FRET monitored over time. The FRET ratio was determined by dividing the FRET emission at 535 nm by the eCFP emission at 475 nm following excitation of samples at 434 nm. Results represent the average values from 3 independent experiments with error bars representing S.D.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.