Abstract
The recent range expansion of human babesiosis in the northeastern United States, once found only in restricted coastal sites, is not well understood. This study sought to utilize a large number of samples to examine the population structure of the parasites on a fine scale to provide insights into the mode of emergence across the region. 228 B. microti samples collected in endemic northeastern U.S. sites were genotyped using published Variable number tandem repeat (VNTR) markers. The genetic diversity and population structure were analysed on a geographic scale using Phyloviz and TESS, programs that utilize two different methods to identify population membership without predefined population data. Three distinct populations were detected in northeastern US, each dominated by a single ancestral type. In contrast to the limited range of the Nantucket and Cape Cod populations, the mainland population dominated from New Jersey eastward to Boston. Ancestral populations of B. microti were sufficiently isolated to differentiate into distinct populations. Despite this, a single population was detected across a large geographic area of the northeast that historically had at least 3 distinct foci of transmission, central New Jersey, Long Island and southeastern Connecticut. We conclude that a single B. microti genotype has expanded across the northeastern U.S. The biological attributes associated with this parasite genotype that have contributed to such a selective sweep remain to be identified.
Introduction
Human babesiosis due to Babesia microti was first recognized on Nantucket Island nearly 50 years ago [1], and a few years later the first cases of Lyme arthritis were described from Old Lyme, Connecticut [2]. Both infections were found to be transmitted by the deer tick (Ixodes dammini; north American clade of I. scapularis), which had started to be locally recognized as a human-biting pest [3]. In the 1970s and 80s, cases of both were restricted to coastal New England sites, as well as foci in Wisconsin, Minnesota and California [4–6]. Over the next 20 years, the number of Lyme disease cases significantly increased and zoonotic risk spread rapidly across the northeastern United States and is now endemic all the way north into Canada, west to Ohio, and south as far as Virginia. Babesiosis, in constrast, lagged behind Lyme disease across these sites in time and in force of transmission [7,8] and most cases were reported from coastal sites in the northeastern U.S. However, in the last two decades, risk for babesiosis has intensified across the northeastern U.S. [9,10].
The 20 year lag between the range expansion of Lyme disease and that of babesiosis is not fully understood but in part relates to the difficulty with which B. microti may be transported. The two key facts that pose a paradox for range expansion are (1) only rodents and insectivores are known to be competent reservoirs of B. microti (may pass infection to uninfected ticks; [11]; and (2) B. microti is not transmitted transovarially [11]. Larval ticks transported long distances by migratory birds, a critical mode of introduction for the agent of Lyme disease (for which certain passerines are competent reservoirs; [12]), are not likely to develop into infected nymphs after they engorge on a bird because birds are not known to be reservoir competent for B. microti. A B. microti-infected nymph (which acquired infection as a larva feeding on a mouse) transported by a bird could develop into an infected adult tick, but because that stage feeds only on medium to large sized mammals, especially deer, would not pass infection to a reservoir competent animal during the adult bloodmeal; deer are not competent reservoirs and carnivores are not likely to be competent. Hence, B. burgdorferi is said to travel on the backs of birds but B. microti on mice. Mice or other small mammals are unlikely to travel large distances. These considerations argue that the range expansion for B. microti babesiosis is not due to introductions of infected ticks by migratory birds.
The existence of silent natural foci of transmission is suggested by early rodent serosurveys for B. microti in Connecticut [13] and the detection of parasites from sites in Maine where human babesiosis had not been recorded [14]. However, ecological surveillance has not been conducted across the northeastern U.S. with sufficient detail to provide much data of utility in understanding the tempo and mode of babesiosis risk. Longitudinal analyses of cases reported to state departments of public health are useful because case reports are based on a standard surveillance case definition and data are comparable between states. In Rhode Island, risk diminished from south to north [15]. In New York, babesiosis case reports gradually expanded from Long Island up the Hudson River valley. Similarly, in Connecticut, case reports expanded through the years from the southeastern coast first extending westward along the coast and then moving inland. [7,16–18]. The expansion of risk has been limited and incremental, with no long-distance introduction events such as those documented for Lyme disease, exemplified by its introduction into Canada. [19] A recent model for the emergence of babesiosis in New England suggests a “stepping-stone” model: a strong predictor of a town reporting babesiosis cases was the presence of a neighboring town reporting cases and that Lyme disease risk was a prerequisite [8]. Two stepping stone scenarios might have been operating concurrently in the last 20 years. (1) The force of B. microti transmission increased slowly across the northeastern landscape with the coastal earliest known zooonotic sites seeding adjacent more northerly sites. (2) Multiple cryptic enzootic sites (natural foci) with little zoonotic risk existed across the region, with local intensification of the force of B. microti transmission as tick densities increased to a threshold (estimated to be more than 20 nymphal deer ticks collected per hour [20]), and subsequent spread to adjacent areas.
The population structure of B. microti may provide evidence for the mode of the expansion of babesiosis risk across the northeast. At the very basic level, new demes, or local populations, will be related genetically to their parent populations. In expanding populations, genetic diversity may be low be due to bottlenecks and founder effects at the expanding front [21,22]. In fact, observed patterns of diversity will vary depending on the process of population expansion, viz., whether the population is being "pushed" or "pulled"[21,23]. A "pulled" expansion occurs when pioneers are seeding new populations ahead of the source population, such as would occur if individual infected ticks are being introduced into a new site. This causes the genetic diversity to be lower at the edge than the main body of the population due to successive founder effects. By contrast, a "pushed" expansion occurs when a population expands at the edges of the source location due to population growth. This expansion is usually slower and allows for diversity in the source population to keep pace with geographical spread. A skewed population diversity can occur near the expanding front of the population due to “allele surfing”, in other words, high rates of reproduction can increase mutation and allow an allele to surf the wave of population growth and become prevalent when it might not have become fixed in a stationary population [21,24–26].
Similar to microsatellite loci, variable number tandem repeats (VNTRs) have been shown to provide high level of discrimination of for strain identification of many organisms due to the high mutation rate of these loci compared to the rest of the genome [27–30]. We have previously described VNTR markers that are capable of identifying strains of B. microti and have shown them to be stable in nature over many years on Nantucket Island. Using these markers, we analyzed the population structure of B. microti and detected 3 distinct populations in ticks and rodents across New England [31]. Whole genome sequencing of ecological and clinical samples determined that these B. microti populations were strongly differentiated, suggesting that they were geographically isolated [32]. However, neither study analyzed sufficient samples to provide detail on the mode of expansion of the range of B.microti in the northeastern U.S. Accordingly, we leveraged >200 diagnostic blood samples from patients suspected of having acute babesiosis presenting to several clinical practices across the northeastern U.S. and analyzed them with the VNTR assay. In particular, we sought to determine the population structure of these parasites, and whether range expansion was best represented by a “pulled” expansion model by introductions into small founder populations, or a “pushed” model consistent with stepping stone expansion.
Materials and methods
B. microti blood samples
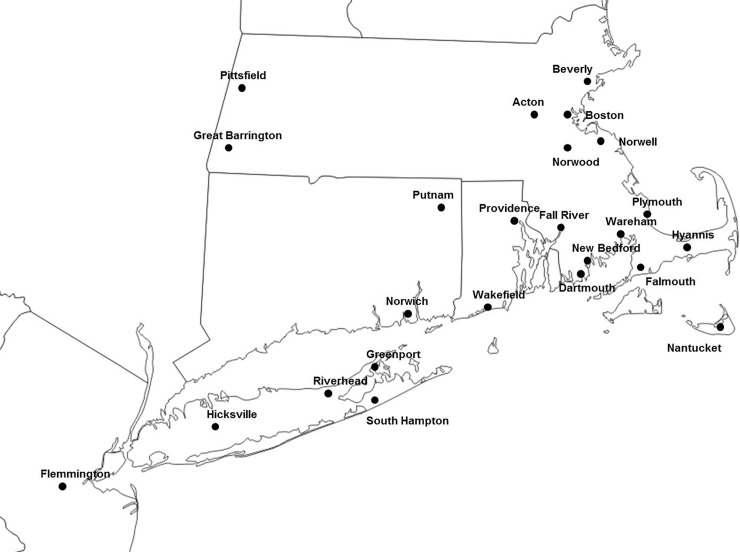
De-identified discarded blood samples were collected from specimens that had been sent to Imugen, Inc. from the northeastern United States for diagnosis of B. microti infection during the transmission season of 2015. The town of the submitting doctor's office or hospital was associated with each sample but no other data was available. (See Fig 1 and Table 1) Samples with a Ct>34 on the diagnostic real time PCR performed at Imugen were excluded from the analysis because they would not have had enough parasite DNA to yield reliable VNTR typing results. This study was considered not to comprise human subjects research by the Tufts University institutional review board.
Fig 1. Map of the Northeastern United States labeled with the sites from which samples were collected.
Table 1. Sites from which samples were collected and the number of haplotype identified from each site.
| Region | No. Samples | No. per region | No. haplotypes |
|---|---|---|---|
| Boston (Bos) | 19 | 11 | |
| Acton, MA | 6 | ||
| Beverly, MA | 1 | ||
| Boston, MA | 3 | ||
| Norwood, MA | 6 | ||
| Norwell, MA | 1 | ||
| Southeastern MA (SEMA) | 40 | 36 | |
| Fall River, MA | 13 | ||
| Plymouth, MA | 8 | ||
| New Bedford, MA | 11 | ||
| Wareham, MA | 4 | ||
| Dartmouth, MA | 4 | ||
| Western MA (WMA) | 7 | 7 | |
| Great Barrington, MA | 1 | ||
| Pittsfield, MA | 6 | ||
| Cape Cod (CC) | 23 | 18 | |
| Falmouth, MA | 6 | ||
| Hyannis, MA | 17 | ||
| Nantucket (N) | 16 | 13 | |
| Nantucket, MA | 16 | ||
| Rhode Island (RI) | 22 | 15 | |
| Providence, RI | 4 | ||
| Wakefield. RI | 18 | ||
| Connecticut (CT) | 27 | 16 | |
| Putnam, CT | 8 | ||
| Norwich, CT | 19 | ||
| Long Island (LI) | 46 | 19 | |
| Greenport, NY | 2 | ||
| Hicksville, NY | 15 | ||
| Riverhead, NY | 12 | ||
| Southhampton, NY | 17 | ||
| New Jersey (NJ) | 15 | 5 | |
| Flemmington, NJ | 15 | ||
| Total: | 228 | 113 |
Genotyping
DNA was extracted using a commercial spin column method (Qiagen Inc.). B. microti was typed using 8 VNTR loci as described [31], with the exception that the hypervariable locus, BMV4, was excluded. Samples were excluded from the final analysis if more than 1 locus failed to amplify. To avoid erroneously scoring stutter peaks, multiple peaks were scored only if the size of the minor peak was almost equal to that of the major peak. B. microti merozoites infecting humans are haploid [33]; so all analyses were done under the assumption of haploidy. Samples that had multiple peaks in more than one locus were excluded, as it was impossible to determine the individual haplotypes needed for assigning a haplotype to a population using Phyloviz (see below). Samples that had multiple peaks in only a single locus were retained in the analysis and treated as two separate haplotypes.
Data analysis
VNTR haplotypes were analyzed with two programs (Phyloviz [34] and TESS [35]) that utilize different algorithms for assigning them to a population. Phyloviz uses the haplotype data to determine mutually exclusive related groups, determines the founder (defined as the haplotype with the highest number of single locus variants) and then predicts the descent from the founder to the other haplotypes via single locus variants without any predefined assumptions of populations or geographic location. TESS uses a Bayesian clustering algorithm to determine population structure from haplotypes and their geographical location without assuming predefined populations, hence TESS requires that a unique geographic location be associated with each sample. Because samples were de-identified and only the location of the contributing clinical practice was known, we created random locations for each sample within a standard deviation of 0.05 degrees longitude and 0.025 degrees latitude from the town associated with each sample using the tool provided by TESS. To ensure that nearest neighbor connections could not occur over the ocean, 23 dummy points, i.e. points at which sampling cannot occur, were added in the Atlantic Ocean along the shoreline. In addition, the spatial network was altered to remove any remaining nearest neighbor connections that spanned the ocean. Geographic distances between each sample point were calculated using TESS. The program was then run for 10 permutations for K populations, from 2 to 8, with allowance for admixture. The mean deviance information criterion (DIC) was calculated across runs for each K population in order to choose the best fit among alternate models. The output from the 10 individual runs of the chosen K was downloaded into CLUMPP [36] which compiled them together. The resulting ancestry coefficients were displayed as a bar graph. An ancestry coefficient of 0.80 or greater for a single population was determined to be a member of that population. Any sample with a coefficient less than 0.80 for any single population was determined to have significant admixture from more than 1 source population. The ancestry coefficients were spatially interpolated onto a map of New England using R [37]. The estimated amount of differentiation between populations was calculated using two different methods, Fst using Genepop on the web [38,39], PhiPT using GenAlEx [40]. The Shannon Index of Diversity was calculated using PAST [41] on samples grouped by region. The Outline map of the northeastern United States was downloaded from dmaps.com (http://dmaps.com/carte.php?num_car=3895&lang=en).
Preprint submitted to bioRxiv (https://www.biorxiv.org/content/early/2017/11/21/223420)
Results
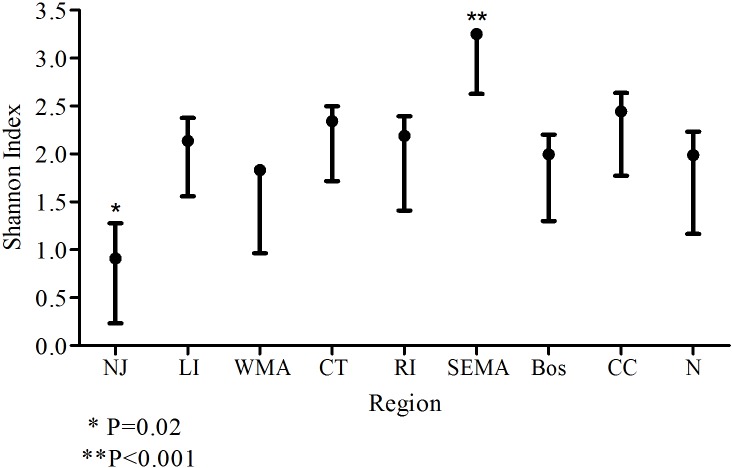
B. microti was typed from 234 specimens from 24 towns throughout New England during 2015 (Fig 1 and Table 1). Of these samples, 42 had multiple alleles in one locus and 6 had multiple alleles for more than 1 locus. The latter were excluded from the analysis because we were unable to accurately determine the haplotype necessary for analysis by Phyloviz. From the 228 samples used in the study, 113 unique haplotypes were obtained. The samples were grouped by geographic region (Table 1) and the Shannon Index (H) was calculated for each region (Fig 2). The diversity for most regions ranged from 1.8–2.5 and was not significantly different from each other. However, the diversity from the New Jersey (NJ) samples was significantly lower (H = 0.9, p = 0.02) and the diversity from southeastern Massachusetts (SeMA) samples was significantly higher (H = 3.3, p<0.001) than the rest. Population differentiation estimates, PhiPT, suggest isolation between some regions and almost none between others (Table 2). Samples from Nantucket (N) and Cape Cod (CC) have significant amounts of population differentiation between each other and each of the other geographic groups. (Table 2) In contrast, there is no evidence of any population differentiation between samples from NJ, Long Island (LI), Connecticut (CT) and Rhode Island (RI). Samples from SeMA and western Massachusetts (WMA) show moderate amounts of population differentiation between each other and those from NJ, LI, CT and RI (Table 2).
Fig 2. Shannon's Index of diversity with standard error for B. microti haplotypes found in each region.
New Jersey (NJ), Long Island (LI), Western Massachusetts (WMA), Connecticut (CT), Rhode Island (RI), southeastern Massachusetts (SEMA), Boston (Bos), Cape Cod (CC) and Nantucket (N).
Table 2. PhiPT estimates for B. microti from human patients by regiona.
| Region | Boston | Cape Cod | Long Island | Nantucket | Connecticut | New Jersey | Rhode Island | SeMAb |
|---|---|---|---|---|---|---|---|---|
| Cape Cod | 0.51 | |||||||
| Long Island | 0.05 | 0.64 | ||||||
| Nantucket | 0.49 | 0.62 | 0.59 | |||||
| Connecticut | 0.07 | 0.62 | 0.02 | 0.54 | ||||
| New Jersey | 0.07 | 0.66 | 0.01 | 0.67 | 0.04 | |||
| Rhode Island | 0.03 | 0.58 | 0.04 | 0.47 | 0.01 | 0.05 | ||
| SeMAb | 0.04 | 0.33 | 0.15 | 0.33 | 0.13 | 0.14 | 0.10 | |
| WMAc | 0.003 | 0.51 | 0.12 | 0.48 | 0.09 | 0.17 | 0.06 | 0.04 |
a Significant population structure PhiPT>0.25 are shown in bold. Moderate population structure PhiPT = 0.1–0.25 is underlined.
b Southeastern Massachusetts
c Western Massachusetts
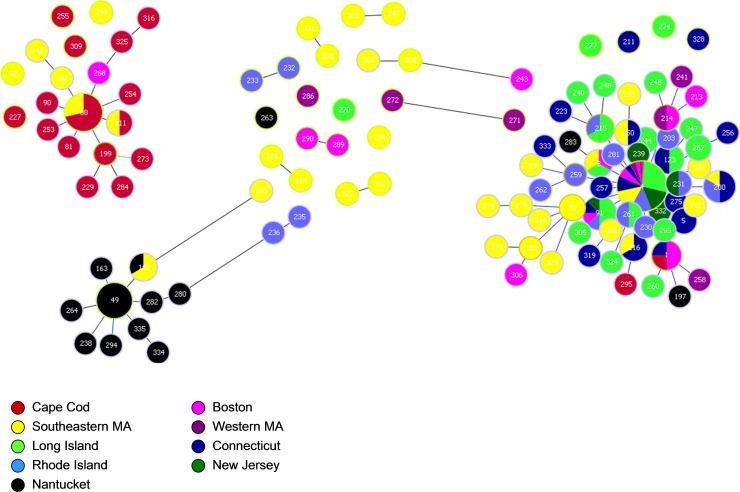
The eBurst algorithm of Phyloviz grouped the samples into 3 main clusters consisting of samples primarily from Nantucket (N population), samples primarily from Cape Cod (CC population) and those from all other sites except for SEMA (Mainland population) (Fig 3). Samples from SEMA were divided among all 3 populations. About 6% of the samples remained unresolved and were not connected to any of the 3 major groups; the majority of these (>75%) were from SEMA and RI.
Fig 3. Cluster analysis of B. microti samples using Phyloviz.
Each small bubble represents a unique haplotype. Bubbles are colored to correspond with the region from which the sample originated. The size is not directly correlated with the number of samples. Haplotypes that differ by a single locus are connected with a gray line. The large circles correspond with the population groupings calculated by TESS; blue is the Nantucket population, red is the Cape Cod population, green is mainland population and pink are the haplotypes that showed significant admixture and could not be placed solely in any of the 3 populations. Bubbles that are unconnected to the major groups are placed in the larger circles according to the ancestry coefficients from TESS.
By plotting the mean DIC for K populations from 2–8 (Fig 1), we determined that 3 populations, K = 3, best fit the data from TESS (Fig 4). Ancestry coefficients from 10 runs for K = 3 were estimated for each sample, and the CLUMPP algorithm was used to combine the data from all the runs (Fig 5). These coefficients indicate the probability of membership into each of the 3 populations and corresponded well with the results from Phyloviz (Fig 3). Many samples that remained unresolved with Phyloviz showed significant amount of admixture, which would explain the inability of that algorithm to decisively place them into any single cluster (Table 3 and Fig 3 inside pink circle). However, the agreement between the two methods was not unanimous. There were a few samples that Phyloviz was unable to assign to a cluster that TESS had >85% certainty of inclusion into one of the populations (see unconnected bubbles inside larger circles Fig 3), as well as samples that Phyloviz connected to major populations that TESS could not determine to >85% probability (see bubbles with grey connections stretched to fit into pink circle Fig 3 and Table 3).
Fig 4. Graph of the mean DIC.
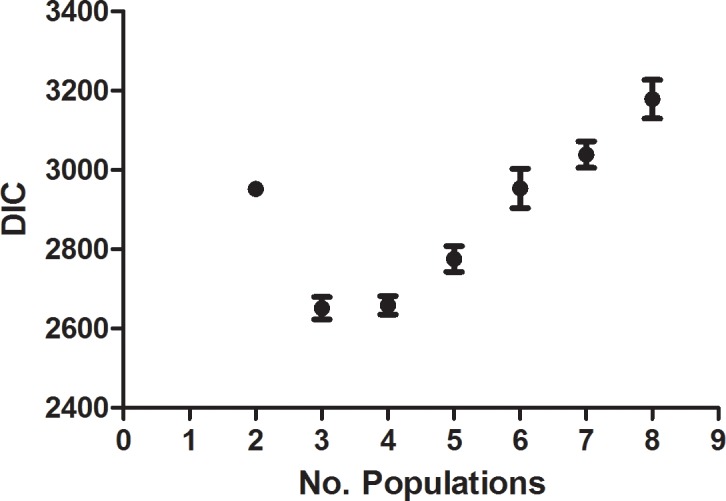
Mean deviance information criterion (DIC) was calculated from 10 individual TESS runs for population size 2–8. Three populations, K = 3, best fit the data.
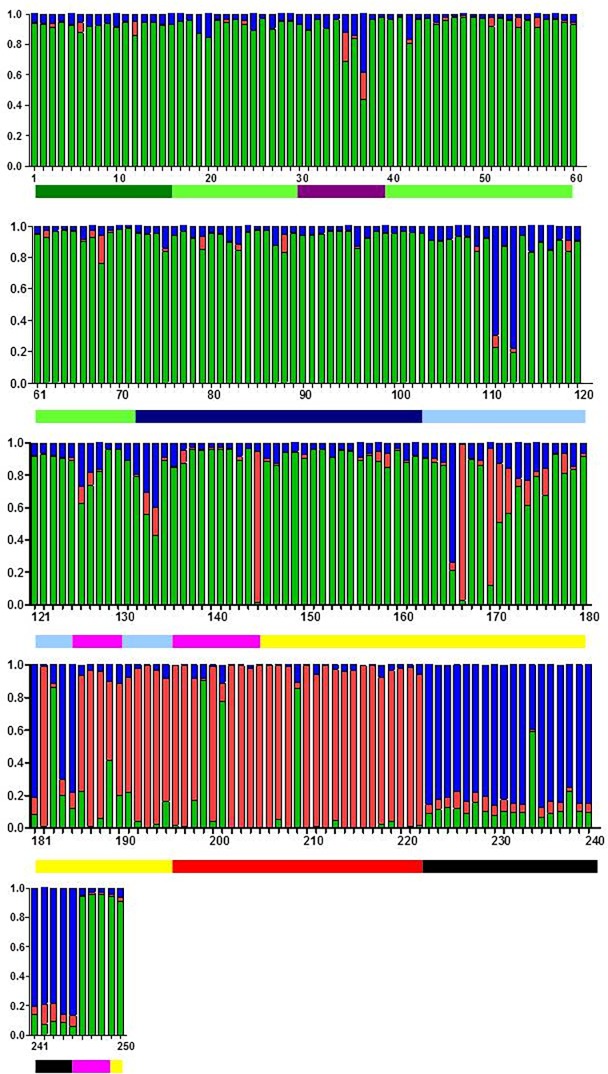
Fig 5. Ancestry coefficients from TESS for K = 3 populations.
Geographic distances between each sample point were calculated using TESS. Green corresponds to the mainland population, red is Cape Cod and blue is Nantucket. Lines beneath the bar chart indicate the source of the sample. Black = Nantucket, light blue = RI, dark blue = CT, purple = WMA, pink = Bos, red = CC, yellow = SeMA, dark green = NJ and light green = LI.
Table 3. Ancestry coefficients from TESS of samples that showed significant admixture.
| Haplotype | Region | M | CC | N |
|---|---|---|---|---|
| 197 | N | 0.59 | 0.01 | 0.39 |
| 272 | WMA | 0.44 | 0.18 | 0.38 |
| 286 | WMA | 0.69 | 0.19 | 0.12 |
| 315 | SEMA | 0.57 | 0.27 | 0.16 |
| 314 | SEMA | 0.51 | 0.37 | 0.13 |
| 327 | SEMA | 0.79 | 0.03 | 0.18 |
| 312 | SEMA | 0.67 | 0.17 | 0.16 |
| 307 | SEMA | 0.73 | 0.05 | 0.22 |
| 308 | SEMA | 0.62 | 0.15 | 0.23 |
| 232 | RI | 0.56 | 0.14 | 0.30 |
| 233 | RI | 0.43 | 0.17 | 0.40 |
| 289 | Bos | 0.56 | 0.14 | 0.30 |
| 290 | Bos | 0.43 | 0.17 | 0.40 |
| 331 | SEMA | 0.20 | 0.68 | 0.11 |
| 330 | SEMA | 0.41 | 0.49 | 0.10 |
| 310 | SEMA | 0.22 | 0.71 | 0.08 |
| 287 | SEMA | 0.22 | 0.71 | 0.06 |
| 235 | RI | 0.22 | 0.08 | 0.70 |
| 283 | N | 0.22 | 0.03 | 0.75 |
| 285 | SEMA | 0.20 | 0.09 | 0.70 |
| 234 | SEMA | 0.21 | 0.05 | 0.74 |
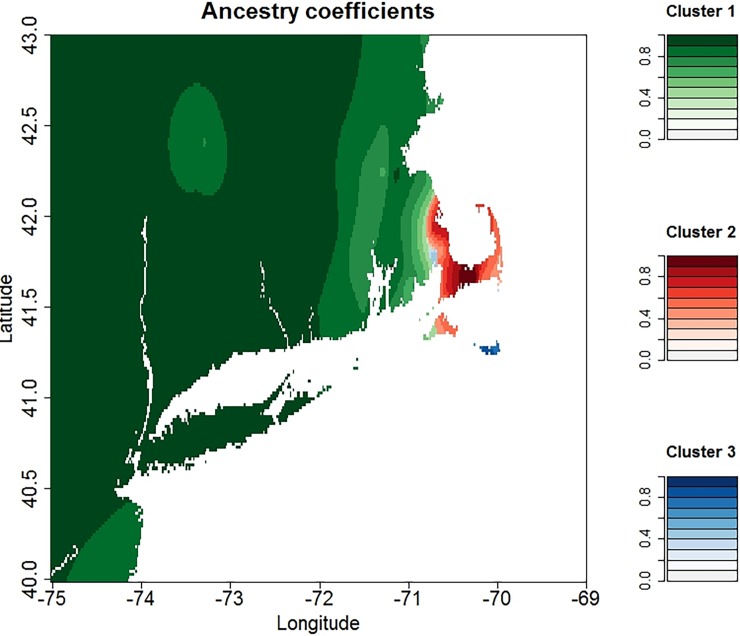
The geographically placed ancestry coefficients produced by TESS were spatially interpolated onto a map of New England (Fig 6). Haplotypes from the Nantucket population are primarily found on Nantucket. There has been some introduction into southeastern MA. The CC population also has limited scope: these haplotypes are found primarily on CC with some extending along the eastern coast of MA south of Boston. Contrary to the limited range of the N and CC populations, the mainland population dominates all of NJ, LI, CT, RI and MA, other than Cape Cod and Nantucket. It should be noted that this study did not include any data from Martha’s Vineyard; so it may be that the predicted populations included in this figure are erroneous.
Fig 6. Geographic interpolation of the ancestry coefficients showing the distribution of each population of B. microti.
Cluster 1(green) = mainland population, cluster 2 (red) = Cape Cod population, and cluster 3 (Blue) = Nantucket population. Areas with samples that have a high admixture coefficient, ie a high probability of membership to that population, are shaded darker. Lighter shades indicate areas where there the ancestry coefficients are lower, indicating areas where mixing is occurring. This study did not include data from Martha’s Vineyard; so the predicted populations on that island may be erroneous.
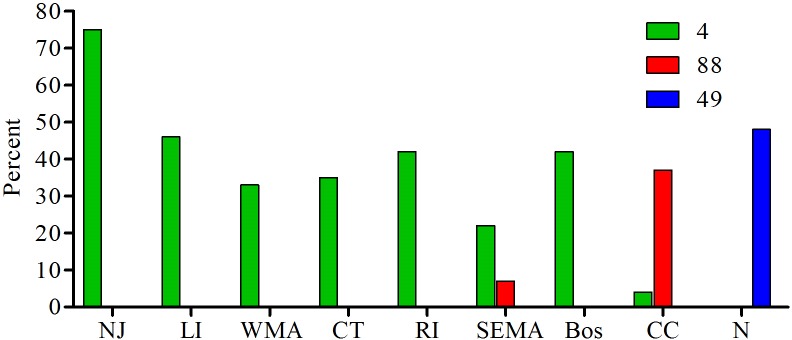
Each of the 3 populations has a dominant haplotype that is also the putative ancestral type (as determined by Phyloviz), type 4 for mainland, type 49 for Nantucket, and type 88 for Cape Cod (Table 4). Type 49 is present in 48% of Nantucket samples; Type 88 is found in 37% of Cape Cod samples, and type 4 ranges from 33% to 75% in the regions included in the mainland population (Fig 7). SEMA is the only region with a mixture of the dominant types; type 4 was detected in 22% of samples and type 88 detected in 7%. All other haplotypes in this study are detected only once or twice from any given region, with the exception of type 91 from LI which was found 4 times (8% of the observed haplotypes). Type 91 differs from the dominant type 4 by only the BMV1 locus (335bp instead of 340bp) of type 4.
Table 4. The microsatellite amplicon sizes of the 3 major haplotypes in base pairs.
| Haplotype | Pop | BMV1 | BMV2 | BMV5 | BMV8 | BMV10 | BMV13 | BMV23 | BMV20 |
|---|---|---|---|---|---|---|---|---|---|
| 4 | M | 340 | 405 | 317 | 241 | 305 | 396 | 243 | 695 |
| 49 | N | 340 | 405 | 317 | 241 | 305 | 520 | 248 | 713 |
| 88 | CC | 346 | 398 | 389 | 271 | 305 | 351 | 243 | 713 |
Fig 7. The percent of the total samples for each region for each of the main haplotypes: type 4, type 88 and type 49.
Discussion
Our analysis provides data to help reconstruct the processes that have led to the current epidemic population structure of B. microti in northeastern US. There are at least 3 distinct populations of B. microti in New England, as we suggested previously [31,32] in analyses of ecological as well as clinical samples, with PhiPT ranging from 0.32–0.67 between them (Table 2). Each of the three populations has a single dominant haplotype that is found in at least 30% of the samples from each site and is the presumed ancestral strain; type 4 for mainland, type 49 for Nantucket and type 88 for CC. Southeastern MA is currently experiencing a natural experiment as the 3 populations, CC, N and M, are zoonotic in this area. The CC population is moving northward and westward along the eastern coast of MA, the N type is invading from the southern coast, and the mainland type is invading from the west. The genetic signature from all 3 populations can be clearly detected in clinical samples from this area, and significant admixture is occurring (Fig 6). For this reason, the diversity of B. microti from SEMA is significantly greater than that from all other regions in our study. Although we do not know when each of the B. microti populations were first introduced into SEMA, nor which one arrived first, type 4 is found more often in this area and the majority of samples harbor loci that originate from type 4. This dominance is clearly represented in the map of the ancestry coefficients from TESS, and suggests that type 4 parasites have some attribute that allows for greater amplification than do the other B. microti populations. It may be that type 4 parasites are more transmissible.
If the expansion of B. microti in New England was caused by individual founders “pulling” the population, we would have expected the diversity estimates from ancestral sites (Nantucket; Cape Cod; Long Island; [11], where cases have been diagnosed since the 1970s, to be greater than those from incipient sites with more recent emergence of cases. However, this was not the case; the diversity estimates of B. microti from the regions we sampled across the northeastern United States were not significantly different. In fact, the diversity of B. microti from ancestral sites, such as Nantucket and Long Island, was no greater than those from more newly established sites. Furthermore, the diversity from coastal CT was not significantly different than that from northern CT where babesiosis cases were first detected 15 years later (data not shown). The maintenance of diversity across New England supports the theory that expansion was the result of a “pushing” population expansion, consistent with the stepping-stone hypothesis inferred by Walter and colleagues [8]. Notably different, however, were samples from NJ; their diversity was significantly less than those from every other site in our study; more than 70% of the parasite samples comprised the dominant type 4. The lack of genetic diversity is consistent with the New Jersey foci representing newly established populations that have experienced significant founder effects. However, B. microti-infected ticks were documented from northern New Jersey in the early 1990s [42] and human cases shortly thereafter [43]. New Jersey became endemic for babesiosis at the same time as northern CT and northern RI, but the diversity of B. microti from those states are similar to those from the rest of the study populations. The biological basis for the limited diversity found in New Jersey B. microti samples remains to be described.
Some patient samples may have been mistakenly assigned to location because we used convenience samples that were de-identified other than for site of the contributing clinical practice. We assumed that a case became exposed near the healthcare provider who provided the sample to Imugen for analysis. Residents of any of our sites are likely to travel within the northeast, and may vacation or visit in sites where risk is similar to where they live. We are confident, for example, that two samples from our Nantucket cohort acquired infection elsewhere. Each of these samples contained parasite haplotypes that grouped with the mainland population. We have analyzed sufficient numbers of ecological samples from Nantucket Island and have never detected the other lineages [31]. Despite this clear example of mistaken assignment, the outcome of our analysis did not appear to be effected; TESS correctly concluded that Nantucket Island is dominated solely by the Nantucket population and the other sites by their respective parasite populations. Accordingly, we believe that our analysis is robust enough to be unaffected by other unknown errors in geographic assignment of samples and that our conclusions about the population structure of B. microti in the northeastern U.S. are reasonable.
It is also possible that focusing our analysis solely on parasites derived from presumably symptomatic patients (those presenting to a healthcare provider who in turn requested analysis of a sample for confirmation of a diagnosis) does not capture variation of all those that may be present in the enzootic cycle of the mainland parasites. There is as yet no published evidence that the diversity of B. microti infectious for humans differs from that in local mice or ticks, i.e., that only a subset of naturally occurring strains are zoonotic. However, such an argument would need to apply across all sites and we note that there is much variation evident in parasites from patients presenting to healthcare providers on Nantucket, Cape Cod, or Southeastern Massachusetts.
Significant differentiation (PhiPT >0.36) between each of the 3 populations implies that they have been isolated from each other and remain so. We have previously speculated that the microbial guild transmitted by the deeer tick had been maintained in relict or refugial foci during glaciation [11]. Then too, postcolonial deforestation likely provided a fragmented landscape that only allowed for perpetuation of ticks and their hosts in small less-disturbed natural foci. The lack of differentiation among parasites from the mainland sites, from central NJ westward to RI, appears to be inconsistent with a scenario of multiple relict foci across the mainland northeastern landscape, with coalescence of the isolated demes occurring as a result of amplification and expansion of the foci as successional habitat increased over the last 100 years. In the 1990s, babesiosis was documented from 3 distinct sites within the area where the mainland population parasites have been detected, viz., Long Island, southeastern CT and central NJ. Each of these foci was isolated from the others; few cases were identified in areas between them. Ecological sampling, where it was done, supports the inference that B. microti was indeed absent or very rare [7,13,15,16,18,44]. We expected to detect a distinct genetic signature of multiple small isolated foci within parasites from the mainland lineages but there is little differentiation among LI, CT, RI and NJ, and our analyses group these sites together into a single population. In fact, the mainland haplotype, type 4, dominates from NJ eastward through NY, CT and RI and northward towards Boston, creating an epidemic population structure.
It may be that these sites were not isolated for sufficient time for genetic drift to operate, thereby explaining the lack of differentiation among mainland parasites. It is also possible that the epidemic population structure occurred purely by chance, i.e. genetic drift has occurred as B. microti has expanded leading to an overabundance of a single haplotype. Some alleles may reach a high frequency because of repeated founder events [22], a process called genetic surfing [26]. We assume that our VNTR loci are neutral or are not linked with loci under selection and thus the observed lack of variation is not due to selective constraints. The alternative hypothesis for the lack of diversity among mainland B. microti is that there were no refugial or relictual sites within fragments of forest, and that the parasite populations have not actually been isolated from each other, allowing sufficient gene flow within the various sites comprising the mainland. However, the population structure of the deer tick suggests otherwise. A seminal study of the population structure of this vector tick and B. burgdorferi infecting them [45] sampled 12 sites in the northeast from Massachusetts to Virginia; 5 of these overlap with our area of study. Mitochondrial 16SrDNA haplotypes demonstrated that the New York-CT region may have contained refugial tick populations that served as a source for expansion of the range of the deer tick. Although tick populations that were sampled were structured, this was not observed for B. burgdorferi, although the borrelial genes that were analyzed were likely to have been influenced by balancing selection [45]. Additional studies are required to identify the relative contributions of selective and demographic processes that serve as the basis for biogeographic variation in northeastern populations of B. microti.
We believe the most likely scenario is that type 4 parasites have selectively swept across the mainland landscape, replacing and erasing historic genetic signatures of other lineages. Such a hypothesis is not without precedent with the microbial guild maintained by I. persulcatus-like ticks. The population structure of B. afzelii (an Eurasian agent of Lyme disease that appears restricted to rodent hosts) in Sweden is essentially clonal, which may be the result of the epidemic spread of a single genotype [46]. Across Europe, however, B. afzelii has significant population structure [47], similar to what we have found in this study. There are likely public health implications of a specific B. microti lineage that appears to be rapidly expanding its range.
Acknowledgments
Many clinicians and clinical practices submit diagnostic samples to Imugen Inc for testing. Samples for this study were de-identified and thus we do not know the identities of their submitters, but we thank them for their contribution to this study.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Imugen Inc. did not play a role in the study design, data analysis, decision to publish, or preparation of the manuscript. Imugen Inc did provide data in the form of the location from where samples were derived. Imugen provided financial support in the form of authors' salaries other than SRT and/or research materials. The funder provided support in the form of salaries for authors [HKG, PJM, KW, VB], but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Western KA, Benson GD, Gleason NN, Healy GR, Schultz MG. Babesiosis in a Massachusetts Resident. N Engl J Med. 1970;283(16):854–6. doi: 10.1056/NEJM197010152831607 [DOI] [PubMed] [Google Scholar]
- 2.Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. An epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20(1):7–17. [DOI] [PubMed] [Google Scholar]
- 3.Spielman A, Wilson ML, Levine JF, Piesman J. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol. 1985;30(1):439–460. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Malawista S. Cases of Lyme Disease in the United States: Locations Correlated with Distribution of Ixodes dammini. Ann Intern Med. 1979. November 1;91(5):730–3. [DOI] [PubMed] [Google Scholar]
- 5.Piesman J, Mather TN, Donahue J, Levine J, Campbell JD, Karakashian SJ, et al. Comparative prevalence of Babesia microti and Borrelia burgdorferi in four populations of Ixodes dammini in eastern Massachusetts. Acta Trop. 1986. September;43(3):263–70. [PubMed] [Google Scholar]
- 6.Lastavica CC, Wilson ML, Berardi VP, Spielman A, Deblinger RD. Rapid Emergence of a Focal Epidemic of Lyme Disease in Coastal Massachusetts. N Engl J Med. 1989. January 19;320(3):133–7. doi: 10.1056/NEJM198901193200301 [DOI] [PubMed] [Google Scholar]
- 7.Joseph JT, Roy SS, Shams N, Visintainer P, Nadelman RB, Hosur S, et al. Babesiosis in Lower Hudson Valley, New York, USA. Emerg Infect Dis. 2011. May;17(5):843–7. doi: 10.3201/eid1705.101334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter KS, Pepin KM, Webb CT, Gaff HD, Krause PJ, Pitzer VE, et al. Invasion of two tick-borne diseases across New England: harnessing human surveillance data to capture underlying ecological invasion processes. Proc R Soc B Biol Sci. 2016. June 15;283(1832):20160834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herwaldt BL, Montgomery S, Woodhall D, Bosserman E. Babesiosis Surveillance—18 States, 2011. Morb Mortal Wkly Rep. 2012;61(27):505–9. [PubMed] [Google Scholar]
- 10.Brinkerhoff RJ, Gilliam WF, Gaines D. Lyme Disease, Virginia, USA, 2000–2011. Emerg Infect Dis. 2014. October;20(10):1661–8. doi: 10.3201/eid2010.130782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telford SR III, Spielman A. Enzootic transmission of Babesia microti In: Tick Borne Pathogens at the Host-Vector Interface. St. Paul, MN: University of Minnesota College of Agriculture; 1992. p. 259–64. [Google Scholar]
- 12.Anderson JF, Johnson RC, Magnarelli LA, Hyde FW. Involvement of birds in the epidemiology of the Lyme disease agent Borrelia burgdorferi. Infect Immun. 1986. February;51(2):394–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson JF, Magnarelli LA, Kurz J. Intraerythrocytic Parasites in Rodent Populations of Connecticut: Babesia and Grahamella Species. J Parasitol. 1979. August 1;65(4):599–604. [PubMed] [Google Scholar]
- 14.Goethert HK, Telford S III. What is Babesia microti? Parasitology. 2003. October;127(04):301–309. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers SE, Mather TN. Human Babesia microti Incidence and Ixodes scapularis Distribution, Rhode Island, 1998–2004. Emerg Infect Dis. 2007. April;13(4):633–5. doi: 10.3201/eid1304.061035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stafford KC, Williams SC, Magnarelli LA, Bharadwaj A, Ertel S-H, Nelson RS. Expansion of Zoonotic Babesiosis and Reported Human Cases, Connecticut, 2001–2010. J Med Entomol. 2014. January 1;51(1):245–52. [DOI] [PubMed] [Google Scholar]
- 17.Xue L, Scoglio C, McVey DS, Boone R, Cohnstaedt LW. Two Introductions of Lyme Disease into Connecticut: A Geospatial Analysis of Human Cases from 1984 to 2012. Vector-Borne Zoonotic Dis. 2015. September;15(9):523–8. doi: 10.1089/vbz.2015.1791 [DOI] [PubMed] [Google Scholar]
- 18.Kogut SJ, Thill CD, Prusinski MA, Lee J-H, Backenson PB, Coleman JL, et al. Babesia microti, Upstate New York. Emerg Infect Dis. 2005. March;11(3):476–8. doi: 10.3201/eid1103.040599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott JD, Anderson JF, Durden LA. Widespread Dispersal of Borrelia burgdorferi–Infected Ticks Collected from Songbirds Across Canada. J Parasitol. 2011. August 24;98(1):49–59. doi: 10.1645/GE-2874.1 [DOI] [PubMed] [Google Scholar]
- 20.Mather TN, Nicholson MC, Hu R, Miller NJ. Entomological correlates of Babesia microti prevalence in an area where Ixodes scapularis (Acari: Ixodidae) is endemic. J Med Entomol. 1996;33(5):866–870. [DOI] [PubMed] [Google Scholar]
- 21.Goodsman DW, Cooke B, Coltman DW, Lewis MA. The genetic signature of rapid range expansions: How dispersal, growth and invasion speed impact heterozygosity and allele surfing. Theor Popul Biol. 2014. December;98:1–10. doi: 10.1016/j.tpb.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 22.Edmonds CA, Lillie AS, Cavalli-Sforza LL. Mutations arising in the wave front of an expanding population. Proc Natl Acad Sci U S A. 2004;101(4):975–979. doi: 10.1073/pnas.0308064100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roques L, Garnier J, Hamel F, Klein EK. Allee effect promotes diversity in traveling waves of colonization. Proc Natl Acad Sci. 2012. June 5;109(23):8828–33. doi: 10.1073/pnas.1201695109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden NH, Mechai S, Margos G. Changing geographic ranges of ticks and tick-borne pathogens: drivers, mechanisms and consequences for pathogen diversity. Front Cell Infect Microbiol [Internet]. 2013. [cited 2014 Apr 16];3 Available from: http://www.frontiersin.org/Journal/10.3389/fcimb.2013.00046/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klopfstein S. The Fate of Mutations Surfing on the Wave of a Range Expansion. Mol Biol Evol. 2005. December 20;23(3):482–90. doi: 10.1093/molbev/msj057 [DOI] [PubMed] [Google Scholar]
- 26.Excoffier L, Foll M, Petit RJ. Genetic Consequences of Range Expansions. Annu Rev Ecol Evol Syst. 2009. December;40(1):481–501. [Google Scholar]
- 27.Goethert HK, Shani I, Telford SR. Genotypic Diversity of Francisella tularensis Infecting Dermacentor variabilis Ticks on Martha’s Vineyard, Massachusetts. J Clin Microbiol. 2004. November 1;42(11):4968–73. doi: 10.1128/JCM.42.11.4968-4973.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farlow J, Postic D, Smith KL, Jay Z, Baranton G, Keim P. Strain Typing of Borrelia burgdorferi, Borrelia afzelii, and Borrelia garinii by Using Multiple-Locus Variable-Number Tandem Repeat Analysis. J Clin Microbiol. 2002. December 1;40(12):4612–8. doi: 10.1128/JCM.40.12.4612-4618.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Llaneza A, Caballero M, Baravalle E, Mesplet M, Mosqueda J, Suarez CE, et al. Development of a tandem repeat-based multilocus typing system distinguishing Babesia bovis geographic isolates. Vet Parasitol. 2010. February 10;167(2–4):196–204. doi: 10.1016/j.vetpar.2009.09.021 [DOI] [PubMed] [Google Scholar]
- 30.Dugat T, Chastagner A, Lagrée A-C, Petit E, Durand B, Thierry S, et al. A new multiple-locus variable-number tandem repeat analysis reveals different clusters for Anaplasma phagocytophilum circulating in domestic and wild ruminants. Parasit Vectors. 2014;7(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goethert HK, Telford SR. Not “out of Nantucket”: Babesia microti in southern New England comprises at least two major populations. Parasit Vectors. 2014. December 10;7(1):546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemieux JE, Tran AD, Freimark L, Schaffner SF, Goethert H, Andersen KG, et al. A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol. 2016. June 13;1(7):16079 doi: 10.1038/nmicrobiol.2016.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudzinska MA, Spielman A, Lewengrub S, Trager W, Piesman J. Sexuality in piroplasms as revealed by electron microscopy in Babesia microti. Proc Natl Acad Sci. 1983. May 1;80(10):2966–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francisco AP, Vaz C, Monteiro PT, Melo-Cristino J, Ramirez M, Carriço JA. PHYLOViZ: phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinformatics. 2012. May 8;13(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caye K, Deist TM, Martins H, Michel O, François O. TESS3: fast inference of spatial population structure and genome scans for selection. Mol Ecol Resour. 2016. March;16(2):540–8. doi: 10.1111/1755-0998.12471 [DOI] [PubMed] [Google Scholar]
- 36.Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007. July 15;23(14):1801–6. doi: 10.1093/bioinformatics/btm233 [DOI] [PubMed] [Google Scholar]
- 37.François O. Running Structure-like Population Genetic Analyses with R. 2016 [cited 2017 May 23]; Available from: http://www-timc.imag.fr/Olivier.Francois/tutoRstructure.pdf
- 38.Raymond M, Rousset F. GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. J Hered. 1995;86(3):248–249. [Google Scholar]
- 39.Rousset F. genepop’007: a complete re-implementation of the genepop software for Windows and Linux. Mol Ecol Resour. 2008. January;8(1):103–6. doi: 10.1111/j.1471-8286.2007.01931.x [DOI] [PubMed] [Google Scholar]
- 40.Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. Bioinformatics. 2012. October 1;28(19):2537–9. doi: 10.1093/bioinformatics/bts460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4(1):9. [Google Scholar]
- 42.Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County. Emerg Infect Dis. 1998;4(1):97 doi: 10.3201/eid0401.980113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herwaldt BL, McGovern PC, Gerwel MP, Easton RM, MacGregor RR. Endemic babesiosis in another eastern state: New Jersey. Emerg Infect Dis. 2003;9(2):184 doi: 10.3201/eid0902.020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prusinski MA, Kokas JE, Hukey KT, Kogut SJ, Lee J, Backenson PB. Prevalence of Borrelia burgdorferi (Spirochaetales: Spirochaetaceae), Anaplasma phagocytophilum (Rickettsiales: Anaplasmataceae), and Babesia microti (Piroplasmida: Babesiidae) in Ixodes scapularis(Acari: Ixodidae) Collected From Recreational Lands in the Hudson Valley Region, New York State. J Med Entomol. 2014. January 1;51(1):226–36. [DOI] [PubMed] [Google Scholar]
- 45.Qiu W-G, Dykhuizen DE, Acosta MS, Luft BJ. Geographic Uniformity of the Lyme Disease Spirochete (Borrelia burgdorferi) and Its Shared History With Tick Vector (Ixodes scapularis) in the Northeastern United States. Genetics. 2002. March 1;160(3):833–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hellgren O, Andersson M, RåBerg L. The genetic structure of Borrelia afzelii varies with geographic but not ecological sampling scale: Genetic structure of Borrelia afzelii. J Evol Biol. 2011. January;24(1):159–67. doi: 10.1111/j.1420-9101.2010.02148.x [DOI] [PubMed] [Google Scholar]
- 47.Vollmer SA, Feil EJ, Chu C-Y, Raper SL, Cao W-C, Kurtenbach K, et al. Spatial spread and demographic expansion of Lyme borreliosis spirochaetes in Eurasia. Infect Genet Evol. 2013. March;14:147–55. doi: 10.1016/j.meegid.2012.11.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.