Abstract
OBJECTIVE
Hemoglobin A1c (HbA1c) level has been associated with increased mortality in middle-aged populations. The optimal intensity of glucose control in older adults with diabetes remains uncertain. We sought to estimate the risk of mortality by HbA1c levels among older adults with and without diabetes.
RESEARCH DESIGN AND METHODS
We analyzed data from adults aged ≥65 years (n = 7,333) from the Third National Health and Nutrition Examination Survey (NHANES III) (1998–1994) and Continuous NHANES (1999–2004) and their linked mortality data (through December 2011). Cox proportional hazards models were used to examine the relationship of HbA1c with the risk of all-cause and cause-specific (cardiovascular disease [CVD], cancer, and non-CVD/noncancer) mortality, separately for adults with diabetes and without diabetes.
RESULTS
Over a median follow-up of 8.9 years, 4,729 participants died (1,262 from CVD, 850 from cancer, and 2,617 from non-CVD/noncancer causes). Compared with those with diagnosed diabetes and an HbA1c <6.5%, the hazard ratio (HR) for all-cause mortality was significantly greater for adults with diabetes with an HbA1c >8.0%. HRs were 1.6 (95% CI 1.02, 2.6) and 1.8 (95% CI 1.3, 2.6) for HbA1c 8.0–8.9% and ≥9.0%, respectively (P for trend <0.001). Participants with undiagnosed diabetes and HbA1c >6.5% had a 1.3 (95% CI 1.03, 1.8) times greater risk of all-cause mortality compared with participants without diabetes and HbA1c 5.0–5.6%.
CONCLUSIONS
An HbA1c >8.0% was associated with increased risk of all-cause and cause-specific mortality in older adults with diabetes. Our results support the idea that better glycemic control is important for reducing mortality; however, in light of the conflicting evidence base, there is also a need for individualized glycemic targets for older adults with diabetes depending on their demographics, duration of diabetes, and existing comorbidities.
INTRODUCTION
Studies in middle-aged adults report that higher levels of glycated hemoglobin (HbA1c) are associated with an increased risk of mortality among individuals with diabetes (1). In a nationally representative sample of adults 20 years of age and older from the National Health and Nutrition Examination Survey (NHANES), higher levels of HbA1c were associated with a higher risk of all-cause and cause-specific (e.g., cardiovascular disease [CVD] and cancer) mortality (1). However, few studies have had sufficient data to assess the association between HbA1c and mortality in an exclusive sample of adults aged 65 years and older with and without diabetes, a population at potentially higher risk of adverse diabetes-related complications.
In the late 1990s, the UK Prospective Diabetes Study (UKPDS) showed a reduction in microvascular complications (e.g., neuropathy and retinopathy) associated with intensive glucose lowering (2,3), which led to the recommendation of more intensive treatment to lower HbA1c (i.e., HbA1c <7.0%) in adults with diabetes. However, in the late 2000s, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group found that very intensive therapy (i.e., HbA1c <6.5%) resulted in increased mortality over 3.5 years (4). The Veterans Affairs Diabetes Trial (VADT) also found that very intensive glucose lowering did not reduce mortality or diabetes-related complications (5). Post hoc analyses of these and other trials have also shown benefits with respect to cardiovascular events and mortality (6,7). Largely, data on the benefits and outcomes associated with HbA1c lowering remain heterogeneous, particularly among populations of older adults (8–11). Therefore, more studies of the HbA1c–mortality relationship are needed to affirm the current clinical practice recommendations and guidelines related to the care of diabetes for older adults (12).
In 2013, the American Geriatrics Society (AGS) Expert Panel on the Care of Older Adults with Diabetes Mellitus published updated guidelines on improving the care of older adults with diabetes (13). General recommendations for glycemic control include the following: 1) target HbA1c goal between 7.5 and 8.0%, 2) target HbA1c may be lowered to between 7.0 and 7.5% if the older adult is considered healthy with few comorbidities and good functional status, and 3) target HbA1c may be increased to between 8 and 9% if the older adult has multiple comorbidities, is in poor health, or has a limited life expectancy. It is recommended that glycemic goals be individualized depending on the patient goals, life expectancy, and overall health status. Consensus panel recommendations from the American Diabetes Association (ADA), as well as the current American Association of Clinical Endocrinologists (AACE) and European Association for the Study of Diabetes (EASD) guidelines and position statements, promote the individualization of glycemic targets in older adults based on health status (14,15).
Given these recent recommendations, we sought to examine the risk of all-cause and cause-specific mortality across HbA1c levels to inform these clinical recommendations in a nationally representative sample of older adults. We analyzed data from the NHANES III (1988–1994) and Continuous NHANES (1999–2004) and their linked mortality data (through December 2011) to determine the risk of mortality by levels of HbA1c in older adults with and without diabetes.
RESEARCH DESIGN AND METHODS
NHANES and Linked Mortality File
The National Center for Health Statistics (NCHS) established the NHANES, a series of cross-sectional examinations that were designed to study diseases and their risk factors among community-dwelling individuals 20 years of age and older in the U.S. The NHANES examinations include interview survey questions on health and nutrition and a physical exam with laboratory testing. We analyzed data from the survey interview and physical examination within NHANES III (1988–1994, n = 33,994) and Continuous NHANES (1999–2004, n = 31,126) and their linked mortality data (through 31 December 2011). In total, 8,969 participants were 65 years of age and older from NHANES III (n = 5,252) and Continuous NHANES (n = 3,717). For this analysis, the study population was limited to adults ≥65 years of age who had available data on physician diagnosis of diabetes, oral and/or insulin medication use, and HbA1c (n = 7,333).
Diabetes and HbA1c Assessment
At the NHANES examination, participants were asked the following questions about their health and diabetes: “Have you ever been told by a doctor or other health professional you had diabetes or sugar diabetes?”; “Are you now taking insulin?”; and “Are you now taking diabetes pills?” Individuals were categorized by diabetes status (no diabetes, undiagnosed diabetes, and diagnosed diabetes) based on their response to these questions and their measured HbA1c at baseline. If participants answered no to 1) a physician diagnosis of diabetes and 2) oral and/or insulin medication use, they were categorized as not having diagnosed diabetes. These participants were further stratified into the following HbA1c categories: <5.0% (excluding participants with HbA1c <4.5% from the analytic sample), 5.0–5.6%, or 5.7–6.4%. Individuals were classified as having undiagnosed diabetes if they met all of the following criteria: 1) no self-report of a physician diagnosis of diabetes, 2) no self-report of oral and/or insulin medication use, and 3) an HbA1c ≥6.5%. Older adults were considered to have diagnosed diabetes if they met any of the following criteria: 1) self-report of a physician diagnosis of diabetes or 2) self-report of oral and/or insulin medication use. These individuals were further stratified into the following HbA1c categories: <6.5% (excluding participants with HbA1c <4.5% from the analytic sample), 6.5–6.9%, 7.0–7.9%, 8.0–8.9%, or ≥9.0%.
Mortality Outcomes
Ascertainment of mortality in NCHS, which includes those survey participants from NHANES, was based on a probabilistic record match between participants in NHANES and the National Death Index (NDI) death certificate records (16). Briefly, the NDI is an NCHS centralized database of all deaths in the U.S. beginning in 1979. Several sources are used to determine vital status, including linkages with the U.S. Social Security Administration and/or through active follow-up of survey participants. Mortality outcomes of interest in this analysis include all-cause and cause-specific (CVD, cancer, and non-CVD/noncancer), based on ICD-10 codes defined in NHANES.
Statistical Analyses
An initial descriptive analysis utilized χ2 and ANOVA tests to examine significant differences in baseline demographic and disease characteristics across levels of HbA1c and diabetes status. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% CI for the association of HbA1c levels with all-cause and cause-specific mortality (CVD, cancer, and non-CVD/noncancer), adjusted for potential confounders of the HbA1c–mortality associations including age, sex, race/ethnicity, education, BMI, smoking status, HDL cholesterol, and hypertension. Models were run separately among 1) participants without diabetes or with undiagnosed diabetes (reference: no diabetes and HbA1c 5.0–5.6%, given a larger sample size and prior literature that suggests an increased risk of mortality for very low HbA1c levels [17]) and 2) participants with diagnosed diabetes (reference: diabetes and HbA1c <6.5%). We also performed stratified analyses for all-cause mortality by age (65–75 years vs. >75 years), sex, race (non-Hispanic white, non-Hispanic black, or Mexican American/other Hispanic), and presence of CVD. Additionally, among individuals with diagnosed diabetes only, we examined all-cause mortality by duration of diabetes and treatment modality (any oral vs. any insulin). Independent of diagnosed diabetes status, a subsidiary analysis was performed to quantify the HR of all-cause mortality across all HbA1c levels (reference: HbA1c 5.0% for all comparisons). All analyses were weighted to represent the U.S. population and to account for the complex survey design. Analyses were performed using STATA 13.0 (STATA Corp, College Station, TX).
Sensitivity Analysis
Because diabetes type is not available within the NHANES data, we are uncertain as to whether the participants with diabetes have type 1 or type 2 diabetes. To address this, we performed a sensitivity analysis excluding participants with a diagnosis of diabetes before the age of 30 years (n = 279).
RESULTS
Baseline demographic and clinical characteristics of the study population are provided in Table 1 by diabetes status and HbA1c level. Participants without diabetes and with a higher HbA1c level were more often non-Hispanic black, had a higher BMI, and had lower HDL cholesterol. As expected, individuals without diabetes and in the highest category of HbA1c (≥6.5%), who would be classified as having undiagnosed diabetes, exhibited common metabolic and sociodemographic risk factors for this group (i.e., these participants were more often non-Hispanic black and had a less than high school education, a higher CVD burden, and lower HDL cholesterol). Among those with diagnosed diabetes, there were a greater proportion of women across higher HbA1c categories. There were also a higher proportion of participants who had a less than a high school education and prevalent CVD across higher HbA1c categories. Mean HDL cholesterol was lower across higher HbA1c categories among those with diabetes. Duration of diabetes was shorter among individuals with diabetes across higher HbA1c categories (P < 0.001). Insulin use was reported more frequently among adults with diabetes in higher HbA1c categories (P < 0.001).
Table 1.
Baseline characteristics of 7,333 NHANES III (1988–1994) and Continuous NHANES (1999–2004) participants aged 65 years and older by HbA1c levels and diabetes status
| Characteristic | No diabetes |
Undiagnosed diabetes |
Diagnosed diabetes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| <5.0% (n = 381) | 5.0–5.6% (n = 3,252) | 5.7–6.4% (n = 2,080) | ≥6.5% (n = 341) | <6.5% (n = 447) | 6.5–7.0% (n = 193) | 7.0–7.9% (n = 272) | 8.0–8.9% (n = 166) | ≥9.0% (n = 201) | |
| Age (years) | 73.5 (0.2) | 72.7 (0.4) | 74.0 (0.2) | 73.8 (0.5) | 73.7 (0.4) | 74.2 (0.6) | 72.7 (0.5) | 72.5 (0.6) | 72.5 (0.6) |
| Female sex* | 59.1 | 54.4 | 57.6 | 44.0 | 57.8 | 56.1 | 49.4 | 53.5 | 65.8 |
| Non-Hispanic black* | 4.4 | 7.3 | 9.2 | 16.4 | 11.1 | 14.5 | 9.7 | 13.5 | 14.1 |
| Less than high school education* | 33.3 | 37.0 | 41.5 | 41.8 | 41.5 | 45.6 | 46.2 | 43.7 | 52.5 |
| Current smoker | 20.3 | 25.3 | 25.0 | 17.9 | 18.1 | 16.4 | 21.7 | 7.4 | 16.7 |
| Cancer | 23.7 | 24.5 | 22.9 | 22.4 | 24.0 | 16.4 | 19.6 | 22.7 | 23.7 |
| CVD* | 26.1 | 31.5 | 31.0 | 33.8 | 42.5 | 48.8 | 47.0 | 52.4 | 58.1 |
| BMI (kg/m2)* | 26.5 (0.1) | 25.9 (0.3) | 27.6 (0.2) | 29.4 (0.3) | 29.4 (0.4) | 30.3 (0.7) | 29.3 (0.5) | 30.0 (0.6) | 29.6 (0.7) |
| HDL cholesterol (mg/dL)* | 55.5 (0.5) | 56.4 (1.4) | 51.0 (0.5) | 45.9 (1.2) | 50.3 (1.1) | 48.9 (1.2) | 44.3 (1.3) | 46.2 (1.4) | 47.1 (1.7) |
| Diabetes duration (years)* | 30.6 (2.7) | 32.8 (2.3) | 25.1 (1.7) | 19.1 (2.7) | 19.1 (1.6) | ||||
| Oral diabetes medication user | 52.4 | 65.3 | 58.2 | 63.2 | 55.0 | ||||
| Insulin user* | 15.8 | 23.1 | 38.7 | 37.0 | 53.0 | ||||
Data are weighted estimates. Data are % or means (SE).
*P for trend <0.05 across HbA1c categories.
The median follow-up was 8.9 years. Among those participants who died (n = 4,729 [64.5%], 73.7 per 1,000 person-years), 1,262 (26.7%) died due to CVD, 850 (18.0%) died due to cancer, and 2,617 (55.3%) died due to non-CVD/noncancer related causes. Table 2 provides the unadjusted mortality rate (per 1,000 person-years) across HbA1c levels and diabetes status for all-cause and cause-specific mortality. The mortality rate increased from 77.5 per 1,000 person-years among individuals with diagnosed diabetes (n = 1,279) who have an HbA1c <6.5%, to 97.1 and 104.4 per 1,000 person-years for individuals with diabetes and an HbA1c of 8.0–8.9% and ≥9.0%, respectively. For persons with diabetes, modest mortality rate increases were observed across HbA1c levels for CVD-related (20.0 per 1,000 person-years for HbA1c <6.5% compared with 25.9 and 31.5 per 1,000 person-years for HbA1c 8.0–8.9% and ≥9.0%, respectively) and cancer-related mortality (11.1 per 1,000 person-years for HbA1c <6.5% compared with 15.6 per 1,000 person-years for HbA1c 8.0–8.9%). The HR for all-cause mortality increased significantly across HbA1c levels among older adults with and without diabetes (both P for trend <0.001). Specifically, an HbA1c >8.0% was associated with increased risk of all-cause and cause-specific mortality in older adults with diabetes. Compared with participants with diabetes and an HbA1c <6.5%, the risk of CVD mortality increased significantly only for those participants with an HbA1c ≥9.0% (HR 2.5 [95% CI 1.1, 5.3]).
Table 2.
Relative HRs (95% CI) for the association between HbA1c cut points among individuals with and without diabetes and all-cause and cause-specific mortality among 7,333 adults aged 65 years and older from NHANES III (1988–1994) and Continuous NHANES (1999–2004) through December 2011
| No diabetes |
Undiagnosed diabetes |
Diagnosed diabetes |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <5.0% (n = 381) | 5.0–5.6% (n = 3,252) | 5.7–6.4% (n = 2,080) | ≥6.5% (n = 341) | <6.5% (n = 447) | 6.5–7.0% (n = 193) | 7.0–7.9% (n = 272) | 8.0–8.9% (n = 166) | ≥9.0% (n = 201) | |||
| All-cause mortality | 0.9 (0.7, 1.2) | 1.0** (reference) | 1.2* (1.01, 1.3) | 1.3* (1.03, 1.8) | 1.0** (reference) | 1.2 (0.8, 1.7) | 1.2 (0.8, 1.7) | 1.6* (1.02, 2.6) | 1.8* (1.3, 2.6) | ||
| Rate per 1,000 person-years | 75.4 (67.1, 84.7) | 61.8 (59.2, 64.6) | 67.7 (64.1, 71.4) | 70.8 (62.3, 80.5) | 77.5 (69.1, 86.9) | 89.6 (75.3, 106.5) | 83.8 (72.5, 96.8) | 97.1 (81.8, 115.2) | 104.4 (89.4, 121.9) | ||
| CVD mortality | 0.9 (0.5, 1.6) | 1.0 (reference) | 1.1 (0.8, 1.4) | 1.4 (0.8, 2.6) | 1.0** (reference) | 1.1 (0.5, 2.6) | 1.7 (0.8, 3.4) | 1.5 (0.6, 4.1) | 2.5* (1.1, 5.3) | ||
| Rate per 1,000 person-years | 18.3 (14.4, 23.1) | 15.9 (14.6, 17.3) | 18.2 (16.5, 20.2) | 20.7 (16.3, 26.2) | 20.0 (16.1, 25.1) | 35.0 (26.5, 46.2) | 21.7 (16.4, 28.8) | 25.9 (18.6, 36.1) | 31.5 (23.7, 41.8) | ||
| Cancer mortality | 0.8 (0.5, 1.3) | 1.0 (reference) | 1.1 (0.8, 1.5) | 0.8 (0.4, 1.6) | 1.0** (reference) | 1.5 (0.4, 5.2) | 1.4 (0.6, 3.4) | 4.7* (1.8, 12.6) | 2.4 (0.8, 6.8) | ||
| Rate per 1,000 person-years | 14.3 (10.9, 18.7) | 11.8 (10.7, 13.0) | 13.3 (11.8, 15.0) | 12.8 (9.4, 17.3) | 11.1 (8.2, 15.0) | 5.6 (2.8, 11.2) | 10.4 (6.9, 15.7) | 15.6 (10.1, 23.9) | 13.1 (8.5, 20.4) | ||
| Non-CVD/noncancer mortality | 1.0 (0.8, 1.3) | 1.0** (reference) | 1.2* (1.0, 1.5) | 1.6* (1.1, 2.4) | 1.0 (reference) | 1.1 (0.7, 1.9) | 1.0 (0.6, 1.5) | 1.0 (0.4, 2.4) | 1.6* (1.01, 2.4) | ||
| Rate per 1,000 person-years | 42.9 (36.7, 50.0) | 34.2 (32.2, 36.3) | 36.1 (33.5, 38.8) | 37.4 (31.3, 44.6) | 46.4 (40.0, 53.8) | 49.0 (38.8, 61.9) | 51.6 (43.0, 62.0) | 55.6 (44.3, 69.7) | 59.7 (48.6, 73.4) | ||
Data are weighted estimates. Models are adjusted for age, sex, education, race, current smoking status, BMI, HDL cholesterol, and hypertension.
*P < 0.05.
**P for trend <0.001.
Among individuals with no diabetes or undiagnosed diabetes (n = 6,054), the HR for all-cause and non-CVD/noncancer mortality increased significantly across HbA1c levels compared with individuals with normoglycemia (HbA1c 5.0–5.6%) (Table 2). However, the HRs for CVD- and cancer-specific mortality did not differ significantly across HbA1c levels.
Stratified analyses were performed to examine possible differences in HRs for all-cause mortality by age strata (65–75 years and >75 years), sex, race, presence of CVD, duration of diabetes, and diabetes treatment modality (Table 3). The risk of all-cause mortality increased across all HbA1c categories among adults aged 65–74 years, while mortality was not significantly higher among adults aged 75 years and older across any HbA1c category. Associations were stronger in non-Hispanic whites and Mexican American/other Hispanic individuals than in non-Hispanic blacks. The risk of all-cause mortality was most pronounced among females and individuals with prevalent CVD and an HbA1c >8.0%. In this sample of older adults, duration of diabetes and diabetes treatment modality did not significantly impact the risk of mortality among individuals with diagnosed diabetes, although this finding may be confounded by indication for disease severity.
Table 3.
Subgroup analysis of relative HRs (95% CI) for the association between HbA1c cut points for individuals with and without diabetes and all-cause mortality among 7,333 adults aged 65 years and older from NHANES III (1988–1994) and Continuous NHANES (1999–2004) through December 2011
| No diabetes |
Undiagnosed diabetes |
Diagnosed diabetes | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <5.0% (n = 381) | 5.0–5.6% (n = 3,252) | 5.7–6.4% (n = 2,080) | ≥6.5% (n = 341) | <6.5% (n = 447) | 6.5–7.0% (n = 193) | 7.0–7.9% (n = 272) | 8.0–8.9% (n = 166) | ≥9.0% (n = 201) | |
| Age | |||||||||
| 65–74 years (n = 3,964) | 0.9 (0.7, 1.2) | 1.0 (reference) | 1.3* (1.1, 1.5) | 1.4* (1.03, 1.9) | 1.0 (reference) | 1.1 (0.7, 2.0) | 1.4 (0.9, 2.1) | 2.1* (1.2, 3.9) | 2.0* (1.0, 3.5) |
| ≥75 years (n = 3,369) | 1.2 (0.9, 1.6) | 1.0 (reference) | 1.0 (0.8, 1.2) | 1.3 (0.9, 1.9) | 1.0 (reference) | 1.2 (0.7, 1.9) | 0.8 (0.5, 1.4) | 1.0 (0.5, 1.9) | 1.5 (0.9, 2.6) |
| Sex | |||||||||
| Male (n = 3,574) | 1.0 (0.8, 1.3) | 1.0 (reference) | 1.1 (1.0, 1.4) | 1.2 (0.9, 1.7) | 1.0 (reference) | 1.4 (0.7, 2.7) | 1.5* (1.03, 2.3) | 1.5 (0.8, 2.7) | 1.9* (1.1, 3.1) |
| Female (n = 3,759) | 0.9 (0.5, 1.5) | 1.0 (reference) | 1.2 (0.9, 1.5) | 1.8* (1.2, 2.6) | 1.0 (reference) | 1.2 (0.6, 2.2) | 1.1 (0.5, 2.7) | 3.0* (1.5, 6.2) | 1.7 (0.6, 4.5) |
| Race/ethnicity | |||||||||
| Non-Hispanic white (n = 4,570) | 0.9 (0.6, 1.2) | 1.0 (reference) | 1.2 (1.0, 1.3) | 1.4* (1.1, 1.9) | 1.0 (reference) | 1.3 (0.8, 2.0) | 1.3 (0.8, 2.1) | 2.0* (1.1, 3.5) | 2.2* (1.4, 3.4) |
| Non-Hispanic black (n = 1,178) | 1.2 (0.8, 1.7) | 1.0 (reference) | 0.9 (0.7, 1.1) | 1.3 (0.9, 1.9) | 1.0 (reference) | 0.7 (0.2, 2.2) | 0.5* (0.3, 0.8) | 0.7 (0.4, 1.3) | 1.1 (0.4, 2.6) |
| Mexican American/other Hispanic (n = 1,302) | 1.6 (0.8, 3.1) | 1.0 (reference) | 1.5* (1.1, 2.1) | 2.6* (1.5, 4.6) | 1.0** (reference) | 1.3 (0.7, 2.6) | 0.9 (0.5, 1.9) | 1.1 (0.5, 2.7) | 1.3 (0.6, 2.8) |
| CVD | |||||||||
| Yes (n = 1,383) | 1.0 (0.5, 2.1) | 1.0 (reference) | 1.3 (1.0, 1.8) | 1.2 (0.7, 2.0) | 1.0 (reference) | 1.6 (0.8, 3.3) | 1.3 (0.7, 2.5) | 4.1* (1.7, 10.0) | 2.6 (0.8, 8.0) |
| No (n = 2,469) | 1.0 (0.6, 1.9) | 1.0 (reference) | 1.0 (0.7, 1.3) | 1.1 (0.7, 1.8) | 1.0 (reference) | 1.3 (0.5, 3.1) | 0.8 (0.4, 1.7) | 0.9 (0.2, 3.3) | 3.9* (1.4, 11.3) |
| Diabetes duration | |||||||||
| ≤10 years (n = 469) | 1.0 (reference) | 1.2 (0.6, 2.3) | 1.7 (0.9, 3.0) | 1.7 (0.9, 3.2) | 2.0 (0.9, 4.4) | ||||
| >10 years (n = 779) | 1.0 (reference) | 1.1 (0.7, 1.9) | 0.9 (0.5, 1.5) | 1.8 (0.9, 3.6) | 1.6* (1.03, 2.6) | ||||
| Diabetes treatment modality | |||||||||
| Any oral (n = 757) | 1.0 (reference) | 1.6 (1.0, 2.8) | 1.2 (0.8, 1.7) | 1.4 (0.7, 2.8) | 1.5 (0.9, 2.5) | ||||
| Any insulin (n = 373) | 1.0 (reference) | 0.9 (0.4, 2.0) | 0.6 (0.3, 1.1) | 1.5 (0.6, 3.7) | 1.0 (0.5, 1.9) | ||||
Data are weighted estimates. Models are adjusted for age, sex, education, race, current smoking status, BMI, HDL cholesterol, and hypertension.
*P < 0.05.
**P for interaction for HbA1c category and risk factor <0.05.
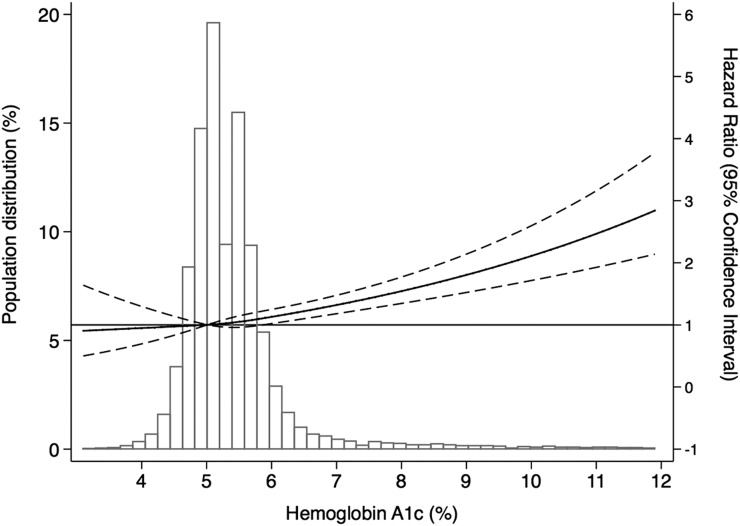
Figure 1 presents the adjusted HR of all-cause mortality by HbA1c, overlaid by the population distribution of HbA1c. This figure indicates that the risk of all-cause mortality appears to increase significantly above an HbA1c of 6.5% compared with the referent HbA1c of 5.0%. The adjusted relative HRs (95% CI) for the association between HbA1c levels among individuals with and without diabetes and all-cause and cause-specific mortality (reference: no diabetes and HbA1c 5.0–5.6% for all comparisons) is provided in Supplementary Table 1. The risk of all-cause mortality was significantly increased among those with diagnosed diabetes and an HbA1c <6.5% (HR 1.3 [95% CI 1.0, 1.6]), HbA1c 6.5–7.0% (HR 1.6 [95% CI 1.1, 2.5]), HbA1c 7.0–7.9% (HR 1.6 [95% CI 1.2, 2.3]), HbA1c 8.0–8.9% (HR 2.2 [95% CI 1.3, 3.7]), and HbA1c ≥9.0% (HR 2.6 [95% CI 2.0, 3.4]) compared with the referent group (no diabetes, HbA1c 5.0–5.6%). The HR for CVD mortality among individuals with diagnosed diabetes was only significant at an HbA1c of ≥9.0% (HR 3.2 [95% CI 1.8, 5.7]) when compared with individuals without diabetes and with an HbA1c between 5.0 and 5.6%. Cancer mortality risk increased significantly at an HbA1c between 8.0 and 8.9% (HR 2.7 [95% CI 1.3, 5.6]) only. The risk of non-CVD/noncancer mortality was significantly increased among those with diagnosed diabetes and HbA1c <6.5% (HR 1.7 [95% CI 1.3, 2.3]), HbA1c 6.5–7.0% (HR 2.1 [95% CI 1.2, 3.5]), HbA1c 7.0–7.9% (HR 1.8 [95% CI 1.2, 2.9]), and HbA1c ≥9.0% (HR 2.9 [95% CI 2.0, 4.4]) compared with the referent group (no diabetes, HbA1c 5.0–5.6%).
Figure 1.
Adjusted HRs of all-cause mortality compared with the reference of 5.0% among persons with and without diabetes, by HbA1c level.
Sensitivity Analysis
Excluding participants with a diagnosis of diabetes before the age of 30 years, we observed a significant association with increased risk of all-cause mortality at an HbA1c ≥9.0% (HR 1.7 [95% CI 1.2, 2.5]); however, the association of HbA1c level and cardiovascular mortality was attenuated and no longer significant across HbA1c levels after this exclusion (P > 0.05). The results and inferences were similar for both mortality due to cancer and non-CVD/noncancer mortality.
CONCLUSIONS
Results from this analysis of a nationally representative sample of adults 65 years and older showed that the risk of all-cause, CVD, and cancer mortality appears to increase significantly above an HbA1c of 8.0% among older adults with diabetes. The current recommendations for older adults with diabetes put forth by the ADA suggest a reasonable HbA1c goal of <7.5% for healthy patients with few comorbidities and intact functional abilities (18). Our findings also suggest a higher risk of mortality among subgroups of participants aged 65–75 years, non-Hispanic white or Mexican American/other Hispanic individuals, and those with prevalent CVD. Particularly for participants with diabetes and prevalent CVD, the risk of all-cause mortality increased significantly for those with an HbA1c ≥8.0%, confirming the ADA’s recommendations of a reasonable HbA1c goal of <8.0% for those patients with existing comorbidities (18). For participants without diabetes or with undiagnosed diabetes, the risk of all-cause and non-CVD/noncancer mortality also increased with a higher HbA1c level, compared with a referent of HbA1c 5.0–5.6%. The mechanisms underlying these associations remain unclear. One explanation is that a higher HbA1c exacerbates cardiovascular complications and therefore subsequently increases the risk of death. Moreover, higher HbA1c may be a marker of suboptimal self-management, resulting in a higher risk of diabetes-related complications, such as nephropathy and microvascular disease, that are associated with all-cause mortality (19). Other potential mechanisms include access to health care/quality of health care, diabetes education, and progressive loss of β-cell function. Unfortunately, the limitations of the NHANES data do not allow us to examine these potential mechanisms.
In general, data on outcomes associated with glycemic control among older adults are sparse. In a retrospective cohort study of 71,092 patients with type 2 diabetes 60 years of age and older enrolled in Kaiser Permanente Northern California, a U-shaped relationship was observed between HbA1c and mortality (20). Compared with the patients with HbA1c <6.0%, mortality risk was lower for HbA1c levels between 6.0 and 9.0% and higher at HbA1c ≥11.0%. In the UK General Practice Research cohort study of patients aged 50 years and older with type 2 diabetes, compared with patients with HbA1c decile (median HbA1c 7.5% [interquartile range (IQR) 7.5, 7.6]), the adjusted HR of all-cause mortality in the lowest HbA1c decile (median 6.4% [IQR 6.1, 6.6]) was 1.52 (95% CI 1.32, 1.76) and in the highest HbA1c decile (median 10.5% [IQR 10.1, 11.2]) was 1.79 (95% CI 1.56, 2.06). Results showed a general U-shaped association, with the lowest HR at an HbA1c of ∼7.5% (21). In a clinic-based setting, the ZODIAC-20 study researchers in the Netherlands showed that among type 2 participants with diabetes aged 75 and older with a duration of disease <5 years, an increase of 1% in HbA1c was associated with an increase in all-cause and CVD mortality risk of 51% (95% CI 17, 95%) and 72% (95% CI 19, 148%), respectively. No association between HbA1c and mortality was observed for patients with diabetes duration >5 years (10). Insurance claim information from the Sharon-Shomron District of Israel found that among 2,994 older adult participants with newly diagnosed diabetes, an HbA1c level ≥7.5% was associated with an increased risk of all-cause mortality, compared with those with HbA1c between 6.5 and 7% (HR 1.4 [95% CI 1.1, 1.6]) (22). Although informative, these studies may not be generalizable to populations of older adults at high risk for adverse outcomes. To the best of our knowledge, our study is the first nationally representative study in the U.S. to examine the associations between HbA1c and mortality specifically in older adults.
In the setting of older adults, the decision to aggressively treat an individual patient’s glucose levels is highly nuanced and cannot be based solely on findings from studies of general populations like NHANES. A post hoc epidemiologic analysis of the ACCORD trial showed that those participants with a higher mortality in the intensive treatment arm were individuals whose HbA1c did not respond to intensive glucose management (23). A lack of positive outcomes associated with intensive glucose management, and therefore differential risk of mortality, may be a result of an individual’s comorbidities and risk-factor profile. In particular, the results from one post hoc analysis of the ACCORD study highlighted the need to characterize and examine comorbid conditions when identifying risk of mortality in the setting of glycemic control, as the overall health of a participant bears a large effect on the mortality risk. Specifically, Papademetriou et al. (24) demonstrated that the excess mortality among the intensively treated group in the ACCORD study was in individuals who exhibited detectable but low levels of renal function that were not large enough to warrant exclusion from study entry. In addition to the ACCORD trial, several other studies have examined relevant factors (i.e., level of comorbidity [25], duration of diabetes [26]) that may modify the potential benefits of intensive blood glucose control. The results from ACCORD and other clinical studies affirm what has been put forth by the ADA, AGS, AACE, and EASD: that glycemic goals be individualized depending on the patient goals, life expectancy, and overall health status. In particular, a highlight from the ADA position statement on intensive glucose treatment asserted that “potential risks of intensive glycemic control may outweigh its benefits in other patients, such as those with a very long duration of diabetes, known history of severe hypoglycemia, advanced atherosclerosis, and advanced age/frailty” (27).
Our study is not without some limitations. Considering the cross-sectional nature of the NHANES data, we were not able to investigate the time-varying changes in HbA1c across the median follow-up of 8.9 years. Second, the smaller sample size of older adults in NHANES resulted in reduced numbers in the highest categories of HbA1c and may introduce a bias into our associations. However, this bias is expected to act on the results conservatively, therefore biasing our results toward the null. Furthermore, diabetes type is not available within the NHANES data. To address this limitation, we performed a sensitivity analysis excluding those participants within our analytic sample to those diagnosed with diabetes before the age of 30 years, and the majority of our findings were robust, with the exception of the association of HbA1c with CVD mortality. For those participants with an HbA1c <5.0%, we are unable to determine whether they truly had normal glycemic control or if they had a preexisting disease (i.e., sickle cell disease) at the time of blood draw, which may alter the red blood cell turnover and therefore the HbA1c–glucose associations due to that particular underlying disease process. Therefore, there is a possibility that inclusion of such participants may bias the results away from the null in those with very low HbA1c. Last, data are not available in NHANES to examine the prospective associations between HbA1c levels and diabetes-related complications such as nephropathy and neuropathy. Despite these limitations, there are several strengths to this study that should be noted. This study utilizes data from a nationally representative sample and, to the best of our knowledge, is the largest nationally representative study of older adults that has examined the association between HbA1c and mortality. For these analyses, we made comparisons across categories of individuals without diabetes and individuals with diabetes (both undiagnosed and diagnosed) among older adults to examine a spectrum of glucose states. Utilizing the large sample size, data were available to examine the relative HRs across important subgroups where mortality risk is expected to be differential, including, age, race, presence of CVD, duration of diabetes, and diabetes treatment modality. Similar to an analysis of adults aged 20 years and older from NHANES III, we observed a greater risk of mortality with increasing HbA1c levels among non-Hispanic white older adults compared with non-Hispanic black older adults (28).
Updated consensus statements have been put forth by both the ADA and AGS based on new evidence between 2002 and 2012 (13,14). The consensus statements make recommendations regarding potentially less aggressive glycemic goals for older adults, but these were largely based on expert opinion given the paucity of existing studies at the time. Our results show that for the general population of U.S. older adults with diabetes, an HbA1c <8.0% is associated with reduced risk of mortality, which is consistent with ADA-recommended goal for healthier older adults. The ADA and AGS both propose that glycemic goals should be individualized and depend on the patient’s health status, life expectancy, and personal goals. This recommendation is supported by our results, which showed differences in the risk of mortality across demographics, history of CVD, duration of diabetes, and type of antidiabetic medication use. Expanding on this current work, future studies should examine prospective associations between HbA1c control and diabetes-related health care utilizations and quality of life.
Supplementary Material
Article Information
Funding. This analysis was supported by a National Institute of Diabetes and Digestive and Kidney Diseases Training Grant in Clinical Research and Epidemiology in Diabetes and Endocrinology (pre-doctoral training grant T32-DK-062707 to P.P.) and a National Heart, Lung, and Blood Institute Training Grant in Cardiovascular Epidemiology, Biostatistics, and Preventive Medicine (post-doctoral training grant T32-HL-007055 to P.P.). E.S.H. received grants from the National Institute of Diabetes and Digestive and Kidney Diseases (K24DK105340 and P30DK092949) and the Agency for Healthcare Research and Quality (RO1HS018542). H.-C.Y. received grants from the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK07963). R.R.K. is supported by a National Institutes of Health Career Development Award (K23-DK-093583).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. P.P. assisted in the study design, performed the analyses, and drafted the manuscript. E.S.H., R.R.K., and S.H.G. assisted in the study design and critically reviewed the manuscript. H.-C.Y. conceptualized the study; assisted in the study design, data analysis, and drafting of the manuscript; critically reviewed the manuscript; and had final responsibility for the decision to submit for publication. All authors approved the final manuscript as submitted. H.-C.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
References
- 1.Saydah S, Tao M, Imperatore G, Gregg E. GHb level and subsequent mortality among adults in the U.S. Diabetes Care 2009;32:1440–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) [published correction appears in Lancet 1998;352:1558]. Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1999;354:602]. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 4.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 7.Hayward RA, Reaven PD, Emanuele NV; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373:978. [DOI] [PubMed] [Google Scholar]
- 8.Miller ME, Williamson JD, Gerstein HC, et al.; ACCORD Investigators . Effects of randomization to intensive glucose control on adverse events, cardiovascular disease, and mortality in older versus younger adults in the ACCORD Trial. Diabetes Care 2014;37:634–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colayco DC, Niu F, McCombs JS, Cheetham TC. A1C and cardiovascular outcomes in type 2 diabetes: a nested case-control study. Diabetes Care 2011;34:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Hateren KJ, Landman GW, Kleefstra N, et al. Glycaemic control and the risk of mortality in elderly type 2 diabetic patients (ZODIAC-20). Int J Clin Pract 2011;65:415–419 [DOI] [PubMed] [Google Scholar]
- 11.Chonchol M, Katz R, Fried LF, et al. Glycosylated hemoglobin and the risk of death and cardiovascular mortality in the elderly. Nutr Metab Cardiovasc Dis 2010;20:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsigos C, Bitzur R, Kleinman Y, et al. Targets for body fat, blood pressure, lipids, and glucose-lowering interventions in healthy older people. Diabetes Care 2013;36(Suppl. 2):S292–S300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno G, Mangione CM, Kimbro L, Vaisberg E; American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus . Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahn A, Raz I, Kleinman Y, et al. Clinical assessment of individualized glycemic goals in patients with type 2 diabetes: formulation of an algorithm based on a survey among leading worldwide diabetologists. Diabetes Care 2015;38:2293–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics Office of Analysis and Epidemiology. NCHS 2011 Linked Mortality Files Matching Methodology, September 2013 [Internet]. Hyattsville, MD, National Center for Health Statistics. Available from https://www.cdc.gov/nchs/data/datalinkage/2011_linked_mortality_file_matching_methodology.pdf. Accessed 22 January 2017
- 17.Carson AP, Fox CS, McGuire DK, et al. Low hemoglobin A1c and risk of all-cause mortality among US adults without diabetes. Circ Cardiovasc Qual Outcomes 2010;3:661–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Diabetes Association Older adults. Sec. 10. In Standards of Medical Care in Diabetes—2016. Diabetes Care 2016;39(Suppl. 1):S81–S85 [DOI] [PubMed] [Google Scholar]
- 19.Fabbian F, De Giorgi A, Monesi M, et al. All-cause mortality and estimated renal function in type 2 diabetes mellitus outpatients: Is there a relationship with the equation used? Diab Vasc Dis Res. 2015;12:46–52 doi: 10.1177/1479164114552656. [DOI] [PubMed] [Google Scholar]
- 20.Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the Diabetes and Aging Study. Diabetes Care 2011;34:1329–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010;375:481–489 [DOI] [PubMed] [Google Scholar]
- 22.Twito O, Ahron E, Jaffe A, et al. New-onset diabetes in elderly subjects: association between HbA1c levels, mortality, and coronary revascularization. Diabetes Care 2013;36:3425–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riddle MC, Ambrosius WT, Brillon DJ, et al.; Action to Control Cardiovascular Risk in Diabetes Investigators . Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 2010;33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papademetriou V, Lovato L, Doumas M, et al.; ACCORD Study Group . Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int 2015;87:649–659 [DOI] [PubMed] [Google Scholar]
- 25.Greenfield S, Billimek J, Pellegrini F, et al. Comorbidity affects the relationship between glycemic control and cardiovascular outcomes in diabetes: a cohort study. Ann Intern Med 2009;151:854–860 [DOI] [PubMed] [Google Scholar]
- 26.Duckworth WC, Abraira C, Moritz TE, et al.; Investigators of the VADT . The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011;25:355–361 [DOI] [PubMed] [Google Scholar]
- 27.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Diabetes Care 2009:32:754]. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kehl KG, Findeisen HM, Fardo DW, Bruemmer D, Mannino DM, Sanderson WT. Race-ethnicity as an effect modifier of the association between HbAlc and mortality in U.S. adults without diagnosed diabetes. Eur J Endocrinol 2011;165:275–281 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.