Abstract
Preeclampsia is associated with hypertension, small-for-gestational-age babies, and increased cytolytic natural killer (NK) cells. The specific role of cytolytic NK cells in the pathophysiology of preeclampsia has not been clearly defined. We hypothesized that Reduced Uterine Perfusion Pressure (RUPP) stimulates proliferation and cytolytic activation of NK cells, and that reducing NK cells in RUPP would prevent hypertension, intrauterine growth restriction, and inflammation in response to placental ischemia. RUPP was induced on gestation day (GD) 14 in pregnant rats. NK cells were depleted by i.p. administration of anti-asialo GM1 antibody on GDs 15 and 17. Placental and circulating NK cells were quantified via flow cytometry, mean arterial pressure (MAP), fetal weights, and cytokines were measured on GD 19. Total placental NK cells were 7.4±2% of gated cells in normal pregnant (NP; n=10) and 16.5± 3% of gated cells in RUPP (n=10) rats. Furthermore, cytolytic placental NK cells also increased in RUPP. Depletion of NK cells in RUPP (RUPP + anti-ASGM1) significantly improved MAP and fetal weights. MAP was 108± 2 mmHg in NP, 125± 2 mmHg in RUPP, and 112± 2 mmHg in RUPP + anti-ASGM1 (n=12). Fetal weight was 2.32 ± 0.05 in NP, 1.8± 0.04g in RUPP, and increased to 2.0± 0.04g in RUPP + anti-ASGM1. Placental interferon-γ (IFN-γ) was 40.4± 5.2 pg/mg in NP, 72.17± 3.2 pg/mg in RUPP, and 44.0± 6.5 pg/mg in RUPP + anti-ASGM1 (P<0.05). Placental tumor necrosis factor-α (TNF-α) was 17.9± 1.7 pg/mg in NP, 23.9± 2.2 pg/mg in RUPP, and 12.9± 2.3 pg/mg in RUPP + anti-ASGM1 (P<0.05). Depletion of NK cells significantly lowered MAP, intrauterine growth restriction, and inflammation in RUPP rats indicating that cytolytic NK cells are important in preeclampsia pathophysiology.
Introduction
Preeclampsia (PE) is a pregnancy specific multisystem hypertensive disorder that is associated with new onset hypertension and proteinuria. It is usually encountered after 20 weeks of gestation and resolves soon after delivery of the fetus and placenta [1]. Other hallmark characteristics of PE include chronic immune activation, fetal growth restriction, and maternal endothelial dysfunction [2,3]. Currently, the only known relief for preeclampsia is delivery making it the leading cause of iatrogenic preterm birth worldwide.
The development of PE is thought to occur beginning with shallow trophoblast invasion of the maternal myometrium leading to insufficient remodeling of uterine spiral arteries, increased uterine artery resistance (UARI), and decreased blood flow to the placenta. This results in placental ischemia, which triggers the release of inflammatory cytokines and increased placental oxidative stress [4,5]. More recently clinical studies have observed that when compared with pregnant controls, preeclamptic woman also manifest an alteration in the population of natural killer (NK) cells [6,7]. This altered population of NK cells may be a culprit of some of the adverse outcomes associated with PE such as decreased fetal weight and elevated blood pressure. However, it is unknown at what gestation time frame NK cells begin to vary between normal pregnant and preeclamptic pregnancies.
During normal pregnancy, regulatory NK cells, which are poorly cytolytic, increase in the periphery and decidua while, cytolytic NK cells decrease [8]. Studies show that the increase in regulatory NK cells is important for the maintenance of pregnancy, as they have important roles in mediating tolerance of fetal antigens, fetal trophoblast invasion, spiral artery remodeling, and angiogenesis to facilitate proper placentation [8–12]. In contrast, PE is associated with a NK population shift to the proinflammatory cytolytic NK phenotype. This altered NK profile may contribute to the pathophysiology associated with PE.
Human NK cells are identified by their expression of CD56 in the absence of CD3 [13], and are divided into two distinct subsets based on expression ofCD56 andCD16: (A)CD56dimCD16+ cytolytic NK cells and (B)CD56brightCD16− regulatory NK cells [10,14]. Human cytolytic NK (cNK) cells produce interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), and display potent cytolytic function upon activation by IL-2 [11,15]. It has also been suggested that IL-17may enhance cytolytic activity of human NK cells [16]. Rodent natural killer cells do not express CD56 and CD16 surface markers; however, they can be identified by surface expression of the NK cell marker named ANK61 [17–21]. Activated rat cNKs are distinguished from nonactivated NK cells by surface expression of ANK44, a protein preferentially expressed on rat cNK cells, and secretion of IFN-γ and TNF-α, similar to human cNK cells [22].
Although an association between PE and increased cNKs in the decidua has been demonstrated clinically, the population of NK cells in the placenta has not been examined in clinical studies or animal models of PE. This population of cells is an important subject of inquiry because the placenta is implicated as the central culprit in the pathophysiology of PE. Furthermore, a direct link between the increased cNKs in PE and pathophysiology such as hypertension and intrauterine growth restriction (IUGR) remains unexplored and requires investigation. In the present study, we utilized the Reduced Uterine Perfusion Pressure (RUPP) model of PE to assess the effects of placental ischemia on NK cell polarization in the circulation and placenta and to examine a role for cNKs in mediating pathophysiology in PE by depleting NK cells in the RUPP rat. Therefore, we hypothesized that RUPP stimulates proliferation and cytolytic activation of NK cells, and that reducing NK cells in RUPP would prevent hypertension, intrauterine growth restriction, and inflammation in response to placental ischemia. To test this hypothesis, we induced RUPP in pregnant rats and depleted the NK cell population using the anti-asialo GM1 antibody (anti-ASGM1), in normal pregnant control and RUPP rats. We then evaluated the PE-associated characteristics of hypertension, intrauterine growth restriction, and inflammation. The results of the present study demonstrate for the first time that cNK cells are directly linked to the hypertension that occurs in response to placental ischemia in an animal model of PE.
Materials and methods
Pregnant Sprague–Dawley rats purchased from Harlan Sprague Dawley (Indianapolis, IN) were used in the present study. At Harlan Sprague Dawley, the animals were maintained on Teklad 2018S. Animals were housed in a temperature-controlled room (23°C) with a 12:12-h light–dark cycle and maintained on Teklad 8640 in the Laboratory Animal Facilities at the University of Mississippi Medical Center. All experimental procedures executed in the present study were in accordance with the National Institutes of Health guidelines for use and care of animals. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center.
Reduction in uterine perfusion pressure
All of our in vivo experiments were performed in rats weighing approximately 250–275 g. On day 14 of gestation, under isoflurane anesthesia (delivered by an anesthesia apparatus, Vaporizer for Florane Anesthetic, Ohio Medical Products, Madison, WI) the RUPP surgery was performed on a subset of normal pregnant (NP) rats. Briefly, a midline incision was made, and a constrictive silver clip (0.203 mm) was placed on the aorta superior to the iliac bifurcation, while ovarian collateral circulation to the uterus was reduced with restrictive clips (0.100 mm) to the bilateral uterine arcades at the ovarian end [23–25]. Animals were administered carprofen (5mg/kg) for 2 days to control postsurgical pain. Rats were excluded from the study when the clipping procedure resulted in total reabsorption of all fetuses.
Natural killer cell depletion
NK cells were depleted in a subset of normal pregnant (NP) and RUPP rats by an intraperitoneal injection of 100 μl (3.5 μg) anti-asialo ganglio-N-tetraosylceramide (anti-asialo-GM1) antibodies (Wako Chemicals, Richmond, VA) in water on days 15 and 17 of gestation [26–28]. Asialo-GM1 (ASGM1) is a glycophospolipid expressed on the surface of NK cells that is a target of immune cell response and acts as a coreceptor for pathogens.
Measurement of mean arterial pressure in conscious rats
On day 18 of gestation, using the isoflurane anesthesia, carotid arterial catheters were inserted for blood pressure measurements. The catheters inserted were V3 tubing (SCI, Scientific Commodities, Inc., Lake Havasu City, AZ), which is tunneled to the back of the neck and exteriorized. On day 19 of gestation, arterial blood pressure was analyzed after placing the rats in individual restraining cages. Arterial pressure was monitored with a pressure transducer (Cobe III tranducer CDX Sema) and recorded continuously for 30min after a 30-min stabilization period. Subsequently, blood samples were collected; placentas were harvested; and litter size and fetal weights were recorded under anesthesia.
Determination of circulating and placental NK cell populations using flow cytometry
The circulating and placental populations of NK cells were quantified by flow cytometry from peripheral blood mononuclear cells (PBMCs) and placental immune cells isolated on day 19 of gestation from NP, RUPP, RUPP + anti-ASGM1, and NP + anti-ASGM1 animals. At the time of harvest, whole blood and placentas were collected and PBMCs and placental lymphocytes were isolated by centrifugation on a cushion of Ficoll-Hypaque (Lymphoprep, Accurate Chemical & Scientific Corp., Westbury, NY) according to the instructions of the manufacturer. For flow cytometric analysis, 1 × 106 cells were incubated for 30 min at 4°C with antibodies against rat Anti-Natural Killer Cell Activation Structures (ANK61) or rat Anti-Natural Killer Cell antibody (ANK44) (AbCam, Cambridge, MA). ANK61 is a killer cell activation structure that is expressed on all NK cells, while ANK44 is a marker of cytolytic activation in NK cells because it only expressed on stimulated, cytotoxic NK cells [22]. After washing, cells were labeled with secondary Fluorescein isothiocyanate (FITC; AbCam) antibody for 30min at 4°C. As a negative control for each individual rat, cells were treated exactly as described above except they were incubated with isotype control antibodies conjugated to FITC alone. Subsequently, cells were washed, fixed, and resuspended in 500 μl of Rosswell Park Memorial Institute (RPMI) medium and analyzed for single staining on a Gallios flow cytometer (Beckman Coulter, Brea, CA). Cells were gated in the forward and side scatter plot. Cells that stained asANK61+ were designated as NK cells. Cells that stained as ANK44+ were designated as cNK cells. The percent of positive stained cells in the gated population above the negative control was collected for individual rats and the mean values for each experimental group were calculated.
Determination of cytokine production
Serumand placental homogenates from pregnant rats in each group were assessed for concentration of IFN-γ, TNF-α, and IL-17 using commercial ELISA kits available from eBiosciences (Santa Clara, CA). Tissue homogenate values were normalized to protein concentration. Protein concentration was determined using the BSA protein analysis. The minimal detectable value for IFN-γ was 9.9 pg/ml, with maximum being 2000 pg/ml with an intra-assay/interassay precision of <5% and <10% coefficient of variation, respectively. The minimal detectable value for TNF-α was 11 pg/ml, with maximum being 2500 pg/ml with an intra-assay/interassay precision of <5% and <10%, respectively. Theminimal detectable value for IL-17 was 1.0 pg/ml, with maximum being 100 pg/ml with an intra-assay/interassay precision of 8.5% and 7.6%, respectively.
Statistical analysis
All of the data are expressed as mean ± SEM. Statistical analyses were performed with a two-way ANOVA (four groups) or one-way ANOVA (three groups) with Tukey’s multiple comparisons test as post hoc analysis. A value of P<0.05 was considered statistically significant.
Results
Cytolytic natural killer cells are stimulated in response to placental ischemia
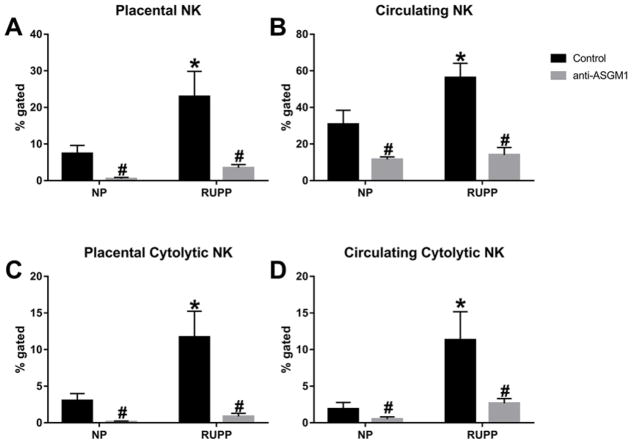
Lymphocytes were isolated from blood and placental samples of animals in each group. Flow cytometry was performed to quantify the % gated of total NK cells and cNKs in the circulation and placenta of pregnant rats. Placental ischemia caused a significant increase in the total NK cell population in the placenta. Total placental NK cells were 7.4 ± 2.2% of gated cells in NP (n=10) versus 16.5± 2.7% in RUPP (n=10; P<0.05, Figure 1A). The circulating NK cell population in RUPP rats was also significantly increased compared with NP rats. Total circulating NK cells were 30.7 ± 7.7% of gated cells in NP (n=10) versus 56.2± 7.8% of gated cells in RUPP (n=10, P<0.05, Figure 1B). Treatment with anti-asialo GM1 (anti-ASGM1) significantly decreased the NK cells in the placenta and circulation of NP and RUPP rats (placental NK cells: NP + anti-ASGM1 0.51± 0.3% (n=6), RUPP + anti-ASGM1 3.5± 0.9% (n=11); P<0.05 versus RUPP, Figure 1A; circulating NK cells: NP + anti-ASGM1 11.5± 1.4 (n=6), RUPP + anti-ASGM1 14.1± 4.0 (n=9); P<0.05 versus RUPP, Figure 1B). Of the total population of cells, the cNK population significantly increased in the placenta of RUPPs compared with NP rats (3.0± 0.96% in NP (n=10) vs 11.7± 3.5% in RUPP (n=10; P<0.05, Figure 1C)). Treatment with anti-ASGM1 effectively depleted cNK cells in the placentas of RUPP and NP rats. The placental cNK population in RUPP + anti-ASGM1 was 0.86± 0.42% (n=7) and 0.14± 0.01% in NP + anti-ASGM1 (n=6; P<0.05 versus RUPP, Figure 1C). The cNK population increased from 1.9± 0.90% in the circulation of NP rats (n=10) to 11.3± 3.9% in RUPP rats (n=10; P<0.05, Figure 1D) and significantly decreased to 2.67±0.64% in the circulation of RUPP + anti-ASGM1 (n=7) and 0.49± 0.31% in NP + anti-ASGM1 (n=6; P<0.05 versus RUPP, Figure 1D). Because anti-ASGM1 is also expressed on CD8+ T cells, we used flow cytometry analysis to assess the effect of anti-ASGM1 on the population of CD8+ T cells in the circulation and placenta of rats from each group. The CD8+ T-cell population was not different in NP, RUPP, RUPP + anti-ASGM1 or NP + anti-ASGM1 rats (placental CD8+ T cells—NP: 12.61± 5.8% (n=9), RUPP: 10.27± 1.3% (n=8), RUPP + anti-ASGM1: 18.48± 5.7% (n=7), NP + anti-ASGM1: 4.06± 2.4% (n=4); Circulating CD8+ T cells—NP: 15.18± 4.4%, RUPP: 28.85± 5.4%, RUPP + anti-ASGM1: 16.61± 4.8%, NP + anti-ASGM1: 5.64± 1.6%). Thus, treatment with anti-ASGM1 specifically causes depletion of NK cells in our animal model.
Figure 1. Natural killer cells are stimulated in response to placental ischemia.
Total NK cells are increased in the placenta (A) and circulation (B) in response to reduced uterine perfusion pressure (RUPP) in pregnant rats. Cytolytic NK cells were significantly increased in the placenta (C) and circulation (D) of RUPP rats. Circulating and placental NK populations were significantly reduced after treatment with anti-asialo GM1 antibody on gestation days 15 and 17 (A–D); *P<0.05 versus NP, #P<0.05 versus RUPP.
Natural killer cell depletion significantly decreased the hypertensive response to placental ischemia
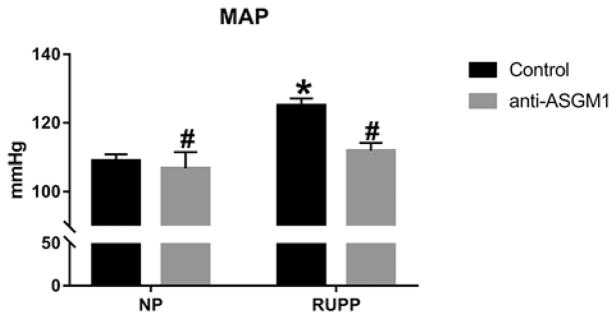
Mean arterial pressure (MAP) was measured on day 19 of gestation in NP, RUPP, RUPP + anti-ASGM1, and NP + anti-ASGM1 rats. Placental ischemia significantly increased blood pressure from 108± 2 mmHg in NP (n=12) to 125± 2 mmHg in RUPP rats (n=12; P<0.05, Figure 2). Depletion of NK cells with anti-ASGM1 significantly lowered the hypertension in response to placental ischemia in RUPP + anti-ASGM1 (112± 2mmHg, n=12; P<0.05 versus RUPP), but did not affect MAP in NP + anti-ASGM1 rats (107± 5mmHg, n=6; n.s. versus NP, P<0.05 versus RUPP).
Figure 2. Reduction in natural killer cells attenuates hypertension in RUPP rats.
Blood pressure is increased in response to reduced uterine perfusion pressure (RUPP) in pregnant rats. Reduction of NK cells inhibited the blood pressure response to RUPP. Reduction of NK cells in NP rats had no effect on blood pressure; *P<0.05 versus NP, #P<0.05 versus RUPP.
Intrauterine growth restriction is blunted with depletion of NK cells during reduced uterine perfusion pressure
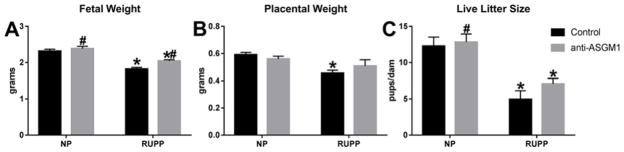
Fetal weight of litters from RUPP rats (1.82± 0.04 g, n=12) was significantly lower than fetal weight of litters from NP rats (2.32± 0.05 g, n=12; P<0.05, Figure 3A). Depletion of NK cells in RUPP animals significantly increased fetal weight to 2.0± 0.04 g in RUPP + anti-ASGM1 (n=12; P<0.05 versus RUPP) and had no effect on fetal weight in litters from NP + anti-ASGM1 rats (2.39± 0.06 g, n=6; n.s. versus NP). Additionally, placental weight in NP rats (0.59 ± 0.02 g, n=12) was significantly decreased in RUPP rats (0.46± 0.02 g, n=12; P<0.05, Figure 3B). NK cell depletion slightly improved placental weight in RUPP + anti-ASGM1 rats to 0.51± 0.05 g (n=12, n.s. versus RUPP), although this did not reach significance. Placental weight in NP + anti-ASGM1 rats (0.55± 0.02 g, n=6) was not significantly different from those in NP rats. Litter size significantly decreased from 12.3± 1.3 pups/dam in NP rats (n=12) to 5.4± 1.2 pups/dam in RUPP rats (n=12, P<0.05). Litter size was 7.1± 0.8 pups/dam in RUPP + anti-ASGM1 rats (n=12; n.s. versus RUPP, P<0.05 versus NP) and 12.8± 1.1 pups/dam in NP + anti-ASGM1 rats (n=6).
Figure 3. Natural killer cell depletion significantly increased fetal weight in RUPP rats.
Fetal and placental weights are decreased in response to placental ischemia in reduced uterine perfusion pressure (RUPP) rats (A and B); however, depletion of NK cells significantly improved weights of offspring in RUPP rats (A). *P<0.05 versus NP, #P<0.05 versus RUPP.
NK cell depletion completely attenuates placental IFN-γ and TNF-α in response to RUPP
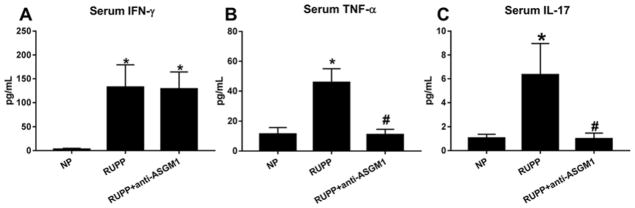
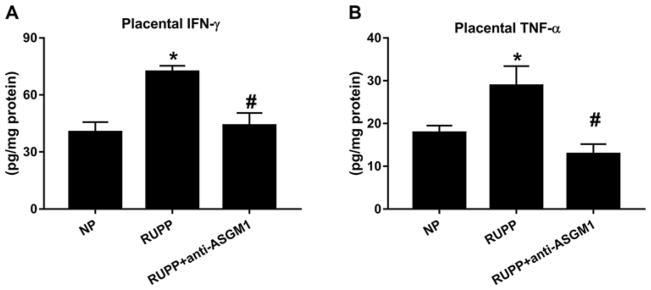
Concentrations of the proinflammatory cytokines IFN-γ and TNF-α were assessed in serum and placental homogenates from NP, RUPP, and RUPP + anti-ASGM1 rats. Serum IFN-γ significantly increased from 2.9± 2 pg/ml in NP rats (n=6) to 132.3±47 pg/ml in RUPP rats (n=4, P<0.05) in response to placental ischemia (Figure 4A). Serum IFN-γ did not change with NK cell depletion and was 128.7± 36 pg/ml in RUPP + anti-ASGM1 rats (n=6). Serum TNF-α in NP rats increased from11.3± 4 pg/ml (n=7) to 45.7± 9 pg/ml in RUPP rats (n=6; P<0.05, Figure 4B). Depletion of NK cells significantly decreased serum TNF-α to 10.9± 4 pg/ml in RUPP + anti-ASGM1 rats (n=6; P<0.05 versus RUPP). Placental IFN-γ significantly increased from 40.4± 5 pg/mg in NP rats (n=7) to 72.2 ± 3 pg/mg in RUPP rats (n=4, P<0.05) in response to placental ischemia (Figure 5A). NK cell depletion normalized placental IFN-γ to 44.0± 6 pg/mg in RUPP + anti-ASGM1 rats (n=6, P<0.05 versus RUPP). Similarly, placental TNF-α in NP rats significantly increased from17.9± 2 pg/mg (n=6) to 28.8± 4 pg/mg in RUPP rats (n=6; P<0.05, Figure 5B). Depletion of NK cells significantly decreased placental TNF-α to 12.9± 2 pg/mg in RUPP + anti-ASGM1 rats (n=6; P<0.05 versus RUPP). Clinical studies of PE report that circulating NK cells from preeclamptic women, but not normal pregnant women, secreted significant amounts of IL-17 [29]. Therefore, we measured IL-17 in the serum of rats from all three groups. Serum IL-17 increased from 1.04± 0.3 pg/ml in NP (n=7) to 6.3± 2.6 pg/ml in RUPP rats (n=6; P<0.05), and significantly decreased to 0.99± 0.5 pg/ml in RUPP + anti-ASGM1 rats (n=7; P<0.05 versus RUPP, Figure 4C). Placental levels of IL-17 were beyond the lower limit of detection in the present study in all animal groups.
Figure 4. Circulating inflammatory cytokines are decreased with NK cell depletion in RUPP rats.
Circulating levels of IFN-γ (A), TNF-α (B), and IL-17 (C) are significantly increased in response to reduced uterine perfusion pressure (RUPP) in pregnant rats. Reduction of NK cells inhibited the increase in circulating proinflammatory cytokines in RUPP rats; *P<0.05 versus NP, #P<0.05 versus RUPP.
Figure 5. Placental inflammatory cytokines are blunted after NK cell depletion in RUPP rats.
Placental levels of IFN-γ (A) and TNF-α (B) are significantly increased in response to reduced uterine perfusion pressure (RUPP) in pregnant rats. Reduction of NK cells attenuated the increase in placental IFN-γ and TNF-α in RUPP rats; *P<0.05 versus NP, #P<0.05 versus RUPP.
Discussion
Natural killer cells play a role in multiple processes pertinent to successful maintenance of a healthy pregnancy. Alterations in the NK cell population are associated with adverse outcomes in pregnancy such as PE. Clinical studies have demonstrated that women with PE have an increased population of cNK cells compared with women with normal, healthy pregnancies [6,7]. However, the involvement of cNK cells in contributing to the pathophysiology associated with placental ischemia during PE has never been examined in vivo. Therefore in the present study, we set out to determine if cNK cells are stimulated in response to RUPP in pregnant rats and how depletion of NK cells would affect inflammation, fetal and placental weight, and hypertension in response to placental ischemia.
We demonstrate a significant increase in total NK and cNK placental populations in response to placental ischemia in pregnant rats (Figure 1). Furthermore, significant increases in total and cytolytic NK cells were also observed in the circulation of pregnant RUPP rats. These data indicate that placental ischemia has a role to stimulate proliferation of NK cells and polarization to cNK cells during pregnancy. These altered populations of NK cells in response to placental ischemia are associated with increases in blood pressure, inflammation, and IUGR. Thus, placental ischemia induced NK cells may have a role in mediating pathophysiology of PE.
Depletion of NK cells in RUPP rats caused a significant decrease in the blood pressure response to placental ischemia (Figure 2). This data demonstrate for the first time that RUPP-stimulated cNK cells contribute to the increase in blood pressure during placental ischemia in an animal model of PE. The possible mechanisms of by which NK cells contribute to this response could be through the release of inflammatory cytokines in the circulation and placenta or through increased cytotoxic activity against maternal or placental cells.
When NK cells were depleted in NP rats, MAP in the NP and NP + anti-ASGM1 groups were unchanged (Figure 2), indicating that depletion of NK cells in late gestation has no effect on maternal blood pressure during normal pregnancy. Furthermore, fetal and placental weights in NP + anti-ASGM1 rats were not significantly different from those in NP rats (Figure 3). These data suggest that the blunted pathophysiology after NK depletion is specifically associated with RUPP and that an altered phenotype in the NK cells exists between NP and RUPP rats.
The increases in the number of total and cNK cells in the circulation and placenta were associated with significantly increased circulating and placental levels of IFN-γ and TNF-α (Figures 4 and 5). These results coupled with the presence of the NK cytolytic activation marker, ANK44, strongly support our interpretation that the NK cells stimulated in response to placental ischemia are cNK cells. Human studies of NK cell subsets have shown that the cNK cell subset is highly cytolytic and produces significant amounts of IFN-γ and TNF-α [11,15]. Small et al. [30] recently published a study demonstrating that NK cells are the major producers of TNF-α in a stroke-prone spontaneous hypertensive rat model. In our current study, depletion of NK cells with anti-ASGM1 also resulted in a depletion of serum and placental TNF-α (Figures 4 and 5) further strengthening the notion of cNK cell involvement in hypertensive pregnancy disorders.
The changes in circulating and placental cytokine levels after NK cell depletion give insight into the major factors that may be utilized by NK cells in contributing to PE pathophysiology. IFN-γ is a TH1-associated cytokine and is secreted by a number of other cell types in addition to NK cells. Circulating IFN-γ in response to placental ischemia was not changed after NK depletion. However, placental IFN-γ significantly decreased in RUPP rats treated with the NK cell depleting antibody. These data could indicate that NK cells are not the major source of IFN-γ in the circulation and possibly that circulating TH1 cells were unaffected by the treatment. In addition, the lowered levels of placental IFN-γ is associated with improved placental and fetal weight indicating that the effects on the placenta may be more profound than that originally anticipated, supporting the important role of cNK cells in fetal demise.
Circulating and placental levels of TNF-α were significantly decreased in response to NK cell depletion in RUPP rats. This suggests that TNF-α may be an important factor by which cNK cells contribute to PE pathophysiology. We have previously demonstrated that infusion of TNF-α into pregnant rats increases mean arterial pressure and renal vascular resistance [31]. Synthesis of the potent vasoconstrictor, endothelin-1, is enhanced by TNF-α [32]. Furthermore, blockade of TNF-α signaling via treatment with etanercept completely attenuated placental ischemia-induced hypertension in RUPP rats [33]. Therefore, the depletion of NK cells in the placental ischemic rats may have yielded the decrease blood pressure response through reduced TNF-α signaling.
Similarly, depletion of NK cells with anti-ASGM1 caused a significant decrease in circulating IL-17, suggesting that cNKs stimulated in response to placental ischemia may be a source of IL-17. This observation is in line with previous clinical studies demonstrating the ability of NK cells from preeclamptic women to secrete IL-17 [29]. We’ve previously demonstrated that TH17 cells and IL-17 play a direct role in facilitating IUGR: late gestation blockade of IL-17 signaling with the soluble IL-17 receptor, IL-17 RC, significantly increased both fetal and placental weights in two different models of PE, the RUPP rat and NP + RUPPTH17s rat [34,35]. In these studies, we did not examine the impact of IL-17 on NK cell proliferation or cytotoxic activation. Importantly in the present study, depletion of NK cells, which correlated with decreased circulating levels of IL-17, improved fetal weight significantly and marginally increased placental weight in RUPP rats (Figure 3). These results implicate the increased population of placental cNKs during RUPP in contributing to IUGR. Therefore, targeting NK cells during late gestation may provide benefit to both the mother and the fetus.
Reduction in cNK cells in the RUPP rat treated with anti-ASGM1 had a profound effect on the blood pressure, IUGR, and circulating and placental inflammatory factors in this model. However, there are limitations of the present study that will require further investigation. The observed effects on inflammation and IUGR cannot be totally attributed to the depletion of cNK cells based on this study alone. It is possible that the decrease in blood pressure after treatment with anti-ASGMI also contributed to the improvements in inflammation and fetal weights. As the focus of the present study was on inflammation and IUGR in response to cNK cell depletion, the contribution of cNK cells in the production of other PE associated mediators such as the vasoconstrictor endothelin-1, the antiangiogenic factor sFlt-1, and oxidative stress molecules in kidney or placenta remain to be investigated. In vitro culture experiments are necessary to gain insight into whether cNK cells are a source of the increased circulating and placental IFN-γ, TNF-α, and IL-17 observed in vivo. Finally, time course studies could help to determine what factors are increased after placental ischemia that stimulate cNK polarization.
In spite of these limitations, the present study does establish cNK cells as contributors to PE pathophysiology. We demonstrate for the first time a direct link between cNK cells and the hypertension in response to placental ischemia in an animal model of PE. Furthermore, the present study is the first to establish an association of cNK cells with IUGR and inflammation in an animal model of PE. On account of the important role of NK cells in the immune response against pathogens, depletion of the total NK cell population may not be the best therapy for PE pathophysiology. However, the importance is that studies elucidating the mechanisms employed by NK cells to mediate pathophysiology of PE could possibly identify therapeutic targets that would not compromise the maternal immune defense against external pathogens. For example, functional inhibition of NK cytotoxic and inflammatory factors via neutralizing antibodies or soluble receptors, or infusion of specific NK cell-associated factors could demonstrate the potential contribution of these factors to PE pathophysiology. Furthermore, in vitro experiments to examine the molecular pathways that may be activated in placental or vascular cells in response to cNK cells and inhibition of NK cell associated factors via gene silencing or splicing in vivo may provide insight into mechanisms or compensatory pathways and molecules that could serve as novel targets. Investigation into the stimulus that instigates the development of the altered NK population in response to placental ischemia and during PE is also warranted. Studies examining placental levels of NK mediating factors such as IL-2, IL-12, and IL-17 (cNK polarizing factors), and IL-4 (regulatory NK polarizing factor) in the presence of placental ischemia and methods to manipulate their levels have not been performed and may provide a target that would prevent the transformation of this cell population. There is a need for novel strategies to better manage PE, and NK cells may be a therapeutic target that could prolong pregnancy and yield beneficial outcomes for mom and baby during PE.
Clinical perspectives.
Preeclampsia, a hypertensive disorder of pregnancy, is a significant cause of maternal and fetal morbidity and mortality and is associated with an altered population of cytolytic NK cells.
Depletion of RUPP-induced cytolytic NK cells in pregnant rats resulted in significant improvements in blood pressure, fetal weight, and decreased inflammation.
The results of the present study identify cytolytic NK cells as a novel target with therapeutic potential for the treatment of preeclampsia in women that may result in improved maternal and fetal health and lower morbidity and mortality associated with this disorder.
Acknowledgments
Funding
This work was supported by American Heart Association grant [16SDG27520000] and National Institutes of Health grants [K99HL130456 and R01HD067541].
Abbreviations
- cNK
cytolytic natural killer
- GD
gestation day
- IFN-γ
interferon-γ
- MAP
mean arterial pressure
- NK
natural killer
- PBMC
peripheral blood mononuclear cell
- PE
preeclampsia
- RPMI
Rosswell Park Memorial Institute
- RUPP
reduced uterine perfusion pressure
- TNF-α
tumor necrosis factor-α
- UARI
uterine artery resistance
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Author Contribution
DCC and BL provided conception and design of research; JE, LMA, MM, JDS, MWC, AG, TI, and DCC performed experiments,; JE, LM and DCC analyzed data; JE, BL, and DCC interpreted results of experiments; JE and DCCC prepared figures; JE and DCC drafted manuscript; JE, LAM, MM, JDS, MWC, AG, TI, BBL, and DCC approved final version of manuscript.
References
- 1.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 2.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: current concepts. Am J Obstet Gynecol. 1998;179:1359–1375. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 3.Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynaecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- 4.Cornelius DC, Lamarca B. TH17- and IL-17- mediated autoantibodies and placental oxidative stress play a role in the pathophysiology of pre-eclampsia. Minerva Ginecol. 2014;66:243–249. [PMC free article] [PubMed] [Google Scholar]
- 5.Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 2013;208:224–233. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, et al. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011;90:105–110. doi: 10.1016/j.jri.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Fukui A, Yokota M, Funamizu A, Nakamua R, Fukuhara R, Yamada K, et al. Changes of NK cells in preeclampsia. Am J Reprod Immunol. 2012;67:278–286. doi: 10.1111/j.1600-0897.2012.01120.x. [DOI] [PubMed] [Google Scholar]
- 8.Saito S, Shiozaki A, Sasaki Y, Nakashima A, Shima T, Ito M. Regulatory T cells and regulatory natural killer (NK) cells play important roles in feto-maternal tolerance. Semin Immunopathol. 2007;29:115–122. doi: 10.1007/s00281-007-0067-2. [DOI] [PubMed] [Google Scholar]
- 9.Saito S, Nakashima A, Myojo-Higuma S, Shiozaki A. The balance between cytotoxic NK cells and regulatory NK cells in human pregnancy. J Reprod Immunol. 2008;77:14–22. doi: 10.1016/j.jri.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Karimi K, Arck PC. Natural killer cells: keepers of pregnancy in the turnstile of the environment. Brain Behav Immun. 2010;24:339–347. doi: 10.1016/j.bbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Vacca P, Moretta L, Moretta A, Mingari MC. Origin, phenotype and function of human natural killer cells in pregnancy. Trends Immunol. 2011;32:517–523. doi: 10.1016/j.it.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013;19:548–556. doi: 10.1038/nm.3160. [DOI] [PubMed] [Google Scholar]
- 13.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev. 2006;20:123–137. doi: 10.1016/j.blre.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 14.Inngjerdingen M, Kveberg L, Naper C, Vaage JT. Natural killer cell subsets in man and rodents. Tissue Antigens. 2011;78:81–88. doi: 10.1111/j.1399-0039.2011.01714.x. [DOI] [PubMed] [Google Scholar]
- 15.Peritt D, Robertson S, Gri G, Showe L, Aste-Amezaga M, Trinchieri G. Cutting edge: differentiation of human NK cells into NK1 and NK2 subsets. J Immunol. 1998;161:5821–5824. [PubMed] [Google Scholar]
- 16.Al Omar S, Flanagan BF, Almehmadi M, Christmas SE. The effects of IL-17 upon human natural killer cells. Cytokine. 2013;62:123–130. doi: 10.1016/j.cyto.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Giezeman-Smits KM, Gorter A, Nagelkerke JF, van Vlierberghe RL, van Eendenburg J, Eggermont AM, et al. Characterization of three new membrane structures on rat NK cells which are involved in activation of the lytic machinery. Immunobiology. 1997;197:429–443. doi: 10.1016/S0171-2985(97)80077-0. [DOI] [PubMed] [Google Scholar]
- 18.Luo DZ, Vermijlen D, Ahishali B, Triantis V, Vanderkerken K, Kuppen PJ, et al. Participation of CD45, NKR-P1A and ANK61 antigen in rat hepatic NK cell (pit cell)mediated target cell cytotoxicity. World J Gastroenterol. 2000;6:546–552. doi: 10.3748/wjg.v6.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wedel J, Hottenrott MC, Bulthuis M, Huitema S, Yard BA, Hillebrands JL. N-octanoyl dopamine attenuates the development of transplant vasculopathy in rat aortic allografts via smooth muscle cell protective mechanisms. Transplantation. 2016;100:80–90. doi: 10.1097/TP.0000000000000870. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Zhou C, Robertson J, Carlock C, Lou YH. Peripheral blood CD8alphaalpha+CD11c+MHC-II+CD3- cells attenuate autoimmune glomerulonephritis in rats. Kidney Int. 2014;85:1078–1090. doi: 10.1038/ki.2013.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altomonte J, Marozin S, De Toni EN, Rizzani A, Esposito I, Steiger K, et al. Antifibrotic properties of transarterial oncolytic VSV therapy for hepatocellular carcinoma in rats with thioacetamide-induced liver fibrosis. Mol Ther. 2013;21:2032–2042. doi: 10.1038/mt.2013.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giezeman-Smits KM, Jonges LE, Chambers WH, Brisette-Storkus CS, Van Vlierberghe RL, Van Eendenburg JD, et al. Novel monoclonal antibodies against membrane structures that are preferentially expressed on IL-2-activated rat NK cells. J Leukoc Biol. 1998;63:209–215. doi: 10.1002/jlb.63.2.209. [DOI] [PubMed] [Google Scholar]
- 23.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, et al. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 24.LaMarca B, Wallace K, Herse F, Wallukat G, Martin JN, Jr, Weimer A, et al. Hypertension in response to placental ischemia during pregnancy: role of B lymphocytes. Hypertension. 2011;57:865–871. doi: 10.1161/HYPERTENSIONAHA.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander BT, Kassab SE, Miller MT, Abram SR, Reckelhoff JF, Bennett WA, et al. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension. 2001;37:1191–1195. doi: 10.1161/01.hyp.37.4.1191. [DOI] [PubMed] [Google Scholar]
- 26.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty D, Rumi MA, Konno T, Soares MJ. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc Natl Acad Sci USA. 2011;108:16295–16300. doi: 10.1073/pnas.1109478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monnier J, Zabel BA. Anti-asialo GM1 NK cell depleting antibody does not alter the development of bleomycin induced pulmonary fibrosis. PLoS One. 2014;9:e99350. doi: 10.1371/journal.pone.0099350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toldi G, Rigo J, Jr, Stenczer B, Vasarhelyi B, Molvarec A. Increased prevalence of IL-17-producing peripheral blood lymphocytes in pre-eclampsia. Am J Reprod Immunol. 2011;66:223–229. doi: 10.1111/j.1600-0897.2011.00987.x. [DOI] [PubMed] [Google Scholar]
- 30.Small HY, Nosalski R, Morgan H, Beattie E, Guzik TJ, Graham D, et al. Role of tumor necrosis factor-alpha and natural killer cells in uterine artery function and pregnancy outcome in the stroke-prone spontaneously hypertensive rat. Hypertension. 2016;68:1298–1307. doi: 10.1161/HYPERTENSIONAHA.116.07933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 32.Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 1992;262:C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- 33.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, et al. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cornelius DC, Hogg JP, Scott J, Wallace K, Herse F, Moseley J, et al. Administration of interleukin-17 soluble receptor C suppresses TH17 cells, oxidative stress, and hypertension in response to placental ischemia during pregnancy. Hypertension. 2013;62:1068–1073. doi: 10.1161/HYPERTENSIONAHA.113.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cornelius DC, Amaral LM, Wallace K, Campbell N, Thomas AJ, Scott J, et al. Reduced uterine perfusion pressure T-helper 17 cells cause pathophysiology associated with preeclampsia during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2016;311:R1192–R1199. doi: 10.1152/ajpregu.00117.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]