Abstract
The eye is an attractive organ for non-invasive discovery and monitoring of disease progression. Traditionally, fluorescein angiography (FA) and indocyanine green angiography (ICGA) have been used for dynamic evaluation of the retina and its vasculature. However, both fluorescein and indocyanine green (ICG) possess considerable disadvantages. FA is limited to assessing superficial retinal blood flow and often results in an unclear view due to fluorescein leakage. This obscures important pathologies such as neovascularization, ischemia and inflammation. ICG, a near-infrared fluorophore (NIRF), has nonspecific binding, high uptake and retention in tissues, as well as detrimental effects on the hepatobiliary tract. Here, we present a potential contrast agent for imaging ocular vascular permeability with ZW800, a heptamethine indocyanine NIRF, conjugated to polystyrene latex beads (ZW800m). ZW800 is an excellent alternative for near-infrared imaging, as it has excellent contrast, superior clearance, and is amendable to conjugation. ZW800m conjugation is an easy, attractive method of in vivo imaging and real-time tracking of ocular vascular pathologies. ZW800m is readily imaged via commercially available laser ophthalmoscope (SLO, HRA OCT, Spectralis) to assess vascular permeability in the mouse retina and choroid. In Type 1 diabetic Ins2Akita mice, ZW800m was observed in mouse retina but not in wild-type mice. After laser-induced choroidal neovascularization (CNV), ZW800m was observed in mouse choroid but not in control. In both CNV and diabetic mice, ZW800 imaging showed increased hyperfluorescence on ICG modality (ICGA) not seen on FA. Presence of ZW800m in respective tissues was confirmed ex vivo with flatmounts visualized with EVOS 800 nm light cube. ZW800 imaging may be easily employed in the research laboratory.
Keywords: ZW800, Polystyrene latex beads, Microspheres, ICG, Neovascularization, Leakage, Retina, Blood flow
1. Introduction
The eye is an attractive organ for non-invasive discovery and monitoring of disease progression. Uniquely situated, the eye allows for non-invasive visualization of the nerves and blood vessels, affording important information about a variety of disorders.
Traditionally, fluorescein angiography (FA) has been used for dynamic evaluation of the retina and its vasculature. However, a considerable disadvantage exists with this approach. Fluorescein angiography is limited, as it only assesses blood flow superficial to the retinal pigmented epithelium (RPE) (Flower and Hochheimer, 1976). Fluorescein exists in an 80% protein bound state where the remaining fraction tends to leak through the fenestrations of the vasculature resulting in an unclear view (Owens, 1996). Near-infrared fluorophores (NIRF) have gained traction due to their ability to be visualized deeper in the eye including the choroid, where many pathologies occur. The infrared wavelength used by this method penetrates deeper into tissues as it is not as readily scattered by the RPE or hemorrhagic lesions (Owens, 1996). These characteristics are important in detecting and monitoring pathologies such as neovascularization, ischemia, and inflammation as they may present subretinally (Yannuzzi et al., 1992).
Indocyanine green (ICG) is a particular NIRF that has become widely used for examining choroidal blood flow and pathology and is routinely used in clinical settings. ICG has also been used in image-guided surgery including cholangiography, sentinel lymph node biopsy, head neck cancer as well as numerous other cancers (Chopra, 2004). Its optical properties and high serum stability make it desirable for percutaneous and intraoperative identification (Choi et al., 2011). However, ICG has several limitations. It has nonspecific binding and high uptake and retention in tissues, leading to considerable background scatter in images of ophthalmic ICG angiography (ICGA). This dye is cleared via the hepatobiliary tract and can cause detrimental damage to the gastrointestinal tract. Furthermore, ICG is very difficult to conjugate to other molecules making it intractable for use in conjunction with biomarkers (Choi et al., 2011).
It has been proposed that NIR nanoparticles existing in a charged state (−1 for ICG) may have slower clearance and thus poorer contrast during imaging. ZW800, a heptamethine indocyanine NIRF, possessing a net charge of zero is rapidly cleared by the kidneys and produces superior imaging (Choi et al., 2011).
Polystyrene microspheres conjugated to molecular probe dyes have been used in combination with the confocal scanning laser ophthalmoscopy (e.g., Heidelberg Spectralis) for real-time tracking of vasculature leakage (Fischer et al., 2013). No microspheres with fluorescent capabilities in the 800 nm range exist commercially; to the best of our knowledge, 715 nm, is the highest reported excitation wavelength for any commercially available microsphere. Common confocal scanning laser ophthalmoscopes are equipped with excitation in the 488 nm range and the 795 nm range with emission filters that range from ~500 to 680 nm and ~800 to 900 nm for the FA and ICGA channels, respectively, which limits the utility of conjugated microspheres outside of those ranges (Seeliger et al., 2005). Furthermore, many strains of mice endogenously express protein in the 488 nm range, predominately secondary to background fluorescence of lipofuscin accumulation in the RPE or genetic manipulation (Sparrow et al., 2013). Hence, ZW800 NHS ester stands as an excellent alternative for NIR imaging in the retina, as it is amenable to conjugation, has better contrast, and possesses superior clearance than ICG (Choi et al., 2011).
In this study, we tested a novel approach to image ocular vascular permeability using 800 nm conjugated polystyrene beads in normal and pathological states. Our goal was to visualize and qualitatively define leakage with bead permeability in the 800 nm range. This offers a very attractive approach to non invasive in vivo visualization of many eye pathologies, and, to our knowledge, this is the first attempt at describing the effectiveness of near infrared fluorescent microsphere imaging for assessing vascular hyper-permeability in the retina.
2. Materials and supplies
2.1. Animals
All animals in this study were treated in accordance with IACUC guidelines.
Five age-matched (six month old) C57Bl6/J mice were compared to heterozygote Ins2Akita mice (C57B16/J background), which were used as a model of diabetes and assessed for retinal vascular leakage. Five age-matched (six month old) C57Bl6/J mice were also compared to C57Bl6/J laser induced Choroidal Neovascularization (CNV) mice and assessed for vascular permeability.
2.2. Scanning laser ophthalmoscope
Heidelberg Spectralis (HRA-OCT Spectralis, Heidelberg Engineering, Germany) running software version 1.7.1.0 was used with excitation laser operating at approximately 488 and 795 nm; detection above 800 nm, and bandpass filters approximately 800 and 900 nm for FA and ICGA channels, respectively.
2.3. Anesthetic
Induction of anesthesia was by an isoflurane/oxygen mixture, which was maintained at 3%. It was dispersed at a flow rate of 1.0 Lpm into an induction chamber. Maintenance anesthesia was continued at a flow rate of 1.0 Lpm and isoflurane/oxygen mixture was lowered to 1.5%.
2.4. Fluorescent agent
ZW800 was purchased from the FLARE Foundation (Boston, MA) and conjugated to 100 nm polystyrene latex beads (Magsphere, Pasadena, CA).
3. Detailed methods
3.1. Fluorophore preparation
Anhydrous coupling of ZW800-NHS ester with aminated polystyrene latex beads was performed by aminating the NIR fluorophore, ZW800, with 100 nm polystyrene latex beads (Choi et al., 2011). This ZW800-NHS product was dissolved in a 10 mM stock solution in >99.9% pure ultra-dry dimethyl sulfoxide (DMSO) (Sigma–Aldrich Co. LLC, St. Louis, MO, USA). Because the beads are pre-made in an aqueous solution of 10% solids, and hydrous coupling would destroy the NHS ester, 500 μL of the bead suspension was spun down by centrifugation (15,000 g × 60 min) and 450 μL of the supernatant was withdrawn, leaving only the 10% bead aliquot. 25 μL of the beads were then re-suspended in 475 μL of DMSO, for a final concentration of 5% beads in DMSO. 5 μL of ZW800 was then added to 5 μL of an amine source reagent, 10× molar excess >99.9% pure N,N-Diisopropylethylamine (DIEA) (Sigma–Aldrich Co. LLC, St. Louis, MO, USA) (e.g., 10 μM ZW800 and 100 μM DIEA). This mixture was combined with the 500 μL mixture of the beads and DMSO for a final concentration of 10 μM. This concentration of ZW800 was used in DMSO, as the organic base yields very high coupling, and at concentrations higher than 10–100 μM, fluorescence is diminished due to quenching.
This combined mixture was shielded from light and vortexed for 120 min. Loss of fluorescence was evidenced when the beads became visibly green from quenching. The ideal target color was a pale green, with minimal quenching.
3.2. Angiographic imaging
Mice were anesthetized and pupils were dilated with a 1% tropicamide solution and periodically dropped with saline solution to prevent corneal desiccation. Mice were placed on a heated water pad (37 °C). The ZW800/microsphere (ZW800m) solution was injected via tail vein (100 uL/20 g mouse). Imaging was accomplished using HRA-OCT Spectralis (Heidelberg Engineering, Germany) with excitation laser and bandpass filter approximately 1–2 min after tail vein injection. Images were acquired from both eyes with a 55 degree lens. These were taken at a standardized length between the mouse eye and lens. The laser intensity was standard for all images. After acquiring images, mice were allowed to recover on a heated water pad and monitored until fully conscious.
3.3. Mouse models of vascular leakage: CNV and diabetes
C57Bl6/J mice were used for laser-induced CNV. Induction of CNV laser photocoagulation (532 nm, 200 mW, 100 ms, 75 μm; OcuLight GL, Iridex, Mountain View, CA) was performed on both eyes of each animal by a single individual. Laser spots were applied in a standardized fashion around the optic nerve, using a slit lamp aiming system and a coverslip as a contact lens. The morphologic end point of the laser injury was the appearance of a cavitation bubble, a sign thought to correlate with the disruption of Bruch’s membrane. Mice were imaged 5 days later.
Six-month-old Ins2Akita mice (on C57Bl6/J background) were used as model of diabetes (>600 mg/dL blood glucose) and assessed for retinal vascular leakage.
3.4. Results
In this study we employed three different mouse models, C57Bl6/J control mice, C57Bl6/J mice treated with CNV laser photocoagulation, and Ins2Akita mice.
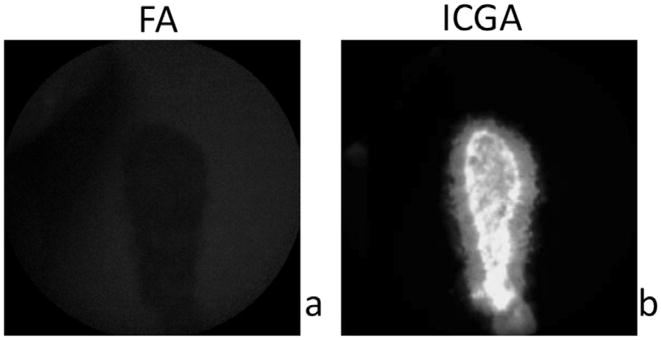
To determine whether ZW800m was specific for ICGA, ZW800m was imaged in vitro using the Heidelberg Spectralis. ZW800m was placed on a coverslip and imaged on FA and ICGA. This established that the nanoparticle was readily imaged using the NIRA capabilities of the Heidelberg Spectralis, ICGA, and did not overlap on FA (Fig. 1).
Fig. 1.
Visualization of ZW800m does not overlap with the FA modality. Hyper-fluorescence is apparent only on ICGA. a. ZW800m placed on coverslips are not seen on FA in vitro. b. ZW800m placed on coverslips fluorescing on ICGA in vitro.
To determine whether ZW800m was specific for ICGA in an animal model, control mice treated with ZW800m were imaged both in on FA and ICGA. It was apparent that the channels did not overlap as fluorescence was detected only on ICGA. In addition, no lesions were noted in these mice when imaged with either modality. This confirmed that ZW800m is an appropriate imaging technique to detect vascular pathologies of the eye.
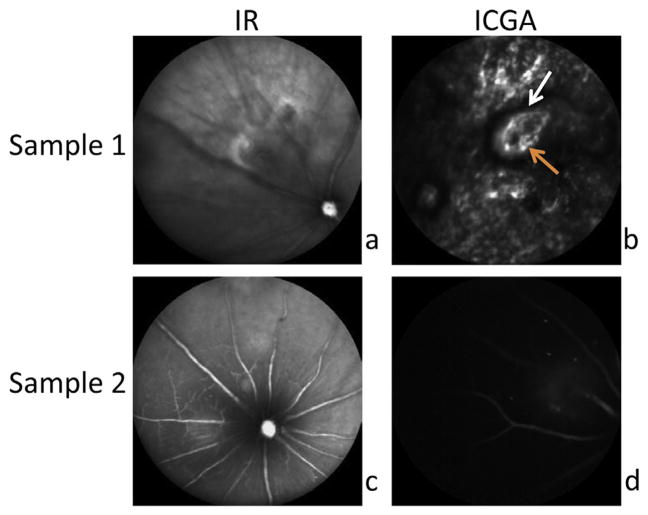
In the C57BI6/J mice treated with CNV laser photocoagulation, CNV lesions were readily apparent in the retina of mice injected with ZW800m (Fig. 2). These lesions corresponded to areas of hyperfluorescence. Fig. 2a and b shows CNV lesion with corresponding leakage 2 min after tail vein injection of ZW800, while Fig. 3a and b shows CNV lesion, with corresponding leakage, 5 min after injection of ZW800m.
Fig. 2.
CNV lesions are apparent in mice imaged with ZW800m on ICGA. Areas of hyperfluorescence are readily apparent (Sample 1 and 2) on ICGA in CNV mouse retina. a. and b. show CNV lesion, with corresponding leakage, within 2 min of tail vein injection of ZW800m. b. White arrow indicates demarcated boundary of hyper-fluorescence. Orange arrow marks abnormal thin vessels typical of CNV. c. and d. show CNV lesion, with corresponding leakage, 5 min after injection of ZW800m.
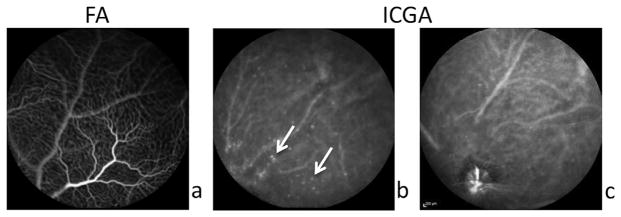
Fig. 3.
ZW800m are useful for visualizing vascular leakage in diabetic retinopathy. a. Diabetic mouse retina shown on FA with prior fluorescein injection. b. Areas of hyper-fluorescence outside of vasculature are apparent in Ins2Akita mouse retina with ZW800m injection on ICGA. White arrows point to distinct circles of hyperfluorescence not seen in control mouse retina. c. ZW800m are seen on ICGA contained within the vasculature in control mouse retina.
To determine whether ZW800m could detect vascular hyper-permeability, we assessed its use in the diabetic retina. Ins2Akita mice injected with ZW800m imaged using FA and ICGA showed hyper-fluorescence indicating possible vascular leakage. Permeability of vasculature was not evident in the Ins2Akita mice when imaged on FA (Fig. 3a.); however, distinct circles of hyperfluorescence outside of the vasculature were noted in Ins2Akita mice injected with ZW800m (Fig. 3b). Fig. 3c shows a non-diabetic littermate injected with ZW800m without the distinctive leakage seen in Fig. 3b.
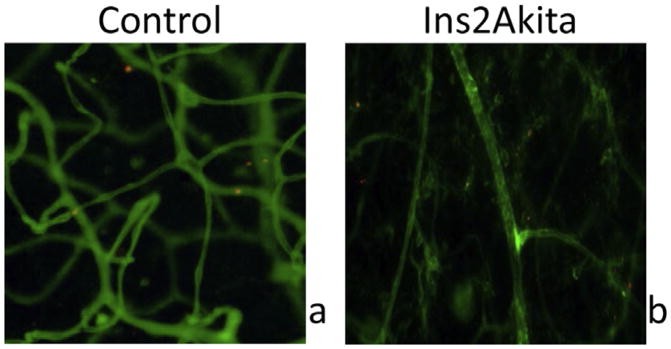
To confirm ZW800m location, retinal flatmounts were imaged via EVOS fluorescence cell imaging system (Life Technologies, Carlsbad, California). Flatmounts were stained with isolectin GS-IB4 (Life Technologies, Carlsbad, California) to assess vasculature. An 800 nm cube was used to detect ZW800. ZW800 fluorophores were located with respect to their presence inside or outside blood vessels. Zw800m outside of the vasculature can be seen in diabetic mouse retinas. In control mouse retinas, ZW800m are seen confined to the vasculature (Fig. 4).
Fig. 4.
Retinal flatmounts confirm ZW800 location. In control mouse retinal flatmount, clusters of ZW800m (red) are seen within the vasculature (green). In diabetic mouse retinal flatmount, ZW800m are seen outside of vasculature. Red = ZW800m, Green = Isolectin 488 staining of vasculature.
4. Potential pitfalls and troubleshooting
4.1. Eye opacity
Evaluation of the retina can only be accomplished by pupil dilation and appropriate levels of anesthesia. Mice tend to develop acute reversible lens opacities under these conditions, which limits visualization and imaging of the retina. To reduce cataract formation we employed several methods: 1) maintaining tear layer and avoiding corneal drying by periodic drops of phosphate buffered saline (Calderone et al., 1986) 2) maintaining body temperature by use of heating water pad (Fraunfelder and Burns, 1970) and, 3) limiting imaging time <5 min. These methods combined allowed for effective imaging (Ridder et al., 2002).
4.2. Tail vein injection
Tail vein collapse and poor tail veins are encountered more frequently in Ins2Akita mice versus control mice. In order to achieve higher rates of success we employed the following methods: 1) use of a heat lamp or warm water bath to dilate tail veins, 2) sterilize tail with 70% isopropyl alcohol, 3) avoidance of air bubbles in syringe, 4) avoidance of aspiration, 5) beginning distally and moving proximally, and, 6) slow injection. These methods are especially important with repetitive tail vein injections.
4.3. Discussion
Here we present a potential contrast agent for visualizing vascular leakage in a mouse model. Vascular leakage not readily imaged with conventional techniques is more readily visualized after a one-time tail vein injection of ZW800m and imaged with HRA-OCT Spectralis. The HRA-OCT Spectralis is common equipment found in many ophthalmic clinics and laboratories allowing for ease of use and accessibility. This makes this a translatable model for assessing vascular leakage in mice and potentially humans.
ZW800 is a zwitterionic nanoparticle, which possesses very unique characteristics. Given the drawbacks of the near infrared fluorophore nanoparticles including primary hepatobiliary clearance, high nonspecific binding, and high tissue uptake and retention, ZW800 is an attractive alternative. Due to its zwitterionic nature, ZW800 possess a net zero charge and is therefore charge neutral. Previous studies involving zwitterionic quantum dots have also shown a better side effect profile. It has no known toxicity, unlike the heavy metals found in quantum dots which could also be used for NIR-wavelength imaging. It is hypothesized that the zero charge allows for renal clearance as well as decreased uptake in vital organs and tissues (Choi et al., 2011). Another important feature of ZW800 is its capacity for superior imaging. ZW800 generated low background interference and therefore better visualization. This was also confirmed in our study as we were able to detect leakage, areas of hyperfluoresence, not seen by other methods i.e. fluorescein angiography. Hyperfluorescence is correlated with increased hyperpermeability, leaky vasculature, as well as incompetent blood vessels seen in neovascularization (Herbort et al., 1998). These images allow for tracking and maintenance of vascular permeability, which becomes very important in diseases such as diabetic retinopathy and choroidal neovascularization. The outcomes of such diseases are devastating and without treatment can lead to irreversible blindness.
Currently, fluorescent microspheres do not exist in the near infrared range. We were able to successfully conjugate ZW800 to microspheres. In addition, ZW800 may be used on its own without conjugation to a microsphere. In this study, we were able to limit the amount of vascular leakage based on the microsphere size. We employed microspheres of 100 nm and it is estimated that healthy retinal endothelial fenestration pores are 60–70 nm long (Rhodin, 1968). Successful control of microsphere diameter can allow us to estimate the size of vascular damage. Future studies should pursue a head-to-head comparison of ZW800m, ICG, and fluorescein in assessing and quantifying vascular leakage. In addition, temporal profiles of vascular leakage with or without treatment as well as explore the use of these conjugates in larger animals. Microspheres are best suited for animal studies, but would not be feasible in the current formulation for clinical translation as glomerular filtration is limited to particles <8 nm in diameter (Longmire et al., 2008). For future clinical development, development of conjugated biodegradable microspheres or use of alternative 80–100 nm particles composed of fullerenes, carbon nanotubes, silica, or dendrimers could be considered (Longmire et al., 2008). Also, the use of DMSO and potential for adverse side effects should investigated as well either through possible dilution or the use of a different solvent.
Acknowledgments
We thank Bonnie Archer for helping to prepare and proofread the manuscript. We thank Michael Frangioni for technical assistance with ZW800. Supported in part by an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NYC, to the Department of Ophthalmology & Visual Sciences, University of Utah.
References
- Calderone L, Grimes P, Shalev M. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Exp Eye Res. 1986 Apr;42(4):331–337. doi: 10.1016/0014-4835(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Choi HS, et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew Chem. 2011;50:6258–6263. doi: 10.1002/anie.201102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A. Zw800-1, a Zwitterionic Near-infrared Fluorophore, and its Cyclic rgd Peptide Derivative Cyclo-(rgdyk)-zw800-1 Molecular Imaging and Contrast Agent Database (micad) Bethesda (MD): 2004. [PubMed] [Google Scholar]
- Fischer MD, et al. Successful subretinal delivery and monitoring of microbeads in mice. PloS One. 2013;8:e55173. doi: 10.1371/journal.pone.0055173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower RW, Hochheimer BF. Indocyanine green dye fluorescence and infrared absorption choroidal angiography performed simultaneously with fluorescein angiography. Johns Hopkins Med J. 1976 Feb;138(2):33–42. [PubMed] [Google Scholar]
- Fraunfelder FT, Burns RP. Acute reversible lens opacity: caused by drugs, cold, anoxia, asphyxia, stress, death and dehydration. Exp Eye Res. 1970 Jul;10(1):19–30. doi: 10.1016/s0014-4835(70)80005-7. [DOI] [PubMed] [Google Scholar]
- Herbort CP, LeHoang P, Guex-Crosier Y. Schematic interpretation of indocyanine green angiography in posterior uveitis using a standard angiographic protocol. Ophthalmology. 1998 Mar;105(3):432–440. doi: 10.1016/S0161-6420(98)93024-X. [DOI] [PubMed] [Google Scholar]
- Longmire M, Choyke PL, Kobayashi H. Clearance properties of nano-sized particles and molecules are imaging agents: considerations and caveats. Nanomedicine (Lond) 2008 Oct;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens SL. Indocyanine green angiography. Br J Ophthalmol. 1996 Mar;80(3):263–266. doi: 10.1136/bjo.80.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968;25:452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Ridder W, 3rd, Nusinowitz S, Heckenlively JR. Causes of cataract development in anesthetized mice. Exp Eye Res. 2002 Sep;75(3):365–370. doi: 10.1006/exer.2002.2007. [DOI] [PubMed] [Google Scholar]
- Seeliger MA, et al. In vivo confocal imaging of the retina in animal models using scanning laser ophthalmoscopy. Vis Res. 2005 Dec;45(28):3519. doi: 10.1016/j.visres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, et al. Quantitative fundus autofluorescence in mice: correlation with HPLC quantitation of RPE lipofuscin and measurement of retina outer nuclear layer thickness. Investig Ophthalmol Vis Sci. 2013 Apr 17;54(4):2812–2820. doi: 10.1167/iovs.12-11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannuzzi LA, et al. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12:191–223. [PubMed] [Google Scholar]