Abstract
OBJECTIVE
Nonalcoholic steatohepatitis (NASH) is increasingly common in obese patients. However, its metabolic consequences in patients with type 2 diabetes mellitus (T2DM) are unknown.
RESEARCH DESIGN AND METHODS
We studied 154 obese patients divided in four groups: 1) control (no T2DM or NAFLD), 2) T2DM without NAFLD, 3) T2DM with isolated steatosis, and 4) T2DM with NASH. We evaluated intrahepatic triglycerides by proton MRS (1H-MRS) and assessed insulin secretion/resistance during an oral glucose tolerance test and a euglycemic-hyperinsulinemic clamp with glucose turnover measurements.
RESULTS
No significant differences among groups were observed in sex, BMI, or total body fat. Metabolic parameters worsened progressively with the presence of T2DM and the development of hepatic steatosis, with worse hyperinsulinemia, insulin resistance, and dyslipidemia (hypertriglyceridemia and low HDL cholesterol) in those with NASH (P < 0.001). Compared with isolated steatosis, NASH was associated with more dysfunctional and insulin-resistant adipose tissue (either as insulin suppression of plasma FFA [33 ± 3 vs. 48 ± 6%] or adipose tissue insulin resistance index [9.8 ± 1.0 vs. 5.9 ± 0.8 mmol/L ⋅ µIU/mL]; both P < 0.03). Furthermore, insulin suppression of plasma FFA correlated well with hepatic steatosis (r = –0.62; P < 0.001) and severity of steatohepatitis (rs = −0.52; P < 0.001). Hepatic insulin sensitivity was also more significantly impaired among patients with T2DM and NASH, both fasting and with increasing insulin levels within the physiological range (10 to 140 µIU/mL), compared with other groups.
CONCLUSIONS
In obese patients with T2DM, the presence of NAFLD is associated with more severe hyperinsulinemia, dyslipidemia, and adipose tissue/hepatic insulin resistance compared with patients without NAFLD. The unfavorable metabolic profile linked to NAFLD should prompt strategies to identify and treat this population early on.
Introduction
Nonalcoholic fatty liver disease (NAFLD) is an increasingly recognized clinical condition that includes a wide spectrum of liver disease. This ranges from isolated steatosis to nonalcoholic steatohepatitis (NASH), which can progress to cirrhosis and eventually hepatocellular carcinoma (1,2). The prevalence of NAFLD in Western countries varies from 24 to 42% (3,4). It is rapidly increasing worldwide in parallel with the increase in obesity, and the prevalence of NAFLD among obese individuals may be as high as ∼65% when measured by proton MRS (1H-MRS) (4). It is believed to be even higher in patients with type 2 diabetes mellitus (T2DM) (3,5), but large studies are lacking. A combination of environmental, lifestyle, genetic, and metabolic factors play a role in the pathogenesis of NAFLD (6).
Prior studies have shown that NAFLD is frequently associated with insulin resistance (7–9), as well as prediabetes and undiagnosed T2DM (10). Patients with T2DM and NAFLD often have poor glycemic control and may require more insulin to control hyperglycemia (11). Several cross-sectional studies have reported that patients with NAFLD and T2DM are at increased risk of developing more aggressive liver disease, such as NASH, advanced fibrosis, cirrhosis, or hepatocellular carcinoma (12–15). There is also a close relationship between dyslipidemia and the presence of adipose tissue and hepatic insulin resistance in patients with NAFLD and T2DM (16,17). Both conditions are characterized by oversecretion of VLDL driven by the high flux of fatty acids to the liver from dysfunctional adipose tissue (18). Increased hepatic VLDL secretion leads to lower HDL cholesterol (HDL-C) and to small, dense LDL cholesterol (LDL-C) particles, the typical triad of NAFLD. It is believed that these metabolic abnormalities contribute significantly to the increased cardiovascular disease of this population (19). Whether the presence of NASH further worsens this unfavorable metabolic profile remains unclear (16).
The aim of our study was to evaluate the metabolic effect of having isolated steatosis or NASH in patients with T2DM well matched for obesity and major clinical variables, using state-of-the-art measurements.
Research Design and Methods
Subjects
We recruited a total of 154 subjects for this study. Of these, 18 subjects without T2DM or NAFLD were used as obese healthy control subjects, while 10 extra nonobese healthy control subjects were included as a reference for metabolic measurements. All underwent a medical history, physical examination, routine chemistries, and electrocardiography. Body weight (±2%) and physical activity were stable for at least 3 months prior to the study as assessed by validated questionnaires (Nutritionquest, Berkeley, CA). Subjects were excluded if they had a history of alcohol abuse (≥30 g/day for men, ≥20 g/day for women, or a standardized Alcohol Use Disorders Identification Test [AUDIT] questionnaire ≥8) or liver (other than isolated steatosis or NASH), heart, pulmonary, and/or renal disease. Informed written consent was obtained from each patient prior to participation. Some of the patients in this cohort have been included in previous reports regarding the role of NAFLD in ethnicity (7) and on plasma aminotransferases (5,20).
Study Design
Metabolic measurements were performed at the clinical research unit and included the following: 1) measurement of fasting plasma glucose, insulin, and free fatty acid (FFA) concentration and A1C, lipid profile, and routine chemistries; 2) 2-h 75-g oral glucose tolerance test (OGTT) with blood drawn every 30 min for plasma glucose, insulin, C-peptide, and FFA concentration to establish the diagnosis of T2DM according to the American Diabetes Association criteria (21) and to calculate insulin secretion and hepatic insulin extraction; 3) total body fat by DXA; 4) measurement of intrahepatic triglycerides by 1H-MRS; 5) measurement of total plasma adiponectin (n = 78); 6) measurement of adipose tissue insulin resistance index (Adipo-IRi: calculated as fasting plasma insulin [FPI] × FFA concentration) and suppression of plasma FFA by low-dose insulin infusion during the euglycemic-hyperinsulinemic clamp; 7) measurement of liver and muscle insulin sensitivity (euglycemic-hyperinsulinemic clamp with [3-3H]glucose): basal and hepatic insulin resistance (suppression of endogenous glucose production [EGP; primarily hepatic] by low-dose insulin infusion) and insulin-stimulated peripheral (muscle) glucose uptake (Rd); 8) an index of hepatic insulin resistance (HIRi: calculated as FPI × EGP); and 9) a liver biopsy to establish the diagnosis of definite NASH by histology.
Liver and Total Body Fat Content by 1H-MRS
For the measurement of intrahepatic triglycerides, we acquired localized proton nuclear magnetic resonance spectra of the liver using methodology previously described (5,22,23). Briefly, three voxels of 30 × 30 × 30 mm were localized in different areas of the liver avoiding vessels and bile ducts. Intrahepatic triglyceride content was calculated as fat fraction (area under the curve [AUC] fat peak/[AUC fat peak + water peak]) using commercial software (NUTS, Acorn NMR Inc.). Measurements were corrected for T1 and T2 relaxation as previously described (24). An intrahepatic triglyceride content of >5.5% was considered diagnostic of NAFLD (4).
Euglycemic-Hyperinsulinemic Clamp
For the euglycemic-hyperinsulinemic clamp (25), after an overnight fast subjects were studied at the research unit as previously described (26) with the infusion of [3-3H]glucose to measure glucose turnover. After the basal equilibration period, insulin was administered as a primed-continuous infusion at 10 mU/m2 ⋅ min for 120 min to assess suppression of endogenous (primarily hepatic) glucose production, followed by an insulin infusion rate of 80 mU/m2 ⋅ min for 120 min to assess insulin-stimulated muscle glucose disposal (Rd). A variable 20% glucose infusion maintained the plasma glucose at ∼90–100 mg/dL. Only patients at San Antonio, TX, underwent this procedure (n = 56).
Liver Biopsy
An ultrasound-guided liver biopsy was performed in patients with a diagnosis of NAFLD by 1H-MRS (n = 86), as all these patients were considered to have high risk of NASH owing to the presence of T2DM. Biopsies were evaluated by a pathologist who was blinded to the subjects’ identity or clinical information. Histologic characteristics for the diagnosis of NASH were determined using standard criteria (27).
Metabolic Index Calculations
EGP and Rd were calculated as previously reported (7,26,28). Indexes of hepatic (HIRi = FPI × EGP) and adipose tissue (Adipo-IRi = FFA × FPI) insulin resistance were calculated based on the linear relationship between the rise in FPI level and inhibition of plasma EGP and FFA in healthy subjects, respectively (29). The higher the rate of EGP and of fasting plasma FFA levels, respectively, the greater the severity of both liver and adipose tissue insulin resistance. Experimental validation has previously been published by our group (7,17,30–32). To estimate hepatic insulin extraction, we used the incremental C-peptide–to–insulin AUC ratio calculated by the trapezoidal rule during the OGTT as previously described (33). Insulin secretion was calculated as the C-peptide–to–glucose AUC ratio (×100) during the OGTT.
Analytical Methods
Plasma glucose was measured by the glucose oxidase method (Analox glucose analyzer; Analox Instruments, Lunenburg, MA). Plasma insulin and C-peptide concentration were measured by radioimmunoassay (Siemens, Los Angeles, CA). A1C was measured by high-performance liquid chromatography (TOSOH G-7). Plasma glucose radioactivity was measured from deproteinized plasma samples precipitated from barium hydroxide/zinc sulfate (26). Finally, total plasma adiponectin was measured by immunoassay (Milliplex MAP, EMD Millipore Corporation, Billerica, MA).
Statistical Analysis
All values are reported as the mean ± SEM for continuous variables and n (%) for categorical variables. Comparison among groups was performed using ANOVA (Bonferroni post hoc analysis for pairwise comparisons) or Kruskal-Wallis for continuous variables according to their distribution or Pearson χ2 or Fisher exact test for categorical variables. Pearson or Spearman correlations were performed based on the characteristics and distribution of the variables. A P value of <0.05 (two-tailed) was considered statistically significant. All statistical calculations were performed using Stata 11.0 (StataCorp, College Station, TX) and JMP, version 11 (SAS Institute, Cary, NC).
Results
Subject Characteristics
The clinical and biochemical characteristics of subjects who participated in the study are shown in Table 1. They were divided in four groups according to the presence or absence of T2DM and the severity of NAFLD: 1) obese control subjects (without T2DM or NAFLD), 2) obese patients with T2DM without NAFLD, 3) obese patients with T2DM and isolated steatosis, and 4) obese patients with T2DM and NASH. As can be observed, there were no significant differences in important clinical characteristics such as sex, BMI, and total body fat among the groups. Age was statistically different among the groups, but this difference was of marginal clinical importance.
Table 1.
Demographic and clinical characteristics of patients
| Obese controls without T2DM or NAFLD (n = 18) | Obese with T2DM |
|||
|---|---|---|---|---|
| No NAFLD (n = 50) | Isolated steatosis (n = 21) | NASH (n = 65) | ||
| Age (years) | 53 ± 2 | 60 ± 1* | 57 ± 1 | 55 ± 1 |
| Sex (male/female), % | 56/44 | 84/16 | 71/29 | 78/22 |
| BMI (kg/m2) | 34.3 ± 0.7 | 34.0 ± 0.5 | 35.4 ± 0.8 | 36.5 ± 0.5 |
| Total body fat by DXA (%) | 36 ± 2 | 35 ± 1 | 38 ± 2 | 37 ± 1 |
| Fasting plasma glucose (mg/dL) | 105 ± 2 | 139 ± 7* | 137 ± 8* | 146 ± 5* |
| A1C (%) | 5.7 ± 0.1 | 7.0 ± 0.2* | 6.8 ± 0.2* | 7.2 ± 0.2* |
| Diabetes medication (%) | ||||
| Metformin | 76 | 58 | 70 | |
| Sulfonylureas | 39 | 11 | 48 | |
| Insulin | 24 | 35 | 24 | |
| FPI (µU/mL) | 8 ± 2 | 9 ± 1 | 12 ± 2 | 19 ± 2*,^ |
| HOMA | 1.9 ± 0.4 | 3.1 ± 0.4 | 4.3 ± 1.0 | 6.8 ± 0.7* |
| Liver fat by 1H-MRS (%) | 3 ± 1 | 3 ± 1 | 15 ± 2*,# | 15 ± 1* |
| AST (IU/L) | 29 ± 4 | 25 ± 2 | 26 ± 2 | 50 ± 4*,^ |
| ALT (IU/L) | 31 ± 6 | 28 ± 3 | 32 ± 3 | 66 ± 6*,^ |
| Systolic BP (mmHg) | 127 ± 4 | 139 ± 3* | 128 ± 2 | 135 ± 2 |
| Diastolic BP (mmHg) | 76 ± 2 | 80 ± 1 | 74 ± 1 | 78 ± 1 |
| On BP medication (%) | 65 | 91 | 72 | 91 |
| Total cholesterol (mg/dL) | 173 ± 7 | 153 ± 4 | 164 ± 11 | 170 ± 6 |
| Triglycerides (mg/dL) | 108 ± 14 | 122 ± 8 | 135 ± 13 | 215 ± 19*,^ |
| LDL-C (mg/dL) | 104 ± 5 | 87 ± 3 | 97 ± 10 | 92 ± 5 |
| HDL-C (mg/dL) | 47 ± 3 | 41 ± 1 | 40 ± 2 | 38 ± 1* |
| Use of statins (%) | 53 | 71 | 67 | 69 |
Data are mean ± SEM unless otherwise indicated. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BP, blood pressure. Symbols represent P values from Bonferroni adjustment for multiple comparisons:
*P ≤ 0.05 compared with control subjects;
#P < 0.05 compared with T2DM without NAFLD;
^P < 0.05 compared with T2DM and isolated steatosis.
Intrahepatic triglyceride content was higher in patients with NAFLD (either isolated steatosis or NASH) compared with the other two groups. However, plasma aspartate aminotransferase levels and alanine aminotransferase levels were only increased in patients with NASH. Of note, there was no difference in intrahepatic triglyceride content between control subjects and patients with T2DM without NAFLD.
Fasting plasma glucose levels and A1C were similarly elevated in patients with T2DM (with or without NAFLD) compared with control subjects (all P ≤ 0.05). Moreover, the use of metformin, sulfonylureas, and insulin was not different between patients with T2DM. The presence of isolated steatosis or NASH in patients with T2DM was not associated with worse blood pressure control, more antihypertensive medication use, or higher total cholesterol or LDL-C. However, patients with NASH and T2DM had higher plasma triglyceride levels and a trend toward lower HDL-C compared with the other patients with T2DM or obese control subjects without T2DM. This occurred despite similar use of statins among groups. Clinical characteristics of nonobese healthy control subjects used as a reference for metabolic measurements were as follows: age 43 ± 4 years, BMI 25.8 ± 0.9 kg/m2, 50% males, and intrahepatic triglyceride content 1.7 ± 0.5%.
Insulin Sensitivity Parameters
Adipose Tissue
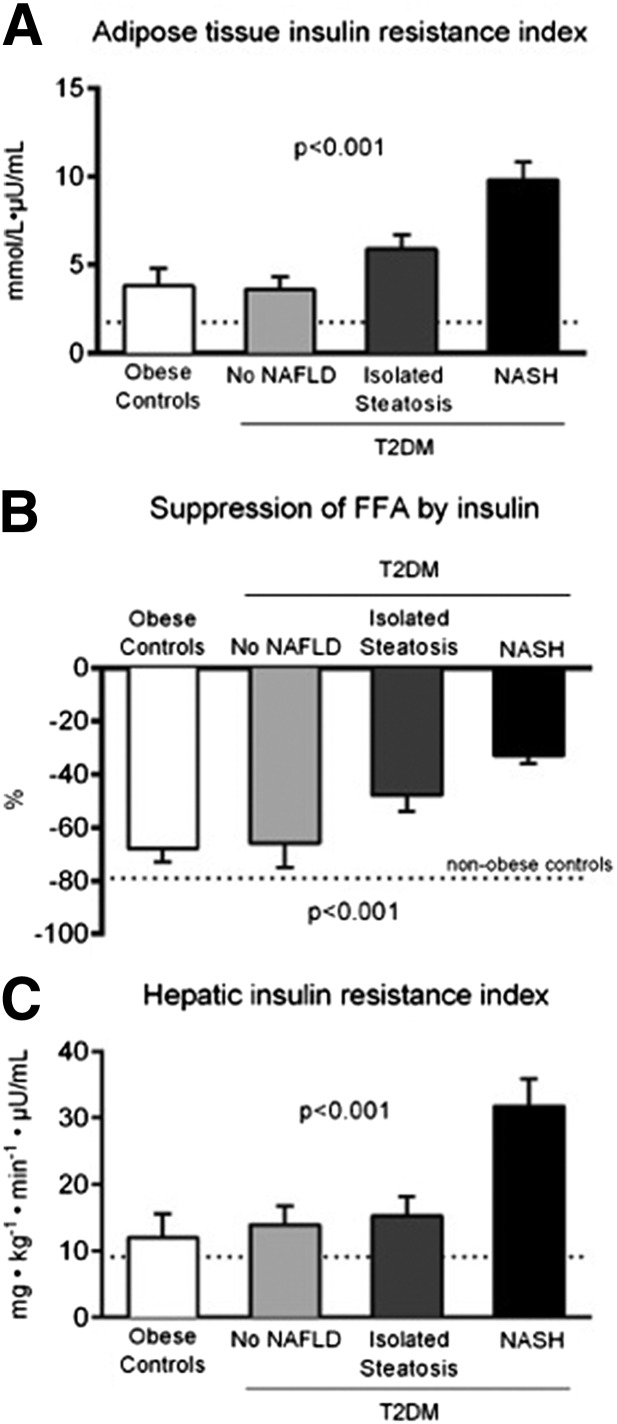
Because adipose tissue has an important role in the pathogenesis of NAFLD, we closely examined adipose tissue insulin sensitivity, expressed either as the Adipo-IRi or the suppression of plasma FFA by low-dose insulin infusion during the euglycemic-hyperinsulinemic clamp. As observed in Fig. 1A and B, patients with T2DM without NAFLD showed adipose tissue insulin resistance similar to that of nondiabetic obese control subjects without NAFLD. In patients with isolated steatosis or NASH, we observed a stepwise worsening of adipose tissue insulin resistance examined either fasting (Fig. 1A) or during a low-dose insulin infusion (Fig. 1B), suggesting an important association between dysfunctional adipose tissue and the severity of liver disease. In support of this, total plasma adiponectin levels showed a trend toward lower values with progression of the severity of liver disease (11.9 ± 1.4 vs. 9.3 ± 1.5 vs. 9.5 ± 1.5 vs. 7.8 ± 0.7 µg/mL; P for trend 0.04).
Figure 1.
A: Adipo-IRi (FPI × fasting FFA concentration). B: Percentage suppression of plasma FFA concentration by low-dose insulin infusion. C: HIRi (FPI concentration × fasting endogenous [primarily hepatic] glucose production). Dotted lines represent mean values for nonobese healthy subjects from the group.
Liver
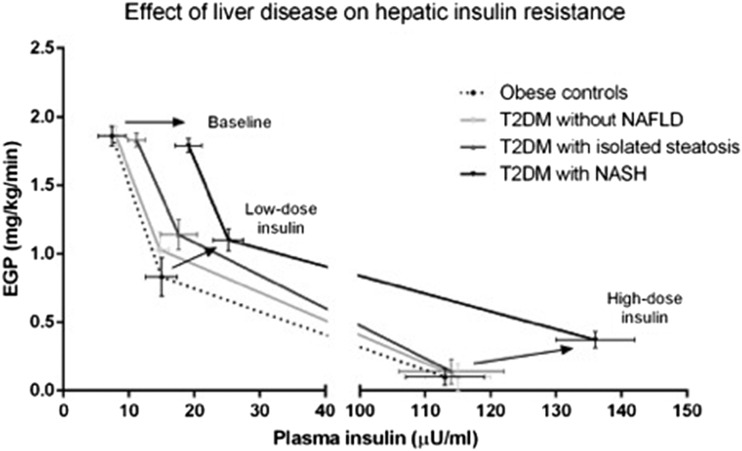
As can be observed in Fig. 1C, fasting insulin resistance at the level of the liver (expressed as the HIRi: EGP × insulin concentration) was worse in all obese groups compared with nonobese subjects without NAFLD. (See dotted line in Fig. 1C.) The most significant increase was seen with the presence of T2DM and NASH. Indeed, as shown in Fig. 2, this was more evident when the changes in EGP were assessed by plasma insulin concentration during fasting and during the euglycemic-hyperinsulinemic clamp. At baseline, all groups showed similar fasting EGP, although FPI was much higher in patients with T2DM and steatosis or NASH, suggesting worse hepatic insulin resistance in both groups. With a relatively small increase in plasma insulin (low-dose insulin clamp), patients with diabetes showed an ∼35% reduction in EGP, while it decreased much more (∼55%) in the control group without diabetes. Finally, during the high-dose step of the euglycemic-hyperinsulinemic clamp, patients with NASH failed to completely suppress EGP as the rest of the groups did despite higher insulin levels.
Figure 2.
Changes in endogenous (primarily hepatic) glucose production and plasma insulin concentration during the low- and high-dose euglycemic-hyperinsulinemic clamp. Arrows represent progression from obese control subjects without NAFLD to obese patients with T2DM and NASH during fasting (top arrow) as well as low-dose (middle arrow) and high-dose (bottom-right arrow) insulin infusion.
Skeletal Muscle
Skeletal muscle insulin sensitivity (Rd) was also found to decrease in a stepwise manner from obese control subjects without NAFLD to patients with T2DM without NAFLD, T2DM with isolated steatosis, or T2DM with NASH (10.4 ± 1.2 vs. 7.7 ± 0.8 vs. 6.4 ± 0.9 vs. 4.8 ± 0.4 mg ⋅ kg LBM−1 ⋅ min−1, respectively; P < 0.001).
Correlations
To further evaluate the role of adipose tissue in the development and progression of NAFLD, we assessed the association between adipose tissue insulin resistance, expressed as suppression of FFA during the low-dose insulin clamp, and hepatic steatosis and liver histology expressed as the composite of steatosis, inflammation, and hepatocyte necrosis or NAFLD activity score. We observed a strong inverse correlation between adipose tissue insulin sensitivity expressed as the suppression of plasma FFA by low-dose insulin and the amount of intrahepatic triglyceride accumulation quantified by 1H-MRS (r = −0.62; P < 0.001) (Fig. 3A), as well as the severity of the NAFLD activity score (rs = −0.52; P < 0.001) (Fig. 3B). Of note, the latter was not only driven by steatosis, as necroinflammation (combination of inflammation and ballooning) was also well correlated with suppression of plasma FFA by low-dose insulin (rs = −0.51; P < 0.001).
Figure 3.
A: Pearson correlation between suppression of plasma FFA levels by low-dose insulin during the euglycemic-hyperinsulinemic clamp and intrahepatic triglycerides measured by 1H-MRS. B: Spearman correlation between suppression of plasma FFA levels by low-dose insulin during the euglycemic-hyperinsulinemic clamp and histological severity of liver disease expressed as the NAFLD activity score.
Insulin Secretion and Hepatic Insulin Clearance
We studied the factors that may lead to the increased FPI in our population, a common abnormality of patients with isolated steatosis or NASH, and which may be the result of increased insulin secretion, decreased insulin clearance, or a combination of both. We observed a ∼1.5- to 2.5-fold increase in the FPI in patients with T2DM and isolated steatosis or NASH (Table 1). We next evaluated insulin secretion and hepatic insulin clearance during the OGTT. Insulin secretion (incremental C-peptide AUC/glucose AUC) was equally impaired in patients with T2DM regardless of the presence or absence of isolated steatosis or NASH (obese control subjects 36.2 ± 14.5, T2DM without NAFLD 5.2 ± 0.9, T2DM with isolated steatosis 6.0 ± 1.0, and T2DM with NASH 5.5 ± 0.8; all P ≤ 0.001 for comparisons against control subjects). Hyperinsulinemia in patients with NASH was mainly driven by reduced hepatic insulin clearance and not by increased insulin secretion, as hepatic insulin clearance, estimated as incremental C-peptide AUC/insulin AUC, was similar between control subjects and patients with T2DM without NAFLD (10.8 ± 1.4 vs. 10.8 ± 1.5; P = 0.99) and only significantly decreased in patients with NASH (6.2 ± 0.6; P = 0.001 against control subjects).
Conclusions
Because NAFLD in T2DM is increasingly common in middle-aged obese patients and carries an increased risk of cirrhosis and cardiovascular disease, we wanted to evaluate the metabolic effect of NAFLD in this population. The advantage of the current study is having combined state-of-the-art metabolic measurements with quantification of intrahepatic triglycerides (1H-MRS) and liver histology. In particular, we wanted to determine whether the presence of NASH was associated with a worse metabolic profile compared with isolated steatosis. Our main finding is that the development and progression of liver disease in patients with T2DM (i.e., without NAFLD → isolated steatosis → NASH) are associated with more severe insulin resistance in adipose tissue (Fig. 1A and B). This finding highlights the important role of lipotoxicity in the development and progression of NAFLD (2,16,17). Supporting this concept, insulin-induced suppression of plasma FFA correlated strongly with the amount of intrahepatic triglycerides measured by 1H-MRS (Fig. 3A), as well as with the severity of liver disease on histology (Fig. 3B). The current findings are consistent with prior reports about the role of adipose tissue insulin resistance in both lean (34) and obese (17) patients with NAFLD. If adipose tissue insulin resistance is indeed a driver of lipotoxicity in NAFLD and NASH, and possibly of mitochondrial dysfunction (35,36), treatment strategies targeting adipose tissue dysfunction (i.e., either by weight loss and/or pharmacological agents, such as thiazolidinediones) will likely play a greater role in the future.
In subjects without diabetes, the hepatic insulin resistance index, a measure of liver resistance in the fasting state, was already ∼30% increased (worse) in obese control subjects versus nonobese subjects without a fatty liver (dotted line in Fig. 1C). However, it was in patients with T2DM and NASH that we observed the most significant impairment in hepatic insulin sensitivity. This was further confirmed when the interaction between changes in EGP and plasma insulin concentration was carefully analyzed during the two-step euglycemic-hyperinsulinemic clamp (Fig. 2). Previous studies assessing insulin sensitivity with the euglycemic-hyperinsulinemic clamp technique reported the association between steatosis and hepatic insulin resistance (11,18,31). However, this study goes further by establishing a close relationship between the severity of liver disease on histology (i.e., steatohepatitis) with worse hepatic insulin resistance and other metabolic abnormalities in patients with NAFLD. Of particular interest was the pronounced hyperinsulinemia associated with the development of steatohepatitis (Table 1). Interestingly, hyperinsulinemia was better explained by impaired hepatic insulin extraction/clearance rather than increased insulin secretion in patients with T2DM and NAFLD. This is in accordance with previous reports by our group (33) and others (37).
The presence of NASH in patients with T2DM was associated with more severe hypertriglyceridemia but with no differences in blood pressure or glycemia (Table 1). These results have important clinical implications, as they suggest that identification of patients with T2DM and NASH may highlight a subgroup of subjects in need of early and/or more aggressive lipid-lowering therapy and cardiovascular risk factor management. However, the cardiovascular consequences of elevated plasma triglycerides in patients with T2DM and NASH remain unclear. VLDL oversecretion and hypertriglyceridemia are well-established abnormalities in NAFLD (2,6,16,18), but the role of steatohepatitis had been less carefully studied. The current work confirms that the severity of NASH on liver histology closely correlates with plasma triglyceride levels and expands on the role of NASH in T2DM. In a recent study by Arulanandan et al. (38), metabolic changes correlated more strongly with increasing levels of intrahepatic triglycerides than liver histological features. However, the authors studied mostly patients without diabetes and did not perform a direct comparison of isolated steatosis versus NASH. In addition, differences in BMI among groups could have confounded the interpretation of these results. However, it is possible that the increased atherogenic risk of obese patients with NAFLD is more closely related to their worse apolipoprotein B–to–A1 ratio (and smaller LDL-C particle size), which correlates better with the severity of hepatic steatosis rather than necroinflammation (39). Clearly more work to understand the role of atherogenic dyslipidemia in NAFLD is much needed, given the considerable debate about the role of NASH in cardiovascular disease (16,19,40).
The presence of NAFLD in T2DM continues to be overlooked by clinicians, but there is an increased awareness about the negative health consequences of steatohepatitis. Several studies have reported that fatty liver disease is common in patients with T2DM (4,12,40,41). In a large Italian study (19), the prevalence of NAFLD was estimated to be as high as 69.5% among patients with T2DM. Of note, cardiovascular disease was much more common compared with occurrence in patients without fatty liver. However, the true prevalence was uncertain, as patients were recruited within a tertiary hospital setting and results confounded by an incomplete screening for secondary causes of hepatic steatosis and the screening limitations of liver ultrasound with suboptimal sensitivity and specificity. More recently, Williamson et al. (42) reported on the prevalence and risk factors for NAFLD from a large, randomly selected study in patients with T2DM from Edinburgh, Scotland. Among 939 patients aged 61–76 years, the prevalence of NAFLD by ultrasound was 42.6%. Higher BMI, A1C, and plasma triglycerides were important factors associated with the development of NAFLD. In our hands, using the gold-standard liver 1H-MRS technique, we have identified that 56% of obese patients with T2DM and normal liver enzymes have NAFLD (5). Therefore, screening and early diagnosis of hepatic steatosis in patients with diabetes may be important to establish timely intervention in this high-risk population.
In summary, in middle-aged obese patients with T2DM, the presence of NAFLD is associated with hyperinsulinemia and more severe adipose tissue and hepatic insulin resistance, as well as worse atherogenic dyslipidemia. The clinical implication is that the presence of NAFLD in patients with T2DM should alert the health care provider to institute a more aggressive lifestyle intervention and consider strategies to minimize high cardiovascular risk.
Supplementary Material
Article Information
Acknowledgments. The authors thank the study volunteers, the Clinical and Translational Science Award nursing staff (in particular, Norma Diaz and Rose Kaminski-Graham), and the nutrition and laboratory staff (University of Texas Health Science Center at San Antonio and University of Florida) for assistance in performing the described studies.
Funding. This work was supported by the Burroughs Wellcome Fund (1006762.01) (to K.C.), by the American Diabetes Association (1-08-CR-08) (to K.C.), by the U.S. Department of Veterans Affairs Medical Research Fund (1 I01 CX000167-01), and by award UL 1RR025767 from the National Center for Research Resources.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.L. contributed to the collection and analysis of the research data and manuscript writing. F.B., P.P.-S., C.O.-L., B.O., D.B., M.L., A.S., and M.H.W. contributed to research data collection, data analysis, and review of the final version of the manuscript. K.C. participated in all aspects of the study, data analysis, and manuscript writing and edited the final version of the manuscript. K.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A slide set summarizing this article is available online.
References
- 1.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–2273 [DOI] [PubMed] [Google Scholar]
- 2.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52:774–788 [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131 [DOI] [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387–1395 [DOI] [PubMed] [Google Scholar]
- 5.Portillo-Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase Levels. J Clin Endocrinol Metab 2015;100:2231–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lomonaco R, Sunny NE, Bril F, Cusi K. Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs 2013;73:1–14 [DOI] [PubMed] [Google Scholar]
- 7.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Role of ethnicity in overweight and obese patients with nonalcoholic steatohepatitis. Hepatology 2011;54:837–845 [DOI] [PubMed] [Google Scholar]
- 8.Bonnet F, Ducluzeau PH, Gastaldelli A, et al.; RISC Study Group . Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 2011;60:1660–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des 2010;16:1941–1951 [DOI] [PubMed] [Google Scholar]
- 10.Ortiz-Lopez C, Lomonaco R, Orsak B, et al. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 2012;35:873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryysy L, Häkkinen AM, Goto T, et al. Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 2000;49:749–758 [DOI] [PubMed] [Google Scholar]
- 12.Adams LA, Harmsen S, St Sauver JL, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community-based cohort study. Am J Gastroenterol 2010;105:1567–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cusi K. Nonalcoholic fatty liver disease in type 2 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2009;16:141–149 [DOI] [PubMed] [Google Scholar]
- 14.Bugianesi E, Vanni E, Marchesini G. NASH and the risk of cirrhosis and hepatocellular carcinoma in type 2 diabetes. Curr Diab Rep 2007;7:175–180 [DOI] [PubMed] [Google Scholar]
- 15.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820–1832 [DOI] [PubMed] [Google Scholar]
- 16.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology 2012;142:711–725.e6 [DOI] [PubMed]
- 17.Lomonaco R, Ortiz-Lopez C, Orsak B, et al. Effect of adipose tissue insulin resistance on metabolic parameters and liver histology in obese patients with nonalcoholic fatty liver disease. Hepatology 2012;55:1389–1397 [DOI] [PubMed] [Google Scholar]
- 18.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 2010;51:679–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targher G, Bertolini L, Padovani R, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007;30:1212–1218 [DOI] [PubMed] [Google Scholar]
- 20.Maximos M, Bril F, Portillo Sanchez P, et al. The role of liver fat and insulin resistance as determinants of plasma aminotransferase elevation in nonalcoholic fatty liver disease. Hepatology 2015;61:153–160 [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association Classification and diagnosis of diabetes. Diabetes Care 2015;38(Suppl. 1):S8–S16 [DOI] [PubMed] [Google Scholar]
- 22.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297–2307 [DOI] [PubMed] [Google Scholar]
- 23.Bril F, Ortiz-Lopez C, Lomonaco R, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int 2015;35:2139–2146 [DOI] [PubMed] [Google Scholar]
- 24.Perman WH, Balci NC, Akduman I. Review of magnetic resonance spectroscopy in the liver and the pancreas. Top Magn Reson Imaging 2009;20:89–97 [DOI] [PubMed] [Google Scholar]
- 25.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 26.Cusi K, Consoli A, DeFronzo R. Metabolic effects of metformin on glucose and lactate metabolism in NIDDM. J Clin Endocrinol Metab 1996;81:4059–4067 [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bril F, Maximos M, Portillo-Sanchez P, et al. Relationship of vitamin D with insulin resistance and disease severity in non-alcoholic steatohepatitis. J Hepatol 2015;62:405–411 [DOI] [PubMed] [Google Scholar]
- 29.Groop LC, Bonadonna RC, DelPrato S, et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 1989;84:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gastaldelli A, Harrison SA, Belfort-Aguilar R, et al. Importance of changes in adipose tissue insulin resistance to histological response during thiazolidinedione treatment of patients with nonalcoholic steatohepatitis. Hepatology 2009;50:1087–1093 [DOI] [PubMed] [Google Scholar]
- 31.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506 [DOI] [PubMed] [Google Scholar]
- 32.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 33.Bril F, Lomonaco R, Orsak B, et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology 2014;59:2178–2187 [DOI] [PubMed] [Google Scholar]
- 34.Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia 2005;48:634–642 [DOI] [PubMed] [Google Scholar]
- 35.Sunny NE, Parks EJ, Browning JD, Burgess SC. Excessive hepatic mitochondrial TCA cycle and gluconeogenesis in humans with nonalcoholic fatty liver disease. Cell Metab 2011;14:804–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koliaki C, Szendroedi J, Kaul K, et al. Adaptation of hepatic mitochondrial function in humans with non-alcoholic fatty liver is lost in steatohepatitis. Cell Metab 2015;21:739–746 [DOI] [PubMed] [Google Scholar]
- 37.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 2008;135:122–130 [DOI] [PubMed] [Google Scholar]
- 38.Arulanandan A, Ang B, Bettencourt R, et al. Association between quantity of liver fat and cardiovascular risk in patients with nonalcoholic fatty liver disease independent of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2015;13:1513–1520.e1 [DOI] [PubMed] [Google Scholar]
- 39.Bril F, Sninsky JJ, Baca AM, et al. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD. J Clin Endocrinol Metab 2016;101:644–652 [DOI] [PubMed] [Google Scholar]
- 40.Lomonaco R, Cusi K. Non-alcoholic fatty liver disease (NAFLD) in diabetes: distraction or impending disaster? In Evidence-Based Management of Diabetes. Shrewsbury, U.K., tfm publishing Ltd., 2012, p. 383–404 [Google Scholar]
- 41.Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int 2009;29:113–119 [DOI] [PubMed] [Google Scholar]
- 42.Williamson RM, Price JF, Glancy S, et al.; Edinburgh Type 2 Diabetes Study Investigators . Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2011;34:1139–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.