Abstract
Objective
Aortic pathologies exhibit sexual dimorphism, with aneurysms in both the thoracic and abdominal aorta (AAA) exhibiting higher male prevalence. Women have lower prevalence of aneurysms, but when they occur, aneurysms progress rapidly. To define mechanisms for these sex differences, we determined the role of sex chromosome complement and testosterone on the location and progression of angiotensin II (AngII)-induced aortic pathologies.
Approach and Results
We used transgenic male mice expressing Sry on an autosome to create low density lipoprotein receptor (Ldlr) deficient male mice with an XY or XX sex chromosome complement. Transcriptional profiling was performed on abdominal aortas from XY or XX males, demonstrating 1746 genes influenced by sex chromosomes or sex hormones. Males (XY or XX) were either sham-operated or orchiectomized prior to AngII infusions. Diffuse aortic aneurysm pathology developed in XY AngII-infused males, while XX males developed focal AAAs. Castration reduced all AngII-induced aortic pathologies in XY and XX males. Thoracic aortas from AngII-infused XY males exhibited adventitial thickening that was not present in XX males. We infused male XY and XX mice with either saline or AngII and quantified mRNA abundance of key genes in both thoracic and abdominal aortas. Regional differences in mRNA abundance existed before AngII infusions, which were differentially influenced by AngII between genotypes. Prolonged AngII infusions resulted in aortic wall thickening of AAAs from XY males, while XX males had dilated focal AAAs.
Conclusions
An XY sex chromosome complement mediates diffuse aortic pathology, while an XX sex chromosome complement contributes to focal AngII-induced AAAs.
Keywords: sex chromosomes, aorta, aneurysm, testosterone, pathology
Introduction
Aortic vascular diseases are life-threatening conditions that are not typically symptomatic, which is of concern since ruptures result in high morbidity and mortality. Amongst the various risk factors that have been identified for aortic vascular diseases, male sex has emerged as a positive risk factor for aneurysmal disease in the ascending aorta (AA)1, distal thoracic aorta (TAA)2, and for abdominal aortic aneurysms (AAAs)3–8. Recent studies analyzing patients within the Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions (GenTAC) registry demonstrated that adult males with Marfan syndrome were more likely than females to have aortic root dilatation (by 8%), aortic regurgitation (by 19%) and the prevalence of previous aortic dissection tended to be higher in males (by 7%).9 Similarly, TAAs are also more prevalent in men.10 Male sex is considered one of the largest non-modifiable risk factors for AAAs, with estimates ranging from a 2–10 fold greater prevalence in men compared to women.6, 8, 11, 12 A higher prevalence of aortic vascular diseases in men is not restricted to aneurysms, as the incidence of acute aortic dissection is also higher in men than women.13–15 Despite a uniformly lower incidence of various forms of aortic vascular diseases in women compared to men, paradoxically, women exhibit more rapid growth rates of both TAAs and AAAs than men, and aneurysms rupture at smaller sizes.2, 16–22 Mechanisms for these sex differences in aortic vascular disease development versus progressive growth have not been defined.
Similar to humans, studies from our laboratory demonstrated that AAAs induced by infusion of angiotensin II (AngII) to hypercholesterolemic mice exhibit a high level of sexual dimorphism, with a 4-fold higher AAA prevalence in male compared to female mice.23 Testosterone was identified as a primary contributor to higher AAA prevalence in AngII-infused male mice.23–26 Moreover, administration of testosterone to neonatal female low density lipoprotein receptor (Ldlr) deficient mice infused with AngII augmented development of aneurysms in the ascending and abdominal aorta.27 Adult female mice exposed to testosterone as neonates maintained a high susceptibility to AngII-induced aortic vascular diseases.27 In contrast, AAA incidence was markedly reduced when adult male mice were castrated.23 These results suggest that while testosterone is a primary mediator of sexual dimorphism of experimental aortic vascular diseases, other differences between males and females may contribute to sexual dimorphism of vascular disease development and severity.
In addition to sex hormones, recent studies demonstrated that an XY sex chromosome complement was sufficient to promote a high level of AAA susceptibility in female Ldlr−/− mice infused with AngII.28 Moreover, XY females exhibited increased aneurysm rupture, which rose to 73% when females were exposed to dihydrotestosterone to mimic an adult male milieu. These results suggest that sex chromosome complement also has profound effects on the vasculature, which is of interest as women with Turner’s Syndrome (monosomy X) exhibit an 100-fold increased risk of aortic dissection.29
The purpose of this study was to define the relative role of sex hormones versus sex chromosome complement on the location and characteristics of aortic vascular diseases following AngII infusions. The four core genotype murine (FCG) model was utilized to assess the relative role of sex hormones versus sex chromosome complement on the formation and progression of AngII-induced AAAs. This model produces mice in which the sex chromosome complement (XX versus XY) is varied independently of gonadal sex (testes versus ovaries). The FCG model was created from a natural mutation of the Sry gene (testis determining gene) on the Y chromosome of mice (XY−), where an Sry transgene was inserted onto an autosome for testes formation and fertility (XY−Sry).30 Breeding of XY−Sry male mice to XX females produces FCG: XX and XY males, XX and XY females. Since testosterone promotes AngII-induced AAs and AAAs in male mice,23, 24 we performed studies on male Ldlr−/− mice with an XY and an XX sex chromosome complement in the presence or absence of male sex hormones Since men experience a higher prevalence of AAs, TAAs, and AAAs than women, we hypothesize that testosterone and an XY sex chromosome complement promote vascular disease along the length of the aorta in male mice infused with AngII.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Testosterone and/or Sex Chromosome Complement Influenced Abdominal Aortic Gene Expression Patterns and Aortic Stiffness
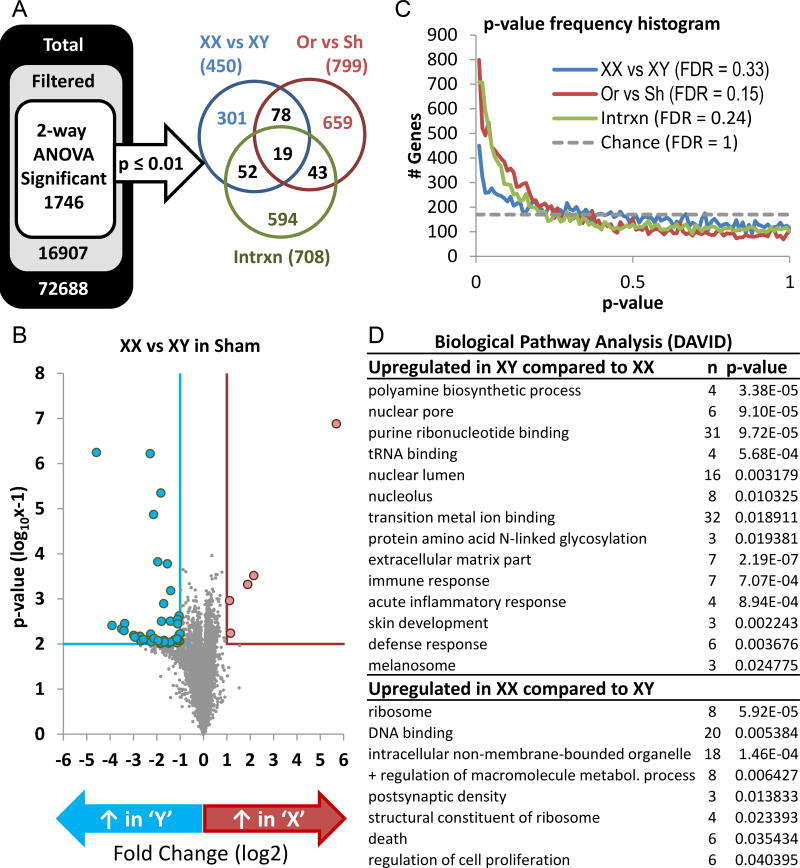
We demonstrated recently that an XY sex chromosome complement promoted expression of inflammatory gene pathways in abdominal aortas from female Ldlr−/− mice.28 Since previous studies focused on abdominal aortas of XX and XY females, in this study we used RNA extracted from abdominal aortas of XY and XX male mice (non-infused) that were gonadally intact (sham-operated) or orchiectomized (ORC) for Affymetrix Mouse Transcriptome Assay 1.0 analysis. A total of 1,746 genes exhibited highly significant differences (two-way ANOVA, P<0.01, Figure 1A, Supplemental Table I). There was a significant main effect of sex chromosome (450 genes, FDR = 0.32), castration (799 genes, ORC vs Sh, FDR = 0.14) and an interaction between sex chromosome and castration (708 genes, FDR = 0.24; Figure 1). A frequency histogram of the number of genes (y-axis) found at different p-value cut-offs (x-axis), with chance depicted by the dashed line, illustrates the number of genes influenced by sex chromosome complement, castration, or by an interaction between sex chromosomes and sex hormones at different p-values (Figure 1). Volcano plots of the chromosome effect with highly stringent cutoffs (> 2-fold change, p ≤ 0.01) demonstrated that, as expected, the expression of genes on sex-chromosomes was influenced strongly (Figure 1). Using this level of stringency, five genes (Xist, Arntl, Npas2, Arhgap20, Ighv14-1) were increased significantly in abdominal aortas of sham-operated XX compared to XY males (Figure 1, Supplemental Table III). In contrast, 51 genes were increased significantly in abdominal aortas of sham-operated XY compared to XX males (Supplemental Table III). RT-PCR on mRNA extracted from abdominal aortas from mice of each genotype and surgical group confirmed microarray results for selected genes enriched in XX (Xist, Npas2, Arntl, Supplemental Figure II) or XY aortas (Cyp2e1, Kap, Supplemental Figure II). Biological pathway analysis revealed several expression patterns, potentially related to aortic vascular diseases, that were increased in abdominal aortas of XY compared to XX males (immune response, acute inflammatory response), while other pathways were increased in abdominal aortas of XX compared to XY males (e.g., DNA binding, regulation of cell proliferation) (Figure 1, Supplemental Table IV).
Figure 1.
Sex chromosome complement, sex hormones, and an interaction between these factors influence gene expression patterns in abdominal aortas of XY and XX males. A, Total number of probe sets on arrays filtered to retain transcripts with reliable signal intensity (FDR). B, Volcano plot illustrating fold change in gene abundance (x-axis) and statistical significance (y-axis). Genes labelled in blue exhibited significant increase in XY compared to XX abdominal aortas; genes labeled in red exhibited significant increase in XX aortas. C, Frequency histogram of the number of genes (y-axis) found at different p-values (x-axis). Chance is illustrated by the dashed line. D, Biological pathway analysis (DAVID) of gene expression comparing pathways upregulated in XY or XX abdominal aortas. Data are mean ± SEM from n = 4–5 mice/genotype.
To determine if differences in gene expression patterns (e.g., extracellular matrix, Figure 1) between aortas of XY and XX males influenced aortic function, we quantified pulse wave velocity (PWV) as an index of aortic stiffness in non-infused XY and XX Ldlr−/− males. Aortas from XY males exhibited significant increases in PWV compared to XX males (Supplemental Figure III).
Aortic Aneurysmal Disease was Diffuse in XY Males and Localized in XX Males
At study endpoint, body weights of castrated (ORC) male mice infused with AngII were decreased significantly compared to sham-operated controls, regardless of genotype (Supplemental Table V; P<0.05). Serum testosterone concentrations were similar in sham-operated XY and XX males infused with AngII (Supplemental Table V; P>0.05). Castration resulted in significant decreases in serum testosterone concentrations of both genotypes, with no differences between genotypes (Supplemental Table V; P<0.05). Systolic blood pressures were not different between genotypes or surgical groups at baseline (XY sham, 111 ± 4; XY ORC, 109 ± 5; XX sham, 110 ± 3; XX ORC, 107 ± 2 mmHg), but increased significantly in AngII-infused XY compared to XX sham-operated males (Supplemental Table V; P<0.05), and this difference was eliminated by castration. Plasma renin concentrations were not significantly different between groups (Supplemental Table V; P>0.05). Atherosclerotic lesion surface area in the aortic arch was not significantly altered by castration or by sex chromosome complement in AngII-infused male mice (Supplemental Table V; P>0.05).
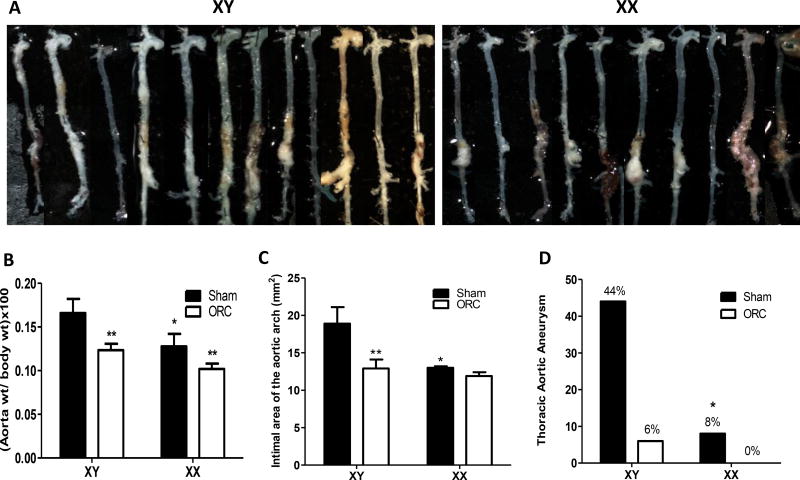
Aortas from XY sham-operated AngII-infused males exhibited diffuse disease that typically extended from the aortic arch to the suprarenal aorta (Figure 2A, top left). In contrast, aortas from XX AngII-infused males exhibited aortic pathology that was generally restricted to the suprarenal portion of the abdominal aorta (Figure 2A, top right). As an index of the extent of aortic pathology, aorta weights were increased significantly in XY compared to XX males, with aortic weights decreased by castration in both genotypes (Figure 2B; P<0.05). The area of the aortic arch was increased significantly in XY compared to XX AngII-infused sham-operated males (Figure 2C; P<0.05), with reductions in arch areas following castration of XY males. The incidence of aneurysms in the aortic arch or thoracic aorta (TAAs) was markedly higher in XY (44%) compared to XX (8%) AngII-infused males (Figure 2D; P<0.05). This difference was abolished by castration, which resulted in almost complete ablation of AngII-induced TAAs in both genotypes.
Figure 2.
An XY sex chromosome complement mediates diffuse aortic vascular disease, while an XX sex chromosome complement is associated with discrete aneurysmal disease in abdominal aortas of male AngII-infused mice. A, Aortas from XY and XX sham-operated males infused with AngII. B, Aorta weight normalized to body weight. C, Area of the aortic arch. D, Incidence of thoracic aortic aneurysms (%) in mice of each genotype and group. Data are mean ± SEM from n = 10–13 mice/genotype/group who survived the 28 day protocol. *, P<0.05 compared to XY within sham-operated determined by two-way ANOVA with sham and genotype as between group factors with Holm-Sidak post hoc analysis (B,C) or by Fisher’s exact test (D). **, P<0.05 compared to sham-operated within genotype determined by two-way ANOVA with sham and genotype as between group factors with Holm-Sidak post hoc analysis.
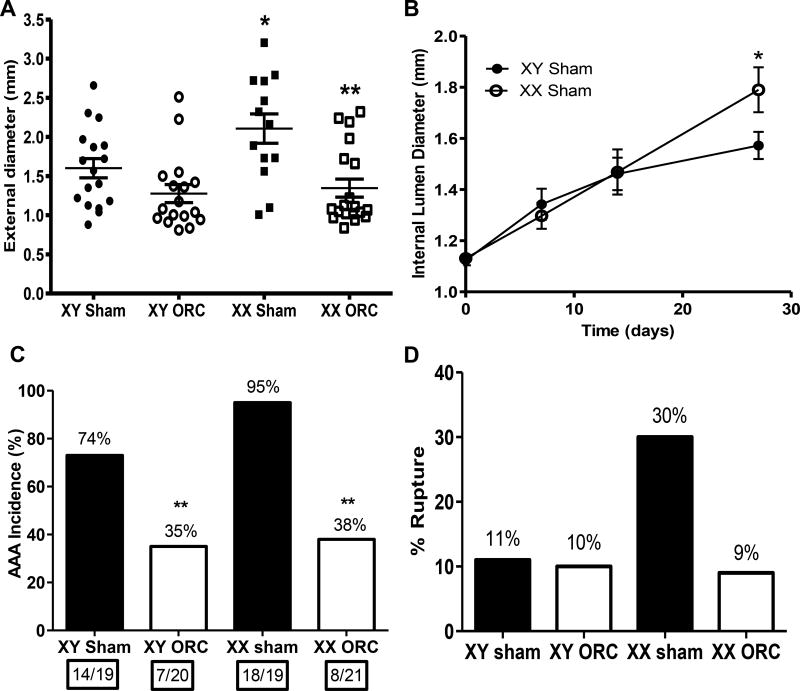
In contrast to diffuse aortic disease of XY males infused with AngII, XX males exhibited focal aortic disease within the suprarenal portion of the abdominal aorta, with significant increases in external AAA diameters that were abolished by castration (Figure 3A; P<0.05). Similarly, abdominal aortic lumen diameters were increased significantly in XX compared to XY sham-operated males infused with AngII (day 28, Figure 3B; P<0.05). The high incidence of AAAs was not significantly different between XX and XY AngII-infused males (Figure 3C; P>0.05), and castration significantly decreased AAA incidences of both genotypes (Figure 3C; P<0.05). Larger AAAs of XX males were associated with slight, but insignificant increases in aneurysmal rupture (Figure 3D; P>0.05), which were also reduced by castration of XX males.
Figure 3.
An XX sex chromosome complement results in focal AAA pathology of increased size compared to XY males infused with AngII. A, Maximal AAA external diameters. Symbols represent individual mice with lines representing mean ± SEM. B, Internal diameters of abdominal aortas at selected intervals during infusion of AngII. Data are mean ± SEM of mice surviving the 28 day infusions. C, AAA incidence (percent above each bar). Numbers in boxes under each bar are mice with an AAA/total number of mice per group. D, % Aneurysm rupture (percent above each bar). *, P<0.05 compared to XY within sham-operated determined by two-way ANOVA with Holm-Sidak post hoc analysis (A,B) or by Fisher’s exact test (C,D). **, P<0.05 compared to sham-operated within genotype determined by two-way ANOVA with Holm-Sidak post hoc analysis (A,B) or by Fisher’s exact test (C,D).
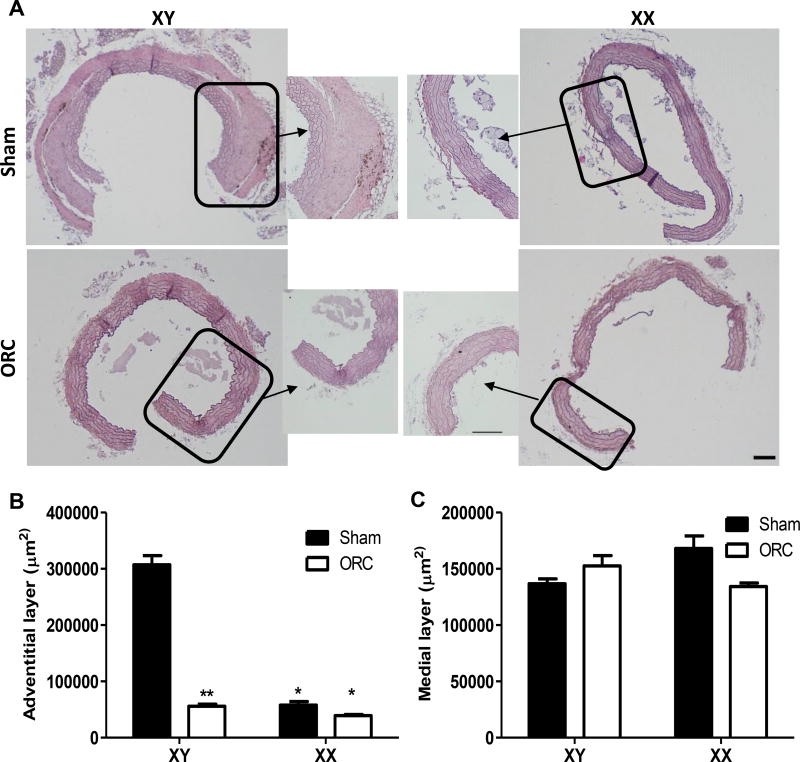
Since XY, but not XX males exhibited considerable aortic pathology in the thoracic aorta, we characterized morphology of tissue sections prepared from thoracic aortas of XY and XX males infused with AngII. Thoracic aorta tissue sections from XY AngII-infused males exhibited pronounced thickening of the adventitia, which was not evident in thoracic aortas of XX males (Figure 4A,B). Adventitial thickening of thoracic aortas from XY males was abolished by castration (Figure 4A,B). In contrast, medial diameters were not significantly different between XY and XX males, and were not influenced by castration (Figure 4A,C; P>0.05).
Figure 4.
Thoracic aortas from XY males, but not XX males, exhibit adventitial thickening in response to AngII infusions. A, Representative thoracic aorta tissue sections from XY and XX sham-operated 51 and orchiectomized (ORC) males infused with AngII for 28 days. Boxes are areas illustrated at higher magnification. B, Quantification of the adventitial layer of thoracic aorta tissue sections from mice of each group. C, Quantification of the medial layer of thoracic aorta tissue sections from mice of each group. Data are mean ± from 6 tissue sections from n = 3 mice/group. *, P<0.05 compared to XY within treatment group determined by two-way ANOVA with surgery and genotype as between group factors, with Holm-Sidak post hoc analysis. **, P<0.05 compared to sham within genotype determined by two-way ANOVA with surgery and genotype as between group factors, with Holm-Sidak post hoc analysis. Scale bar represents 100 µm.
Aortic Genes Related to AAA Development Exhibited Region-specific and AngII-induced Differences in Abundance
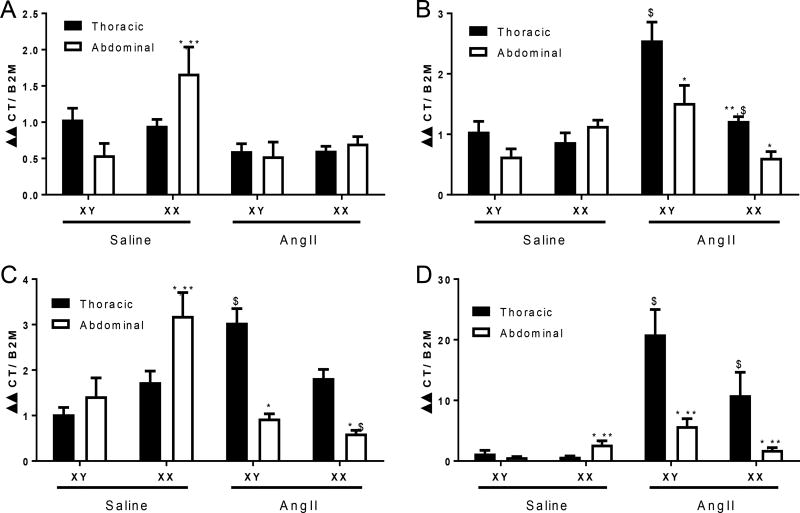
Initial studies defined gene expression patterns in abdominal aortas from non-infused male XY and XX mice (Figure 1). To determine if differences in aortic disease development between XY and XX male mice are associated with regional differences in aortic gene expression, and whether AngII regulates aortic genes in a region-specific manner, we contrasted effects of short-term (1 day) infusions of saline versus AngII on abundance of genes within the thoracic versus abdominal aortas of male XY and XX Ldlr−/− mice. We chose short-term AngII infusions to limit the extent of overt aneurysm pathology between genotypes. Abundance of angiotensin converting enzyme (ACE), collagen 1a1, and thrombospondin (Thbs1) mRNAs were increased significantly in abdominal compared to thoracic aortas of XX, but not XY saline-infused male mice (Figure 5A–D; P<0.05). Moreover, abdominal aortas of saline-infused XX males exhibited significantly increased mRNA abundance of ACE, collagen 1a1, and Thbs1 compared to abdominal aortas of saline-infused XY males (Figure 1A,C,D; P<0.05). Infusion of AngII resulted in significant elevations in mRNA abundance of matrix metalloproteinase 2 (MMP2), collagen 1a1, and Thbs1 in thoracic, but not abdominal aortas of XY male mice compared to XY saline-infused controls (Figure 5B–D; P<0.05). In contrast, abdominal aortas of XX males did not respond to AngII with increased expression of MMP2 or Thbs1. However, mRNA abundance of collagen 1a1 was decreased significantly by AngII in abdominal aortas of XX males. As a result, following AngII infusions, marked regional differences in expression levels of MMP2, collagen 1a1, and Thbs1 were evident, with increased expression levels in thoracic compared to abdominal aortas of XY and XX male mice (Figure 5B–D; P<0.05). The magnitude of AngII-induced changes in the regional expression levels of these genes were more pronounced in XY than XX male mice.
Figure 5.
mRNA abundance of key genes implicated in aneurysm development in thoracic versus abdominal aortas from XY and XX male mice infused with either saline or AngII-infused (1 day). A, Angiotensin converting enzyme (ACE) mRNA abundance. B, Matrix metalloproteinase 2 mRNA abundance. C, Collagen 1a1 mRNA abundance. D, Thrombospondin 1 mRNA abundance. Data are mean ± SEM from n = 5 mice/group/sex. *, P<0.05 compared to thoracic within genotype determined by three-way ANOVA with region, treatment or genotype as between group factors and Holm-Sidak post hoc analysis. **, P<0.05 compared to XY within aortic region determined by three-way ANOVA with region, treatment or genotype as between group factors and Holm-Sidak post hoc analysis. $, compared to saline within region and genotype determined by three-way ANOVA with region, treatment or genotype as between group factors and Holm-Sidak post hoc analysis.
Regional Differences in AngII-induced Aortic Vascular Diseases Between XY and XX Males Persisted with Aneurysm Progression
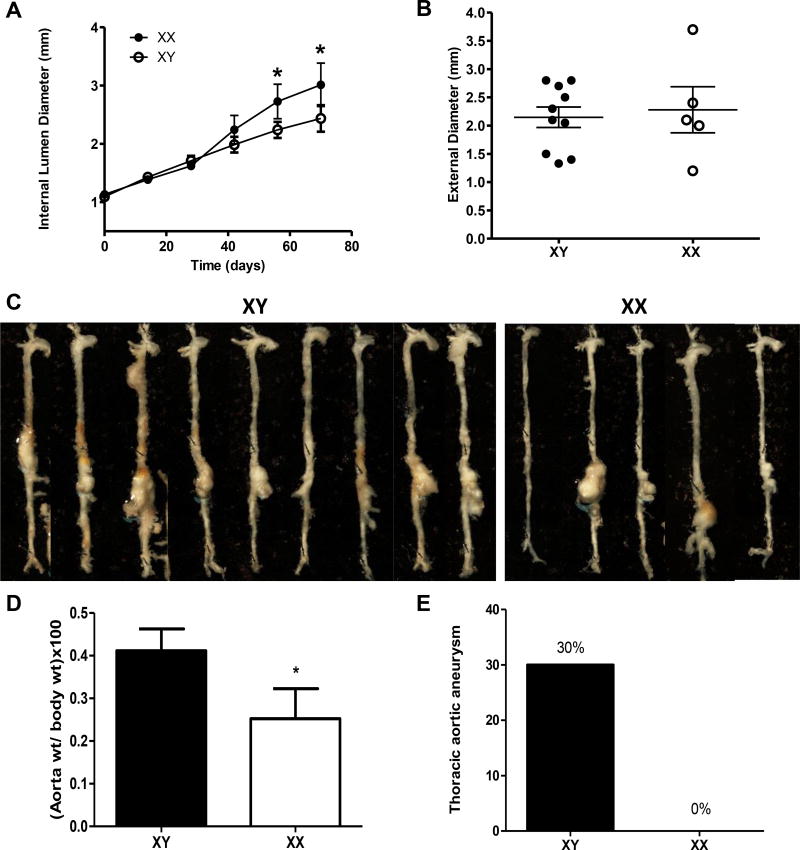
Despite a lower prevalence of TAA and AAA development in women compared to men, previous studies demonstrated that aneurysm growth rates are more aggressive in women, and aneurysms may rupture at smaller sizes.2, 16–22, 31 Therefore, we examined progression of aortic vascular diseases in response to 3 months of prolonged AngII infusions in XY and XX males. Increased abdominal aortic lumen diameters of XX males persisted with prolonged AngII infusions (Figure 6A; P<0.05). Surprisingly, dilated abdominal aortas of XX males were not associated with significantly larger AAA diameters at study endpoint (Figure 6B; P>0.05). These results suggest divergent aneurysm remodeling between genotypes. Similar to 28 day AngII infusions, XY males infused with AngII for 3 months exhibited diffuse aortic disease (Figure 6C), resulting in significant increases in aorta weight (Figure 6D; P<0.05) and incidence of TAAs compared to XX males (Figure 6E; P<0.05).
Figure 6.
XX males exhibit dilated AAAs while XY males exhibit diffuse aortic disease with prolonged AngII infusions (3 months). A, Internal lumen diameters of suprarenal aorta at selected intervals of AngII infusions. B, Maximal AAA external diameters at study endpoint. Symbols represent individual mice with lines mean ± SEM. C, Aortas from mice in each group at study endpoint. D, Aorta weight normalized to body weight. E, Incidence of thoracic aortic aneurysms in mice of each genotype. Data are mean ± SEM from n = 5–10 mice/group. *, P<0.05 compared to XY determined by Student t-test (A-D) or Fisher’s exact test (E).
Since maximal AAA diameters were not different between XY and XX males, but aneurysms of XX males were more dilated, we performed ex vivo ultrasound analysis of AAA 3-D structures following prolonged AngII infusions (Supplemental Figure IV, representative 3-D images of AAA from each group in C). AAA wall volumes were increased in XY compared to XX males (Supplemental Figure IVA), while AAA lumen volumes were increased significantly in XX compared to XY males (Supplemental Figure IVB).
Discussion
The key novel findings of this study are: (1) Male sex hormones, coupled with an XY sex chromosome complement result in development of diffuse aortic disease associated with adventitial thickening in response to AngII infusions. (2) In contrast, an XX sex chromosome complement in phenotypic male mice results in focal aneurysmal disease within the suprarenal abdominal aorta. (3) These regional differences in aortic vascular disease development are associated with differences in abundance of genes implicated in aneurysmal disease between thoracic versus abdominal aortas of XY and XX male mice. (4) Short-term infusion of AngII superimposes pronounced regional differences in aortic gene expression in both genotypes, with greater differences in XY males. (5) Focal suprarenal AAAs of AngII-infused XX males progress to exhibit pronounced aortic lumen dilation, while progressing AAAs of XY males infused chronically with AngII have thickened vascular wall volumes and aortas exhibit diffuse disease.
It is well established that male sex is a significant non-modifiable risk factor for development of AAAs, with men exhibiting a 2–10-fold higher prevalence than women.6, 8, 11, 12 Recent results, performed on a large population of well-characterized patients with Marfan syndrome, also exhibited a higher prevalence of aortic root dilatation and regurgitation in men compared to women.1 Moreover, using data from the Swedish national healthcare registers from 1987 to 2002, the incidence of TAA was 52% in men compared to 28% in women.10 Collectively, these studies indicate that men exhibit a higher prevalence of vascular disease localized to different regions of the aorta compared to women. A variety of mechanisms for these differences have been proposed, ranging from influences of sex hormones and their receptors,32 differences in aortic stiffness,33 hemodynamic influences, and basic differences in aortic size between sexes.34–37 Of interest, sex chromosome abnormalities, such as Turners syndrome (monosomy X), are associated with a higher risk of aortic dissection,29 another aortic vascular disease that exhibits higher prevalence in men than women.38 Recent studies from our laboratory demonstrated that an XY sex chromosome complement, when inserted in phenotypic females with low circulating testosterone, was sufficient to increase the incidence and severity of AngII-induced AAAs.28 We also noted that exposure of XY females to dihydrotestosterone, to mimic a male-like milieu, resulted in a striking level of aneurysm rupture.28 In this study, we used male mice with an XY or XX sex chromosome complement, as males experience testosterone exposures throughout life and testosterone has been demonstrated to increase AngII-induced AAAs in male or female mice.23, 24, 27 Our results demonstrate that testosterone, through a sex chromosome-dependent (XY) mechanism, promotes the presence of diffuse aortic vascular disease in male mice infused with AngII. In contrast, aortas from XX males exhibit focal aortic pathology manifest within the suprarenal region of the abdominal aorta, as has been typically described for AngII-induced AAAs that occur in females.39 These striking differences in regional location of AngII-induced aortic pathology were both sex chromosome and sex hormone-mediated.
Several potential mechanisms have been suggested to contribute to sex differences in aortic vascular disease development.40 In this study, gene expression analysis exhibited 1746 genes that were differentially expressed in abdominal aortas of non-infused XY and XX males according to sex hormone (e.g., influenced by castration), sex chromosome complement, or by an interaction between these factors. Pathway analysis of gene array data identified genes related to extracellular matrix, acute inflammatory response, regulation of cell proliferation and the immune response may relate to differences in AAA susceptibility between males and females. Of the aortic genes exhibiting pronounced differences based on sex chromosome complement, Xist, the RNA gene within the X chromosome that is the effector of the X-inactivation process, confirms the validity of sex chromosome manipulation in XX males. Arntl (BMAL1) and Npas2, genes enriched in aortas from XX compared to XY males, are genes expressing proteins that are core components of the circadian clock apparatus. To our knowledge, there is minimal information related to influences of circadian rhythm on AAA formation and/or rupture. Abdominal aortas from XY males had higher mRNA abundance of Cyp2e1 (a member of the cytochrome p450 enzyme family) and Kap (kidney androgen-regulated protein) compared to XX males. It is unclear if these differences in abdominal aortic gene expression between XY and XX males are related to aneurysm outcomes of the current study. An intriguing difference between XY and XX males infused with AngII was the large adventitial thickening that occurred in the thoracic aorta of XY, but not XX males. Increased adventitial thickening in response to AngII infusions in thoracic aortas may have contributed to the observed augmented aortic stiffening of XY males.41 Since blood pressures were increased significantly in XY compared to XX males infused with AngII, it is possible that hemodynamic differences contributed to these divergent morphologic responses and susceptibility to AngII-induced TAAs between genotypes. However, blood pressure was similar in AngII-infused sham and castrated males, even though TAA incidence was markedly reduced by castration. These results suggest that blood pressure may not be the primary contributor to the development of diffuse aortic vascular pathologies of XY males.
Previous investigators demonstrated a large number of genes (1475) that are differentially expressed in the ascending versus descending aortas of female mice.42 These differences were ascribed to developmental origins of cells within distinct regions of the aorta. Specifically, smooth muscle cells of the ascending aorta develop from cardiac neural crest and second heart field43, descending aorta cells develop from somites while those of the abdominal aorta are derived from mesodermal origin.44 These differences have been suggested to contribute to diverging physiologic and pathophysiologic responses of the aorta, including regulation of the extracellular matrix.26, 44, 45 Notably, previous studies indicate that cells of mesodermal origin that populate the abdominal aorta express androgen receptors,46 and recent studies indicate increased expression of androgen receptors in abdominal aortas of patients with an AAA compared to control aorta.32 In this study, with the exception of collagen 1, XY and XX males exhibited the opposite patterns of gene expression in abdominal versus thoracic aortas, with increased thoracic gene expression in XY males, and increased abdominal gene expression in XX males. Notably, infusion of AngII resulted in robust regional differences in expression patterns of several genes with more robust regional differences (e.g., higher in thoracic than abdominal) in aortas from XY males exhibiting diffuse disease in response to the peptide. It is unclear if embryonic origins of vascular wall cells contribute to the observed regional differences in aortic gene expression patterns or the regional responses to AngII.
Despite a lower prevalence of aortic vascular disease in women than men, women with AAAs or TAAs appear to exhibit more progressive aneurysmal growth, with a propensity to rupture at smaller aneurysm sizes.2, 21, 22, 35, 47–49 Previous results from our laboratory demonstrated that castration of male mice with established AngII-induced AAAs resulted in aneurysm remodeling, with thin walled AAAs of castrated males compared to muscularized aneurysms of intact males.50 Results of the current study agree with previous findings, in that AAAs of XY males had large wall volumes as they continued to grow. In addition, the diffuse nature of aortic vascular pathology of XY males persisted with prolonged AngII infusions. In contrast, XX males exhibited dilated thin walled AAAs with prolonged AngII infusions, suggesting an increased propensity of weakened aortas to rupture. These results indicate that sex chromosome-mediated influences on aortic vascular biology may contribute to differences in the progression of aortic vascular disease between sexes.
A limitation of the model used in these studies is that it does not define whether the observed phenotype results from genes residing on the Y or second X chromosome (that escape X-inactivation). Moreover, XX males that are derived from this breeding strategy are infertile and have small testes because they lack the Y chromosome genes that are responsible for spermatogenesis. However, as XX mice have serum testosterone concentrations that are not significantly different from male XY mice (Supplemental Table V), it is unlikely that these differences influenced experimental findings.
In conclusion, this is the first demonstration that sex chromosome complement, coupled with sex hormones (e.g., testosterone), modulate the region-specific development of aortic pathology in response to AngII. An XY sex chromosome complement in males resulted in diffuse aneurysmal disease along the aorta, while a male XX sex chromosome complement mediated focal abdominal aortic aneurysmal disease. These regional differences in aortic vascular disease according to sex chromosome complement were dependent on the presence of male sex hormones. Regional differences in expression levels of genes implicated in aneurysm development between thoracic versus abdominal aortas from XY and XX males may contribute to divergent aneurysm susceptibility and regional location of aortic pathology between sexes in response to AngII. Finally, aneurysm progression and remodeling were differentially regulated by sex chromosome complement, with XX males exhibiting thin walled AAAs and XY males exhibiting thickened AAAs and diffuse aortic disease from prolonged AngII infusions. These studies suggest that sex differences between men and women in development and progression of aortic vascular diseases may arise from the complex interplay between sex hormones and sex chromosome complement in defining regional aortic gene expression, diffuse versus focal aortic pathology, and differential aneurysm remodeling of aortic vascular diseases.
Supplementary Material
Highlights.
Sex chromosome complement, and sex hormones, interact to influence gene expression profiles of abdominal aorta.
In male mice infused with AngII, an XY sex chromosome complement promotes diffuse aortic aneurysmal disease associated with adventitial thickening, while an XX sex chromosome complement contributes to focal aneurysms in the abdominal aorta. These effects are dependent on the presence of male sex hormones.
Sex chromosome complement regulates the expression levels of key genes implicated in aneurysm formation in an aortic region-specific (thoracic versus abdominal) manner, and these differences are regulated by AngII.
Following prolonged AngII infusions, AAAs of XX males exhibit dilated lumens and thin aortic walls, while AAAs of XY males have increased aortic wall volumes and diffuse aortic disease.
These results suggest that sex chromosome complement, and male sex hormones, contribute to aortic region-specific development and progression of aneurysmal disease.
Acknowledgments
We are grateful to the technical support of Victoria English for measurement of plasma renin concentrations, and Wendy Katz for sectioning of aortic tissues.
Sources of Funding. This work was funded by the National Institutes of Health (HL107326, LC), with cores services support from P20GM103527 (LC), and from a predoctoral fellowship from the American Heart Association (14PRE20030018, YA).
Abbreviations
- AngII
angiotensin II
- AAA
abdominal aortic aneurysm
- Ldlr
low density lipoprotein receptor
- ORC
orchiectomized
- MMP2
matrix metalloproteinase 2
- TAA
thoracic aortic aneurysm
Footnotes
Disclosures. None.
References
- 1.Roman MJ, Devereux RB, Preiss LR, et al. Gen TACI. Associations of age and sex with marfan phenotype: The national heart, lung, and blood institute gentac (genetically triggered thoracic aortic aneurysms and cardiovascular conditions) registry. Circulation. Cardiovascular genetics. 2017;10 doi: 10.1161/CIRCGENETICS.116.001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheung K, Boodhwani M, Chan KL, Beauchesne L, Dick A, Coutinho T. Thoracic aortic aneurysm growth: Role of sex and aneurysm etiology. Journal of the American Heart Association. 2017;6 doi: 10.1161/JAHA.116.003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pleumeekers HJ, Hoes AW, van der Does E, van Urk H, Hofman A, de Jong PT, Grobbee DE. Aneurysms of the abdominal aorta in older adults. The rotterdam study. American journal of epidemiology. 1995;142:1291–1299. doi: 10.1093/oxfordjournals.aje.a117596. [DOI] [PubMed] [Google Scholar]
- 4.Zarrouk M, Holst J, Malina M, Lindblad B, Wann-Hansson C, Rosvall M, Gottsater A. The importance of socioeconomic factors for compliance and outcome at screening for abdominal aortic aneurysm in 65-year-old men. Journal of vascular surgery. 2013;58:50–55. doi: 10.1016/j.jvs.2012.12.080. [DOI] [PubMed] [Google Scholar]
- 5.Svensjo S, Bjorck M, Wanhainen A. Current prevalence of abdominal aortic aneurysm in 70-year-old women. The British journal of surgery. 2013;100:367–372. doi: 10.1002/bjs.8984. [DOI] [PubMed] [Google Scholar]
- 6.Weiss N, Rodionov RN, Mahlmann A. Medical management of abdominal aortic aneurysms. VASA. Zeitschrift fur Gefasskrankheiten. 2014;43:415–421. doi: 10.1024/0301-1526/a000388. [DOI] [PubMed] [Google Scholar]
- 7.Vardulaki KA, Prevost TC, Walker NM, Day NE, Wilmink AB, Quick CR, Ashton HA, Scott RA. Growth rates and risk of rupture of abdominal aortic aneurysms. The British journal of surgery. 1998;85:1674–1680. doi: 10.1046/j.1365-2168.1998.00946.x. [DOI] [PubMed] [Google Scholar]
- 8.Pleumeekers HJ, Hoes AW, van der Does E, van Urk H, Grobbee DE. Epidemiology of abdominal aortic aneurysms. European journal of vascular surgery. 1994;8:119–128. doi: 10.1016/s0950-821x(05)80446-3. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Almarzooq Z, Codell NCF, Wang Y, Roman MJ, Devereux RB, Weinsaft JW. Cine-cmr partial voxel segmentation demonstrates increased aortic stiffness among patients with marfan syndrome. Journal of thoracic disease. 2017;9:S239–S245. doi: 10.21037/jtd.2017.04.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olsson C, Thelin S, Stahle E, Ekbom A, Granath F. Thoracic aortic aneurysm and dissection: Increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006;114:2611–2618. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 11.Wilmink AB, Quick CR. Epidemiology and potential for prevention of abdominal aortic aneurysm. The British journal of surgery. 1998;85:155–162. doi: 10.1046/j.1365-2168.1998.00714.x. [DOI] [PubMed] [Google Scholar]
- 12.Morris GE, Hubbard CS, Quick CR. An abdominal aortic aneurysm screening programme for all males over the age of 50 years. European journal of vascular surgery. 1994;8:156–160. doi: 10.1016/s0950-821x(05)80451-7. [DOI] [PubMed] [Google Scholar]
- 13.Clouse WD, Hallett JW, Jr, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ., 3rd Acute aortic dissection: Population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clinic proceedings. 2004;79:176–180. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 14.Wenger NK, Speroff L, Packard B. Cardiovascular health and disease in women. The New England journal of medicine. 1993;329:247–256. doi: 10.1056/NEJM199307223290406. [DOI] [PubMed] [Google Scholar]
- 15.Maitusong B, Sun HP, Xielifu D, Mahemuti M, Ma X, Liu F, Xie X, Azhati A, Zhou XR, Ma YT. Sex-related differences between patients with symptomatic acute aortic dissection. Medicine. 2016;95:e3100. doi: 10.1097/MD.0000000000003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juvonen T, Ergin MA, Galla JD, Lansman SL, Nguyen KH, McCullough JN, Levy D, de Asla RA, Bodian CA, Griepp RB. Prospective study of the natural history of thoracic aortic aneurysms. The Annals of thoracic surgery. 1997;63:1533–1545. doi: 10.1016/s0003-4975(97)00414-1. [DOI] [PubMed] [Google Scholar]
- 17.Davies RR, Goldstein LJ, Coady MA, Tittle SL, Rizzo JA, Kopf GS, Elefteriades JA. Yearly rupture or dissection rates for thoracic aortic aneurysms: Simple prediction based on size. The Annals of thoracic surgery. 2002;73:17–27. doi: 10.1016/s0003-4975(01)03236-2. discussion 27-18. [DOI] [PubMed] [Google Scholar]
- 18.Nienaber CA, Fattori R, Mehta RH, et al. International Registry of Acute Aortic D. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C. [DOI] [PubMed] [Google Scholar]
- 19.Grootenboer N, Bosch JL, Hendriks JM, van Sambeek MR. Epidemiology, aetiology, risk of rupture and treatment of abdominal aortic aneurysms: Does sex matter? European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2009;38:278–284. doi: 10.1016/j.ejvs.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Skibba AA, Evans JR, Hopkins SP, Yoon HR, Katras T, Kalbfleisch JH, Rush DS. Reconsidering gender relative to risk of rupture in the contemporary management of abdominal aortic aneurysms. Journal of vascular surgery. 2015;62:1429–1436. doi: 10.1016/j.jvs.2015.07.079. [DOI] [PubMed] [Google Scholar]
- 21.Sweeting MJ, Thompson SG, Brown LC, Powell JT, collaborators R Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. The British journal of surgery. 2012;99:655–665. doi: 10.1002/bjs.8707. [DOI] [PubMed] [Google Scholar]
- 22.Thompson SG, Brown LC, Sweeting MJ, Bown MJ, Kim LG, Glover MJ, Buxton MJ, Powell JT. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: Implications for surveillance intervals and their cost-effectiveness. Health technology assessment. 2013;17:1–118. doi: 10.3310/hta17410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin ii-induced vascular diseases in apolipoprotein e-deficient mice. Endocrinology. 2004;145:3866–3872. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 24.Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen increases at1a receptor expression in abdominal aortas to promote angiotensin ii-induced aaas in apolipoprotein e-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1251–1256. doi: 10.1161/ATVBAHA.107.160382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thatcher SE, Zhang X, Howatt DA, Yiannikouris F, Gurley SB, Ennis T, Curci JA, Daugherty A, Cassis LA. Angiotensin-converting enzyme 2 decreases formation and severity of angiotensin ii-induced abdominal aortic aneurysms. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2617–2623. doi: 10.1161/ATVBAHA.114.304613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thatcher SE, Zhang X, Woody S, Wang Y, Alsiraj Y, Charnigo R, Daugherty A, Cassis LA. Exogenous 17-beta estradiol administration blunts progression of established angiotensin ii-induced abdominal aortic aneurysms in female ovariectomized mice. Biology of sex differences. 2015;6:12. doi: 10.1186/s13293-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circulation research. 2012;110:e73–85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alsiraj Y, Thatcher SE, Charnigo R, Chen K, Blalock E, Daugherty A, Cassis LA. Female mice with an xy sex chromosome complement develop severe angiotensin ii-induced abdominal aortic aneurysms. Circulation. 2017;135:379–391. doi: 10.1161/CIRCULATIONAHA.116.023789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong SC, Cheung M, Zacharin M. Aortic dilatation and dissection in turner syndrome: What we know, what we are unclear about and what we should do in clinical practice? International journal of adolescent medicine and health. 2014;26:469–488. doi: 10.1515/ijamh-2013-0336. [DOI] [PubMed] [Google Scholar]
- 30.Itoh Y, Mackie R, Kampf K, Domadia S, Brown JD, O'Neill R, Arnold AP. Four core genotypes mouse model: Localization of the sry transgene and bioassay for testicular hormone levels. BMC Res Notes. 2015;8:69. doi: 10.1186/s13104-015-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pape LA, Tsai TT, Isselbacher EM, Oh JK, O'Gara PT, Evangelista A, Fattori R, Meinhardt G, Trimarchi S, Bossone E, Suzuki T, Cooper JV, Froehlich JB, Nienaber CA, Eagle KA, International Registry of Acute Aortic Dissection I Aortic diameter >or = 5.5 cm is not a good predictor of type a aortic dissection: Observations from the international registry of acute aortic dissection (irad) Circulation. 2007;116:1120–1127. doi: 10.1161/CIRCULATIONAHA.107.702720. [DOI] [PubMed] [Google Scholar]
- 32.Villard C, Eriksson P, Kronqvist M, Lengquist M, Jorns C, Hartman J, Roy J, Hultgren R. Differential expression of sex hormone receptors in abdominal aortic aneurysms. Maturitas. 2017;96:39–44. doi: 10.1016/j.maturitas.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Tong J, Schriefl AJ, Cohnert T, Holzapfel GA. Gender differences in biomechanical properties, thrombus age, mass fraction and clinical factors of abdominal aortic aneurysms. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2013;45:364–372. doi: 10.1016/j.ejvs.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Lindquist Liljeqvist M, Hultgren R, Siika A, Gasser TC, Roy J. Gender, smoking, body size, and aneurysm geometry influence the biomechanical rupture risk of abdominal aortic aneurysms as estimated by finite element analysis. Journal of vascular surgery. 2017;65:1014–1021 e1014. doi: 10.1016/j.jvs.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 35.Lo RC, Lu B, Fokkema MT, Conrad M, Patel VI, Fillinger M, Matyal R, Schermerhorn ML, Vascular Study Group of New E Relative importance of aneurysm diameter and body size for predicting abdominal aortic aneurysm rupture in men and women. Journal of vascular surgery. 2014;59:1209–1216. doi: 10.1016/j.jvs.2013.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sweet MP, Fillinger MF, Morrison TM, Abel D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. Journal of vascular surgery. 2011;54:931–937. doi: 10.1016/j.jvs.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 37.Lederle FA, Johnson GR, Wilson SE, Gordon IL, Chute EP, Littooy FN, Krupski WC, Bandyk D, Barone GW, Graham LM, Hye RJ, Reinke DB. Relationship of age, gender, race, and body size to infrarenal aortic diameter. The aneurysm detection and management (adam) veterans affairs cooperative study investigators. Journal of vascular surgery. 1997;26:595–601. doi: 10.1016/s0741-5214(97)70057-0. [DOI] [PubMed] [Google Scholar]
- 38.Meszaros I, Morocz J, Szlavi J, Schmidt J, Tornoci L, Nagy L, Szep L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117:1271–1278. doi: 10.1378/chest.117.5.1271. [DOI] [PubMed] [Google Scholar]
- 39.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makrygiannis G, Courtois A, Drion P, Defraigne JO, Kuivaniemi H, Sakalihasan N. Sex differences in abdominal aortic aneurysm: The role of sex hormones. Ann Vasc Surg. 2014;28:1946–1958. doi: 10.1016/j.avsg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Bersi MR, Bellini C, Wu J, Montaniel KRC, Harrison DG, Humphrey JD. Excessive adventitial remodeling leads to early aortic maladaptation in angiotensin-induced hypertension. Hypertension. 2016;67:890–896. doi: 10.1161/HYPERTENSIONAHA.115.06262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfaltzgraff ER, Shelton EL, Galindo CL, Nelms BL, Hooper CW, Poole SD, Labosky PA, Bader DM, Reese J. Embryonic domains of the aorta derived from diverse origins exhibit distinct properties that converge into a common phenotype in the adult. J Mol Cell Cardiol. 2014;69:88–96. doi: 10.1016/j.yjmcc.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sawada H, Rateri DL, Moorleghen JJ, Majesky MW, Daugherty A. Smooth muscle cells derived from second heart field and cardiac neural crest reside in spatially distinct domains in the media of the ascending aorta-brief report. Arteriosclerosis, thrombosis, and vascular biology. 2017;37:1722–1726. doi: 10.1161/ATVBAHA.117.309599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 45.Thieszen SL, Dalton M, Gadson PF, Patterson E, Rosenquist TH. Embryonic lineage of vascular smooth muscle cells determines responses to collagen matrices and integrin receptor expression. Exp Cell Res. 1996;227:135–145. doi: 10.1006/excr.1996.0258. [DOI] [PubMed] [Google Scholar]
- 46.Cunha GR, Shannon JM, Neubauer BL, Sawyer LM, Fujii H, Taguchi O, Chung LW. Mesenchymal-epithelial interactions in sex differentiation. Hum Genet. 1981;58:68–77. doi: 10.1007/BF00284152. [DOI] [PubMed] [Google Scholar]
- 47.Larsson E, Labruto F, Gasser TC, Swedenborg J, Hultgren R. Analysis of aortic wall stress and rupture risk in patients with abdominal aortic aneurysm with a gender perspective. Journal of vascular surgery. 2011;54:295–299. doi: 10.1016/j.jvs.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 48.Mofidi R, Goldie VJ, Kelman J, Dawson AR, Murie JA, Chalmers RT. Influence of sex on expansion rate of abdominal aortic aneurysms. The British journal of surgery. 2007;94:310–314. doi: 10.1002/bjs.5573. [DOI] [PubMed] [Google Scholar]
- 49.Solberg S, Singh K, Wilsgaard T, Jacobsen BK. Increased growth rate of abdominal aortic aneurysms in women. The tromso study. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005;29:145–149. doi: 10.1016/j.ejvs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Zhang X, Thatcher S, Wu C, Daugherty A, Cassis LA. Castration of male mice prevents the progression of established angiotensin ii-induced abdominal aortic aneurysms. Journal of vascular surgery. 2015;61:767–776. doi: 10.1016/j.jvs.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.