Abstract
OBJECTIVE
To investigate whether addition of three different doses of liraglutide to insulin in patients with type 1 diabetes (T1D) results in significant reduction in glycemia, body weight, and insulin dose.
RESEARCH DESIGN AND METHODS
We randomized 72 patients (placebo = 18, liraglutide = 54) with T1D to receive placebo and 0.6, 1.2, and 1.8 mg liraglutide daily for 12 weeks.
RESULTS
In the 1.2-mg and 1.8-mg groups, the mean weekly reduction in average blood glucose was −0.55 ± 0.11 mmol/L (10 ± 2 mg/dL) and −0.55 ± 0.05 mmol/L (10 ± 1 mg/dL), respectively (P < 0.0001), while it remained unchanged in the 0.6-mg and placebo groups. In the 1.2-mg group, HbA1c fell significantly (−0.78 ± 15%, −8.5 ± 1.6 mmol/mol, P < 0.01), while it did not in the 1.8-mg group (−0.42 ± 0.15%, −4.6 ± 1.6 mmol/mol, P = 0.39) and 0.6-mg group (−0.26 ± 0.17%, −2.8 ± 1.9 mmol/mol, P = 0.81) vs. the placebo group (−0.3 ± 0.15%, −3.3 ± 1.6 mmol/mol). Glycemic variability was reduced by 5 ± 1% (P < 0.01) in the 1.2-mg group only. Total daily insulin dose fell significantly only in the 1.2-mg and 1.8-mg groups (P < 0.05). There was a 5 ± 1 kg weight loss in the two higher-dose groups (P < 0.05) and by 2.7 ± 0.6 kg (P < 0.01) in the 0.6-mg group vs. none in the placebo group. In the 1.2- and 1.8-mg groups, postprandial plasma glucagon concentration fell by 72 ± 12% and 47 ± 12%, respectively (P < 0.05). Liraglutide led to higher gastrointestinal adverse events (P < 0.05) and ≤1% increases (not significant) in percent time spent in hypoglycemia (<55 mg/dL, 3.05 mmol/L).
CONCLUSIONS
Addition of 1.2 mg and 1.8 mg liraglutide to insulin over a 12-week period in overweight and obese patients with T1D results in modest reductions of weekly mean glucose levels with significant weight loss, small insulin dose reductions, and frequent gastrointestinal side effects. These findings do not justify the use of liraglutide in all patients with T1D.
Introduction
Although the discovery of insulin dramatically transformed the clinical outcomes in patients with type 1 diabetes (T1D), a relevant proportion of patients do not reach their individual glycemic treatment targets and are thus vulnerable to microvascular complications and excess mortality (1). This is in spite of the introduction of self-monitoring of blood glucose (BG) and improved methods of insulin delivery like insulin pens and continuous subcutaneous insulin infusion (CSII) of insulin through insulin pumps. Recent observations suggest that the addition of liraglutide to insulin improves glycemic control significantly (2,3), as reflected in the reduction of mean glucose concentrations, glycemic excursions, and HbA1c. In the first study, the patients already had well-controlled diabetes (2), while the second included those whose diabetes was poorly controlled and who were obese or overweight (3). In both studies, the reduction of HbA1c was ∼0.5% over a period of 6 months. The changes in glycemic control and the reduction in glycemic variability occurred within the first few days of the institution of liraglutide treatment, as observed with continuous glucose monitoring in the first study (2,3). These retrospective studies also demonstrated the effects of liraglutide on weight loss (2,3) and the lowering of systolic blood pressure (SBP) in obese or overweight (3). The third study was prospectively randomized and demonstrated a reduction in BG concentrations and HbA1c over a period of just 4 weeks (4). Nonrandomized studies from other groups have also confirmed similar clinical and metabolic benefits of glucagon-like peptide 1 (GLP-1) receptor agonists in T1D (4–7).
Based on our previous studies, we have now conducted a prospectively randomized study in patients with T1D investigating the effects of three different doses of liraglutide, using a continuous glucose-monitoring system (CGMS). This trial also investigated the comparative effects of three doses of liraglutide and placebo. We hypothesized that treatment with liraglutide in T1D would decrease overall mean glucose, fasting and postprandial glucose concentrations, and postprandial glucagon and increase postprandial C-peptide concentrations. In addition, we also investigated the effects of liraglutide on SBP, insulin requirements, carbohydrate intake, body weight, and plasma CRP concentrations.
Research Design and Methods
This study is a single-center randomized, placebo-controlled, parallel-group, and double-blind phase IV study conducted from November 2012 to April 2014 at the Diabetes and Endocrinology Center of Western New York at University at Buffalo. The local institutional review board approved the study protocol. All patients provided written informed consent.
Patients were eligible for enrollment in the trial if they were adults 18–75 years of age with T1D, had fasting C-peptide <0.1 nmol/L, were on insulin therapy (via CSII [also known as insulin pump]) or multiple (four or more) injections of insulin per day for >12 months with or without a history of diabetic ketoacidosis, had HbA1c of ≤8.5% (69 mmol/mol), and were well versed with carbohydrate counting. Exclusion criteria were T1D for <12 months; coronary event or procedure (myocardial infarction, unstable angina, coronary artery bypass, surgery, or coronary angioplasty) in the previous 3 months; hepatic disease (transaminase >3 times normal) or cirrhosis; renal impairment (serum creatinine >1.5 mg/dL); HIV or hepatitis C–positive status; any other life-threatening, noncardiac disease; history of pancreatitis; history of gastroparesis; history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 (MEN 2) syndrome; family history of MEN 2, medullary thyroid cancer, or familial medullary thyroid cancer; pregnancy or of childbearing potential without use of adequate contraception; participation in any other concurrent clinical trial; use of an investigational agent or therapeutic regimen within 30 days of study; and inability to give informed consent.
Subjects who met the inclusion and exclusion criteria were block randomized to receive subcutaneous injection daily of liraglutide 0.6 mg (18 subjects), 1.2 mg (18 subjects), or 1.8 mg (18 subjects) or placebo (18 subjects) for 12 weeks. The subjects, study coordinators and investigators who were involved in adjusting insulin and liraglutide doses were blinded to the treatment. Liraglutide and placebo administered via a pen kit (obtained from Novo Nordisk Pharmaceuticals) were indistinguishable from each other. The effects of 0.6 mg liraglutide were investigated, as our first retrospective study suggested that this dose when given in 14 patients with T1D (mean BMI 24 kg/m2, HbA1c of 6.6%) for 1 week significantly improved glycemia, with reduced insulin doses within 24 and 48 h with reduction in glycemic variability by ∼50% with only 0.5 kg weight loss (2). It thus suggested that this might benefit normal-weight patients with T1D and those who may not tolerate higher doses of the drug.
All subjects were instructed by the study staff and certified diabetes educator in the dosing and administration of the study medication. They were seen by a registered dietitian who ensured that they are well versed with their carbohydrate counting. They were advised to monitor their capillary BG by finger-stick before and 2 h after each meal and to wear their CGMS constantly for 12 weeks. Meal challenge studies were carried out at baseline and at the end of the study. All patients were started on 0.6 mg of study drug per day to minimize side effects, with subsequent titration to 1.2 and 1.8 mg on a weekly basis until they reached the maximal tolerated or target dose. Prior to the initiation of liraglutide, if the HbA1c was ≥7.5% (58 mmol/mol), then no reductions were made in dose of insulin, while if the HbA1c ranged from 7 to 7.5% (53 to 58 mmol/mol) and was ≤7% (53 mmol/mol) then the basal and preprandial insulin doses were reduced by 10% and 25%, respectively. Patients were seen every week for the first 4 weeks after the initiation of study drug and then every 2 weeks until the completion of the study. Insulin doses were titrated during study visits based on finger-stick BG and CGMS to attain preprandial BG concentrations between 4.9 and 6.6 mmol/L (90 and 120 mg/dL) and 2-h postprandial <7.7 mmol/L (140 mg/dL).
Medication adherence was evaluated by counting used pens. All patients were blinded to their CGMS (Dexcom SEVEN PLUS) including the patients who were already using unblinded CGMS, as CGMS use alone has been shown to improve glycemic control (8). All patients were provided sufficient training with CGMS use during the trial period including troubleshooting support. All patients were allowed to see the CGMS reports at the end of their visits.
Average weekly glucose, fasting glucose, SD, and percent time spent in different glycemic thresholds were obtained from CGMS. Insulin doses, carbohydrate intake (in grams), and carbohydrate helpings (frequency of eating) were obtained from insulin pump and patient food/insulin dose/BG diaries for patients on CSII and multiple daily injections (MDI), respectively. These parameters were measured every week, while blood pressure (average of three readings) was measured manually in the fasting state after 10 min in the sitting position at study visits. SD in CGMS represents the variability of BG concentrations. All patients were instructed to document total carbohydrate intake including correctional carbohydrates during hypoglycemia. The carbohydrate helpings per day were calculated from number of carbohydrate entries from the insulin pump for patients on insulin pump and from the food/insulin dose/BG log for patients on MDI. The weekly average carbohydrate intake in grams and frequency were estimated every week over a period of 12 weeks.
The primary end point of the study was the difference from baseline in mean weekly BG concentrations before and after 12 weeks of treatment in each of the liraglutide groups compared with placebo. The difference in mean weekly BG concentrations was calculated every week during the 12 weeks of treatment. The change in HbA1c, insulin doses, percent time spent in different glycemic ranges (3.8–8.8, 8.8–13.3, 13.3–22.25, 3.05–3.88, and <3.05 mmol/L), SD (measure of variability in BG concentrations), body weight, SBP, carbohydrate intake, and postprandial glucagon were secondary end points.
Meal Challenge Test and Plasma Measurements
Meal Challenge Test
In order to assess the postprandial changes induced by liraglutide, a meal challenge was carried out before starting liraglutide or placebo and at the end of the study period on days 0 and 84. We used a 910-cal; high-fat, high-carbohydrate meal, which we have used previously (9–11). The ingestion of the meal was completed in 15 min. Insulin bolus was injected subcutaneously immediately before the meal based on the insulin-to-carbohydrate ratio and correction factor for each individual subject. Study subjects continued to receive their basal insulin (unchanged basal rates for patients on CSII or long-acting insulin at their usual time for patients on MDI). Liraglutide was omitted on day 0 but was injected on day 84 (45 min prior to the meal). Sequential blood samples were obtained at 0, 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, and 300 min. Blood sample was collected from an indwelling intravenous cannula in a superficial forearm vein.
Plasma Measurements
HbA1c was measured at Quest Diagnostics by immunoturbidimetric assay. ELISAs were used to measure total GLP-1, gastrointestinal polypeptide (GIP) (EMD Millipore, Billerica, MA), and glucagon (R&D Systems, Minneapolis, MN). CRP was measured using ELISA assay (American Diagnostica, Inc., Stamford, CT). Free fatty acid (FFA) levels were measured by a colorimetric assay (Wako Chemicals, Richmond, VA).
Statistical Methods
There are no previous randomized studies that have examined the effect of liraglutide on mean weekly BG concentrations in subjects with T1D. With a conservative estimate of a difference in mean weekly BG concentrations of 20 mg/dL before and after treatment with liraglutide (clinically significant difference based on our preliminary study [2]), a sample size of 15 patients per treatment group should provide adequate power (β = 0.2) to detect a significant difference (α = 0.05), provided the SD of the residuals is not >25. Thus, 60 subjects will be needed for the study. Sample size was increased by 20% to 72 to compensate for potential dropout.
Data are presented as mean ± SEM. Final analysis was done based on the intention-to-treat principle. One-way ANOVA was used to compare baseline characteristics (Table 1) of all four groups (three liraglutide groups and one placebo group). Student t test was used to compare the change in mean weekly BG concentrations, HbA1c, percent time spent in different glycemic thresholds, insulin doses, body weight, BMI, carbohydrate intake, and blood pressure in liraglutide groups compared with placebo. ANOVA was used to compare changes in postprandial glucose and glucagon between groups. Student t test was used to compare changes in area under the curve (AUC) in postprandial glucose and glucagon in liraglutide groups compared with placebo. χ2 test was used to test difference in proportions and frequency of gastrointestinal adverse events and hypoglycemic episodes. Changes in these end points were calculated by averaging the differences in weekly average values from baseline. Pearson correlation was used to test relationships among variables. All end points were normally distributed. A P value of <0.05 was considered significant. SPSS software (SPSS, Chicago, IL) was used for analysis.
Table 1.
Baseline characteristics of patients in the study groups
| Placebo | 0.6 mg | 1.2 mg | 1.8 mg | P | |
|---|---|---|---|---|---|
| n | 17 | 14 | 16 | 16 | |
| Age (years) | 50 ± 3 | 45 ± 4 | 42 ± 3 | 42 ± 3 | 0.27 |
| Age of T1D diagnosis (years) | 19 ± 3 | 19 ± 3 | 21 ± 3 | 21 ± 3 | 0.92 |
| Duration of T1D (years) | 30 ± 3 | 25 ± 2 | 21 ± 3 | 20 ± 3 | 0.04 |
| Sex (male/female) | 7/10 | 9/9 | 8/5 | 4/11 | 0.29§ |
| HbA1c, % (mmol/mol) | 7.69 ± 0.17 (61 ± 2) | 7.46 ± 0.19 (58 ± 2) | 7.84 ± 0.17 (62 ± 2) | 7.41 ± 0.15 (57 ± 1.6) | 0.24 |
| Average glucose (CGMS), mmol/L (mg/dL) | 9.37 ± 0.44 (169 ± 8) | 8.99 ± 0.38 (162 ± 7) | 9.32 ± 0.33 (168 ± 6) | 9.43 ± 0.27 (170 ± 5) | 0.93 |
| Fasting glucose (CGMS), mmol/L (mg/dL) | 9.54 ± 0.66 (172 ± 12) | 8.71 ± 0.66 (157 ± 12) | 8.43 ± 0.55 (152 ± 10) | 9.21 ± 0.44 (166 ± 8) | 0.61 |
| SD (CGMS), mmol/L (mg/dL) | 4.2 ± 0.22 (76 ± 4) | 4.05 ± 0.22 (73 ± 4) | 4.16 ± 0.16 (75 ± 3) | 3.9 ± 0.16 (70 ± 3) | 0.77 |
| Body weight (kg) | 80 ± 6 | 80 ± 4 | 96.0 ± 4 | 83 ± 4 | 0.08 |
| BMI (kg/m2) | 28 ± 2 | 26 ± 3 | 33 ± 2 | 28 ± 4 | 0.20 |
| Total insulin dose (units) | 46.1 ± 9.5 | 52.8 ± 3.7 | 71.2 ± 5.5¶$ | 48.1 ± 4.3 | 0.01 |
| Basal insulin dose (units) | 26.0 ± 5.1 | 30.70 ± 2.9 | 42.6 ± 4.5¶$ | 27.0 ± 2.3 | 0.01 |
| Bolus insulin dose (units) | 24.2 ± 5.1 | 21.9 ± 2.2 | 28.0 ± 3.2 | 20.9 ± 2.8 | 0.46 |
| Daily carbohydrate intake (g) | 160 ± 15 | 161 ± 29 | 171 ± 17 | 153 ± 18 | 0.95 |
| Daily carbohydrate helpings (meals/day) | 4.1 ± 0.3 | 3.7 ± 0.4 | 3.5 ± 0.3 | 3.3 ± 0.3¶ | 0.18 |
| SBP (mmHg) | 122 ± 4 | 125 ± 4 | 121 ± 3 | 120 ± 2 | 0.78 |
| Diastolic blood pressure (mmHg) | 75 ± 3 | 75 ± 3 | 75 ± 2 | 77 ± 2 | 0.92 |
| Pulse rate | 76 ± 2 | 75 ± 2 | 75 ± 2 | 75 ± 2 | 0.16 |
| % time spent at BG concentrations (CGMS) | |||||
| <3.05 mmol/L (55 mg/dL) | 4 ± 1 | 3 ± 1 | 3 ± 1 | 2 ± 1 | 0.60 |
| 3.05–3.88 mmol/L (55–70 mg/dL) | 5 ± 1 | 5 ± 1 | 4 ± 1 | 4 ± 1 | 0.32 |
| 3.8–8.8 mmol/L (70–160 mg/dL) | 43 ± 3 | 49 ± 3 | 45 ± 2 | 44 ± 3 | 0.74 |
| 8.8–13.3 mmol/L (160–240 mg/dL) | 29 ± 2 | 27 ± 2 | 30 ± 2 | 33 ± 2 | 0.39 |
| 13.3–22.25 mmol/L (240–400 mg/dL) | 18 ± 3 | 15 ± 2 | 18 ± 2 | 18 ± 3 | 0.94 |
| Episodes of hypoglycemia/total number of SMBG readings (incidence %) | |||||
| <3.05 mmol/L (55 mg/dL) | 1/36 (2) | 1/37 (2) | 1/36 (2) | 1/37 (2) | 0.84 |
| 3.05–3.88 mmol/L (55–70 mg/dL) | 2/36 (5) | 4/37 (10) | 3/36 (8) | 1/37 (2) | 0.31 |
| Glucagon levels (ng/L) | 97 ± 14 | 106 ± 12 | 112 ± 18 | 95 ± 13 | 0.38 |
| Total GLP-1 (pmol/L) | 23 ± 9 | 21 ± 3 | 28 ± 5 | 20 ± 3 | 0.24 |
| Total GIP (pg/mL) | 78 ± 14 | 73 ± 18 | 91 ± 20 | 73 ± 12 | 0.41 |
| FFAs (mmol/L) | 0.51 ± 0.08 | 0.58 ± 0.11 | 0.49 ± 0.12 | 0.55 ± 0.07 | 0.52 |
| CRP (g/L) | 3.05 ± 0.57 | 3.17 ± 1.0 | 3.01 ± 0.92 | 3.53 ± 0.67 | 0.73 |
Data are means ± SEM unless otherwise indicated.
¶Significant compared with placebo (P < 0.05).
§χ2 test.
$Significant compared with 1.8-mg group (P < 0.05).
Results
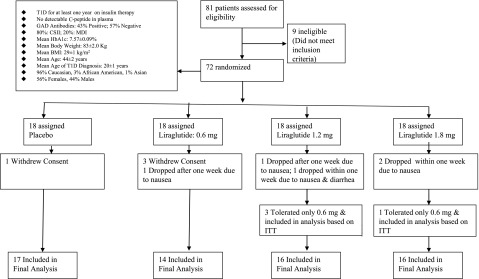
Sixty-three subjects of the 72 randomized patients completed the study (12.5% dropout rate) (Fig. 1). These patients were recruited between 2 November 2012 and 5 January 2014, with follow-up until April 2014. Baseline characteristics of study subjects are presented in Table 1. All the groups had similar age, BMI, and glycemic control. The total and basal insulin doses were higher in the 1.2-mg liraglutide group by 23–25 units compared with the placebo and 1.8-mg liraglutide groups (P < 0.05 for both). All patients had a history of at least one episode of diabetic ketoacidosis. Approximately 80% and 20% of randomized patients were on CSII with insulin pumps and MDI, respectively (Fig. 1).
Figure 1.
Trial profile. ITT, intention-to-treat principle.
Effect of Liraglutide on Glycemic Control
In the 1.2-mg and 1.8-mg groups, the mean weekly reduction in average BG concentrations (primary end point) was −0.55 ± 0.11 mmol/L (10 ± 2 mg/dL) and −0.55 ± 0.05 mmol/L (10 ± 1 mg/dL), respectively (P < 0.0001), while it remained unchanged in the 0.6-mg and placebo groups (Table 2). The change in average glucose was −0.01 ± 0.11 (P = 0.51) and 0.04 ± 0 mmol/L in the 0.6-mg and placebo groups, respectively.
Table 2.
Effect of liraglutide treatment on glycemic control, body weight, insulin dose, blood pressure, glucagon, FFA, and CRP concentrations
| Change over 12 weeks |
P (vs. placebo) |
||||||
|---|---|---|---|---|---|---|---|
| Placebo | 0.6 mg | 1.2 mg | 1.8 mg | 0.6 mg | 1.2 mg | 1.8 mg | |
| Average glucose, mmol/L (mg/dL) (CGMS) | 0.04 ± 0 (0.72 ± 0) | −0.01 ± 0.11 (0.18 ± 1.9) | −0.55 ± 0.11 (10 ± 2)* | −0.55 ± 0.05 (10 ± 1)* | 0.51 | <0.001 | <0.001 |
| HbA1c, % (mmol/mol) | −0.3 ± 0.15 (−3.3 ± 1.6)* | −0.26 ± 0.17 (−2.8 ± 1.9) | −0.78 ± 0.15 (−8.5 ± 1.6)* | −0.42 ± 0.15 (−4.6 ± 1.6)* | 0.81 | <0.01 | 0.39 |
| Glucose SD (CGMS), mmol/L (mg/dL) | −0.02 ± 0.04 (0 ± 1) | −0.04 ± 0.03 (1 ± 0) | −0.23 ± 0.04 (4 ± 1) | −0.12 ± 0.04 (2 ± 1) | 0.65 | <0.01 | 0.13 |
| Total insulin dose (units) | −1.9 ± 0.7 | −2.8 ± 0.7 | −12.1 ± 0.7* | −10.0 ± 0.5* | 0.76 | <0.001 | <0.001 |
| Basal insulin dose (units) | 0.4 ± 0.4 | −1.4 ± 0.3 | −6.3 ± 0.4 | −1.7 ± 0.3 | <0.01 | <0.001 | <0.001 |
| Bolus insulin dose (units) | −1.5 ± 0.4 | −1.4 ± 0.5 | −6.1 ± 0.6 | −8.2 ± 0.3* | 0.87 | <0.001 | <0.001 |
| % time spent at BG concentrations (CGMS) | |||||||
| <3.05 mmol/L (55 mg/dL) | −0.2 ± 0.2 | 0.7 ± 0.3 | 1.0 ± 0.3 | 1.3 ± 0.2 | <0.01 | <0.01 | <0.001 |
| 3.05–3.88 mmol/L (55–70 mg/dL) | −1.0 ± 0.2 | 0.1 ± 0.3 | 1.2 ± 0.4 | 1.1 ± 0.2 | <0.01 | <0.001 | <0.001 |
| 3.8–8.8 mmol/L (70–160 mg/dL) | 1.4 ± 0.6 | −1.1 ± 0.8 | 2.7 ± 0.7 | 4.8 ± 0.8 | <0.05 | 0.19 | <0.01 |
| 8.8–13.3 mmol/L (160–240 mg/dL) | −0.6 ± 0.5 | 0.5 ± 0.6 | −3.5 ± 0.7* | −3.8 ± 0.5* | 0.13 | <0.001 | <0.001 |
| 13.3–22.25 mmol/L (240–400 mg/dL) | 0.8 ± 0.7 | 0.8 ± 0.6 | −2.1 ± 0.9 | −3.5 ± 0.5 | 1.00 | <0.05 | <0.001 |
| Episodes of hypoglycemia/total number of SMBG readings (incidence %)¶ | |||||||
| <3.05 mmol/L (55 mg/dL) | 14/419 (3) | 21/495 (5) | 21/426 (4) | 14/472 (2) | 0.47 | 0.82 | 0.65 |
| 3.05–3.88 mmol/L (55–70 mg/dL) | 21/419 (5) | 35/495 (7) | 24/426 (5) | 27/472 (5) | 0.61 | 0.85 | 0.74 |
| Number of patients with hypoglycemia/total number of patients¶ | |||||||
| <3.05 mmol/L (55 mg/dL) | 14/17 | 13/14 | 16/16 | 13/16 | 0.76 | 0.42 | 0.85 |
| 3.05–3.88 mmol/L (55–70 mg/dL) | 16/17 | 14/14 | 16/16 | 16/16 | 0.86 | 0.89 | 0.91 |
| Carbohydrate intake (g) | −13.4 ± 2.6 | −23.7 ± 2.5 | −47.6 ± 2.6* | −46.4 ± 1.6* | <0.01 | <0.001 | <0.001 |
| Daily carbohydrate helpings (meals per day) | 0.0 ± 0.6 | −0.3 ± 0.2 | −0.9 ± 0.3* | −0.4 ± 0.1 | 0.14 | <0.001 | <0.001 |
| Body weight (kg) | −0.3 ± 0.5 | −2.7 ± 0.6* | −5.0 ± 1.2* | −4.8 ± 0.7* | <0.05 | <0.01 | <0.001 |
| BMI (kg/m2) | −0.1 ± 0.2 | −1.1 ± 0.4* | −1.7 ± 0.5* | −1.5 ± 0.3* | <0.05 | <0.01 | <0.001 |
| SBP (mmHg)† | −0.5 ± 0.7 | −3.0 ± 1.1 | −1.5 ± 1.0 | −3.3 ± 1.1* | 0.07 | 0.89 | <0.05 |
| DBP (mmHg) | 0.9 ± 0.5 | 0.5 ± 0.70 | −0.1 ± 0.4 | −1.0 ± 0.7 | 0.52 | 0.34 | 0.053 |
| Pulse rate | 0 ± 2 | −1 ± 3 | 2.0 ± 1 | 2 ± 2 | 0.62 | 0.48 | 0.43 |
| Glucagon (ng/L) | 5 ± 3 | 7 ± 4 | −6 ± 9 | 9 ± 6 | 0.57 | 0.38 | 0.66 |
| GLP-1 (pmol/L) | 5 ± 7 | 5 ± 2 | 7 ± 5 | 13 ± 4* | 0.52 | 0.44 | 0.17 |
| GIP (pg/mL) | −10 ± 8 | 22 ± 21 | 39 ± 13* | 38 ± 11* | 0.27 | <0.05 | <0.05 |
| FFA (mmol/L) | 0.03 ± 0.03 | 0.05 ± 0.04 | 0.02 ± 0.02 | −0.1 ± 0.03* | 0.54 | 0.88 | <0.05 |
| CRP (g/L) | −0.14 ± 0.11 | −0.21 ± 0.15 | −0.48 ± 0.15* | −0.96 ± 0.19* | 0.41 | <0.05 | <0.05 |
Data are means ± SEM unless otherwise indicated. DBP, diastolic blood pressure.
*P < 0.05 for change compared with baseline within group (paired t test).
¶χ2 test for comparison.
†Two normotensive subjects in 1.8-mg group taking ACE inhibitors for renal protection had to stop or reduce the dose of the drugs because of a fall in SBP to <100 mmHg. Two known hypertensive subjects in this group also had the doses of ACE inhibitor and β-blocker reduced because of a fall in SBP. In the 0.6-mg group, in one normotensive patient the dose of valsartan was reduced to 160 mg from 320 mg at day 56 and to 80 mg at day 70 owing to fall in SBP from 112 to 100 mmHg.
HbA1c fell by 0.78 ± 0.15% (−8.5 ± 1.6 mmol/mol) in the 1.2-mg group (from 7.84 ± 0.17% [62 ± 2 mmol/mol] to 7.06 ± 0.15% [54 ± 1.6 mmol/mol]; P < 0.0001) and by 0.42 ± 15% (4.6 ± 1.6 mmol/mol) in the 1.8-mg group (from 7.41 ± 0.15% [57 ± 1.6 mmol/mol] to 6.99 ± 0.15% [53 ± 1.6 mmol/mol]; P = 0.001). HbA1c fell by 0.26 ± 0.17% (2.8 ± 1.9 mmol/mol; P = 0.81) in the 0.6-mg group and by 0.3 ± 0.15% (3.3 ± 1.6 mmol/mol) in the placebo group. Only the decline in the 1.2-mg group was statistically significant compared with placebo (Table 2). The change in HbA1c was related to baseline HbA1c in the 1.2-mg and 1.8-mg groups (r = 0.55, P = 0.052, for 1.2 mg, and r = 0.56, P = 0.03, for 1.8 mg) but not to change in BMI (r = 0.26, P = 0.39, for 1.2 mg, and r = 0.31, P = 0.27, for 1.8 mg). Basal and bolus insulin doses fell in both 1.2-mg and 1.8-mg groups. Change in HbA1c was related to change in bolus (r = 0.50, P = 0.008) and total insulin doses (r = 0.52, P = 0.005). Change in HbA1c was not related to sex.
The glycemic variability (SD of CGM readings) fell by 0.23 ± 0.04 mmol/L (4 ± 1 mg/dL) (P < 0.01) in the 1.2-mg group, while it remained unchanged in other liraglutide and placebo groups. Percent time spent in hyperglycemia (8.8–13.3 mmol/L, i.e., 160–240 mg/dL) decreased in both the 1.2- and 1.8-mg groups by 3–4% (P < 0.001 for both). Percent time spent in hyperglycemia (13.3–22.25 mmol/L, i.e., 240–400 mg/dL) decreased by 2% (P < 0.05) and 3% (P < 0.001), respectively, in the 1.2-mg and 1.8-mg liraglutide groups. Percent time spent in glycemic threshold (3.8–8.8 mmol/L, i.e., 70–160 mg/dL) increased by 1% (P < 0.05) in the 0.6-mg group and by ∼5% (P < 0.001) in the 1.8-mg group. Percent time spent in different glycemic thresholds was unchanged in the placebo group. All liraglutide groups had ≤1% increase in time spent in hypoglycemia (<3.8 mmol/L, i.e., 70 mg/dL, and <3.05 mmol/L, i.e., 55 mg/dL), equivalent to 10 min to 2 h per week. The incidence of hypoglycemia based on self-monitoring of blood glucose (SMBG) readings was essentially unchanged. There was no severe hypoglycemic episode requiring hospitalization or urgent medical attention in the placebo or liraglutide-treated groups. There were no changes in C-peptide concentrations in any groups.
Effect of Liraglutide on Carbohydrate Intake and Body Weight
The total daily carbohydrate intake fell by 30% (∼47 g) in the 1.2-mg and 1.8-mg groups. This effect occurred within the first week (decrease by 34 g in both groups) and continued to the end of the study. The frequency of carbohydrate helpings was reduced in the 1.2-mg and 1.8-mg groups from 3.5 ± 0.3 to 2.6 ± 0.3 meals per day, P < 0.001, and from 3.3 ± 0.3 to 2.9 ± 0.3, P < 0.01, respectively.
Mean body weight in the 1.2-mg and 1.8-mg groups fell by 5 ± 1 kg (96 ± 4 kg to 91 ± 4 kg and 83 ± 4 kg to 78 ± 5 kg, respectively, P < 0.001 for both). The reduction in body weight was most impressive (3.63 kg in the 1.2-mg group and 2.27 kg in the 1.8-mg group) in the first 2 weeks after the initiation of liraglutide treatment. There was a further fall of 1.37 kg in the 1.2-mg and 2.53 kg in the 1.8-mg groups thereafter over 10 weeks. In the 0.6-mg group, there was a reduction in mean body weight by 3 kg (80 ± 4 to 77 ± 4 kg, P = 0.006). Of subjects treated with liraglutide, 89% lost weight. There was no weight loss in the placebo group.
Effect of Liraglutide on Blood Pressure, FFAs, and CRP
There was a fall in SBP by 3 ± 1 mmHg (P < 0.05) in the 1.8-mg group only. The change in blood pressure was unrelated to weight loss (r = 0.247, P = 0.376). Fasting plasma FFA fell significantly in the 1.8-mg group from 0.55 ± 0.07 to 0.45 ± 0.05 mmol/L (P < 0.05) (Tables 1 and 2).
CRP concentrations fell significantly by 15 ± 6% (from 3.01 ± 0.92 to 2.53 ± 0.83 g/L, P < 0.05) in the liraglutide 1.2-mg group and by 19 ± 8% (from 3.53 ± 0.67 to 2.57 ± 0.52 g/L, P < 0.05) in the liraglutide 1.8-mg group (Tables 1 and 2), with no changes in other groups.
Effect of Liraglutide on Glucose and Glucagon Excursion After a Mixed Meal
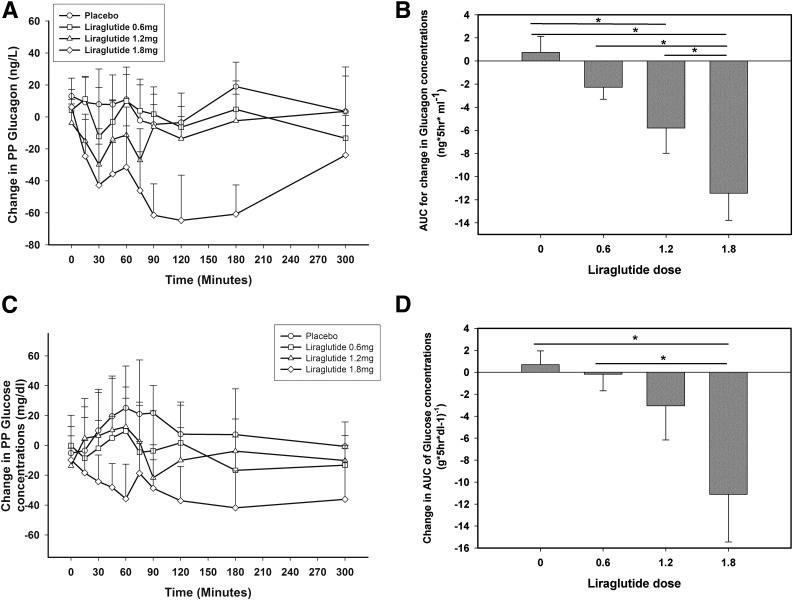
The increase in plasma glucagon concentrations after a high-fat, high-carbohydrate meal was reduced in patients taking 1.2 mg and 1.8 mg liraglutide (Fig. 2A and Supplementary Fig. 1A) at 12 weeks: the AUC of glucagon was lowered by 47 ± 12% and 72 ± 12% in the 1.2 mg and 1.8 mg groups, respectively, and thus was dose dependent (P < 0.05 for both compared with baseline) (Fig. 2B and Supplementary Fig. 1A). This was associated with lower glucose excursion by 21 ± 8% at 12 weeks in the 1.8-mg liraglutide group (AUC from 67 ± 5 to 52 ± 5 g * 5 h * dL−1, P = 0.017) (Fig. 2C and D and Supplementary Fig. 1B), which was also significantly lower compared with change in the placebo group (Fig. 2D).
Figure 2.
Change in glucagon concentrations after meal challenge before and after 12 weeks of liraglutide or placebo treatments (A) and AUC for glucagon change at 12 weeks compared with 0 weeks in all groups (B) in patients with T1D. *P < 0.05 compared with 0 weeks. Change in glucose concentrations after meal challenge before and after 12 weeks of liraglutide or placebo treatments (C) and AUC for glucose change at 12 weeks (W) compared with 0 weeks in all groups (D) in patients with T1D. *P < 0.05 compared with 0 weeks. hr, hours; PP, postprandial.
Adverse Effects
The cumulative incidence of nausea was 65% (35 of 54) (P = 0.001) in the liraglutide groups vs. 17% (3 of 18) in placebo. Eleven patients in the 0.6-mg group; 10 in the 1.2-mg group, 9 in the 1.8-mg group, and 3 in the placebo group complained of transient nausea. Self-reported moderate nausea was reported for the first 2–5 days after the initiation of liraglutide and then for another 2–4 days at the time of escalation of the dose. These patients reported having either mild nausea or intermittent nausea during the remaining study duration. The dropout rate resulting from nausea was 9% (5 of 54) in all liraglutide groups, and the breakdown in each group is shown in Fig. 1 (Trial profile). One patient in the 1.2-mg group had to drop out owing to both nausea and diarrhea for 1 week. One patient each in the placebo and 0.6-mg groups reported one episode of vomiting, while two patients in 1.2-mg and 1.8-mg group reported two episodes of vomiting. Of 16 patients in the 1.2-mg group, 6 (37.5%) reported appetite suppression vs. 8 of 16 (50%) patients in the 1.8-mg group and none in the placebo group.
Conclusions
Our data show that after liraglutide, glycemic control improved rapidly, and this improvement persisted until the end of study in the groups given 1.2 and 1.8 mg liraglutide. This improvement was reflected in the reduction of mean glucose concentration in both higher-dose liraglutide groups, with reduction in glycemic variability only in the 1.2-mg group over the duration of the study. Percent time spent in hyperglycemia (>8.8 mmol/L, i.e., 160 mg/dL) decreased significantly in the 1.2- and 1.8-mg groups in association with a reduction in the dose of total and prandial insulin. There was an increase of 1% in hypoglycemia (both <3.05 mmol/L, i.e., 55 mg/dl, and <3.8 mmol/L, i.e., 70 mg/dL) in the 1.2- and 1.8-mg groups. There was higher incidence of mild gastrointestinal adverse events. There was no significant change in any of these indices in patients treated with the placebo or on 0.6 mg liraglutide.
Over the period of 12 weeks, HbA1c fell significantly from baseline in the 1.2- and 1.8-mg groups. However, the decrease in the group on 1.8 mg was not significantly different from that in the placebo group. It is not clear why the magnitude of the fall in HbA1c was greater in the 1.2-mg group than that in the 1.8-mg group. It is possible that the higher baseline HbA1c in the 1.2-mg group may have contributed to this, since the magnitude of change in HbA1c was shown to be related to baseline HbA1c. This difference is intriguing, since other indices changed either equally or more in the group taking 1.8 mg liraglutide. It is possible that a 12-week study is not sufficient to stabilize HbA1c levels. Furthermore, the reduction of 0.42% (4.6 mmol/mol) in HbA1c in the 1.8-mg group was obtained in combination with 5 kg weight loss and a reduction in the insulin dose by 10 units (21%), while the body weight and insulin dose remain unchanged in the placebo group. Although the 1.2-mg group was heavier than the 1.8-mg group by 13 kg, the change in HbA1c was not related to change in BMI. Large and longer-duration randomized clinical trials in overweight and obese patients with T1D with similar baseline body weight, insulin dose, and HbA1c will clarify the heterogeneous HbA1c response with two higher-dose liraglutide groups.
In addition, there was a reduction in body weight of 5 kg in the 1.2- and 1.8-mg groups in 12 weeks. Protein, fat, and total calorie intake were not measured, and this is a limitation of our study. Few patients may not have documented correctional carbohydrates consumed during hypoglycemia, but this will be true for all groups, and therefore bias resulting from this is likely to be small. However, they were asked to record their daily carbohydrate intake, which fell by 47 g in the 1.2-mg and 1.8-mg groups.
In the absence of β-cell function, there are three likely mechanisms that contribute to improved glycemia in these patients. Firstly, there was a reduction in the magnitude of postprandial increase of glucagon by 72% after 1.8 mg liraglutide and 47% after the 1.2-mg dose, consistent with the effect seen in patients with type 2 diabetes (12). Secondly, the reduction in carbohydrate intake by nearly one-third associated with appetite suppression induced by liraglutide contributed to the glycemic control. Thirdly, there may be an insulin-sensitizing effect, which has recently been shown to occur in patients with T1D after the administration of exenatide, a GLP-1 receptor agonist (7), especially in association with weight loss.
There was a significant reduction in SBP in spite of an adequate blood pressure control at baseline. A mean reduction of 3 mm in the 1.8-mg liraglutide group occurred in spite of the reduction in the dose of ACE inhibitors and β-blockers in some patients. The fall in SBP is consistent with our original observation of such an effect with exenatide in type 2 diabetes (13) and with our recently published data on liraglutide in obese patients with T1D (3). It is important that this effect is not related to weight loss. It is possible that this effect is due to a direct action on the vasculature.
A recent 12-week randomized study in normal-weight patients with T1D with 1.2 mg liraglutide and a 24-week randomized study from the same group in overweight and obese patients with poorly controlled T1D with 1.8 mg liraglutide demonstrated significant reduction in body weight and insulin dose without any additional effect on HbA1c compared with placebo (14,15). The latter study also demonstrated reduction in hypoglycemic events (15). Novo Nordisk has withdrawn their intent to seek a regulatory indication for the use of liraglutide in T1D in view of no additional difference in HbA1c in two large phase-3 trials despite weight loss and reduced insulin doses (16). Considering the above in light of the results seen in our study, further research should be directed toward overweight and obese patients with T1D with a composite primary end point of change in HbA1c, body weight, insulin dose, and possible reduction in hypoglycemia and also weigh the expense of the additional therapeutic intervention against these benefits. Whether patients newly diagnosed with T1D and/or patients with T1D with detectable C-peptide are more likely to benefit is also of interest.
Our study has some limitations. High frequency of gastrointestinal adverse effects can easily unmask who was likely to be on liraglutide. But this would be true for any randomized study of liraglutide with placebo or other comparator group that does not have gastrointestinal side effects. This is a single-center study with a relatively small sample size of only 72 patients randomized to four different groups for a short duration of 12 weeks, which is not sufficient for HbA1c to stabilize to a new level. Nevertheless, it clearly shows that while the two higher doses have beneficial effects, the lowest dose of 0.6 mg is not likely to be effective in in patients with T1D. One of the strengths of our study is that we used CGM throughout the entire duration of the trial for 12 weeks. This allowed us to capture ∼1% increase in percent time spent in hypoglycemia (both <3.05 mmol/L, i.e., 55 mg/dL, and <3.8 mmol/L, i.e., 70 mg/dL) in the 1.2- and 1.8-mg liraglutide groups despite unchanged incidence of hypoglycemia based on SMBG.
In conclusion, the two higher doses of liraglutide are effective in improving various indices of glycemic control, reducing postprandial glucagon increase, total insulin dose, carbohydrate intake, and body weight. Our study paves the way for larger multicenter clinical trials over longer periods in overweight and obese patients with T1D to establish the durability and consistency of effects of liraglutide in T1D.
Supplementary Material
Article Information
Acknowledgments. The authors thank the following for their help in execution of the study: Howard Lippes, MD (endocrinologist, Catholic Health System, State University of New York at Buffalo), and Manisha Garg, Sargam Kapoor, Abdul Rafeh, Shyam Mohan, and Aishwarya Utkosh (research volunteers, Department of Endocrinology, Diabetes and Metabolism, State University of New York at Buffalo). The authors thank Dexcom for providing 20 Starter kits of Dexcom SEVEN PLUS Continuous Glucose Monitoring System.
Funding. N.D.K. received an Endocrine Fellows Foundation grant. P.D. has received grants from the National Institutes of Health, the Centers for Disease Control and Prevention, the John R. Oishei Foundation, the William G. McGowan Charitable Fund, and the Millard Fillmore Foundation.
The Endocrine Fellows Foundation was not involved in the conduct of the trial, data collection, statistical analysis, or preparation of the manuscript.
Duality of Interest. This research was supported by a grant from Novo Nordisk to P.D. N.D.K. and A.C. have served on the speaker panel for Novo Nordisk. S.D. has served on the speaker panel for AbbVie. P.D. has received research support from GlaxoSmithKline, Novo Nordisk, Bristol-Myers Squibb, Takeda Pharmaceuticals, Allergan, Sanofi, ConjuChem, Dainippon Pharmaceuticals, Procter & Gamble Pharmaceuticals, Mitsubishi, Quigley Pharma, AbbVie, Transition Therapeutics, and Tolerx; has received honorarium from Eli Lilly, Novartis, GlaxoSmithKline, Merck, Novo Nordisk, Takeda, and Sanofi; and has received grants from GlaxoSmithKline, Bristol-Myers Squibb, Novartis Pharmaceuticals, Abbott Laboratories, Takeda, Sankyo Pharmaceuticals North America, the citrus industry of Florida, and Solvay Pharmaceuticals. No other potential conflicts of interest relevant to this article were reported.
Novo Nordisk was not involved in the conduct of the trial, data collection, statistical analysis, or preparation of the manuscript.
Author Contributions. N.D.K. and P.D. wrote the first draft of the manuscript. All of the authors participated in subsequent revisions of the manuscript. N.D.K., S.D., H.G., A.Ma., A.C., and P.D. conceived the study concept and design. N.D.K. spearheaded the conduct of the study and contributed heavily in recruitment of study participants, titration of insulin doses, and management of patients. N.D.K. and H.G. performed the statistical analysis and interpretation of data. N.D.K., H.G., and P.D. wrote the manuscript. S.D., A.Ma., M.B., and A.C. reviewed and edited the manuscript and contributed to discussion. S.D., A.Me., S.S., J.H., K.G., and N.B. were involved heavily with the conduct of the study and the management of the patients including the handling of the continuous glucose-monitoring data on glycemia. M.Y. conducted the quality-of-life aspect of the study. N.D.K. and P.D. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014.
Footnotes
Clinical trial reg. no. NCT01722266, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1136/-/DC1.
References
- 1.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med 2014;371:1972–1982 [DOI] [PubMed] [Google Scholar]
- 2.Varanasi A, Bellini N, Rawal D, et al. Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol 2011;165:77–84 [DOI] [PubMed] [Google Scholar]
- 3.Kuhadiya ND, Malik R, Bellini NJ, et al. Liraglutide as additional treatment to insulin in obese patients with type 1 diabetes mellitus. Endocr Pract 2013;19:963–967 [DOI] [PubMed] [Google Scholar]
- 4.Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta-cell function. Diabetes Care 2011;34:1463–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison LB, Mora PF, Clark GO, Lingvay I. Type 1 diabetes treatment beyond insulin: role of GLP-1 analogs. J Investig Med 2013;61:40–44 [DOI] [PubMed]
- 6.Traina AN, Lull ME, Hui AC, Zahorian TM, Lyons-Patterson J. Once-weekly exenatide as adjunct treatment of type 1 diabetes mellitus in patients receiving continuous subcutaneous insulin infusion therapy. Can J Diabetes 2014;38:269–272 [DOI] [PubMed] [Google Scholar]
- 7.Sarkar G, Alattar M, Brown RJ, Quon MJ, Harlan DM, Rother KI. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care 2014;37:666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bode B, Beck RW, Xing D, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Sustained benefit of continuous glucose monitoring on A1C, glucose profiles, and hypoglycemia in adults with type 1 diabetes. Diabetes Care 2009;32:2047–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aljada A, Mohanty P, Ghanim H, et al. Increase in intranuclear nuclear factor kappaB and decrease in inhibitor kappaB in mononuclear cells after a mixed meal: evidence for a proinflammatory effect. Am J Clin Nutr 2004;79:682–690 [DOI] [PubMed] [Google Scholar]
- 10.Ghanim H, Abuaysheh S, Sia CL, et al. Increase in plasma endotoxin concentrations and the expression of toll-like receptors and suppressor of cytokine signaling-3 in mononuclear cells after a high-fat, high-carbohydrate meal: implications for insulin resistance. Diabetes Care 2009;32:2281–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghanim H, Sia CL, Upadhyay M, et al. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr 2010;91:940–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horowitz M, Flint A, Jones KL, et al. Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract 2012;97:258–266 [DOI] [PubMed] [Google Scholar]
- 13.Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P, Dandona P. Exenatide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract 2007;13:444–450 [DOI] [PubMed] [Google Scholar]
- 14.Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S. Twelve-week treatment with liraglutide as add-on to insulin in normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled, double-blind parallel study. Diabetes Care 2015;38:2250–2257 [DOI] [PubMed] [Google Scholar]
- 15.Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (Lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2016;4:221–232 [DOI] [PubMed] [Google Scholar]
- 16.Novo Nordisk completes second and final phase 3a trial with liraglutide as adjunct therapy to insulin for people with type 1 diabetes (NN9211) [Internet], 2015. Available from http://globenewswire.com/news-release/2015/08/24/762981/0/en/Novo-Nordisk-completes-second-and-final-phase-3a-trial-with-liraglutide-as-adjunct-therapy-to-insulin-for-people-with-type-1-diabetes-NN9211.html. Accessed 26 March 2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.