Abstract
OBJECTIVE
This study aimed to analyze the relationship of variability in hemoglobin A1c (HbA1c) over years with subsequent depressive symptoms.
RESEARCH DESIGN AND METHODS
Subjects (n = 837) were participants of the Israel Diabetes and Cognitive Decline (IDCD) study, which aimed to examine the relationship of characteristics of long-term type 2 diabetes with cognitive decline. All pertain to a diabetes registry established in 1998, which contains an average of 18 HbA1c measurements per subject. The results presented here are based on the IDCD baseline examination. Symptoms of depression were assessed using the 15-item version of the Geriatric Depression Scale (GDS). To quantify the association between variability in glycemic control (measured as the SD of HbA1c measurements [HbA1c-SD]) since 1998 with the number of depression symptoms at IDCD baseline, incidence rate ratios (IRRs) and corresponding 95% CIs were estimated via negative binomial regression modeling and used to account for the overdispersion in GDS scores.
RESULTS
Subjects’ ages averaged 72.74 years (SD 4.63 years), and the mean number of years in the diabetes registry was 8.7 (SD 2.64 years). The mean GDS score was 2.16 (SD 2.26); 10% of subjects had a GDS score ≥6, the cutoff for clinically significant depression. Mean HbA1c significantly correlated with HbA1c-SD (r = 0.6625; P < 0.0001). The SD, but not the mean, of HbA1c measurements was significantly associated with the number of subsequent depressive symptoms. For each additional 1% increase in HbA1c-SD, the number of depressive symptoms increased by a factor of 1.31 (IRR = 1.31 [95% CI 1.03–1.67]; P = 0.03).
CONCLUSIONS
Variability in glycemic control is associated with more depressive symptoms.
Introduction
Type 2 diabetes and depression both are highly prevalent among the elderly population and are associated with increased risk for morbidity and mortality. Major depression is a severely debilitating disease associated with significant burden and disability (1). Even subsyndromal depression, the most prevalent clinical presentation among the elderly (2), is associated with disability, functional limitations (3), and poorer psychiatric and functional longitudinal outcomes. The risk for depression is doubled in the presence of type 2 diabetes (4), which itself reaches a prevalence of 22–33% in those ≥65 years old (5). In turn, in the presence of depression, individuals with type 2 diabetes adhere less to medical treatments, have worse glycemic control, and show increased risk for diabetes-related complications (4). Optimal interventions for reducing depression in type 2 diabetes could therefore not only ameliorate depression but also contribute to better results in other type 2 diabetes–related outcomes.
Degree of glycemic control has a central role in preventing some type 2 diabetes–related complications (6) and may therefore be relevant for preventing depression. Compared with younger subjects with type 2 diabetes, however, the elderly are at increased risk for hypoglycemia and other adverse effects of antidiabetes treatments (7,8). Therefore, despite the high susceptibility of older individuals to type 2 diabetes complications (e.g., myocardial infarction, visual impairment, and renal disease [7]) compared with all other age groups, guidelines generally agree that the potential benefits of achieving tight glycemic control in this population should be weighed against the risk of hypoglycemia (7,8) and that antidiabetes treatment should be personalized. Treatment guidelines for depression or its prevention are lacking in the context of diabetes in this older and growing segment of the population (8). Development of such guidelines may be hampered by the apparent inconsistencies regarding the association of glycemic control and risk for depression (9). These inconsistencies may partly be driven by the cross-sectional design applied in many studies, in which depression and hemoglobin A1c (HbA1c), the gold standard measurement of glycemic control, are assessed simultaneously, thereby preventing an understanding of the true nature of the relationship between these factors or its directionality. Compared with a single HbA1c measurement or mean HbA1c, long-term variability in glycemic control, expressed as the SD of all HbA1c measurements (HbA1c-SD), better reflects changes in glycemic control over time and, accordingly, has been shown to be associated with disease complications in type 1 diabetes (10) and in type 2 diabetes (11,12). However, scarce information describes the relationship of HbA1c variability with depression.
In this report we analyze the relationship of long-term variability in HbA1c with subsequent depressive symptoms in elderly subjects with type 2 diabetes participating in the Israel Diabetes and Cognitive Decline (IDCD) study.
Research Design and Methods
The IDCD is a collaboration among the Icahn School of Medicine at Mount Sinai, NY, the Sheba Medical Center, Israel, and Maccabi Health Services (MHS), Israel. The study was approved by all three institutional review board committees.
Sample
This study consists of 1,288 elderly patients (≥65 years old) with type 2 diabetes who are engaged in the IDCD study, a longitudinal investigation assessing the relationship of long-term type 2 diabetes characteristics with cognitive decline and other outcomes of type 2 diabetes. The design and detailed methods have been published elsewhere (13). Briefly, subjects were randomly selected from the approximately 11,000 individuals with type 2 diabetes who are in the diabetes registry of MHS, the second largest health maintenance organization (HMO) in Israel. The MHS diabetes registry is an integral part of the MHS Electronic Patient Record system and was established in 1998 to facilitate disease management and to improve treatment. Criteria for addition to the registry are any of the following: 1) HbA1c >7.25%; 2) glucose >200 mg/dL on two exams more than 3 months apart; 3) purchase of antidiabetes medication twice within 3 months, supported by HbA1c >6.5% or glucose >125 mg/dL within half a year; or 4) diagnosis of type 2 diabetes (ICD-9 code) by a general practitioner, internist, endocrinologist, ophthalmologist, or type 2 diabetes advisor, supported by HbA1c >6.5% or glucose >125 mg/dL within half a year. These criteria have been validated by 20 physicians in MHS against their own practice records (14). In addition, age-specific prevalences were similar to those of a diabetes registry of another large HMO in Israel (14). The MHS diabetes registry collects detailed laboratory, medication, and medical diagnoses information of its subjects since 1998 or since the type 2 diabetes diagnosis (if after 1998) (14). Thus we have access to all data in the diabetes registry since 1998 (or since the type 2 diabetes diagnosis if after 1998) and until initiation of the IDCD study in 2009. This analysis presents relationships between historical data from the MHS and the baseline data of the IDCD. The longitudinal component of the IDCD is ongoing.
Eligibility Criteria for the IDCD Study
Subjects were eligible for the study if they were listed in the MHS diabetes registry; living in the central area of Israel; diagnosed with type 2 diabetes; aged ≥65 years; assessed as cognitively normal at baseline (based on a weekly multidisciplinary consensus conference); not suffering from major medical, psychiatric, or neurological conditions that affect cognitive performance; had three or more HbA1c measurements in the diabetes registry; spoke Hebrew fluently; and had an informant for the study. The latter criterion was implemented to ensure data were obtained regarding the existence of functional impairments secondary to cognitive changes and of changes in behavior.
Subject Recruitment Process
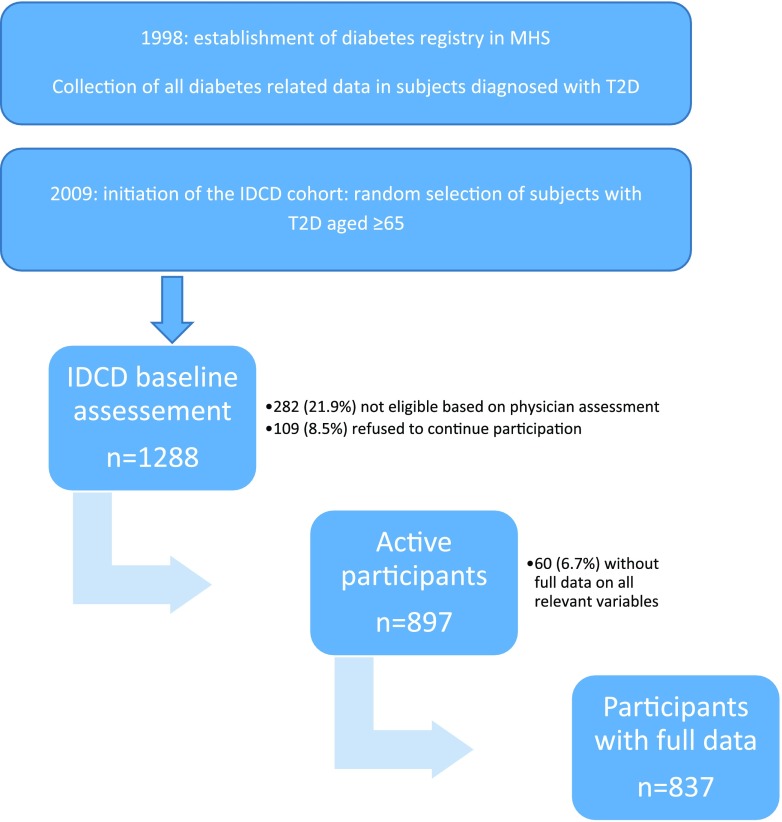
The electronic records for patients in the MHS diabetes registry were thoroughly reviewed to identify potential subjects (Fig. 1). Patients with an ICD-9 code for dementia or its subtypes, those treated with prescribed cholinesterase inhibitors, and those with a major psychiatric or neurological condition (such as schizophrenia or Parkinson disease) that could affect cognitive performance were excluded. Potential subjects were randomly selected, contacted by mail and then by phone, and, after determining that they were fluent in Hebrew and had a family member or caregiver who was willing to be an informant for the study, asked whether they were willing to participate. Those who were willing to participate in the study were assessed in two phases. First they were visited by a study physician who obtained signed informed consent; performed medical, neurological, and geriatric assessments; and drew blood for inflammatory markers (interleukin-6, C-reactive protein), and haptoglobin and apolipoprotein E genotypes. In the second phase (optimally within 2 weeks from the physician’s visit), the potential subjects were visited by a neuropsychologist who administered a comprehensive cognitive battery (described in detail elsewhere [15]) and administered questionnaires to the subject and informant to assess cognitive, mood, and functional impairment. All potential subjects’ cognitive data were discussed by a multidisciplinary team during consensus conferences in order to define the subjects’ cognitive status (as cognitively normal, mild cognitive impairment, or dementia and their subtypes). To be eligible, subjects had to be cognitively normal at baseline.
Figure 1.
Study flowchart. T2D, type 2 diabetes.
HbA1c
HbA1c values were extracted from the diabetes registry. The data on HbA1c are historical prospective data, that is, starting in 1998 or at the time of type 2 diabetes diagnosis (if after 1998) until the IDCD baseline assessment (2009). HbA1c was measured with standard methods of high-performance liquid chromatography using an ion exchange column. Participants were assessed under fasting conditions approximately annually at the MHS (the mean and median of two assessments per year, with the 25th percentile of one and the 75th percentile of three yearly assessments). Variability of glycemic control is defined in this study as the HbA1c-SD for each subject.
Symptoms of Depression
Symptoms of depression at entry into the IDCD study (2009) were assessed at the time of the cognitive assessment using the 15-item version of the Geriatric Depression Scale (GDS) (16), in which subjects answer a series of 15 questions regarding their recent mood. Higher scores represent more depressive symptoms.
Statistical Analyses
Sample characteristics are summarized as the mean (SD) and median (range) for continuous variables and number (percentage) for categorical variables. To quantify the association between the SD of HbA1c measures taken while in the IDCD registry (starting in 1998 or later, at the time of type 2 diabetes diagnosis, until IDCD baseline in 2009) and GDS scores at time of entry into the IDCD study (2009), incidence rate ratios (IRRs) and corresponding 95% CIs were estimated via negative binomial regression modeling. Negative binomial regression was used to account for the overdispersion of GDS scores. These models were adjusted for sociodemographic, cardiovascular, and diabetes-related covariates (described below). Examination of the relationship of HbA1c variability with depression is new, so to enable comparison with analyses of other cohorts, we applied six distinct models controlling for covariates similar to those used in previous studies that addressed the relationship of HbA1c variability with other diabetes-related complications (10,11). Model A was unadjusted; model B adjusted for age; model C also adjusted for number of years in the registry (17), years of education, and sex; model D additionally adjusted for HDL, LDL, and total cholesterol, systolic and diastolic blood pressures, glomerular filtration rate, and diabetes medication group; and model E also adjusted for mean HbA1c. Finally, in model F, the Mini-Mental State Examination (MMSE) total score was added to the model. Our primary model was model C (adjusting for age, number of years in the registry, years of education, and sex). However, because of the novelty of the associations of HbA1c variability with depression, we believe that including all models will deepen our understanding regarding the association between HbA1c variability and depression and the factors contributing to this relationship, thus stimulating a larger number of comparable replication studies.
All hypotheses testing was two-sided and conducted at the 5% level of significance. Statistical analyses were conducted using SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Description of the Sample
A total of 1,288 subjects completed the preliminary screening, expressed interest in participating, were approached by a study physician, and signed informed consent. Of them, 282 (21.1%) were excluded from the study because of incompatibility with eligibility criteria (primarily clinical impairment) and 109 (8.5%) refused to continue their participation in the study, so 897 subjects remained active participants. The analyses include the 837 subjects who had complete data on sociodemographic, cardiovascular, and diabetes-related covariates.
Table 1 describes the sample characteristics of the final 837 subjects included in the analysis. The mean age of the patients in the sample was 72.74 years (SD 4.63 years), and the mean number of years in the diabetes registry was 8.7 (SD 2.64 years). Mean number of HbA1c measurements per subject was 17.83 (SD 9.56). Mean MMSE score was 28.02 (SD 1.79), consistent with normal cognitive status, and mean GDS score was 2.16 (SD 2.26); 10% of subjects had a GDS score ≥6, the cutoff for clinically significant depression. Most subjects (87%) were treated with antidiabetes medications: oral medications alone, insulin alone, or a combination of both. Other demographic and health-related characteristics are presented in Table 1.
Table 1.
Characteristics of the sample (n = 837) at IDCD baseline
| Mean (SD) or n (%) | Median (min–max) | |
|---|---|---|
| HbA1c-SD (%) | 0.52 (0.38) | 0.40 (0.04–3.32) |
| Mean HbA1c (%) | 6.82 (0.77) | 6.70 (4.88–10.14) |
| HbA1c measures (n) | 17.83 (9.56) | 16 (2–60) |
| Years in the diabetes registry | 8.7 (2.64) | 9.89 (0.99–15.07) |
| Age (years) | 72.74 (4.63) | 72 (66–88) |
| Years of education | 13.17 (3.45) | 12 (0–26) |
| Mean HDL (mg/dL) | 47.82 (10.93) | 45.89 (25.04–104.6) |
| Mean LDL (mg/dL) | 101.43 (19.88) | 101.02 (24.7–169.41) |
| Mean cholesterol (mg/dL) | 180.57 (25.18) | 179.06 (93.93–264.97) |
| Systolic BP (mmHg) | 134.74 (9.38) | 134.20 (103.09–170.25) |
| Diastolic BP (mmHg) | 77 (4.85) | 77.21 (56.06–96.19) |
| GFR (mL/min/1.73 m2) | 81 (26.26) | 78.54 (13.51–185.11) |
| GDS | 2.16 (2.26) | 1 (0–14) |
| MMSE total score | 28.02 (1.79) | 28 (13–30) |
| Female sex | 501 (60%) | |
| Diabetes medications | ||
| Hypoglycemic only | 652 (78%) | |
| Insulin only | 8 (1%) | |
| Insulin + hypoglycemic | 71 (8%) | |
| None | 106 (13%) | |
| GDS score | ||
| 0 | 219 (26%) | |
| 1 | 200 (24%) | |
| 2 | 145 (17%) | |
| 3 | 82 (10%) | |
| 4 | 72 (8%) | |
| 5 | 42 (5%) | |
| 6 | 31 (4%) | |
| 7 | 15 (2%) | |
| 8 | 14 (2%) | |
| ≥9 | 17 (2%) |
BP, blood pressure; GFR, glomerular filtration rate.
Relationship of Mean HbA1c and HbA1c-SD Since 1998 (or Since Time of Entry into the Diabetes Registry) and Number of Depressive Symptoms at IDCD Baseline (2009)
Mean HbA1c was significantly correlated with HbA1c-SD (r = 0.6625; P < 0.0001). Mean HbA1c was not associated with the number of depressive symptoms in any of the statistical models (IRR 0.93 [95% CI 0.81–1.06]; P = 0.26, for the fully adjusted model F). By contrast, greater HbA1c-SD was significantly associated with a larger number of depressive symptoms in all statistical models, such that for each additional 1% increase in HbA1c-SD, the number of depressive symptoms increased by a factor of 1.29, representing a 29% increase (IRR 1.29 [95% CI 1.03–1.55]; P = 0.0078) in the basic model adjusting for age, number of years in the diabetes registry, years of education, and sex (model C; Table 2). In the fully adjusted model (model F), for each additional 1% increase in HbA1c-SD, the number of depressive symptoms increased by a factor of 1.31, representing a 31% increase (IRR 1.31 [95% CI 1.03–1.67]; P = 0.03). Table 2 presents IRRs for the unadjusted and partially adjusted statistical models.
Table 2.
Association between variability in HbA1c and GDS
| HbA1c-SD | IRR (95% CI) | P value |
|---|---|---|
| Overall (n = 837) | ||
| Model A | 1.20 (1.001–1.44) | 0.0485* |
| Model B | 1.22 (1.02–1.47) | 0.0285* |
| Model C | 1.29 (1.07–1.55) | 0.0078* |
| Model D | 1.24 (1.02–1.51) | 0.0318* |
| Model E | 1.33 (1.04–1.70) | 0.0230* |
| Model F | 1.31 (1.03–1.67) | 0.0273* |
| Sample stratified by mean HbA1c ≤7 (n = 555) | ||
| Model A | 1.17 (0.82–1.67) | 0.3939 |
| Model B | 1.18 (0.82–1.68) | 0.3728 |
| Model C | 1.31 (0.90–1.89) | 0.1550 |
| Model D | 1.26 (0.86–1.83) | 0.2341 |
| Model E | 1.30 (0.87–1.93) | 0.1979 |
| Model F | 1.30 (0.88–1.92) | 0.1943 |
| Sample stratified by mean HbA1c >7 (n = 282) | ||
| Model A | 1.22 (0.93–1.61) | 0.1516 |
| Model B | 1.30 (0.98–1.71) | 0.0650 |
| Model C | 1.41 (1.07–1.86) | 0.0139* |
| Model D | 1.40 (1.06–1.86) | 0.0185* |
| Model E | 1.41 (1.02–1.94) | 0.0351* |
| Model F | 1.41 (1.03–1.93) | 0.0331* |
IRR (95% CI) data were estimated using a negative binomial regression modeling score for the entire sample (n = 837) and stratified by mean HbA1c. Model A was unadjusted; model B was adjusted for age; model C was adjusted for age, years in the registry, years of education, and sex; model D was adjusted for age, years in the registry, years of education, sex, total cholesterol, systolic blood pressure, diastolic blood pressure, glomerular filtration rate (GFR), HDL, LDL, and diabetes medication group; model E was adjusted for age, years in the registry, years of education, sex, total cholesterol, systolic blood pressure, diastolic blood pressure, GFR, diabetes medication group, HDL, LDL, and mean HbA1c; and model F was adjusted for age, years in the registry, years of education, sex, total cholesterol, systolic blood pressure, diastolic blood pressure, GFR, diabetes medication group, mean HbA1c, HDL, LDL, and MMSE total score.
*Indicates statistical significance.
Clinical trials have demonstrated that HbA1c ≤7% is associated with reduced risk for development and progression of type 2 diabetes–related microvascular complications compared with HbA1c >7%, and thus is considered a reasonable treatment goal (18). To facilitate translation of the current results to the clinical setting, we repeated the analyses, stratifying the sample by HbA1c above and below 7%, the HbA1c target for most patients with type 2 diabetes (19). The relationship of HbA1c-SD with depressive symptoms had the same direction in both groups (i.e., higher variability was associated with more depression symptoms) but was stronger in the group with HbA1c >7% in all statistical models (Table 2).
During the first year after type 2 diabetes diagnosis, high variability in HbA1c may reflect treatment initiation and decreased glucose levels in the blood thereafter; therefore, we repeated the analysis excluding HbA1c values from the first year after entry into the diabetes registry. The results remained essentially unchanged in that higher HbA1c-SD was significantly associated with a larger number of depressive symptoms in statistical models 1–4 (Table 3).
Table 3.
Association of variability in HbA1c and GDS score, excluding HbA1c values from the first year after entry into the diabetes registry
| Model | Mean HbA1c-SD |
IRR (95% CI) | P value | |
|---|---|---|---|---|
| 1 | 2 | |||
| 0 | 2.29 | 2.61 | 1.14 (0.96–1.35) | 0.1293 |
| 1 | 2.15 | 2.61 | 1.21 (1.02–1.45) | 0.0321 |
| 2 | 2.09 | 2.48 | 1.18 (0.99–1.42) | 0.0633 |
| 3 | 3.26 | 3.91 | 1.20 (1.01–1.43) | 0.0422 |
| 4 | 3.26 | 3.89 | 1.19 (0.98–1.45) | 0.0811 |
Model 0 was unadjusted; model 1 was adjusted for age, years in registry, years of education, and sex; model 2 was adjusted for age, years in registry, years of education, sex, total cholesterol, systolic blood pressure, diastolic blood pressure, and MMSE score; model 3 was adjusted for age, years in registry, years of education, sex, total cholesterol, systolic blood pressure, diastolic blood pressure, MMSE score, and depression status at baseline; and model 4 was adjusted for age, years in registry, years of education, sex, total cholesterol, systolic blood pressure, diastolic blood pressure, MMSE score, depression status at baseline, and mean HbA1c.
The primary goal of the study was to examine whether HbA1c variability predicts depressive symptoms. To rule out the possibility of reverse causality, that is, that depression predicts HbA1c variability (20), we examined the relationship of depression at entry into the diabetes registry with variability in HbA1c thereafter. The GDS, the primary depression questionnaire used by the IDCD study, is not used by MHS. Thus we exploited from the diabetes registry data related to depression within ≤1 year of entry into the diabetes registry (1998 or the time of type 2 diabetes diagnosis if after 1998) to predict variability in HbA1c. We operationalized depression diagnosis based on ICD-9 codes for depression or treatment with antidepressants within ≤1 year of entry into the diabetes registry (data relating to HbA1c values from the first year after entry into the diabetes registry were not included in the analysis). In all models adjusting for demographic, health-related, and cognitive factors, the association between depression at entry into the diabetes registry and subsequent HbA1c-SD was not significant (Table 4).
Table 4.
Association of depression* at entry into the diabetes registry with variability in HbA1c thereafter**
| Model† | No baseline depression | Baseline depression | Ratio of geometric means (95% CI) | P value |
|---|---|---|---|---|
| 0 | 0.41 | 0.28 | 1.50 (1.28–1.76) | <0.0001 |
| 1 | 0.40 | 0.35 | 1.14 (0.98–1.32) | 0.0947 |
| 2 | 0.40 | 0.35 | 1.13 (0.97–1.31) | 0.1038 |
| 3 | 0.39 | 0.38 | 1.05 (0.91–1.20) | 0.5111 |
*Defined as a depression diagnosis or antidepressant medication prescription within 1 year of entry into the diabetes registry.
**HbA1c-SD measurements following 1 year of entry to registry until IDCD baseline assessment (2009).
†Model 0 was unadjusted; model 1 was adjusted for age, years in the diabetes registry, years of education, and sex; model 2 was adjusted for age, years in the diabetes registry, years of education, sex, cholesterol, systolic blood pressure, diastolic blood pressure, and MMSE score; and model 3 was adjusted for age, years in the diabetes registry, years of education, sex, cholesterol, systolic blood pressure, diastolic blood pressure, MMSE score, and mean HbA1c.
Conclusions
The results of this study demonstrate that variability in HbA1c over time (mean 8.7 years) is associated with the subsequent number of depressive symptoms in elderly subjects with type 2 diabetes. This relationship is particularly relevant for subjects with poor glycemic control (mean HbA1c >7%). We cannot rule out that the opportunity for detecting significance in this group was greater, because higher mean levels were associated with greater SD and the group with mean HbA1c >7% was substantially larger. Results were not affected by adjustment for sociodemographic, type 2 diabetes, and health-related characteristics that may be associated with depression, nor by adjustment for a global cognitive score. Larger variability in HbA1c was associated with higher mean HbA1c; nevertheless, we found no correlation between mean HbA1c and the number of depression symptoms. To exclude the potential contribution of variability in HbA1c close to type 2 diabetes diagnosis—when adequate glycemic control is being instantiated—to the overall HbA1c variability over years, we repeated the analyses, excluding HbA1c measurements from the first year after the type 2 diabetes diagnosis. Results remained mostly unchanged. Though long-term effects of initially uncontrolled diabetes on brain biology and mood cannot be ruled out, our results suggest that long-term variability in glycemic control, rather than initial variability after type 2 diabetes diagnosis, is associated with symptoms of depression. These results stress the potential value of HbA1c variability, which may better reflect the course of type 2diabetes over time, in predicting depression.
The directionality of the association of type 2 diabetes with depression is not clear, and some view it as bidirectional (21); most studies suggest that prediabetes states and HbA1c predict depression at follow-up, including in subjects who were not depressed at baseline (22). However, in subjects already suffering from type 2 diabetes, lack of positive affect has also been associated with future higher levels of HbA1c (23). Within type 2 diabetes, the relationship of glycemic control (a core contributor to other type 2 diabetes–related complications [6]) with depression, and the directionality of this relationship, remains to be elucidated. In this study we considered reverse causality, that is, that depression at the time of entry into the diabetes registry (1998 or at time of type 2 diabetes diagnosis if later than 1998) may be associated with subsequent variability in HbA1c thereafter, but such a relationship was not detected. Although, in the context of an HMO, defining depression based on an ICD-9 code or on prescription of antidepressant medications may underestimate its true prevalence, our results provide evidence for a possible causal path from poor glycemic control to a higher risk for depression, rather than the opposite.
Previous studies have shown that poor glycemic control at baseline was associated with increased risk for the presence (9,24) and persistence of depressive symptoms (25) at follow-up in old community-dwelling subjects. One cross-sectional study demonstrated a positive association between poor glycemic control and anxiety, but not depression scores, in a sample including both patients with type 1 and patients with type 2 diabetes, with a wide age range (20–75 years) (26). In summary, previous studies generally demonstrated that worse glycemic control is associated with a higher risk for incident depression; however, there is still no consensus regarding the nature of the emotional outcome and the characteristics of the population at risk. Differences in methodologies used to measure glycemic control and depression, as well as differences in study design (cross-sectional vs. longitudinal), may explain, at least partially, the inconsistent findings regarding the relationship of glycemic control with depression.
Mean HbA1c is recognized as a predictor of some diabetes-related complications (11); however, HbA1c variability, independent of mean HbA1c levels, has been shown to be positively associated with micro- and macrovascular complications and mortality in diabetes (27), and to outweigh the predictive value of the mean for cardiovascular disease (11,28,29) and retinopathy (11,12,30). The relationship of HbA1c variability and brain-related outcomes has been demonstrated in elderly patients with type 2 diabetes, in whom higher HbA1c variability was significantly associated with poorer cognitive function, even after adjusting for mean HbA1c values (31).
Late-life depression is strongly associated with cognitive status and may result from common underlying mechanisms (32). Nevertheless, in this study, the relationship of HbA1c variability with depression was unaltered after adjusting for cognitive performance, reflecting the robustness of the relationship.
Several mechanisms may explain the relationship of glycemic control with depression. Periods of sustained hyperglycemia have detrimental long-term effects and therefore, even if HbA1c levels are lower thereafter (a process that would affect HbA1c variability), episodes of uncontrolled type 2 diabetes, expressed as increased variability in HbA1c, carry a high risk for long-term complications (33). The risk for microvascular complications of type 2 diabetes increases exponentially rather than linearly with increasing HbA1c (34,35). Vascular pathology, in turn, has been proposed as an underlying mechanism for vascular depression, which is highly prevalent in late-life diabetes (36). In addition, high glucose levels, which require more aggressive treatment regimens, specifically insulin injections, may also be more strongly associated with adverse psychological states and with increased type 2 diabetes disease burden and distress (37). In this study, however, adjustment for type of antidiabetes treatment did not affect the results. The population participating in our study comprised elderly subjects. Older brains, specifically areas involved in depression, may be most vulnerable to the effects of worse glycemic control, as demonstrated in a study examining the association of fasting serum glucose with cerebral glucose metabolism, a measure of neural circuity (assessed with fluorodeoxyglucose positron emission tomography), whereby increasing fasting plasma glucose levels were associated with lower cerebral glucose metabolism in frontal and parietal association cortices in normal elderly and even more so in elderly subjects with late-life depression, but not in young controls (38).
The elderly are also at higher risk for the detrimental effects of hypoglycemic episodes on the brain, as demonstrated by the increased risk for cognitive decline and dementia in the elderly with type 2 diabetes who experience episodes of severe hypoglycemia (39). The mechanism underlying this relationship is unclear; some reports suggest neuronal death and changes in brain structure (40). Such effects could also lead to depression. In this regard, in contrast to mean HbA1c, variability of glycemic control better reflects the presence of larger HbA1c fluctuations over time from hyper- and hypoglycemia.
Strengths of this study include the large sample; validated type 2 diabetes diagnoses for each subject; an average of 18 HbA1c measurements; strong validity for risk factor levels and medical diagnosis; and a thorough cognitive evaluation, permitting the verification of intact cognition at the time of GDS assessment and examining overall cognition as a potential confounder.
This study is observational, and at this point only cross-sectional cognitive data are available, preventing conclusions regarding the causality and directionality of the association between variability in glycemic control and symptoms of depression. Depression diagnosis at entry into the diabetes registry was not associated with subsequent variability in HbA1c. However, we cannot rule out the possibility that subsyndromal symptoms of depression that were not captured by ICD-9 codes for depression or by the use of antidepressant treatments lead to more erratic self-management of type 2 diabetes via poor lifestyle characteristics and worse adherence to medications, factors that we could not control for in this study. Brain imaging was not available, thus limiting our ability to evaluate the contribution of brain volume and vasculature to the association of variability in HbA1c with depression score. We used time since entry into the diabetes registry, rather than time of type 2 diabetes diagnosis, as a measure of diabetes duration. For those who entered the diabetes registry at the time of its establishment, in 1998, we have no access to data regarding duration of diabetes prior to 1998. In addition, the definition of depression outcome in the study is based on the GDS score rather than clinical diagnosis. A relatively small number of subjects were treated with insulin (9%), possibly limiting our ability to examine the role of this treatment in the relationship of HbA1c variability and symptoms of depression. Finally, we do not have information on episodes of severe hypoglycemia, which may substantially contribute to variability in HbA1c and may be associated with depression (41), thereby potentially affecting the relationship found in this study.
In conclusion, these results indicate that higher variability in glycemic control is associated with more depressive symptoms, suggesting that long-term stability of glycemic control may help prevent depressive symptoms in elderly individuals with type 2 diabetes.
Article Information
Funding. This study was supported by the National Institute on Aging (grants R01 AG034087 [to M.S.B.] and P50 AG05138 [to M.S.]), and the Helen Bader Foundation, the Irma T. Hirschl Scholar Award, and the Leroy Schecter Foundation Award (to M.S.B.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Authors Contributions. R.R.-S. and M.S.B. designed the study, researched and collected data, and wrote the manuscript. A.H. researched data and contributed to the discussion. J.S., E.G.-B., and L.S. reviewed the manuscript. E.M. performed statistical analysis and reviewed the manuscript. M.S., D.L., and J.M.S. reviewed the manuscript and contributed to the discussion. R.P. and R.T. researched the data. R.R.-S., A.H., and M.S.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.Chisholm D, Sanderson K, Ayuso-Mateos JL, Saxena S. Reducing the global burden of depression: population-level analysis of intervention cost-effectiveness in 14 world regions. Br J Psychiatry 2004;184:393–403 [DOI] [PubMed] [Google Scholar]
- 2.Goldney RD, Fisher LJ, Dal Grande E, Taylor AW. Subsyndromal depression: prevalence, use of health services and quality of life in an Australian population. Soc Psychiatry Psychiatr Epidemiol 2004;39:293–298 [DOI] [PubMed] [Google Scholar]
- 3.Lyness JM, Kim J, Tang W, et al. The clinical significance of subsyndromal depression in older primary care patients. Am J Geriatr Psychiatry 2007;15:214–223 [DOI] [PubMed] [Google Scholar]
- 4.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Number (in millions) of civilian, noninstitutionalized persons with diagnosed diabetes, United States, 1980–2014. Diabetes is becoming more common in the United States. From 1980 through 2014, the number of Americans with diagnosed diabetes has increased fourfold (from 5.5 million to 22.0 million) [Internet]. Available at https://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm. Accessed 13 June 2017
- 6.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghissi E. Management of type 2 diabetes mellitus in older patients: current and emerging treatment options. Diabetes Ther 2013;4:239–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbatecola AM, Paolisso G, Sinclair AJ. Treating diabetes mellitus in older and oldest old patients. Curr Pharm Des 2015;21:1665–1671 [DOI] [PubMed] [Google Scholar]
- 9.Hamer M, Batty GD, Kivimaki M. Haemoglobin A1c, fasting glucose and future risk of elevated depressive symptoms over 2 years of follow-up in the English Longitudinal Study of Ageing. Psychol Med 2011;41:1889–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care 2008;31:2198–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penno G, Solini A, Zoppini G, et al.; Renal Insufficiency and Cardiovascular Events (RIACE) Study Group . Hemoglobin A1c variability as an independent correlate of cardiovascular disease in patients with type 2 diabetes: a cross-sectional analysis of the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Cardiovasc Diabetol 2013;12:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penno G, Solini A, Bonora E, et al.; Renal Insufficiency And Cardiovascular Events Study Group . HbA1c variability as an independent correlate of nephropathy, but not retinopathy, in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care 2013;36:2301–2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravona-Springer R, Heyman A, Schmeidler J, et al. Haptoglobin 1-1 genotype is associated with poorer cognitive functioning in the elderly with type 2 diabetes. Diabetes Care 2013;36:3139–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heymann AD, Chodick G, Halkin H, et al. The implementation of managed care for diabetes using medical informatics in a large preferred provider organization. Diabetes Res Clin Pract 2006;71:290–298 [DOI] [PubMed] [Google Scholar]
- 15.Beeri MS, Ravona-Springer R, Moshier E, et al. The Israel Diabetes and Cognitive Decline (IDCD) study: design and baseline characteristics. Alzheimers Dement 2014;10:769–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999;14:858–865 [DOI] [PubMed] [Google Scholar]
- 17.West RK, Ravona-Springer R, Schmeidler J, et al. The association of duration of type 2 diabetes with cognitive performance is modulated by long-term glycemic control. Am J Geriatr Psychiatry 2014;22:1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbatecola AM, Paolisso G. Diabetes care targets in older persons. Diabetes Res Clin Pract 2009;86(Suppl 1):S35–S40 [DOI] [PubMed] [Google Scholar]
- 19.Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA 2014;311:2315–2325 [DOI] [PubMed] [Google Scholar]
- 20.Chen PC, Chan YT, Chen HF, Ko MC, Li CY. Population-based cohort analyses of the bidirectional relationship between type 2 diabetes and depression. Diabetes Care 2013;36:376–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan A, Lucas M, Sun Q, et al. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med 2010;170:1884–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mast BT, Miles T, Penninx BW, et al. Vascular disease and future risk of depressive symptomatology in older adults: findings from the Health, Aging, and Body Composition Study. Biol Psychiatry 2008;64:320–326 [DOI] [PubMed] [Google Scholar]
- 23.Nefs G, Pouwer F, Denollet J, Kramer H, Wijnands-van Gent CJ, Pop VJ. Suboptimal glycemic control in type 2 diabetes: a key role for anhedonia? J Psychiatr Res 2012;46:549–554 [DOI] [PubMed] [Google Scholar]
- 24.Maraldi C, Volpato S, Penninx BW, et al. Diabetes mellitus, glycemic control, and incident depressive symptoms among 70- to 79-year-old persons: the Health, Aging, and Body Composition Study. Arch Intern Med 2007;167:1137–1144 [DOI] [PubMed] [Google Scholar]
- 25.Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with type 2 diabetes. Diabet Med 2008;25:1096–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins MM, Corcoran P, Perry IJ. Anxiety and depression symptoms in patients with diabetes. Diabet Med 2009;26:153–161 [DOI] [PubMed] [Google Scholar]
- 27.Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 2015;38:2354–2369 [DOI] [PubMed] [Google Scholar]
- 28.Prince CT, Becker DJ, Costacou T, Miller RG, Orchard TJ. Changes in glycaemic control and risk of coronary artery disease in type 1 diabetes mellitus: findings from the Pittsburgh Epidemiology of Diabetes Complications Study (EDC). Diabetologia 2007;50:2280–2288 [DOI] [PubMed] [Google Scholar]
- 29.Yang HK, Kang B, Lee SH, et al. Association between hemoglobin A1c variability and subclinical coronary atherosclerosis in subjects with type 2 diabetes. J Diabetes Complications 2015;29:776–782 [DOI] [PubMed] [Google Scholar]
- 30.Hsu CR, Chen YT, Sheu WH. Glycemic variability and diabetes retinopathy: a missing link. J Diabetes Complications 2015;29:302–306 [DOI] [PubMed] [Google Scholar]
- 31.Kim C, Sohn JH, Jang MU, et al. Association between visit-to-visit glucose variability and cognitive function in aged type 2 diabetic patients: a cross-sectional study. PLoS One 2015;10:e0132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebedeva A, Borza T, Håberg AK, et al. Neuroanatomical correlates of late-life depression and associated cognitive changes. Neurobiol Aging 2015;36:3090–3099 [DOI] [PubMed] [Google Scholar]
- 33.Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002;287:2563–2569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manschot SM, Biessels GJ, de Valk H, et al.; Utrecht Diabetic Encephalopathy Study Group . Metabolic and vascular determinants of impaired cognitive performance and abnormalities on brain magnetic resonance imaging in patients with type 2 diabetes. Diabetologia 2007;50:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos M, Kövari E, Hof PR, Gold G, Bouras C, Giannakopoulos P. The impact of vascular burden on late-life depression. Brain Res Brain Res Rev 2009;62:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aikens JE, Perkins DW, Piette JD, Lipton B. Association between depression and concurrent type 2 diabetes outcomes varies by diabetes regimen. Diabet Med 2008;25:1324–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marano CM, Workman CI, Lyman CH, et al. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res 2014;222:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinkohl I, Aung PP, Keller M, et al.; Edinburgh Type 2 Diabetes Study (ET2DS) Investigators . Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care 2014;37:507–515 [DOI] [PubMed] [Google Scholar]
- 40.Northam EA, Lin A. Hypoglycaemia in childhood onset type 1 diabetes--part villain, but not the only one. Pediatr Diabetes 2010;11:134–141 [DOI] [PubMed] [Google Scholar]
- 41.Pathak RD, Schroeder EB, Seaquist ER, et al.; SUPREME-DM Study Group . Severe hypoglycemia requiring medical intervention in a large cohort of adults with diabetes receiving care in U.S. integrated health care delivery systems: 2005–2011. Diabetes Care 2016;39:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]