Abstract
OBJECTIVE
The purpose of this study was to rigorously explore psychosocial factors associated with automated insulin delivery systems among people living with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Across four sites in the U.S. and U.K., 284 participants completed structured interviews or focus groups on expectations, desired features, potential benefits, and perceived burdens of automated insulin delivery systems. Recorded audio files were transcribed and analyzed using NVivo.
RESULTS
Three themes were identified as critical for uptake of automated insulin delivery: considerations of trust and control, system features, and concerns and barriers to adoption. Children and adolescents with type 1 diabetes primarily identified needs specific to their life stage and social contexts (e.g., school). Adults with type 1 diabetes, parents of youth with type 1 diabetes, and partners of adults with type 1 diabetes were most concerned about the accuracy, adaptability, and algorithm quality alongside expectations that systems stabilize glucose levels and reduce risk for long-term complications.
CONCLUSIONS
Incorporating stakeholder perspectives on use of automated insulin delivery systems will improve the adoption of devices, quality of life, and likelihood of optimal health. Efforts to build trust in systems, optimize user-system interactions, and provide clear guidance about device capabilities and limitations may help potential users achieve optimal glycemic outcomes.
Introduction
Automated insulin delivery systems represent an innovation that will change the landscape of diabetes self-management by combining three previously distinct elements of diabetes care: single (insulin) or dual (insulin and glucagon) hormone delivery via pump(s), continuous glucose monitoring (CGM), and predictive algorithms that use glucose data to generate automated decisions about hormone timing and doses. In the U.S., 40–62% of adults with type 1 diabetes use pump therapy and ∼21% of individuals above age 25 years use CGM, with rates of CGM use increasing as device performance has improved and nonadjunctive indication has been realized (1–6). Thus, there are ready users and others with type 1 diabetes not using diabetes devices who may opt for automated insulin delivery if there are glycemic and quality of life benefits. However, people with type 1 diabetes who initiate technologically advanced alternatives or supplements to traditional insulin injections frequently report discontinuous or interrupted use of these devices (1–5). Automated insulin delivery systems represent a promising frontier in the management of type 1 diabetes; this study provides preliminary data to help researchers and clinicians understand and address the lack of interest in technological advancements as well as barriers to initiating and maintaining consistent use of such systems (7).
Seeking to optimize benefits for automated insulin delivery system users and resources for regulatory bodies and technology developers, our research sought to translate the experiences and expectations of selected patient groups into tailored measures to help assess type 1 diabetes management experiences in using these systems. As part of a multisite study to identify and describe perspectives on psychosocial factors relevant to automated insulin delivery from various stakeholders, researchers used qualitative research methods to understand patient and family expectations, attitudes, emotions, and experiences with automated insulin delivery. The purpose of the study was to deliver experience-based findings to inform medical device regulatory reviews and the development of validated psychosocial measures for use during imminent clinical trials by various device developers.
Research Design and Methods
Qualitative methods were selected to capture a broad range of factors describing how diabetes management devices fit into the lives of people with diabetes (PWD) and why diabetes management devices might be initiated, continued, or discontinued. Qualitative methods are most appropriate for developing more complete understandings of phenomena through depth as opposed to breadth and for generating new measures (8). The goal of this research was to identify and explore topics related to interest in, uptake of, sustained use of, discontinuance of, and concerns about automated insulin delivery systems. The depth offered by this type of study seemed most appropriate for designing new survey measures to accompany the rise of automated insulin delivery systems. Findings will also be used to help communicate realistic expectations about emerging systems to potential users. Interviews were conducted January 2015 to December 2015, and data were analyzed December 2015 to April 2016.
Sampling and Recruitment
In order to learn from a variety of experiences and capitalize on existing research efforts, this study was implemented by four research teams in the U.S. (Joslin Diabetes Center, Lurie Children’s Hospital, and Stanford Children’s Health) and the U.K. (Bournemouth University). Research teams included psychologists, diabetes physicians, nurses, diabetes educators, and representatives from diabetes advocacy and family support organizations. Five patient populations were sampled: children, adolescents, and adults diagnosed with type 1 diabetes, as well as parents and partners of PWD. Participants were recruited using existing type 1 diabetes research networks and infrastructure (e.g., clinics, clinical trials, and support groups) with access to PWD with and without automated insulin delivery knowledge or experience. Across all four sites, every effort was made to recruit diverse participants with respect to socioeconomic background, sex, duration of diabetes, and technology use. In addition, we attempted to enroll equal numbers of participants based on the five patient populations sampled. Common recruitment strategies across sites included advertisements on social media and posted flyers at pediatric and adult clinics where participants could self-select by responding to the advertisement. Two sites offered either semistructured interviews or focus groups (Stanford Children’s Health and Bournemouth University). At Stanford Children’s Health, patients were assigned to the interview modality that worked best in their schedules, and at Bournemouth University, patients were given the choice between individual and focus groups based on personal preference. Joslin Diabetes Center only offered semistructured interviews, whereas Lurie Children’s Hospital offered only focus groups; at both sites, researchers used electronic medical records to identify eligible patients, who were later approached in clinic, with the exception of the adults recruited at Lurie Children’s Hospital, where they only used social media and posted flyers in the hospital. Parents and partners were recruited through the PWD. Research protocols were approved by ethical review boards at each site, and participants received $100 at U.S. sites and £20 at the U.K. site for their participation. Prior to their participation, adults and parents provided consent for study participation; youth additionally provided assent.
Data Collection and Analysis Plan
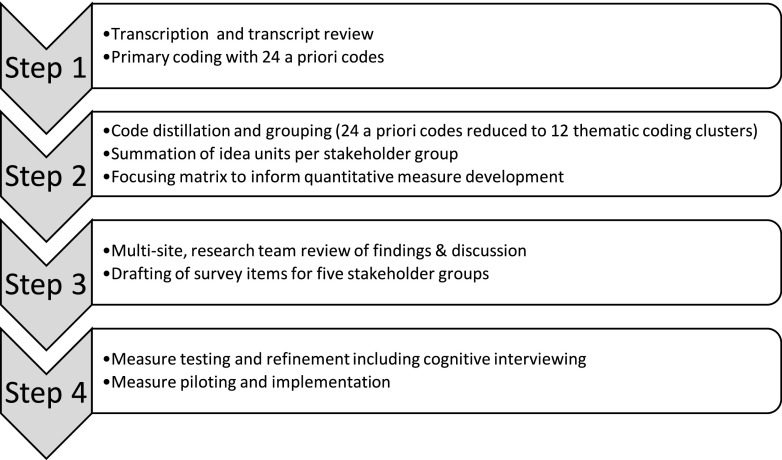
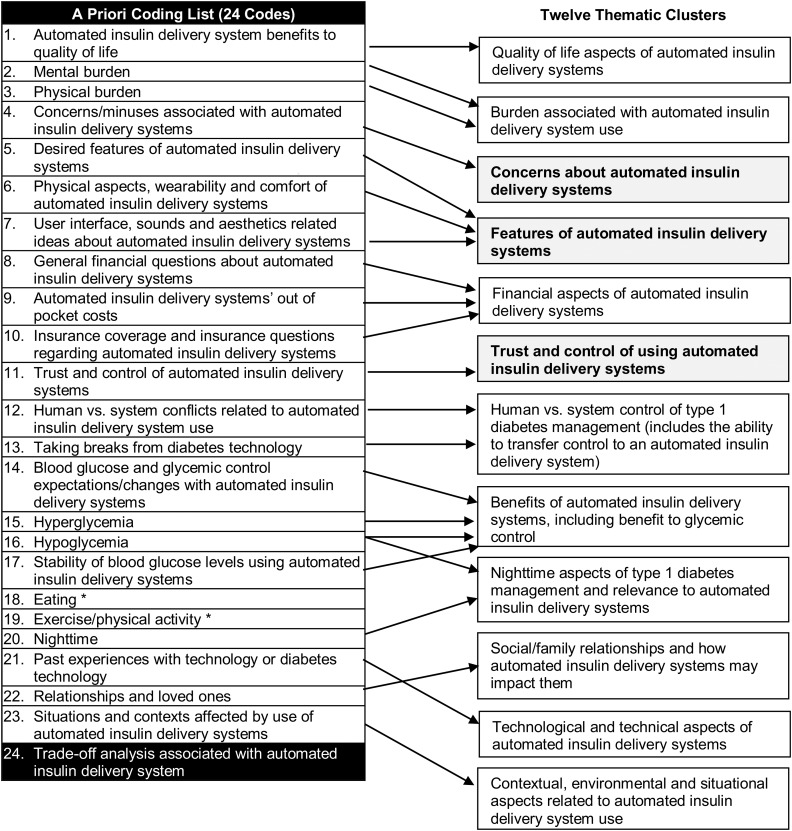
Interview and focus group questions explored the following themes: expectations, hopes and anxieties, perceived benefits and barriers, impact on daily function, self-management, health care interactions, and costs associated with automated insulin delivery and considerations of comfort, wearability, and user-technology interfaces. Sample questions from research discussions included the following: 1) “What would be some of the tasks that would be involved in using an automated insulin delivery system?,” 2) “What are some of the possible benefits from the system?,” 3) “What are your expectations about what the system might do?,” 4) “Are there any aspects of automated insulin delivery systems that you think might hurt your diabetes management or worry you?,” 5) “Are there particular times of day or situations when you might find an automated insulin delivery system particularly useful?,” and 6) “What would stop you from wanting to try or use one of these systems or what might get in the way?” Data on experiences and expectations of both single hormone and dual hormone systems were collected; we refer to both types of systems as automated insulin delivery systems for simplicity. Findings were intended to help develop empirically grounded, self-administered quantitative measures to assess characteristics associated with effective initiation and use of automated insulin delivery systems across age groups and family stakeholders. Investigators used hypotheses about themes that might emerge to identify 24 codes prior to data analysis (Fig. 1, step 1, and Fig. 2).
Figure 1.
The data analysis and subsequent measure development procedures implemented for this study. Four steps and nine substeps have been identified in the sequential list.
Figure 2.
The condensing of 24 investigator-identified a priori codes into 12 thematic clusters deemed most salient by a multistep, qualitative analysis and consensus process. *Eating and exercise/physical activity were related to several clusters. Trade-off analysis (#24) coding was used for other analytical purposes and is not represented in the 12 clusters here. Clusters discussed in this manuscript are in bold print.
Focus groups and interviews were led by facilitators who received advanced training on conducting mixed-methods research. Selected demographic data and notes on the interview/focus group sessions were recorded by facilitators. Data analysis and measure development procedures were divided into four steps (summarized in Fig. 1). In step 1, all focus groups and interviews were audiorecorded and audio data were transcribed by an independent, Health Insurance Portability and Accountability Act (HIPAA)-compliant service provider (Medikin, New York, NY). Transcripts were reviewed, de-identified, and uploaded to NVivo software version 11.2 for analysis (QSR International Pty Ltd.) (9).
Next, transcripts were analyzed using thematic analysis based around the 24 codes identified by investigators a priori. Thematic analysis offers a flexible method for identifying, analyzing, and presenting patterns across qualitative research methods (10). Beyond “giving voice” to study participants, our thematic analysis acknowledged the expertise and experiences of our researchers (10).
Over 9 weeks, nine coders representing all four sites reviewed and assigned codes to data from 137 transcripts consisting of >3,500 pages of data. Codes are words or phrases that succinctly capture what a segment of text is about. The coding process consisted of multiple steps designed to support the replicable identification of themes and quality monitoring of data and analytic products. A qualitative data coordinator worked with site teams to ensure that initial coding combined a priori codes as well as emergent codes identified by coders as they read and analyzed transcripts. One transcript was coded by all coders and discussed using a consensus process to build similar understandings of concepts and themes before coders were assigned groups of transcripts for independent coding. Transcripts were assigned to coders by the qualitative data coordinator based on transcript length, transcript variety, and coder availability. Coders met weekly via HIPAA-compliant BlueJeans Video Conferencing to discuss criteria used in selection and nonselection of codes as well as challenges encountered when analyzing transcripts. At each stage of analysis, coders were able to highlight memorable quotations and outliers or recommend alternative language or interpretations of data. Emergent codes were grouped into clusters with related a priori codes or set aside for future analyses. Attitudes toward technology in society, rather than diabetes technology in particular, is one example of an emergent code; some data relevant to this code were captured under the thematic cluster named “technological and technical aspects of automated insulin delivery systems,” other aspects of that code were not. Twenty-five percent of transcripts were randomly selected for double coding. In order to quantitatively and qualitatively compare interrater agreement, pairs of coders were randomly selected each week from among those assigned double-coded transcripts. Rather than seeking statistical guideposts, researchers relied on levels of agreement and consensual identification of themes for in-depth analysis. Measures developed from this research would later be subjected to cognitive interviews and validity testing. By the 4th of 9 weeks of coding, no new themes arose and thematic saturation was achieved around the research questions of interest.
Next, in step 2, 24 a priori codes as well as emergent codes were condensed to 12 thematic clusters (see Fig. 2). Clusters comprised closely related codes grouped under thematic headings derived from iterative research dialogues. Two additional analytic methods were used. First, coded data were synthesized into cluster-specific “idea units” to represent the range of ideas discussed by each of the participant groups (children, adolescents, and adults with type 1 diabetes and their parents and partners). Second, a data matrix was designed to distill analyses into possible survey items for the measures to be developed. In step 3, summaries of analyses were presented to the entire research team during a face-to-face study meeting. Integration of qualitative results and expertise with PWD enabled the team to harness the power of participants’ narrative and meaning while ensuring that distillations for quantitative items did not miss critical factors influencing the use of diabetes management devices. Further, data volume contributed to both theoretical saturation and cross-participant verification of themes. Step 4 consisted of measure development and piloting and is not discussed in this manuscript.
Results
Demographic Characteristics of Sample
Table 1 summarizes demographic characteristics of participants and data sources. Interview and focus group data were obtained from 284 participants across the four sites. Participants were predominantly white, non-Hispanic (92.0%) and 75.0% reported current pump use for the person diagnosed with type 1 diabetes. Race/ethnicity and pump information were not available for children and teens at all sites.
Table 1.
Characteristics of participants and qualitative data sources
| Descriptive characteristics of participants and data sources | Value (range) |
|---|---|
| Overall number of focus groups | 48 |
| Overall number of semistructured interviews | 89 |
| Adults, n | 113 |
| Data from semistructured interview | 31.0 |
| Age, years | 39.5 (18–77) |
| Female | 70.8 |
| Race/ethnicity | |
| Black/African American | 1.8 |
| Hispanic/Latino | 0.9 |
| Asian/Pacific Islander American | 3.5 |
| White, non-Hispanic | 92.0 |
| Other | 0.9 |
| Bachelor's degree or higher education | 73.5 |
| Current pump use | 72.6 |
| Current CGM use | 54.5 |
| Hemoglobin A1c | 7.5% (5.0–11.8%) |
| Adolescent/young adult with type 1 diabetes, n | 35 |
| Data from semistructured interview | 45.7 |
| Age, years | 14.7 (12–20.8) |
| Children with type 1 diabetes, n | 16 |
| Data from semistructured interview | 43.8 |
| Age, years | 10.3 (9–11) |
| Parents, n | 65 |
| Data from semistructured interview | 25.1 |
| Relationship to child | |
| Mothers | 79.7 |
| Fathers | 17.2 |
| Other | 1.5 |
| Responding for female child | 61.7 |
| Child's race/ethnicity | |
| Black/African American | 1.5 |
| Hispanic/Latino | 5.3 |
| Asian/Pacific Islander American | 0.0 |
| White, non-Hispanic | 89.9 |
| Other racial group | 3.3 |
| Child's current pump use | 71.8 |
| Child's current CGM use | 53.8 |
| Child's hemoglobin A1c | 8.1% (6.4–13.0%) |
| Partners of people with type 1 diabetes, n | 55 |
| Data from semistructured interview | 20.0 |
| PWD race/ethnicity | |
| Black/African American | 0.0 |
| Hispanic/Latino | 1.8 |
| Asian/Pacific Islander American | 1.9 |
| White, non-Hispanic | 94.5 |
| Other racial group | 1.9 |
| PWD current pump use | 83.6 |
| PWD current CGM use | 74.1 |
| PWD hemoglobin A1c | 6.9% (5.0–9.2%) |
| Total participants, n | 284 |
Values are presented as percentage or mean (range) unless otherwise indicated.
Twelve thematic clusters represented the majority of data analyzed and are shown in Fig. 2. Current analyses focus on three prevailing themes: trust and control, features, and concerns and trade-offs. These themes were most discussed throughout the data across stakeholder subgroups; differences noted between subgroups were primarily related to emphasis. The trust and control theme was focused on user-system interaction and contained subtopics related to user control versus automation and thoughts about taking breaks from technology over long-term diabetes management. The features cluster included preidentified concepts related to potential features of automated systems, as well as features identified by the participants as desirable. Concerns and trade-offs deliberated changes in self-management burden, the adaptability/flexibility of these systems, as well as the socially embedded nature of type 1 diabetes management and device use. Quotes representing each of these themes are provided in Table 2.
Table 2.
Sample participant data for three emergent themes
| Exemplar quotes representing three major themes | |
|---|---|
| Trust and control |
“I think that’s a critical part of a closed-loop system…the accuracy and dependability. And there are a lot of pieces that can fail. Just infusion sites are so—one day they work and the next they don’t. So, there are a lot of human factors in there. I think that a lot of those could be alleviated, but that’s what I worry about is if they don’t address at least the ones that are the most critical, then I think the systems just won’t be accurate. You won’t have faith in them. You won’t be able to trust them. And then, you probably spent a lot of money on something that’s just not working.” (Adult with type 1 diabetes) |
| “If I was able to take over, manually, the action of the pump, then I wouldn’t feel worried. The worry is letting it work automatically and it not doing it correctly and I go too low or too high, particularly at nighttime if I’m asleep and it makes the wrong decision in terms of a dose. That would be a worry.” (Adult with type 1 diabetes) | |
| “Having seen some data now and being exposed to it a little bit more like actually seeing those nondiabetic blood sugars on the screen and showing patient after patient after patient that are on these systems that have zero variation and they all stay between maybe 80 and 120 for some of the more advanced studies, I am a believer now. I am a closed-loop convert. I can’t wait to not have to make those decisions.” (Adult with type 1 diabetes) | |
| “Inevitably with any new type of medical treatment or technology, there is going to be a bit of a teething period where you are just kind of getting your head around how it works.” (Adult with type 1 diabetes) | |
| “My ultimate aim would be to forget that I have diabetes in the first place. [Other participants in the room agree.] That has to be everybody’s dream that we become whatever is called normal...that is my ultimate expectation within my lifetime. I don’t think they’ll find a cure but I would like to forget that I’ve got it.” (Adult with type 1 diabetes who wanted to trust a new system) | |
| Features |
“Would it be great to have a system that you just plug it in and just live your life and you don’t have to do your fingerpricks and you don’t have to worry about telling it what you’re going to do because it activates and it makes decisions on the spot?” (Adult with type 1 diabetes) |
| “Well, this is not scientific, but sort of what [automated insulin delivery] means to me is with state-of-the-art technology to try to imitate what the pancreas does for the human body by using several devices and/or drugs in combination so that a person with type 1 diabetes can live a normal life.” (Parent of youth with type 1 diabetes) | |
| “I kind of think it would be quite big, especially if there were wires and stuff connecting the pump to the cannula, and you have a sensor and a pump and the other gadget to carry around. I kind of think it would be quite heavy.” (Adolescent with type 1 diabetes) | |
| “Is it discreet? Is it bulky? Is it going to draw attention to itself with alarms and things like that?” (Parent of youth with type 1 diabetes) | |
| [Device companies] “forget that it is old-school looking. . .this ugly device [becomes] part of our wardrobe, it is part of our daily wear. For me, especially, as a female who dresses up in business wear every day, I want something that is attractive on my body and I think sometimes that gets forgotten in the discussion.” (Adult with type 1 diabetes) | |
| Concerns and trade-offs | “If it means less interaction and better control, yeah. If it means more interaction and better control, no. If it means the same interaction and the same control, no. Why change? Why learn a new system?” (Adult with type 1 diabetes) |
| “If I had to carb count, I don’t know if that’s really alleviating it, because it’s the mental stuff that I don’t want to deal. I don’t really care about the physical stuff as much. I’ll wear it, I’ll change it, and whatever. It’s the mental stuff I am tired of doing.” (Adult with type 1 diabetes) | |
| “I don’t want to be wearing ten different things on me. It’s kind of like already burdensome as it is.” (Adult with type 1 diabetes) | |
| “Well, it should be customizable. You should be able to choose what your alarm of choice is and how often you want it. It needs to be responsive to—it needs to be customizable for the user so they can set it up the way they want. I mean a cell phone is. This should be also.” (Adult with type 1 diabetes) | |
| “Well, I think that varying blood sugars and also the fact that we carry this burden and live so close to death, I think that has a lot of effect on the kind of person that you are and how you relate to other people and the highs and the lows and your moods and that sort of stuff. So I think the better blood sugars that you can have then the less weight that you have to carry on your shoulders every day. So I think that perhaps that could have a positive effect on relationships with other people because you just are not having that burden all the time.” (Adult with type 1 diabetes) | |
| “I became obsessed with the idea of being normal again and having something that would make you normal to an extent where you feel normal and I mean what is normal obviously; but being in a situation where you don’t have to worry about everything, being in a situation where you don’t have to carb count, being in a situation where you can go out with your mates and not really care or just have it in the back of your mind or something.” (Adult with type 1 diabetes) |
Overall, negative themes such as device concerns and areas of uncertainty were more endorsed than positive themes such as device benefits. Ideas about potential benefits of automated insulin delivery systems in specific situations, relationships, or contexts were also mentioned frequently, such as nighttime and eating routines. Emphasis on the negative or unknown in participant expressions may reflect both the developmental stage of the systems as well as low levels of knowledge about such systems among the majority of participants interviewed.
Trust and Control
Trust and control data discuss user-system interactions, including one’s ability to trust systems to operate effectively, suspend and restart usage, override system miscalculations, and share system data with caregivers. All groups mentioned difficulties they might have trusting new systems and questions about whether the system or the user would have ultimate decision-making authority.
In order to consider using automated insulin delivery systems, participants stated that systems had to be trusted to do what they “promise” to do (eliminate high and low blood glucose variability while subsequently reducing risks of complications). Participants desired systems that would diminish the daily prominence of type 1 diabetes self-care activities and recognized that automated insulin delivery systems might be able to relieve some of the burden of type 1 diabetes management. Further, approximately half of the transcripts contained discussion about how automated insulin delivery systems might reduce human error in self-management. Automated insulin delivery systems were perceived as being able to stabilize glycemic control while improving quality of life. More specifically, participants indicated that systems should be automatic, accurate, efficient, and adaptive and should be associated with improved mood, sleep, relationships, and greater normalcy of eating and exercising behaviors. Finally, participant narratives described automated insulin delivery systems that could lead to reductions in monitoring, stress, conflict, hospitalizations, and future complications.
Reflecting a common uncertainty about how much trust one could place in automated insulin delivery systems and their algorithms, PWD of all ages and parents wanted to be able to override decision-making functions of the systems. Other participants were cautious about trusting automated insulin delivery systems in general, and early generation systems in particular, due to their novelty. Despite skepticism about the functioning of various system components, most participants were hopeful about the advancement of automated insulin delivery systems and the future of diabetes devices. Participants expected that developers would make efforts to build user trust and confidence but believed that part of the incremental research and development process meant that new devices would still entail new learning and a period of adjustment for PWD and their loved ones.
In terms of subgroup emphasis, stakeholders discussed their beliefs and concerns about system trust and control differently. Children were more concerned with how automated insulin delivery systems might affect specific social situations, school, and time with friends; they hoped that system adaptability would ease these concerns. Adults with type 1 diabetes and parents of youth with type 1 diabetes were more worried about device safety than others. Similarly, partners raised each of the aforementioned trust and control themes as well as their partners’ past experiences with diabetes-related technology. Participants experienced with advanced type 1 diabetes devices such as pumps and CGM were more likely to presage the potential benefits of using automated delivery systems than people who followed lower tech management regimens. “High-tech” participants discussed trust and control, physical features, and past experiences (positive and negative) with technology more than their “low-tech” counterparts.
In sum, participants described a range of expectations that would help them trust the automated insulin delivery systems and feel comfortable with the process of controlling such systems.
Features
Potential system features were of great interest to study participants. Data about features emerged in a qualitatively different manner than other themes; participants listed desired physical, mechanical, or technical features and described how the identified feature(s) could make a difference in their daily lives. Seven categories of features were identified: features borrowed from prior technologies, physical features, comfort and wearability, the algorithm and computing architecture, user interface features, device education and support programs, and other features. First and foremost, participants wanted a device they could “set and forget.” Participants thought automated insulin delivery systems should approximate their understanding of a biologic pancreas.
Various configurations and components of automated insulin delivery systems were envisioned, such as one adolescent’s belief that systems should simply have “glucagon, insulin, CGM, no tubes, [and be] waterproof.” In addition to preferring wireless or tubeless and waterproof devices, the majority of participants worried about the size, weight, and appearance of possible devices. Other participants described the neglected importance of aesthetics in the development of type 1 diabetes devices; the ever-present nature of these devices makes them part of the “daily uniform” of PWD. Children and teens wanted devices that came in colors, could visually function as extensions of their personalities, and were built on their generation’s immersion in technological devices and applications. Adolescents spent a great deal of their interviews/focus groups discussing their concerns about the physical features, wearability, and comfort of potential systems. Finally, some parents sought devices to help lessen the sense of difference that their child experiences; many parents believed that less visible and less intrusive devices would improve their children’s self-esteem and socializing. This example demonstrates how common interests in less visible systems may stem from different beliefs (children and teens also wanted less visible systems, but so that they could control to whom their diagnosis was revealed). Youth were not explicitly concerned with enhancing self-esteem but shared parents’ interest in systems that may help improve socializing.
Concerns
Participants were most concerned about two areas of uncertainty and the ability of automated insulin delivery systems to diminish social challenges related to type 1 diabetes and its management. First, participants were uncertain about the types and intensity of changes in management burden that would result from using automated insulin delivery systems. Would the management burden be reduced? Or would burden simply be shifted so that users were now responsible for different, technology-oriented tasks such as device charging or maintenance? Lack of knowledge about automated insulin delivery systems and what they will require from users raised anxiety and wariness among participants. For the majority of participants, the thought of possibly increasing and not decreasing their self-management burden seemed untenable. Even among those with “out of control” blood glucose levels, status quo equilibria have been achieved through investments of time and effort. Quality of life or health improvements are critical for PWD to embrace a new system and way of managing their diabetes.
Similar uncertainty existed about the adaptability and customizability of automated insulin delivery systems. Participants wanted systems that could adapt to the specifics of their lives. Descriptions of personalization preferences for systems included the ability to be temporarily removed, devices that “learn” from one’s habits and routines over time, and customizable alarms and sounds. Participant interest in customization parallels societal advancement of personalized medicine and routinization of on-demand access to technological devices. Social trends in personal health management through data quantification and monitoring were also reflected in participant interest in using glucose data to improve insights about their own self-management. Despite these developments, teens and children described potential prohibitions against devices in some settings. Youth mentioned rules against insulin pumps in some sports and against phones in some schools. For school-aged youth, systems must be recognizable medical devices clearly distinguishable from other technological devices, and yet many children expressed a desire for the technologies to blend in and not alert others to their diabetes. When type 1 diabetes devices are suitable for individuals’ distinctive lifestyles, participants believed that PWD would be more inclined to use the devices.
Study participants across subgroups wanted devices that would improve social and situational aspects of diabetes management. Participants extensively discussed how self-management contributed to stress, conflict, worry, and dependency in their relationships and interfered with many situations and activities of daily life. Their hope was that a system could automate, replace, or postpone some of the management tasks and help them regain some sense of normalcy; yet, they were concerned that the promise of automated insulin delivery systems might be exaggerated.
Conclusions
Our research reveals multiple psychosocial factors that influence initial and persistent use of automated insulin delivery systems. Our findings expand previous research findings about psychosocial factors in medical device use (11–14) and offer new insights. First, psychosocial factors are influential in PWD’s considerations of changes to their self-management regimens. Participants seek technical innovations that will improve blood glucose stability and reduce the likelihood of long-term complications without increasing their management-related burden. Releasing clear expectations and potential benefits of systems, achieving high levels of satisfaction in published trials, and including technical support and fail-safes that place system control in users’ hands may help engender trust.
Second, device features figure prominently in users’ daily experiences with type 1 diabetes devices. Possible system features were related to participants’ trust, expectations, and concerns. Features like being wireless and waterproof were almost unanimously requested and could improve uptake. Supporting findings by Alsaleh et al. (12), participants were concerned about the size, visibility, and attention demands of automated insulin delivery systems. Being able to customize device features and attributes based on one’s lifestyle was also described as important. Device features were more than specifications and materials; they were facilitators with the power to lessen stigma and improve user experiences and trust while supporting PWD’s interest in control, independence, and data-driven responsiveness of devices.
Third, participants expressed concerns about whether automated insulin delivery systems would add to or complicate regimen burden and how systems would fit into specific aspects of participants’ lives. This finding extends the conclusion of Low et al. (13) that PWD were most concerned about the size, portability, and technical problems associated with their insulin pumps. Participants were interested in how such systems would adapt to help manage diabetes in the presence of moderate or intense physical activity. Concerns were also linked to low levels of information about automated insulin delivery systems among participants. In fact, many participant concerns may be addressed as more information about automated insulin delivery systems is revealed and developers communicate the capabilities of their devices. Despite concerns, participants were cautiously optimistic about the potential impact of automated insulin delivery systems and their interest in trying such systems as they become available. Across the researchers’ experiences with diabetes technology trials, patients almost unanimously affirm their interest in continuing the use of test devices even when patients experience a number of difficulties throughout trial implementation.
Limitations of this study include a study design where participants were not always classified by characteristics that might have allowed for more in-depth comparative analysis as well as voluminous qualitative data that required resource-intensive analytic procedures in order to distill important patterns and themes. Although site data collection was coordinated and questions were standardized, differences in site implementation and procedures limit our ability to compare across groups based on demographic and behavioral characteristics, e.g., sex or pump users. Some sites reported aggregated demographic data, and different demographic variables were captured across sites. Our samples appear to differ from the broader population of people affected by type 1 diabetes in that CGM usage is higher in our samples than in other studies, possibly indicating differential access, motivation, and/or technological sophistication than the general population of those affected by type 1 diabetes (1). Furthermore, in an effort to present the most salient themes, not all themes discussed in participant data are mentioned, just those most commonly brought up. Moreover, the interview and focus group guides focused on experiences, expectations, and potential effects on quality of life, so the absence of some themes may be artifactual. For example, concerns about insurance coverage and additional supplies were raised, but discussions about direct costs of automated insulin delivery systems were infrequent. Overall, study data depict cross-geography and cross-stakeholder verification of themes related to stakeholder understanding of how automated insulin delivery systems might change their lives. We hope that the insights provided by the study outweigh the study’s limitations.
By unveiling the importance of psychosocial factors in the early stages of automated insulin delivery system development, this research affirms a critical role for qualitative approaches to clinical questions in diabetes research. This study contributes to limited literature about the psychosocial factors that support or detract from medical device utilization. As a qualitative study, this research illuminates these factors in the words and experiences of five critical groups, including three developmentally distinct groups of PWD and their loved ones. Reinforcing research on other type 1 diabetes devices, our study also found social and emotional elements of diabetes management and care to be critically important in considerations of device “fit” within one’s life (7,12–15). Although study participants may differ from the overall population of people living with type 1 diabetes in terms of both their glycemic control and use of diabetes technological devices, we believe the variety of experiences across geographies, payer types, and disease management perspectives adequately outlines domains of experience that should be examined with future quantitative studies. Our group has recently used this study’s results to guide the development of comprehensive survey measures to predict uptake and sustained use of automated insulin delivery systems. These survey measures will be deployed in future studies where statistical analyses, including those dependent upon individual-level characteristics, are planned to examine the distribution of specific factors and experiences associated with automated insulin delivery system interest and uptake, across diverse groups affected by type 1 diabetes. In preparation for broader use of automated insulin delivery systems and related experience surveys, researchers should include items that assess device features and patient expectations and concerns, as well as potential changes in regimens, management behaviors, vigilance, glycemic control, and physical and emotional burdens.
Article Information
Acknowledgments. The authors acknowledge the research contributions of the site research teams, including Annie Astle (Bournemouth University), Sarah Collard (Bournemouth University), Persis Commissariat (Joslin Diabetes Center), Kimberly Garza (Lurie Children's Hospital), Pramod Regmi (Bournemouth University), Danielle Slaughter (Lurie Children's Hospital), and Amanda Whitehouse (Joslin Diabetes Center). Marisa Hilliard (Baylor College of Medicine), Molly Tanenbaum (Stanford School of Medicine), and Sarah Hanes (Stanford School of Medicine) also provided invaluable assistance.
Funding. This study was supported by funding from The Leona M. and Harry B. Helmsley Charitable Trust.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.N. designed the qualitative methods, helped coordinate data collection, led implementation of the data analysis, managed data, and contributed to manuscript writing and post hoc measure development. S.C.S. designed the qualitative methods, analyzed data, and wrote the manuscript. E.I. assisted in qualitative analysis design, wrote the first list of codes used by coders, and collected, managed, and analyzed data. K.D.B., J.W.-B., L.L., and K.K.H. served as site principal investigators and were involved in the review and implementation of ethical approvals, research design, data collection, guidance of analytic discussions, and post hoc measure development. All authors reviewed and edited the manuscript. K.K.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 2.Pickup J. Insulin pumps. Int J Clin Pract Suppl 2011;65(Suppl. 170:16–19 [DOI] [PubMed] [Google Scholar]
- 3.Renard E. Insulin pump use in Europe. Diabetes Technol Ther 2010;12(Suppl. 1):S29–S32 [DOI] [PubMed] [Google Scholar]
- 4.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 5.White HD, Goenka N, Furlong NJ, et al. The U.K. service level audit of insulin pump therapy in adults. Diabet Med 2014;31:412–418 [DOI] [PubMed] [Google Scholar]
- 6.Wong JC, Foster NC, Maahs DM, et al.; T1D Exchange Clinic Network . Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care 2014;37:2702–2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naranjo D, Tanenbaum ML, Iturralde E, Hood KK. Diabetes technology: uptake, outcomes, barriers, and the intersection with distress. J Diabetes Sci Technol 2016;10:852–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creswell JW. Qualitative Inquiry and Research Design: Choosing Among Five Approaches. Thousand Oaks, CA, SAGE Publications, Inc., 2012 [Google Scholar]
- 9.NVivo Qualitative Data Analysis Software. Melbourne, Australia, QSR International Pty Ltd., 2015
- 10.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006;3:77–101 [Google Scholar]
- 11.Barnard KD, Hood KK, Weissberg-Benchell J, Aldred C, Oliver N, Laffel L. Psychosocial assessment of artificial pancreas (AP): commentary and review of existing measures and their applicability in AP research. Diabetes Technol Ther 2015;17:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alsaleh FM, Smith FJ, Taylor KM. Experiences of children/young people and their parents, using insulin pump therapy for the management of type 1 diabetes: qualitative review. J Clin Pharm Ther 2012;37:140–147 [DOI] [PubMed] [Google Scholar]
- 13.Low KG, Massa L, Lehman D, Olshan JS. Insulin pump use in young adolescents with type 1 diabetes: a descriptive study. Pediatr Diabetes 2005;6:22–31 [DOI] [PubMed] [Google Scholar]
- 14.Ritholz MD, Smaldone A, Lee J, Castillo A, Wolpert H, Weinger K. Perceptions of psychosocial factors and the insulin pump. Diabetes Care 2007;30:549–554 [DOI] [PubMed] [Google Scholar]
- 15.Barnard KD, Wysocki T, Allen JM, et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res Care 2014;2:e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]