Abstract
Whole body studies using long-lived growth hormone receptor gene disrupted or knock out (GHR-KO) mice report global GH resistance, increased insulin sensitivity, reduced insulin-like growth factor 1 (IGF-1), and cognitive retention in old-age, however, little is known about the neurobiological status of these mice. The aim of this study was to determine if glutamatergic and inflammatory markers that are altered in aging and/or age-related diseases and disorders, are preserved in mice that experience increased healthspan. We examined messenger ribonucleic acid (mRNA) expression levels in the brain of 4- to 6-, 8- to 10-, and 20- to 22-month GHR-KO and normal aging control mice. In the hippocampus, glutamate transporter 1 (GLT-1) and anti-inflammatory nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB)-p50 were elevated in 8- to 10-month GHR-KO mice compared with age-matched controls. In the hypothalamus, NFκB-p50, NFκB-p65, IGF-1 receptor (IGF-1R), glutamate/aspartate transporter (GLAST), and 2-amino-3-(5-methyl-3-oxo 2,3-dihydro-1,2 oxazol-4-yl) propanoic acid receptor subunit 1 (GluA1) were elevated in 8- to 10- and/or 20- to 22-month GHR-KO mice when comparing genotypes. Finally, interleukin 1-beta (IL-1β) mRNA was reduced in 4- to 6- and/or 8- to 10-month GHR-KO mice compared with normal littermates in all brain areas examined. These data support the importance of decreased brain inflammation in early adulthood and maintained homeostasis of the glutamatergic and inflammatory systems in extended longevity.
Keywords: Glutamate, Inflammation, Brain, Aging, Cognition, Insulin-like growth factor.
Specific areas of the brain are essential for several processes that play a key role in extended longevity, including hippocampus (cognition), hypothalamus (insulin-glucose homeostasis and energy expenditure), and striatum (motor learning and movements). These regions are interconnected through neuronal pathways, have an extensive glutamatergic component, and their dysfunction can lead to accelerated aging as well as several age-related diseases and disorders including dementia, Alzheimer’s disease, diabetes, and Parkinson’s diseases ( 1–13 ). Many of these illnesses occur as the result of a slow build-up of harmful material that occurs over several years. For instance, there is a build-up of plaques, tangles, and inflammation coupled with neurodegeneration in the glutamate (Glu) rich hippocampal region in Alzheimer’s disease. Diabetes may be the result of chronic inflammation, likely in the hypothalamus, which is responsible for insulin-glucose homeostasis and has an abundance of glucose-sensing neurons that are primarily glutamatergic in nature ( 12 , 14 , 15 ). Taken together these data support the necessity for preservation of neuronal function in key brain areas resulting in increased healthspan.
Two of the main interrelated systems thought to be involved in age-and metabolic-related cognitive disorders are the glutamatergic and inflammatory systems. Glu is the predominant excitatory neurotransmitter in the mammalian central nervous system and its dysregulation has been associated with decreased cognition, increased inflammation, and several age-related neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases ( 1 , 3 , 4 , 10 , 16–22 ). In the brain, glial cells, composed of astrocytes and microglia, are the primary contributors to clearance of Glu from the extracellular space through surface expression of excitatory amino acid transporters. One such transporter, GLT-1 in rodents (excitatory amino acid transporters 2 in humans), is responsible for up to 90% of Glu clearance from the extracellular space in the brain, and has been shown to decrease in abundance and function with age, leading to increased extracellular Glu and excitotoxicity ( 19 ). Additionally, glia are the main producers of inflammatory (pro- and anti-inflammatory) mediators in the brain and play an important role in innate immunity. For instance, activated microglia enhance production of inflammatory cytokines, such as IL-1β ( 23–25 ), and decrease anti-inflammatory cytokines, such as IκB kinase β (IKKβ), NFκB-p50, and NFκB-p65 ( 26 , 27 ). During aging, microglia have an increased inflammatory response and may contribute to the onset of chronic neurodegenerative diseases ( 27–29 ). Furthermore, IGF-1 plays a role in information processing in the brain that may be independent of circulating IGF-1 ( 30 ). These data support an interrelated mechanism whereby increased neuronal extracellular Glu and elevated neuroinflammation may be responsible for the cognitive decline associated with aging.
The aim of this study was to determine if glutamatergic and inflammatory markers that are known to be altered with normal aging and/or age-related diseases and disorders are preserved in mice that experience successful aging. We used long-lived GHR-KO mice that live 35%–70% longer than their normal littermates. GHR-KO mice are GH resistant with low levels of IGF-1 (which may offer protection for disease associated neuronal loss ( 31 ) and be involved in extended life-span ( 32) ), and have improved insulin signaling, decreased proinflammatory and increased anti-inflammatory activity in the periphery, and decreased oxygen consumption and energy cost of locomotor activity, all of which may contribute to their increased longevity ( 33–37 ). Additionally, GHR-KO mice are GH resistant, which may alter neurotransmission and the levels of other hormones, thereby delaying brain aging and cognitive decline ( 30 ). We examined the hypothalamus, hippocampus, and striatum in 4- to 6-, 8- to 10-, and 20- to 22-month GHR-KO mice that experience successful aging compared with normal aging littermate control mice by assessing mRNA expression levels of brain markers involved in age and metabolic disorders including inflammatory and glutamatergic markers. Anti-inflamatory markers included IKKβ, NFκB-p50, and NFκB-p65, whereas the proinflammatory marker IL-1β was examined. Growth factor markers included IGF-1 and its receptor. Finally, glutamatergic transporters (vesicular Glu transporter [VGLUT] 1, VGLUT3, GLAST, GLT-1 and receptor subunits (N-methyl D-aspartate receptor subunit 2b [GluN2B] and GluA1) were measured.
Methods
Animals
Four- to six-, eight- to ten-, and twenty- to twenty-two-month-old female GHR-KO and normal littermate control mice were obtained from a colony at Southern Illinois University School of Medicine originally developed from breeders provided by Dr. John J. Kopchick and used for all experiments ( 35 , 38 ). Protocols for animal use were approved by the Laboratory Animal Care and Use Committee at Southern Illinois University School of Medicine. Animals were housed according to approved guidelines, and food and water were available ad libitum.
RNA Purification and Polymerase Chain Reaction Analysis
All animals were decapitated under isoflurane anesthetic and their brains were rapidly removed. The hippocampus, hypothalamus, and striatum were dissected on wet ice and tissue was immediately frozen on dry ice. Samples were stored at −70°C until RNA extraction. RNA was purified using the miRNeasy Mini Kit (Qiagen, Boston, MA) following the manufacturer’s protocol for purification of total RNA from animal tissue. The quantity of total RNA was determined using an ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE) and complimentary DNA (cDNA) was synthesized from 2 µg of total RNA using a cDNA Synthesis Kit (Bio-Rad, Hercules, CA) following the manufacturer’s protocol.
Real-time polymerase chain reaction was performed on individual samples as previously described ( 39 ). Briefly, each reaction contained 12.5-µL iQ SYBR Green Supermix (Bio-Rad, Hercules), 0.4 µL each of backward and forward primer (see Table 1 ), and 2-µL diluted cDNA (3 H 2 O: 1 cDNA). The reaction included 2 minutes at 94°C (denaturing), 30 seconds at 62°C (annealing), and 30 seconds at 72°C (extension). Β-2-microglobulin was used as the housekeeping gene control, based on prior studies involving altered inflammatory states, and was used to determine relative expression of the mRNA of interest as previously described ( 39 ).
Table 1.
Primers Used for mRNA Analysis
| Target mRNA | Forward Primer | Reverse Primer |
|---|---|---|
| IKKβ | GCTGTCCTTACCCTGCTGAG | TCCTTGCTGCAGAACGATGT |
| NFκB-p50 | GCCAGAAGAGGGTGTCAGAG | ACATTTGCCCAGTTCCGTAG |
| NFκB-p65 | TCTGCTTCCAGGTGACAGTG | ATCTTGAGCTCGGCAGTGTT |
| IL-1β | CATCTCGGAGCCTGTAGTGC | CGTGGACCTTCCAGGATGAG |
| IGF-1 | CTGAGCTGGTGGATGCTCTT | CACTCATCCACAATGCCTGT |
| IGF-1R | TGACTCGGGACTGTTCAACG | TCCTGTATACCACTCCGCCA |
| VGLUT1 | CTCAGCCCGCCTACTTTGAA | GTGACGACTGCGCAAAAAGT |
| VGLUT3 | CCTTCCTGGTGCTTGCTGTAG | GGCATATCGTGGAGCAATGTC |
| GLT-1 | GCGGGTGATGTCAGCTCT | CCGAAGAGGGATTGCAAGGT |
| GLAST | TTTCTCTCTAGGGGCAGGCT | CAGAAGGGAGGGCCTCTAGT |
| GluN2B | GGAGCTGGCATCCGAATACA | GGGTGTCGAGGGTTTGAGAC |
| GluA1 | AGCCTTGCACCGTCTGATTT | CCATAAGCTGGACGCTGAGT |
Data Analysis
All data were generated from individual mice. Mean and standard error of the mean were determined for each group ( n = 5–9 mice per group). A two-way analysis of variance with a Fisher’s least significant difference post hoc test was used to determine age-related changes in mRNA levels within genotypes and age-matched alterations between genotypes. Statistical significance was determined at p < .05.
Results
Altered mRNA Expression Levels in the Hippocampus
Inflammatory markers
We observed significant age-related decreases in IKKβ in the hippocampus of control and GHR-KO mice ( F (2,36) = 17.28; p < .0001). In control mice, there was a significant decrease in mRNA expression in 8- to 10-month ( p < .001) and 20- to 22-month ( p < .0001) mice compared with 4- to 6-month mice ( Figure 1A ). In the GHR-KO mice, 20- to 22-month mice were significantly decreased compared with 4- to 6-month ( p < .001) and 8- to 10-month ( p < 0.05) mice ( Figure 1A ). There was no difference in IKKβ between age-matched genotypes ( Figure 1A ). There was significant age-related decreases ( F (2, 36) = 3.782; p = .0323) and a trend in genotypic differences ( F (1,36) = 3.712; p = 0.0620) and no overall interaction in NFκB-p50 mRNA expression in the hippocampus. NFκB-p50 was decreased in 8- to 10-month control mice compared with 4- to 6-month control mice ( p < 0.05) and in 20- to 22-month GHR-KO mice compared with 8- to 10-month GHR-KO mice ( p < 0.05; Figure 1B ). Additionally, 8- to 10-month GHR-KO mice had significantly elevated NFκB-p50 mRNA levels compared with age-matched controls ( p < 0.01; Figure 1B ). There were no significant age- or genotype-related changes in NFκB-p65 in the hippocampus ( Figure 1C ). We observed significant age-related alterations in IL-1β mRNA expression in the hippocampus ( F (2,33) = 4.492; p = .0188). IL-1β was significantly decreased in 4- to 6-month GHR-KO mice compared with 8- to 10-month ( p < 0.01) and 20- to 22-month ( p < 0.05) GHR-KO mice ( Figure 1D ) and in 4- to 6-month-old mice when comparing genotypes ( p < 0.05). There were no age-related changes in IL-1β mRNA expression in the hippocampus of control mice ( Figure 1D ).
Figure 1.
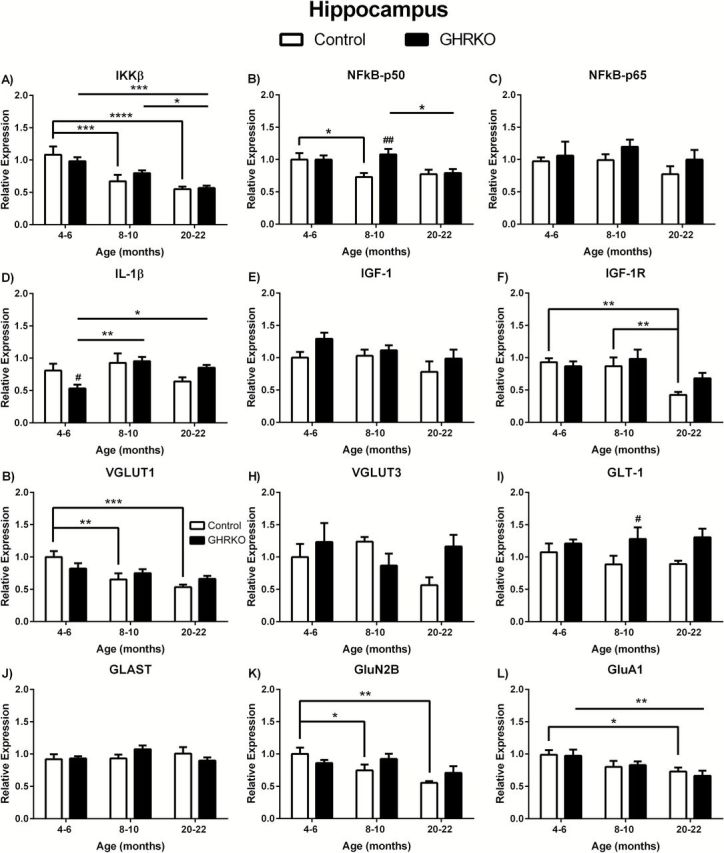
Messenger ribonucleic acid (mRNA) levels in hippocampus of growth hormone receptor gene disrupted or knock out (black bars) and normal aging (white bars) mice. mRNA expression levels of IKKβ, NFκB-p50, NFκB-p65, IL-1β, IGF-1, IGF-1R, VGLUT1, VGLUT3, GLAST, GLT-1, GluN2B, and GluA1 in the hippocampus of 4- to 6-, 8- to 10-, and 20- to 22-month-old mice. Two-way analysis of variance with a Fisher’s least significant difference post hoc analysis ( n = 5–9 mice per group). * p < .05, ** p < .01, *** p < .001, and **** p < .0001 indicate significance between ages within the same genotype. # p < .05 and ## p < .01 indicate significance between age-matched genotypes.
Growth factors
There were no age- or genotype-related changes in IGF-1 in the hippocampus ( Figure 1E ). However, IGF-1R mRNA expression was significantly decreased with age ( F (2,36) = 6.568; p = 0.0037). Twenty- to twenty-two-month control mice expressed less IGF-1R compared with 4- to 6-month ( p < .01) and 8- to 10-month ( p < 0.01) control mice ( Figure 1F ). There were no genotype-related changes in IGF-1R in the hippocampus ( Figure 1F ).
Glutamatergic markers
We observed altered VGLUT1 with age ( F (2,38) = 8.136; p = .0011) in the hippocampus. The expression of VGLUT1 was significantly decreased in 8- to 10-month ( p < .01) and 20- to 22-month ( p < .001) normal control mice compared with 4- to 6-month mice ( Figure 1G ). There were no age-related changes in GHR-KO mice or genotype-related alterations in VGLUT1 ( Figure 1G ). There was significance difference between genotypes in GLT-1 mRNA expression in the hippocampus ( F (1,37) = 7.481; p = .0095). GLT-1 was significantly ( p < 0.05) elevated in 8- to 10-month GHR-KO mice compared with age-matched control mice ( Figure 1I ). There were no age-associated alterations in GLT-1 expression levels ( Figure 1I ). GluN2B was significantly altered with age ( F (2,39) = 5.970; p = .0055) in the hippocampus, with 8- to 10-month ( p < 0.05) and 20- to 22-month olds ( p < .01) being significantly decreased in normal aging control mice ( Figure 1K ). There were no age-related changes in GHR-KO mice or genotype-related alterations in GluN2B ( Figure 1K ). GluA1 was also significantly altered with age ( F (2,34) = 6.080; p = 0.0055) with 20- to 22-month control and GHR-KO mice being significantly decreased ( p < 0.05 and p < 0.01, respectively) compared with 4- to 6-month mice of the same genotype ( Figure 1L ). No genotype-associated changes were observed in GluA1 levels ( Figure 1L ). No age- or genotype-related changes were observed in VGLUT3 or GLAST ( Figure 1H and J , respectively).
Altered mRNA Expression Levels in the Hypothalamus
Inflammatory markers
We observed significant age-related decreases in IKKβ in the hypothalamus ( F (2.39) = 7.003; p = 0.0025) with 20- to 22-month control mice having significantly lower IKKβ mRNA expression compared with 4- to 6-month ( p < .001) and 8- to 10-month ( p < .05) mice of the same genotype ( Figure 2A ). There were no significant changes related to age in GHR-KO mice or between genotypes in the hypothalamus ( Figure 2A ). There were no significant age-related changes in NFκB-p50 in the hypothalamus of control or GHR-KO mice ( Figure 2B ). However, NFκB-p50 was significantly elevated ( F (1,39) = 13.31; p = .0008) in 8- to 10- and 20- to 22-month (both p < .01) GHR-KO mice compared to age-matched controls ( Figure 2B ). NFκB-p65 expression levels were altered in regards to age ( F (2,37) = 3.624; p = .0365) and genotype ( F (1,37) = 5.590; p = .0234), but with no overall interaction ( F (2,37) = 1.784; p = .1821). NFκB-p65 was decreased in the hypothalamus of 8- to 10-month control mice compared with 4- to 6-month control mice ( p < .01; Figure 2C ). Additionally, NFκB-p65 was elevated in 20- to 22-month GHR-KO mice compared with age-matched controls ( p < .05; Figure 2C ). There were no significant age-related changes in IL-1β in the hypothalamus of control or GHR-KO mice ( Figure 2D ). However, IL-1β was significantly decreased ( F (1,37) = 14.51; p = .0005) in 4- to 6- and 8- to 10-month (both p < .05) GHR-KO mice compared with age-matched controls ( Figure 2D ).
Figure 2.
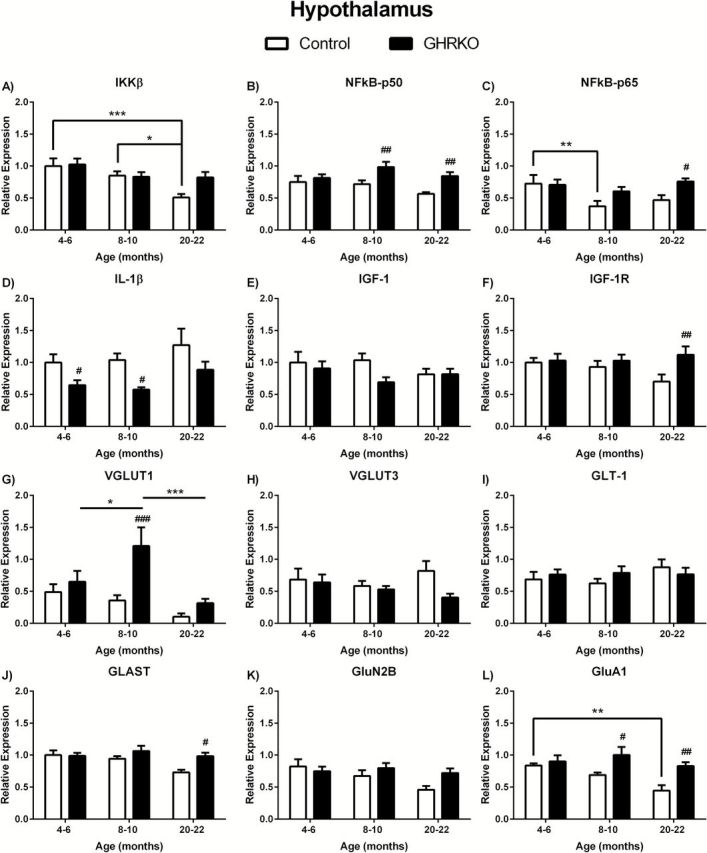
Messenger ribonucleic acid (mRNA) levels in hypothalamus of growth hormone receptor gene disrupted or knock out (black bars) and normal aging (white bars) mice. mRNA expression levels of IKKβ, NFκB-p50, NFκB-p65, IL-1β, IGF-1, IGF-1R, VGLUT1, VGLUT3, GLAST, GLT-1, GluN2B, and GluA1 in the hypothalamus of 4- to 6-, 8- to 10-, and 20- to 22-month-old mice. Two-way analysis of variance with a Fisher’s least significant difference post hoc analysis ( n = 5–9 mice per group). * p < .05, ** p < .01, and *** p < .001 indicate significance between ages within the same genotype. # p < 0.05, ## p < .01, and ### p < .001 indicate significance between age-matched genotypes.
Growth factors
There were no age- or genotype-related changes in IGF-1 in the hypothalamus ( Figure 2E ). There were also no age-related changes in IGF-1R in the hypothalamus ( Figure 2F ). However, IGF-1R mRNA expression was significantly altered in relation to genotype ( F (1,39) = 4.873; p = .0332). Twenty- to twenty-two-month old GHR-KO mice expressed more IGF-1R compared with age-matched control mice ( p < .01; Figure 2F ).
Glutamatergic markers
We observe altered age-related ( F (2,34) = 5.451; p = .0088) and genotype-related ( F (1.34) = 8.598; p = .0060) changes in VGLUT1 in the hypothalamus, with no significant interaction ( Figure 2G ). Eight- to ten-month GHR-KO mice had significantly elevated VGLUT1 expression when compared to 4- to 6-month ( p < .05) and 20- to 22-month ( p < 0.001) GHR-KO mice ( Figure 2G ). Additionally, VGLUT1 in 8- to 10-month GHR-KO mice was significantly increased compared to age-matched control mice ( p < .001; Figure 2G ). There was a significant difference in GLAST expression in the hypothalamus between genotypes ( F (1,39) = 5.545; p = .0237) with 20- to 22-month GHR-KO mice being significantly increased compared with age-matched control mice ( p < 0.05; Figure 2J ). No age-related differences were observed in GLAST expression levels. Finally, there were age- ( F (2,38) = 4.107; p = .0243) and genotype- ( F (1,38) = 12.44; p = .0011) related changes in GluA1 expression levels in the hypothalamus with no significant interaction ( Figure 2L ). There was a significant decrease in GluA1 expression in 20- to 22-month control mice compared with 4- to 6-month control mice ( p < .01), but not in GHR-KO mice ( Figure 2L ). Additionally, both 8- to 10-month ( p < 0.05) and 20- to 22-month-old GHR-KO ( p < 0.01) mice had elevated GluA1 expression in the hypothalamus compared to age-matched controls ( Figure 2L ). There were no age- or genotype-related changes in expression levels of VGLUT3, GLT-1, or GluN2B in the hypothalamus ( Figure 2H , I , and K , respectively).
Altered mRNA Expression Levels in the Striatum
Inflammatory markers
There were no age- or genotype-related changes in mRNA expression levels in any of the anti-inflammatory markers examined ( Figure 3A–C , respectively). However, we observed genotype-related changes in proinflammatory IL-1β ( F (1,29) = 4.265; p = .0480), which was significantly decreased in 8- to 10-month GHR-KO mice compared with age matched controls ( p < .05; Figure 3D ).
Figure 3.
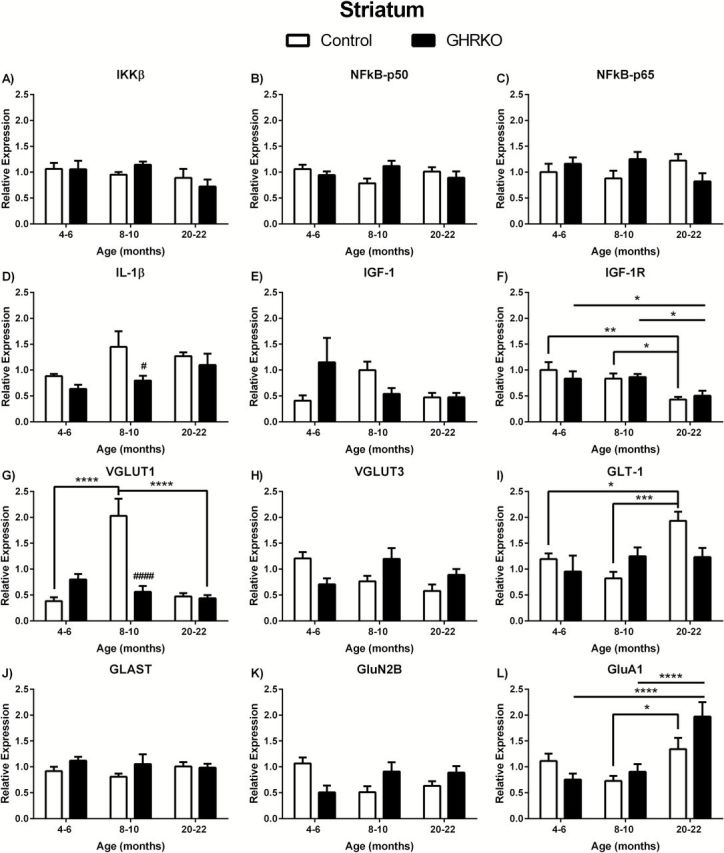
Messenger ribonucleic acid (mRNA) levels in striatum of growth hormone receptor gene disrupted or knock out (black bars) and normal aging (white bars) mice. mRNA expression levels of IKKβ, NFκB-p50, NFκB-p65, IL-1β, IGF-1, IGF-1R, VGLUT1, VGLUT3, GLAST, GLT-1, GluN2B, and GluA1 in the striatum of 4- to 6-, 8- to 10-, and 20- to 22-month-old mice. Two-way analysis of variance with a Fisher’s least significant difference post hoc analysis ( n = 5–9 mice per group). * p < .05, ** p < .01, *** p < .001, and **** p < .0001 indicate significance between ages within the same genotype. # p < .05, ## p < .01, ### p < .001, and #### p < .0001 indicate significance between age-matched genotypes.
Growth factors
As was the case in the hippocampus and hypothalamus, we did not observe any significant differences in IGF-1 mRNA expression levels in the striatum ( Figure 3E ). However, we did observe age-related changes in IGF-1R expression in the striatum ( F (2,37) = 7.834; p = .0015). IGF-1R expression was significantly decreased in 20- to 22-month mice compared with both 4- to 6- and 8- to 10-month-old mice for both genotypes (GHR-KO: p < .01 and p < .05, respectively; control: p < .05 and p < .05, respectively).
Glutamatergic markers
We observed age-associated ( F (2,28) = 18.92; p < .0001) and genotype-associated ( F (1,28) = 8.343 and p < .0074) changes in VGLUT1 mRNA expression levels in the striatum. VGLUT1 in 8- to 10-month control mice was significantly elevated compared with 4- to 6-month ( p < .0001) and 20- to 22-month ( p < .0001) control mice and compared with age-matched GHR-KO mice ( p < .0001; Figure 3G ). There was a significant effect of age ( F (2,31) = 5.356; p = .0100) on GLT-1 expression in the striatum with levels in 20- to 22-month control mice being significantly elevated compared with 4- to 6-month ( p < .05) and 8- to 10-month ( p < .001) mice of the same genotype ( Figure 3I ). Additionally, we observed age-related changes in GluA1 expression in the striatum ( F (2,34) = 13.28; p < .0001). GluA1 levels in 20- to 22-month control mice were significantly elevated compared to 8- to 10-month control mice ( p < .05; Figure 3L ). GluA1 expression was also elevated in 20- to 22-month GHR-KO mice compared with 4- to 6-month ( p < .0001) and 8- to 10-month old ( p < .0001) GHR-KO mice. There were no significant changes in striatal VGLUT3, GLAST, or GluN2B ( Figure 3H , J , and K , respectively).
Discussion
Neurobiological components and functions have been extensively examined as they relate to aging diseases and disorders. However, few studies have examined the brain in relation to increased healthspan and little is known about the neurological factors that might influence cognitive function and how they change with aging. However, Masser and colleagues ( 40 ) recently reported on an existing correlation between mRNA expression and cognitive function in rats. The aim of this study was to determine if glutamatergic and inflammatory markers, that are known to be altered in aging and/or age-related diseases and disorders, are preserved in GHR-KO mice that experience successful aging. The majority of age- and genotype-related differences were detected in the hippocampus and hypothalamus of the 20- to 22-month-old mice.
We focused on the NFκB family due to its involvement in learning and synaptic plasticity ( 41–43 ), which is often affected in aging and age-related disorders. Inactive NFκB is usually expressed with three subunits (IKKβ, p50, and p65). In the active state, IKKβ is ubiquinated, p50 and p65 form dimers and bind to NFκB sites in the promoter region of target genes, thereby activating transcription and/or expression. In the hippocampus, we observed significantly elevated NFκB-p50 mRNA levels in 8- to 10-month-old GHR-KO mice compared to littermate controls, but no other genotype-associated changes in IKKβ, NFκB-p50, or NFκB-p65 at any of the ages studied. This may be indicative of a mechanism whereby NFκB-p50 is involved in the sustained cognition in GHR-KO mice. In the hippocampus, NFκB p65/p50 heterodimers are localized to the cytoplasm and synapses and are activated by excitation, such as that produced by Glu ( 43–45 ). Interestingly, Boersma and colleagues ( 46 ) postulate that transcriptional regulation via NFκB is required for the induction of changes in excitatory synapses and spine density, but not for maintenance. Furthermore, deletion of either the NFκB-p50 or NFκB-p65 gene in mice has been associated with decreased cognition ( 43 , 46 , 47 ).
We also evaluated a proinflammatory cytokine, IL-1β, due to its link to glutamatergic neurotransmission. Glu is the predominant excitatory neurotransmitter in the mammalian central nervous system and under normal conditions, it plays an important role in several brain functions including learning and memory, energy expenditure, and insulin-glucose homeostasis, and other higher-level functions ( 7 ). However, when Glu is present in excess, it can lead to neuroinflammation, excitotoxicity, and cell death. We observed significantly reduced IL-1β mRNA in 4/6 month and/ or 8- to 10-month GHR-KO mice compared to normal littermates in all three brain areas examined. This elevated IL-1β mRNA expression in the brains of control mice may lead to an increase in extracellular Glu by increasing the velocity of the cystine-Glu exchanger (xCT) as has been observed by Hewett’s group ( 17 ). We also observed significantly decreased GLT-1 expression in the hippocampus of middle-aged control mice compared with age-matched GHR-KO mice. Interestingly, previous studies have shown that elevated IL-1β decreases Glu uptake via decreasing the surface expression of GLT-1 on astrocytes, possibly leading to increased extracellular Glu ( 48 ). Taken together, these data provides a mechanism whereby decreased IL-1β early in life could provide protection from Glu-related cognitive decline later in life.
Contrary to previous reports on IGF-1 plasma and liver levels observed in GHR-KO mice ( 38 , 49 ), we did not observe any age- or genotype-associated alterations in IGF-1 mRNA expression in any of the three brain regions examined. This finding is also supported by recent data that showed circulating IGF-1 levels did not alter hippocampal Igf1 or its receptor ( 50 ). However, our data is consistent with observations from another long-lived mouse, the Ames dwarf, where no difference was observed in hippocampal IGF-1 mRNA in aged Ames mice compared with age-matched controls ( 51 ). Interestingly, Sun and colleagues ( 51 ) observed an increase in IGF-1 protein levels in the hippocampus of the Ames mice, which may be a result of the ability of IGF-1 to cross the blood brain barrier and contribute to brain IGF-1 protein levels ( 52 ). Additionally, we observed an increase in IGF-1R expression in the hypothalamus of 20- to 22-month GHR-KO mice compared with age-matched controls. In the brain, IGF-1 promotes neurogenesis and long-term memory consolidation in the hippocampus which requires limbic activation of IGF-1R, specifically the hypothalamus and amygdala ( 27 , 30 ). Our data support a role for late-life IGF-1R involvement in cognitive retention and successful aging.
Finally, we examined glutamatergic markers in the brain of GHR-KO mice. It is well known that elevated Glu levels in the hippocampus can lead to excitotoxicity, neurodegeneration, and decreased cognition associated with aging and age-related disorders. This elevation could be due to an increase in Glu release through increased packaging or stimulated release, a decrease in Glu clearance (fewer transporters), or a combination of the two. GLT-1 was significantly decreased in the hippocampus in 8- to 10-month control mice compared with age-matched GHR-KO mice. However, sustained Glu neurotransmission in the hippocampus throughout life may be required for cognitive retention in old age. In support of this, hippocampal mRNA levels of VGLUT3 and GluN2B decreased with age in control mice, but sustain levels in GHR-KO mice, contrary to what was previously reported on GluN1 mRNA levels in the hippocampus of GHR-KO mice ( 53 ). In the hypothalamus, mRNA levels of GluA1 was significantly decreased with age in control mice, sustained in GHR-KO mice, and significantly elevated in 20- to 22-month GHR-KO mice when comparing the two genotypes, suggesting that Glu may play a role in insulin-glucose homeostasis. Additionally, VGLUT1 expression was significantly elevated in GHR-KO 8- to 10-month hypothalamus, which coincided with decreased VGLUT1 levels in GHR-KO striatum at the same age. In the striatum, we observed age-related changes in VGLUT1, GLT-1, and GluA1 in normal aging mice. In GHR-KO mice, the only significant change with age was observed in mRNA expression levels of GluA1. Additionally, when comparing the two genotypes, mRNA levels of VGLUT1 in the striatum were decreased in 8- to 10-month GHR-KO mice. Taken together, our results support that sustained glutamatergic markers throughout aging in the hippocampus, hypothalamus, and striatum of GHR-KO mice may play a role in the retained cognition, energy expenditure, insulin-glucose homeostasis, the sleep–wake cycle, and neuroendocrine output of the pituitary gland previously observed in these mice.
In conclusion, we observed age-related alterations in neuroinflammation, growth factor, and glutamatergic markers in normal aging mice that were rescued in GHR-KO mice. Of major importance was the observed decreased IL-1β expression in all three brain areas in 4- to 6- and/or 8- to 10-month GHR-KO mice, sustained glutamatergic neurotransmission/regulation, and sustained IGF-1R expression in the hippocampus and hypothalamus in 20- to 22-month GHR-KO mice. These data support the importance of brain inflammation in early life and maintained homeostasis of the glutamatergic, growth factor, and inflammatory systems in successful aging. Future studies will address how these mRNA expression levels relate to protein levels.
Funding
Support was contributed by the Center for Alzheimer’s Disease and Related Disorders at Southern Illinois University School of Medicine and National Institute on Aging at the National Institutes of Health (grant numbers R01AG019899 and P01 AG31736).
References
- 1. Callaerts-Vegh Z, Moechars D, Van Acker N, et al. Haploinsufficiency of VGluT1 but not VGluT2 impairs extinction of spatial preference and response suppression . Behav Brain Res . 2013. ; 245 : 13 – 21 . [DOI] [PubMed] [Google Scholar]
- 2. Haglund L, Swanson LW, Köhler C . The projection of the supramammillary nucleus to the hippocampal formation: an immunohistochemical and anterograde transport study with the lectin PHA-L in the rat . J Comp Neurol . 1984. ; 229 : 171 – 185 . [DOI] [PubMed] [Google Scholar]
- 3. Kashani A, Betancur C, Giros B, Hirsch E, El Mestikawy S . Altered expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in Parkinson disease . Neurobiol Aging . 2007. ; 28 : 568 – 578 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirvell SL, Esiri M, Francis PT . Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer’s disease . J Neurochem . 2006. ; 98 : 939 – 950 . [DOI] [PubMed] [Google Scholar]
- 5. Nickell J, Pomerleau F, Allen J, Gerhardt GA . Age-related changes in the dynamics of potassium-evoked L-glutamate release in the striatum of Fischer 344 rats . J Neural Transm . 2005. ; 112 : 87 – 96 . [DOI] [PubMed] [Google Scholar]
- 6. Stephens ML, Quintero JE, Pomerleau F, Huettl P, Gerhardt GA . Age-related changes in glutamate release in the CA3 and dentate gyrus of the rat hippocampus . Neurobiol Aging . 2011. ; 32 : 811 – 820 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tong Q, Ye C, McCrimmon RJ, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia . Cell Metab . 2007. ; 5 : 383 – 393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tordera RM, Totterdell S, Wojcik SM, et al. Enhanced anxiety, depressive-like behaviour and impaired recognition memory in mice with reduced expression of the vesicular glutamate transporter 1 (VGLUT1) . Eur J Neurosci . 2007. ; 25 : 281 – 290 . [DOI] [PubMed] [Google Scholar]
- 9. Tsamis KI, Mytilinaios DG, Njau SN, Baloyannis SJ . Glutamate receptors in human caudate nucleus in normal aging and Alzheimer’s disease . Curr Alzheimer Res . 2013. ; 10 : 469 – 475 . [DOI] [PubMed] [Google Scholar]
- 10. Vertes RP, McKenna JT . Collateral projections from the supramammillary nucleus to the medial septum and hippocampus . Synapse . 2000. ; 38 : 281 – 293 . [DOI] [PubMed] [Google Scholar]
- 11. Villa RF, Ferrari F, Gorini A . Energy metabolism of rat cerebral cortex, hypothalamus and hypophysis during ageing . Neuroscience . 2012. ; 227 : 55 – 66 . [DOI] [PubMed] [Google Scholar]
- 12. Ziegler DR, Cullinan WE, Herman JP . Distribution of vesicular glutamate transporter mRNA in rat hypothalamus . J Comp Neurol . 2002. ; 448 : 217 – 229 . [DOI] [PubMed] [Google Scholar]
- 13. Hascup KN, Hascup ER . Altered neurotransmission prior to cognitive decline in AβPP/PS1 mice, a model of Alzheimer’s disease . J Alzheimers Dis . 2015;44:771–776. [DOI] [PubMed] [Google Scholar]
- 14. Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI . Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats . J Clin Invest . 1997. ; 99 : 361 – 365 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI . Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release . Diabetes . 1995. ; 44 : 180 – 184 . [DOI] [PubMed] [Google Scholar]
- 16. Felger JC, Miller AH . Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise . Front Neuroendocrinol . 2012. ; 33 : 315 – 327 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fogal B, Li J, Lobner D, McCullough LD, Hewett SJ . System x©- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury . J Neurosci . 2007. ; 27 : 10094 – 10105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gegelashvili G, Robinson MB, Trotti D, Rauen T . Regulation of glutamate transporters in health and disease . Prog Brain Res . 2001. ; 132 : 267 – 286 . [DOI] [PubMed] [Google Scholar]
- 19. Maragakis NJ, Dykes-Hoberg M, Rothstein JD . Altered expression of the glutamate transporter EAAT2b in neurological disease . Ann Neurol . 2004. ; 55 : 469 – 477 . [DOI] [PubMed] [Google Scholar]
- 20. Miller AH, Haroon E, Raison CL, Felger JC . Cytokine targets in the brain: impact on neurotransmitters and neurocircuits . Depress Anxiety . 2013. ; 30 : 297 – 306 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitew S, Kirkcaldie MT, Dickson TC, Vickers JC . Altered synapses and gliotransmission in Alzheimer’s disease and AD model mice . Neurobiol Aging . 2013. ; 34 : 2341 – 2351 . [DOI] [PubMed] [Google Scholar]
- 22. Sziklas V, Petrides M . Memory and the region of the mammillary bodies . Prog Neurobiol . 1998. ; 54 : 55 – 70 . [DOI] [PubMed] [Google Scholar]
- 23. Ma XC, Gottschall PE, Chen LT, Wiranowska M, Phelps CP . Role and mechanisms of interleukin-1 in the modulation of neurotoxicity . Neuroimmunomodulation . 2002. ; 10 : 199 – 207 . [DOI] [PubMed] [Google Scholar]
- 24. Moraes CA, Santos G, Spohr TCL. de SE, et al. Activated microglia-induced deficits in excitatory synapses through IL-1β: Implications for Cognitive Impairment in Sepsis . Mol Neurobiol . 2014. . doi:10.1007/s12035-014-8868-5 [DOI] [PubMed] [Google Scholar]
- 25. Ramírez G, Rey S, von Bernhardi R . Proinflammatory stimuli are needed for induction of microglial cell-mediated AbetaPP_{244-C} and Abeta-neurotoxicity in hippocampal cultures . J Alzheimers Dis . 2008. ; 15 : 45 – 59 . [DOI] [PubMed] [Google Scholar]
- 26. Hsiao HY, Chen YC, Chen HM, Tu PH, Chern Y . A critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in Huntington’s disease . Hum Mol Genet . 2013. ; 22 : 1826 – 1842 . [DOI] [PubMed] [Google Scholar]
- 27. Yirmiya R, Goshen I . Immune modulation of learning, memory, neural plasticity and neurogenesis . Brain Behav Immun . 2011. ; 25 : 181 – 213 . [DOI] [PubMed] [Google Scholar]
- 28. Liu B, Hong JS . Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention . J Pharmacol Exp Ther . 2003. ; 304 : 1 – 7 . [DOI] [PubMed] [Google Scholar]
- 29. von Bernhardi R, Tichauer JE, Eugenín J . Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders . J Neurochem . 2010. ; 112 : 1099 – 1114 . [DOI] [PubMed] [Google Scholar]
- 30. Deak F, Sonntag WE . Aging, synaptic dysfunction, and insulin-like growth factor (IGF)-1 . J Gerontol A Biol Sci Med Sci . 2012. ; 67 : 611 – 625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cohen E, Paulsson JF, Blinder P, et al. Reduced IGF-1 signaling delays age-associated proteotoxicity in mice . Cell . 2009. ; 139 : 1157 – 1169 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lorenzini A, Salmon AB, Lerner C, et al. Mice producing reduced levels of insulin-like growth factor type 1 display an increase in maximum, but not mean, life span . J Gerontol A Biol Sci Med Sci . 2014. ; 69 : 410 – 419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartke A, Bonkowski M, Masternak M . THow diet interacts with longevity genes . Hormones (Athens) . 2008. ; 7 : 17 – 23 . [DOI] [PubMed] [Google Scholar]
- 34. Masternak MM, Bartke A . Growth hormone, inflammation and aging . Pathobiol Aging Age Relat Dis . 2012. ; 2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) . Proc Natl Acad Sci U S A . 1997. ; 94 : 13215 – 13220 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Westbrook R, Bonkowski MS, Arum O, Strader AD, Bartke A . Metabolic alterations due to caloric restriction and every other day feeding in normal and growth hormone receptor knockout mice . J Gerontol A Biol Sci Med Sci . 2014. ; 69 : 25 – 33 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hong SL, Longo KA, Gosney E, Kopchick JJ . Increased metabolic flexibility and complexity in a long-lived growth hormone insensitive mouse model . J Gerontol A Biol Sci Med Sci . 2014. ; 69 : 274 – 281 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Panici JA, Wang F, Bonkowski MS, et al. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A Biol Sci Med Sci . 2009. ; 64 : 1126 – 1133 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice . J Gerontol A Biol Sci Med Sci . 2005. ; 60 : 1238 – 1245 . [DOI] [PubMed] [Google Scholar]
- 40. Masser DR, Bixler GV, Brucklacher RM, et al. Hippocampal subregions exhibit both distinct and shared transcriptomic responses to aging and nonneurodegenerative cognitive decline . J Gerontol A Biol Sci Med Sci . 2014. ; 69 : 1311 – 1324 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahn HJ, Hernandez CM, Levenson JM, Lubin FD, Liou HC, Sweatt JD . c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation . Learn Mem . 2008. ; 15 : 539 – 549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaltschmidt B, Ndiaye D, Korte M, et al. NF-kappaB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling . Mol Cell Biol . 2006. ; 26 : 2936 – 2946 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D . NF-kappa B functions in synaptic signaling and behavior . Nat Neurosci . 2003. ; 6 : 1072 – 1078 . [DOI] [PubMed] [Google Scholar]
- 44. Kaltschmidt C, Kaltschmidt B, Baeuerle PA . Brain synapses contain inducible forms of the transcription factor NF-kappa B . Mech Dev . 1993. ; 43 : 135 – 147 . [DOI] [PubMed] [Google Scholar]
- 45. Suzuki T, Mitake S, Okumura-Noji K, Yang JP, Fujii T, Okamoto T . Presence of NF-kappaB-like and IkappaB-like immunoreactivities in postsynaptic densities . Neuroreport . 1997. ; 8 : 2931 – 2935 . [DOI] [PubMed] [Google Scholar]
- 46. Boersma MC, Dresselhaus EC, De Biase LM, Mihalas AB, Bergles DE, Meffert MK . A requirement for nuclear factor-kappaB in developmental and plasticity-associated synaptogenesis . J Neurosci . 2011. ; 31 : 5414 – 5425 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oikawa K, Odero GL, Platt E, et al. NF-κB p50 subunit knockout impairs late LTP and alters long term memory in the mouse hippocampus . BMC Neurosci . 2012. ; 13 : 45 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prow NA, Irani DN . The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis . J Neurochem . 2008. ; 105 : 1276 – 1286 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bartke A, Chandrashekar V, Turyn D, et al. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice . Proc Soc Exp Biol Med . 1999. ; 222 : 113 – 123 . [DOI] [PubMed] [Google Scholar]
- 50. Yan H, Mitschelen M, Bixler GV, et al. Circulating IGF1 regulates hippocampal IGF1 levels and brain gene expression during adolescence . J Endocrinol . 2011. ; 211 : 27 – 37 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A . Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice . Neurobiol Aging . 2005. ; 26 : 929 – 937 . [DOI] [PubMed] [Google Scholar]
- 52. Carro E, Nuñez A, Busiguina S, Torres-Aleman I . Circulating insulin-like growth factor I mediates effects of exercise on the brain . J Neurosci . 2000. ; 20 : 2926 – 2933 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Magnusson KR, Das SR, Kronemann D, Bartke A, Patrylo PR . The effects of aging and genotype on NMDA receptor expression in growth hormone receptor knockout (GHRKO) mice . J Gerontol A Biol Sci Med Sci . 2011. ; 66 : 607 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]


