Abstract
Background.
The motoric cognitive risk (MCR) syndrome, characterized by slow gait and cognitive complaints, is a simple and easily accessible clinical approach to identify older adults at high risk for transitioning to dementia. This study aims to define subtypes of MCR based on individual quantitative gait variables and to compare their neuropsychological profiles and risk factors as well risk for incident cognitive impairment.
Methods.
MCR was diagnosed in 314 community-residing, nondemented, older adults aged 65 and older (56% women) based on the presence of cognitive complaints and slow gait velocity (MCRv). Four new subtypes of MCR were defined by substituting slow gait with short stride length (MCRsl), slow swing time (MCRsw), high stride length variability (MCRslv), and high swing time variability (MCRswv). MCR subtypes were not mutually exclusive.
Results.
A total of 25 participants (8%) met criteria for MCRv, 20 for MCRsl (6.4%), 15 for MCRsw (4.8%), 16 for MCRslv (5.1%), 12 for MCRswv (3.8%), and 266 participants (84.7%) did not meet criteria for any MCR subtype. At baseline, MCRv was associated with deficits in attention and language as well as in overall cognitive status. MCRswv was associated with deficits in all cognitive domains including memory. Obesity and sedentariness were risk factors of MCRv, MCRsl, and MCRsw. MCRv status predicted incident cognitive impairment in global cognition (odds ratio: 3.59, p = .016), whereas MCRswv status predicted incident cognitive impairment in memory (odds ratio: 4.24, p = .048).
Conclusions.
MCR subtypes based on individual gait parameters show commonalities and differences in cognitive profiles and risk factors. Future studies should investigate whether the MCR subtypes predict different subtypes of dementia.
Keywords: Motoric cognitive risk syndrome, Gait, Neuropsychological assessment, Dementia.
The recently described motoric cognitive risk (MCR) syndrome is characterized by cognitive complaints and slow gait and identifies nondemented older individuals at high risk for transitioning to dementia ( 1–3 ). MCR syndrome relies on the strong relationship between gait, cognitive function, and the risk of dementia ( 4 ). The worldwide prevalence of MCR was estimated at 9.7% based on a sample of 26,082 older adults (age range: 60–114 years) from 17 countries and was associated with an increased risk of dementia ( 2 ). The incidence rate of MCR was reported to be 65.2 per 1000 person-years (age range: 60–100 years), with Parkinson’s disease, stroke, depressive symptoms, obesity, and sedentariness identified as the major risk factors ( 3 ).
Gait velocity can be easily measured in clinical settings without requiring burdensome equipment or procedures. Hence, the use of gait velocity as a criterion of MCR enhances the accessibility of MCR as a dementia risk assessment tool in a wide variety of clinical settings. However, it is unknown whether gait velocity represents the optimal gait measure to be used in combination with cognitive complaints to predict future cognitive decline and dementia. Different quantitative gait parameters show differential associations with various cognitive domains ( 5–9 ). For instance, swing time was reported to predict decline in memory domain, whereas stride length predicted decline in executive domain ( 5 ), highlighting that quantitative gait parameters can predict cognitive decline ( 5 , 10 , 11 ) and dementia ( 5 , 12 ) independently from cognitive performances.
In this study, we proposed to adapt the current operational definition of MCR to define four new subtypes of MCR syndrome by substituting the slow gait criterion with other quantitative gait variables (stride length, swing time, stride length variability, and swing time variability) with established links to cognitive impairment in older adults ( 5 , 6 ). We examined and compared the cognitive profiles and risk factors of each MCR subtype in healthy, nondemented, older adults. Further, we explored the predictive value of each MCR subtype for incident cognitive impairment. Based on previous evidence showing that individual gait measures are linked to different cognitive domains ( 5 ), we hypothesized that MCR subtypes are differentially associated with specific cognitive domains and will predict different cognitive trajectories. This approach is similar to the concept of amnestic and nonamnestic mild cognitive impairment, which are characterized by impairments in different domains of cognitive function, and have been shown to predict Alzheimer’s and non-Alzheimer’s dementias, respectively ( 13 ). Defining different subtypes of MCR using alternate quantitative gait parameters may provide new insights into preclinical markers of dementia and help improve identification of patients at high risk for dementia.
Methods
Participants
The study population included 314 participants from the “Central Control of Mobility in Aging” study, a prospective cohort study of community-dwelling adults aged 65 and older recruited from lower Westchester County, NY. Study design has been previously reported ( 14 ). In brief, after completing a telephone screening interview, eligible individuals were scheduled for two in-person visits at the research center. During the study visits, participants received neuropsychological, cognitive, psychological, and mobility assessments as well as a neurological examination. Exclusion criteria were inability to speak English, inability to ambulate independently, presence of dementia (previous diagnosis or failing cognitive screeners) or progressive neurological diseases, such as Parkinson’s disease, significant loss of vision and/or hearing, current diagnosis or history of psychiatric disorders, recent or anticipated medical procedures that may affect mobility, and patients on hemodialysis. Comprehensive in-person assessments were completed at baseline and follow-up visit (median interval between baseline and follow-up visits: 728.0 days [interquartile range: 392–770 days]) in all participants included in this study. Written informed consents were obtained at clinic visits according to study protocols, and the study was approved by the Einstein Institutional Review Board.
Gait Assessment
Gait was measured using an instrumented walkway with embedded pressure sensors (GAITRite, CIR systems, Havertown, PA). The walkway measures 8.5 m × 0.9 m × 0.01 m ( L × W × H ) with an active recording area of 6.1 m × 0.61 m ( L × W ). Participants were asked to walk for one trial on the instrumented walkway at their usual pace in a quiet, well-lit room wearing comfortable footwear and without any attached monitors. Based on the results from previous quantitative gait studies ( 5 ), velocity (cm/s) as well as stride length (cm), swing time (s), stride length variability ( SD ), and swing time variability ( SD ) were selected to define new MCR subtypes. The four new gait variables were selected as they had the highest loading in the three previously described domains of gait (pace, rhythm, and variability) ( 5 , 6 ).
MCR Syndrome and Subtypes
Participants were diagnosed with MCR, if they met all the following four criteria at baseline evaluations:
Cognitive complaint assessed by one or more of the following: a score of ≥0.5 on the Clinical Dementia Rating scale rated by the study clinician ( 15 ), a ‘yes’ response to the memory impairment item on the 30-item Geriatric Depression Scale ( 16 ), or a score of ≥1 on the AD8-dementia screener ( 17 ).
Slow gait defined as 1 SD or more below age- and sex-appropriate mean values established within the same cohort (MCRv) and may include participants with neurological as well as nonneurological gait abnormalities. For the four new subtypes of MCR, we substituted the slow gait criterion with abnormalities in the alternative gait variables: MCR stride length (MCRsl), MCR swing time (MCRsw), MCR stride length variability (MCRslv), and MCR swing time variability (MCRswv). Short stride length and slow swing time were defined as 1 SD or more below age- and sex-appropriate mean values and high stride length variability and high swing time variability as 1 SD or more above age- and sex-appropriate mean values. The MCR subtypes were not mutually exclusive.
Preserved activities of daily living based on a standardized scale ( 18 ) as well clinical interview.
Absence of dementia. Dementia diagnoses were assigned after review of all available clinical and neuropsychological information at consensus case conferences.
Cognitive and Behavioral Assessment
A neuropsychological test battery validated in our and other aging populations was administered at all visits to all participants ( 19 ). We focused on the performances on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which consists of 12 subtests making up five indices: immediate memory, visuospatial/constructional, language, attention, and delayed memory ( 20 ). For the assessment of memory, we focused only on the delayed memory index. Of note, the neuropsychological test information was not used to assign MCR diagnosis.
Risk Factors for MCR
We examined the association of recently published potentially modifiable risk factors for incident MCRv ( 3 ) Parkinson’s disease, stroke, depressive symptoms, sedentariness, and obesity—with each MCR subtype controlling for age, gender, and education. Stroke was self-reported during the medical interview. Depressive symptoms were defined as a previously validated cutscore of more than 5 on the 30-item Geriatric Depression Scale ( 16 ); sedentariness was self-reported difficulty in walking less than quarter mile or negotiating stairs ( 21 , 22 ), and obesity was defined as a body mass index of ≥30kg/m 2 . Only two participants reported a diagnosis of Parkinson’s disease; therefore, it was not included in the risk factors analysis.
Data Analysis
Two-sample t -tests or Fisher’s exact test, as appropriate, were used to assess differences in MCR status between participants meeting criteria for each of the MCR subtypes and those who did not meet criteria for each subtype ( Table 1 and Supplementary Tables for the detailed performance of the others). Linear regressions adjusted for age, gender, and education were performed to assess the association between the different MCR subtypes (independent variable) and RBANS indexes (dependent variable) at baseline. Logistic regressions adjusted for age, gender, and education were performed to assess the association between risk factors (independent variable) and baseline MCR subtype diagnosis (dependent variable) at baseline. Logistic regressions adjusted for age, gender, and education were performed to assess the association between baseline MCR status (independent variable) and RBANS indexes (dependent variable) at follow-up. In addition, we examined incident cognitive impairment at follow-up as a dichotomous outcome, which was defined as RBANS index scores at follow-up visits that were 1 SD or more below scores of the sample at baseline. Participants with scores 1.5 SD or more below the mean at baseline were excluded from this analysis to avoid including participants with prevalent significant cognitive decline at baseline. All analyses were conducted using SPSS version 21 (SPSS Inc., Chicago, IL).
Table 1.
Clinical Characteristics of MCR Subtypes † ( n = 314)
| MCR Velocity ( n = 25) | MCR Stride Length ( n = 20) | MCR Swing Time ( n = 15) | MCR Stride Length Var ( n = 16) | MCR Swing Time Var ( n = 12) | |
|---|---|---|---|---|---|
| Age, y (range) | 79.4±8.1 (69.7–95.4) | 78.5±7.3 (69.9–95.4) | 78.0±6.9 (68.5–91.7) | 79.1±7.3 (69.9–91.8) | 83.9±7.1* (73.3–95.4) |
| Female, % | 32* | 30* | 47 | 38 | 58 |
| Education, y | 14.2±2.4 | 14.9±2.5 | 14.9±2.1 | 13.9±2.1 | 15.9±2.3 |
| Gait | |||||
| Velocity, cm/s | 66.1±13.4* | 67.5±18.1* | 98.6±31.1 | 79.1±24.0* | 70.1±17.5* |
| Stride length, cm | 92.9±20.2* | 87.9±18.3* | 104.3±25.1* | 98.6±22.0* | 88.8±22.1* |
| Stride length SD , cm | 3.81±1.65* | 4.74±2.75* | 3.37±3.21 | 6.16±3.32* | 3.73±1.34 |
| Swing time, s | 0.43±0.07* | 0.39±0.06 | 0.34±0.03* | 0.42±0.05 | 0.38±0.07 |
| Swing time SD , s | 0.03±0.01* | 0.03±0.01* | 0.02±0.01 | 0.03±0.01* | 0.04±0.01* |
| Risk factors | |||||
| Stroke, n (%) | 3 (12) | 5 (25)* | 2 (13) | 4 (25)* | 1 (8) |
| Obesity, n (%) | 13 (52)* | 14 (70)* | 9 (60)* | 7 (44) | 5 (42) |
| Depres sympt, n (%) | 12 (45) | 13 (65)* | 9 (60)* | 8 (50) | 8 (67)* |
| Sedentariness, n (%) | 14 (56)* | 12 (60)* | 8 (53)* | 4 (25) | 8 (67)* |
Notes : Obesity: body mass index ≥ 30; Depressive symptoms: score >5 at the 30-item Geriatric Depression Scale; Sedentariness: walking less than quarter mile or difficulty negotiating stairs. Depres sympt = depressive symptoms; MCR = motoric cognitive risk; stride length var: stride length variability; swing time var: swing time variability.
*Difference with non-MCR group ( p < .05).
† MCR subtypes are not mutually exclusive.
Results
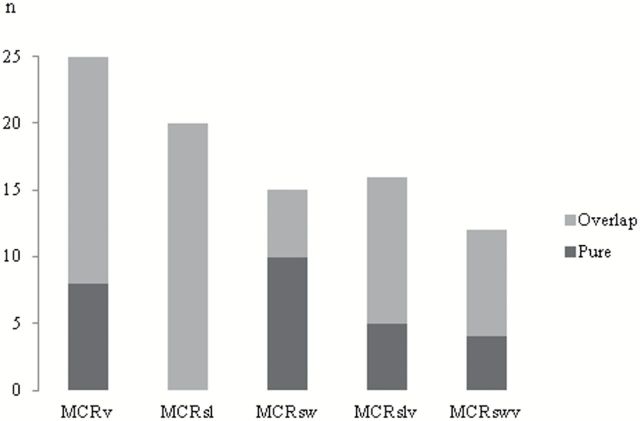
At baseline, the mean age of the 314 participants was 76.9±6.8 years (range: 65.1–95.9 years), with 56% women and a mean education of 14.6±3.1 years. The clinical characteristics of each MCR subtype are presented in Table 1 , and demographics of their non-MCR counterparts are provided in Supplementary Tables . A total of 25 participants (8.0%) met criteria for MCRv at baseline, 20 for MCRsl (6.4%), 15 for MCRsw (4.8%), 16 for MCRslv (5.1%), and 12 for MCRswv (3.8%). As MCR subtypes were not mutually exclusive, 48 participants (15.3%) met criteria for any one of the five MCR subtypes, and among them, 22 participants (7.0%) met criteria for more than one MCR subtype. Overlap between MCR subtypes was seen in 68% of participants meeting criteria for MCRv; 100% of MCRsl, 33% of MCRsw, 69% of MCRslv, and 67% of MCRswv ( Figure 1 ). Only MCRswv participants were significantly older than their healthy counterparts ( p < .01). More men received a MCRv ( p = .02) and MCRsl ( p = .02) diagnosis than their healthy counterparts. All participants with MCR diagnoses walked significantly slower than their healthy counterparts except for participants with MCRsw.
Figure 1.
Degree of overlap among MCR subtypes. Illustration of the repartition of participants meeting criteria for each MCR subtypes (MCRsl = MCR stride length; MCRslv = MCR stride length variability; MCRsw = MCR swing time; MCRswv = MCR swing time variability; and MCRv = MCR velocity; ) between pure MCR (without overlap with any other MCR subtypes) and overlap MCR (participant meeting criteria for at least another MCR subtype). n = number of participants.
MCR Subtypes and Baseline Cognitive Profiles
The associations between MCR subtypes and baseline cognitive profiles are presented in Table 2 . MCRv, MCRsl, and MCRswv were associated with lower global cognitive function, with the highest association seen for MCRswv (β: −13.62, 95% confidence interval [CI]: −20.6 to −6.7, p < .001). MCRswv was the only subtype associated with all the four cognitive domains at baseline including impaired memory (β: −6.73, 95% CI: −12.8 to −0.7, p < .001). The 48 participants meeting criteria for any one of the five MCR subtypes were associated with impairments in global cognitive function (β: −5.70, 95% CI: −9.4 to −2.0, p = .003), in attention (β: −5.46, 95% CI: −10.1 to −0.8, p = .021), and in language (β: −6.69, 95% CI: −10.0 to −3.4, p < .001).
Table 2.
Linear Regression—Adjusted for Age, Gender, and Education—Showing the Association Between MCR Subtypes* (independent variable) and Cognition (RBANS indexes) at Baseline (dependent variable)
| Global β (95% CI), p value | Memory β (95% CI), p value | Attention β (95% CI), p value | Language β (95% CI), p value | Visuospatial β (95% CI), p value | |
|---|---|---|---|---|---|
| MCR velocity | −8.05 (−12.9 to −3.2), .001 | −3.62 (−7.9 to 0.6), .094 | −9.75 (−15.9 to −3.6), .002 | −9.16 (−13.5 to −4.8), <.001 | −1.83 (−7.5 to 3.8), .523 |
| MCR stride length | −8.31 (−13.7 to −2.9), .003 | −4.42 (−9.1 to 0.3), .064 | −8.04 (−14.8 to −1.3), .020 | −9.40 (−14.2 to −4.6), <.001 | −0.94 (−7.2 to 5.3), .766 |
| MCR swing time | 0.12 (−6.1 to 6.3), .969 | 1.88 (−3.4 to 7.2), .487 | 1.38 (−6.4 to 9.1), .727 | −3.69 (−9.3 to 1.9), .194 | 3.59 (−3.5 to 10.6), .317 |
| MCR stride length var | −4.97 (−11.0 to 1.1), .107 | −4.26 (−9.4 to 0.9), .107 | −3.94 (−11.5 to 3.6), .307 | −4.94 (−10.4 to 0.5), .075 | −1.94 (−8.8 to 4.9), .579 |
| MCR swing time var | −13.62 (−20.6 to −6.7), <.001 | −6.73 (−12.8 to −0.7), .029 | −10.81 (−19.6 to −2.0), .016 | −11.24 (−17.5 to −5.0), <.001 | −11.11 (−19.1 to −3.2), .006 |
Notes: Boldface values indicate p values <.05. CI = confidence interval; MCR = motoric cognitive risk; Stride length var = stride length variability; Swing time var = swing time variability.
*The comparison group is constituted by the participants, who did not meet MCR subtype criteria.
MCR Subtypes and Incident Cognitive Impairment
All 314 participants had one or more annual follow-up visits. MCR subtypes presented different profiles of incident cognitive impairment ( Table 3 ). MCRv (odds ratio [ OR ]: 3.59, 95% CI: 1.3–10.1, p = .016) and MCRsl ( OR : 3.44, 95% CI: 1.1–11.0, p = .037) predicted risk of incident global cognitive impairment. Incident memory impairment was predicted by MCRswv ( OR : 4.24, 95% CI: 1.0–17.8, p < .048). Participants with MCRsw and MCRslv did not present any increased risk of incident cognitive impairment for any of the cognitive domains.
Table 3.
Logistic Regression—Adjusted for Age, Gender, and Education—Showing Associations Between MCR Subtypes* (independent variable) and Incident Impairment † on Different Cognitive Domains (dependent variable)
| Global OR (95% CI), p value | Memory OR (95% CI), p value | Attention OR (95% CI), p value | Language OR (95% CI), p value | Visuospatial OR (95% CI), p value | |
|---|---|---|---|---|---|
| MCR velocity | 3.59 (1.3–10.1), .016 | 0.96 (0.3–3.8), .954 | 0.45 (0.1–3.7), .456 | 3.51 (1.2–10.2), .020 | 1.83 (0.6–5.5), .282 |
| MCR stride length | 3.44 (1.1–11.0), .037 | 1.69 (0.4–6.8), .454 | 1.24 (0.3–5.9), .786 | 2.47 (0.7–8.2), .140 | 4.10 (1.4–12.5), .013 |
| MCR swing time | 0.55 (0.1–4.5), .574 | 0.59 (0.1–4.8), .624 | 3.04 (0.8–11.7), .107 | 0.58 (0.1–5.6), .602 | 2.65 (0.8–9.3), .129 |
| MCR stride length var | 1.52 (0.4–5.9), .542 | 0.48 (0.1–3.9), .493 | 1.74 (0.4–8.4), .492 | 1.08 (0.2–5.0), .922 | 1.86 (0.5–7.5), .382 |
| MCR swing time var | 2.61 (0.6–12.2), .221 | 4.24 (1.0–17.8), .048 | 2.16 (0.4–11.7), .371 | 2.024 (0.4–12.0), .346 | 5.09 (1.3–19.5), .018 |
Notes: Boldface values indicate p values <.05. Global = total score of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); Attention = attention index of the RBANS; Language = language index of the RBANS; MCR = motoric cognitive risk; Memory = delayed memory index of the RBANS; Stride length var = stride length variability; Swing time var = swing time variability; Visuospatial = visuospatial/constructional index of the RBANS.
*The comparison group is constituted by the participants, who did not meet MCR subtype criteria at baseline.
† Incident cognitive impairment is defined as a score of 1 SD or more below the mean baseline RBANS index scores at follow-up visits, after excluding participants with RBANS index scores of ≥ 1.5 SD below the mean at baseline.
MCR Subtypes and Risk Factors
MCRsl and MCRslv were both associated with a history of strokes ( Table 4 ). All of the MCR subtypes, except MCRslv, were associated with sedentariness. MCRsl was associated with all the four risk factors.
Table 4.
Logistic Regression—Adjusted for Age, Gender, and education—Showing Associations Between Risk Factors (independent variable) and MCR Subtypes (dependent variable)
| MCR Velocity OR (95% CI), p value | MCRsl OR (95% CI), p value | MCRsw OR (95% CI), p value | MCRslv OR (95% CI), p value | MCRswv OR (95% CI), p value | |
|---|---|---|---|---|---|
| Stroke | 1.95 (0.5–7.4), .324 | 5.53 (1.7–17.6), .004 | 2.14 (0.5–10.3), .340 | 6.08 (1.7–22.0), .006 | 0.95 (0.1–8.9), .961 |
| Obesity | 3.00 (1.2–7.2), .015 | 7.61 (2.6–22.1), <.001 | 4.55 (1.4–14.5), .010 | 2.20 (0.7–6.6), .161 | 2.52 (0.7–9.0), .156 |
| Depressive symptoms | 2.03 (0.9–4.8), .106 | 5.06 (1.9–13.7), .001 | 3.71 (1.2–11.7), .020 | 2.12 (0.8–6.0), .158 | 3.95 (1.1–14.7), .040 |
| Sedentariness | 4.16 (1.7–10.0), .001 | 5.96 (2.2–16.6), .001 | 3.73 (1.2–11.4), .021 | 0.99 (0.3–3.3), .983 | 4.38 (1.2–16.3), .028 |
Notes: Boldface values indicate p values <.05. Obesity: body mass index ≥ 30; Depressive symptoms: score >5 at the 30-item Geriatric Depression Scale; Sedentariness: walking less than quarter mile or difficulty negotiating stairs.
MCR = motoric cognitive risk; MCRsl = MCR stride length; MCRslv = MCR stride length variability; MCRsw = MCR swing time; MCRswv = MCR swing time variability; OR = odds ratio.
Discussion
We investigated cognitive and risk factors profiles of five different subtypes of MCR and their respective risk of incident cognitive impairment. Each MCR subtype was associated with specific cognitive and risk factor profiles at baseline. MCRv and MCRsl were associated with incident global cognitive impairment, whereas the MCRswv was associated with incident memory impairment.
The MCR conceptual framework tested in this analysis aimed to better identify older adults at high risk for cognitive disorders. We also sought to highlight differences in cognitive profiles of different MCR subtypes, which may indicate different underlying dementia pathologies. Similar to the classification of mild cognitive impairment into amnestic and nonamnestic subtypes ( 13 ), which are predictive of different types of dementia, three of the five subtypes of MCR predicted incident cognitive impairment in specific cognitive domains. MCRv was associated with incident global cognitive impairment, suggesting its ability to predict dementia overall ( 1 , 2 ). On the other hand, MCRsl predicted incident cognitive impairment in overall cognition as well as in visuospatial domain. Decreased stride length is the hallmark of synucleinopathies (ie Parkinson’s disease) ( 23 , 24 ), and visuospatial domains are typically affected in these pathologies ( 25 ). MCRswv was associated with impairments in all cognitive domains and with an increased risk of incident memory impairment ( OR : 4.24). Disturbed control of temporal variability, as measured by swing time variability, has been associated with Alzheimer’s disease ( 12 , 26 ). Hence, the MCRswv subtype could represent a preclinical marker of Alzheimer’s disease and may reflect the observation that swing time variability represents a marker of higher level of gait control ( 9 , 27 ) and is strongly associated with cognitive function ( 5 , 27 , 28 ). As objective cognitive deficits are not required to meet criteria for MCR, we found that two MCR subtypes (MCRsw and MCRslv) were not associated with baseline cognitive function or incident cognitive impairment. MCRv, MCRsl, and MCRswv appear to capture different dementia pathologies, but further clinicopathological studies are needed to build on these findings. Note that the individual gait parameters used to define each MCR subtype load highest on the previously identified gait domains (ie pace, rhythm, or variability) ( 5 ).
The association between the selected gait parameters and brain structures could help to understand the link between MCR subtypes and cognitive functions. Slow gait was associated with global cerebral atrophy and subcortical white matter hyperintensities ( 29 ) that represents by itself a main contributor of cognitive decline ( 30 ). Stride length was related to reduced gray matter volume; however, this relationship has not been found in other gait parameters, like cadence ( 31 ). These observations have been supported by animal models showing that stride length and velocity are controlled supraspinally by phasic outputs from the basal ganglia to the supplementary motor area ( 32 )—essential network involved in cognitive functioning, especially involved in attention and executive function processing ( 33 )— highlighting the relationship of MCRv and MCRsl with cognition. Hippocampal regions—typically affected in early Alzheimer’s disease ( 34 )—have been associated with control of gait in aging ( 35 ) and especially with the variability of the temporal gait parameters ( 36 ), supporting our hypothesis that MCRswv could represent an early marker of Alzheimer’s disease. This insight into the neural correlates of gait supports the idea that the different MCR subtypes could represent novel clinical markers of different dementia syndromes. Based on the cognitive profile of the MCR subtypes and the close relationship between gait parameters and brain regions, we hypothesized that MCRv could represent a transitional state between normal aging and dementia in general, whereas MCRswv is specifically associated with Alzheimer’s disease, and MCRsl with synucleinopathies, like Parkinson’s disease or dementia with Lewy’s bodies.
The specificity of MCR subtypes for distinct dementia pathologies is also supported by the differences in risk factor profiles. Stroke was associated with only two subtypes, MCRslv ( OR : 6.08) and MCRsl ( OR : 5.53). Sedentariness has been linked to increased risk of dementia ( 37 ) and was associated with all MCR subtypes except MCRslv. Increased level of depressive symptoms (using the Geriatric Depression Scale as a continuous measure) was associated with worse velocity, stride, and swing time variability ( 38 ). MCRsl, MCRsw, and MCRswv, but not MCRv, were associated with depressive symptoms in this analysis, confirming the strong relationship between gait and depression ( 38–40 ). Obesity was a main risk factor for MCRsl ( OR : 7.61), MCRsw ( OR : 4.55), and MCRv subtypes ( OR : 3.00). Obesity has been reported to be a risk factor for cognitive decline ( 41 ) and dementia ( 42 , 43 ) and is associated with temporal atrophy ( 44 ).
A number of potential limitations are noted. The prevalence of various MCR subtypes was lower than the recently reported global prevalence of MCR ( 2 ), which might reflect the healthier nature of this volunteer sample, but resulted in a lower prevalence of risk factors and a small number of participants meeting criteria for some MCR subtypes. These results should be confirmed in different and more diverse populations and with longer term longitudinal studies. Furthermore, the MCR subtypes were not mutually exclusive, 45% of the participants meeting criteria for any MCR subtype showed overlap between MCR subtypes. Although we followed the published guidelines to quantify gait in older adults ( 45 ), measuring gait on a longer distance would increase the accuracy of gait parameters. A longer follow-up period would help verify our hypothesis regarding the differential prediction of MCR subtypes for different dementia subtypes. Only a very small percentage of the participants had brain imaging studies done as part of the Central Control of Mobility in Aging study protocol, which prevented further biological examination. A major advantage of the MCR concept is its simple, clinically relevant, and inexpensive approach; however, the new MCR subtypes require the access to an instrumented walkway or similar devices that might limit its use in many clinical settings. Nonetheless, these MCR subtypes have the potential of providing valuable dementia insights in research settings that could be then translated to clinical settings.
MCR subtypes are able to identify older adults with different cognitive and risk factors profiles and predict incident cognitive impairment. The classical MCRv subtype predicts incident global cognitive impairment, whereas MCRsl and MCRswv seem to prelude incident cognitive impairment due to synucleinopathies and Alzheimer pathology, respectively. Using these expanded MCR criteria, clinicians will be able to anticipate older adults at risk of cognitive decline in different domains by combining cognitive complaints with different quantitative gait parameters.
Funding
This study was supported by funds from the National Institutes of Health, National Institute on Aging (R01AG036921-01A1 to R.H. and R01AG044007-01A1 to J.V.). G.A. is supported by a grant from the Geneva University Hospitals and the Resnick Gerontology Center, Albert Einstein College of Medicine, Yeshiva University.
Supplementary Material
Acknowledgments
We thank all of the Central Control of Mobility in Aging study clinicians and research students for their assistance with data collection.
References
- 1. Verghese J, Wang C, Lipton RB, Holtzer R . Motoric cognitive risk syndrome and the risk of dementia . J Gerontol A Biol Sci Med Sci . 2013. ; 68 : 412 – 418 . doi:10.1093/gerona/gls191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk . Neurology . 2014. ; 83 : 718 – 726 . doi:10.1212/WNL.0000000000000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verghese J, Ayers E, Barzilai N, et al. Motoric cognitive risk syndrome: multicenter incidence study . Neurology . 2014. ; 83 : 2278 – 2284 . doi:10.1212/WNL.0000000000001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H . Abnormality of gait as a predictor of non-Alzheimer’s dementia . N Engl J Med . 2002. ; 347 : 1761 – 1768 . doi:10.1056/NEJMoa020441 [DOI] [PubMed] [Google Scholar]
- 5. Verghese J, Wang C, Lipton RB, Holtzer R, Xue X . Quantitative gait dysfunction and risk of cognitive decline and dementia . J Neurol Neurosurg Psychiatry . 2007. ; 78 : 929 – 935 . doi:10.1136/jnnp.2006.106914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lord S, Galna B, Verghese J, Coleman S, Burn D, Rochester L . Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach . J Gerontol A Biol Sci Med Sci . 2013. ; 68 : 820 – 827 . doi:10.1093/gerona/gls255 [DOI] [PubMed] [Google Scholar]
- 7. Allali G, Assal F, Kressig RW, Dubost V, Herrmann FR, Beauchet O . Impact of impaired executive function on gait stability . Dement Geriatr Cogn Disord . 2008. ; 26 : 364 – 369 . doi:10.1159/000162358 [DOI] [PubMed] [Google Scholar]
- 8. Allali G, Kressig RW, Assal F, Herrmann FR, Dubost V, Beauchet O . Changes in gait while backward counting in demented older adults with frontal lobe dysfunction . Gait Posture . 2007. ; 26 : 572 – 576 . doi:10.1016/j.gaitpost.2006.12.011 [DOI] [PubMed] [Google Scholar]
- 9. Hausdorff JM . Gait variability: methods, modeling and meaning . J Neuroeng Rehabil . 2005. ; 2 : 19 . doi:10.1186/1743-0003-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ben Assayag E, Shenhar-Tsarfaty S, Korczyn AD, et al. Gait measures as predictors of poststroke cognitive function: evidence from the TABASCO study . Stroke . 2015. ;46:1077–1083. doi:10.1186/1743-0003-2-19 [DOI] [PubMed] [Google Scholar]
- 11. Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging . J Gerontol A Biol Sci Med Sci . 2013. ; 68 : 929 – 937 . doi:10.1093/gerona/gls256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM . Gait and cognition: a complementary approach to understanding brain function and the risk of falling . J Am Geriatr Soc . 2012. ; 60 : 2127 – 2136 . doi:10.1111/j.1532-5415.2012.04209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen RC . Clinical practice. Mild cognitive impairment . N Engl J Med . 2011. ; 364 : 2227 – 2234 . doi:10.1056/NEJMcp0910237 [DOI] [PubMed] [Google Scholar]
- 14. Holtzer R, Wang C, Verghese J . Performance variance on walking while talking tasks: theory, findings, and clinical implications . Age (Dordr) . 2014. ; 36 : 373 – 381 . doi:10.1007/s11357-013-9570-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morris JC . The Clinical Dementia Rating (CDR): current version and scoring rules . Neurology . 1993. ; 43 : 2412 – 2414 . doi:10.1212/WNL.43.11.2412-a 1526-632X [DOI] [PubMed] [Google Scholar]
- 16. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report . J Psychiatr Res . 1982. ; 17 : 37 – 49 . doi:10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- 17. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia . Neurology . 2005. ; 65 : 559 – 564 . doi:10.1212/01.wnl.0000172958.95282.2a [DOI] [PubMed] [Google Scholar]
- 18. Gill TM, Allore HG, Holford TR, Guo Z . Hospitalization, restricted activity, and the development of disability among older persons . JAMA . 2004. ; 292 : 2115 – 2124 . doi:10.1001/jama.292.17.2115 [DOI] [PubMed] [Google Scholar]
- 19. Holtzer R, Verghese J, Wang C, Hall CB, Lipton RB . Within-person across-neuropsychological test variability and incident dementia . JAMA . 2008. ; 300 : 823 – 830 . doi:10.1001/jama.300.7.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Randolph C, Tierney MC, Mohr E, Chase TN . The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity . J Clin Exp Neuropsychol . 1998. ; 20 : 310 – 319 . doi:10.1076/jcen.20.3.310.823 [DOI] [PubMed] [Google Scholar]
- 21. Verghese J, Katz MJ, Derby CA, Kuslansky G, Hall CB, Lipton RB . Reliability and validity of a telephone-based mobility assessment questionnaire . Age Ageing . 2004. ; 33 : 628 – 632 . doi:10.1093/ageing/afh210 [DOI] [PubMed] [Google Scholar]
- 22. Verghese J, Wang C, Xue X, Holtzer R . Self-reported difficulty in climbing up or down stairs in nondisabled elderly . Arch Phys Med Rehabil . 2008. ; 89 : 100 – 104 . doi:10.1016/j.apmr.2007.08.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stolze H, Kuhtz-Buschbeck JP, Drücke H, Jöhnk K, Illert M, Deuschl G . Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease . J Neurol Neurosurg Psychiatry . 2001. ; 70 : 289 – 297 . doi:10.1136/jnnp.70.3.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grabli D, Karachi C, Welter ML, et al. Normal and pathological gait: what we learn from Parkinson’s disease . J Neurol Neurosurg Psychiatry . 2012. ; 83 : 979 – 985 . doi:10.1136/jnnp-2012-302263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Calabresi P, Picconi B, Parnetti L, Di Filippo M . A convergent model for cognitive dysfunctions in Parkinson’s disease: the critical dopamine-acetylcholine synaptic balance . Lancet Neurol . 2006. ; 5 : 974 – 983 . doi:10.1016/S1474-4422(06)70600-7 [DOI] [PubMed] [Google Scholar]
- 26. Sheridan PL, Solomont J, Kowall N, Hausdorff JM . Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease . J Am Geriatr Soc . 2003. ; 51 : 1633 – 1637 . doi:10.1046/j.1532-5415.2003.51516.x [DOI] [PubMed] [Google Scholar]
- 27. Beauchet O, Launay CP, Annweiler C, Allali G . Hippocampal volume, early cognitive decline and gait variability: which association? Exp Gerontol . 2015. ; 61 : 98 – 104 . doi:10.1016/j.exger.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 28. Beauchet O, Annweiler C, Montero-Odasso M, Fantino B, Herrmann FR, Allali G . Gait control: a specific subdomain of executive function? J Neuroeng Rehabil . 2012. ; 9 : 12 . doi:10.1186/1743-0003-9-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Starr JM, Leaper SA, Murray AD, et al. Brain white matter lesions detected by magnetic resonance [correction of resosnance] imaging are associated with balance and gait speed . J Neurol Neurosurg Psychiatry . 2003. ; 74 : 94 – 98 . doi:10.1136/jnnp.74.1.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Garde E, Lykke Mortensen E, Rostrup E, Paulson OB . Decline in intelligence is associated with progression in white matter hyperintensity volume . J Neurol Neurosurg Psychiatry . 2005. ; 76 : 1289 – 1291 . doi:10.1136/jnnp.2004.055905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Callisaya ML, Beare R, Phan TG, Chen J, Srikanth VK . Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people . PLoS One . 2014. ; 9 : e84909 . doi:10.1371/journal.pone.0084909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Drew T, Prentice S, Schepens B . Cortical and brainstem control of locomotion . Prog Brain Res . 2004. ; 143 : 251 – 261 . doi:10.1016/S0079-6123(03)43025-2 [DOI] [PubMed] [Google Scholar]
- 33. Leunissen I, Coxon JP, Caeyenberghs K, Michiels K, Sunaert S, Swinnen SP . Task switching in traumatic brain injury relates to cortico-subcortical integrity . Hum Brain Mapp . 2014. ; 35 : 2459 – 2469 . doi:10.1002/hbm.22341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fox NC, Schott JM . Imaging cerebral atrophy: normal ageing to Alzheimer’s disease . Lancet . 2004. ; 363 : 392 – 394 . doi:10.1016/S0140-6736(04)15441-X [DOI] [PubMed] [Google Scholar]
- 35. Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F . The neural basis of age-related changes in motor imagery of gait: an fMRI study . J Gerontol A Biol Sci Med Sci . 2014. ; 69 : 1389 – 1398 . doi:10.1093/gerona/glt207 [DOI] [PubMed] [Google Scholar]
- 36. Annweiler C, Montero-Odasso M, Bartha R, Drozd J, Hachinski V, Beauchet O . Association between gait variability and brain ventricle attributes: a brain mapping study . Exp Gerontol . 2014. ; 57 : 256 – 263 . doi:10.1016/j.exger.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 37. Rovio S, Kåreholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease . Lancet Neurol . 2005. ; 4 : 705 – 711 . doi:10.1016/S1474-4422(05)70198-8 [DOI] [PubMed] [Google Scholar]
- 38. Brandler TC, Wang C, Oh-Park M, Holtzer R, Verghese J . Depressive symptoms and gait dysfunction in the elderly . Am J Geriatr Psychiatry . 2012. ; 20 : 425 – 432 . doi:10.1097/JGP.0b013e31821181c6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Demakakos P, Cooper R, Hamer M, de Oliveira C, Hardy R, Breeze E . The bidirectional association between depressive symptoms and gait speed: evidence from the English Longitudinal Study of Ageing (ELSA) . PLoS One . 2013. ; 8 : e68632 . doi:10.1371/journal.pone.0068632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanders JB, Bremmer MA, Deeg DJ, Beekman AT . Do depressive symptoms and gait speed impairment predict each other’s incidence? A 16-year prospective study in the community . J Am Geriatr Soc . 2012. ; 60 : 1673 – 1680 . doi:10.1111/j.1532-5415.2012.04114.x [DOI] [PubMed] [Google Scholar]
- 41. Anstey KJ, Kingston A, Kiely KM, Luszcz MA, Mitchell P, Jagger C . The influence of smoking, sedentary lifestyle and obesity on cognitive impairment-free life expectancy . Int J Epidemiol . 2014. ;43:1874–1883. doi:10.1093/ije/dyu170 [DOI] [PubMed] [Google Scholar]
- 42. Loef M, Walach H . Midlife obesity and dementia: meta-analysis and adjusted forecast of dementia prevalence in the United States and China . Obesity (Silver Spring) . 2013. ; 21 : E51 – E55 . doi:10.1002/oby.20037 [DOI] [PubMed] [Google Scholar]
- 43. Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K . Central obesity and increased risk of dementia more than three decades later . Neurology . 2008. ; 71 : 1057 – 1064 . doi:10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- 44. Gustafson D, Lissner L, Bengtsson C, Björkelund C, Skoog I . A 24-year follow-up of body mass index and cerebral atrophy . Neurology . 2004. ; 63 : 1876 – 1881 . doi:10.1212/01.WNL.0000141850.47773.5F [DOI] [PubMed] [Google Scholar]
- 45. Kressig RW, Beauchet O ; European GAITRite Network Group . Guidelines for clinical applications of spatio-temporal gait analysis in older adults . Aging Clin Exp Res . 2006. ; 18 : 174 – 176 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.