Abstract
High and low rates of ammonium supply are believed to favour ammonia-oxidising bacteria (AOB) and archaea (AOA), respectively. Although their contrasting affinities for ammonium are suggested to account for these differences, the influence of ammonia concentration on AOA and AOB has not been tested under environmental conditions. In addition, while both AOB and AOA contribute to nitrous oxide (N2O) emissions from soil, N2O yields (N2O–N produced per NO2−–N generated from ammonia oxidation) of AOA are lower, suggesting lower emissions when AOA dominate ammonia oxidation. This study tested the hypothesis that ammonium supplied continuously at low rates is preferentially oxidised by AOA, with lower N2O yield than expected for AOB-dominated processes. Soil microcosms were supplied with water, urea or a slow release, urea-based fertiliser and 1-octyne (inhibiting only AOB) was applied to distinguish AOA and AOB activity and associated N2O production. Low ammonium supply, from mineralisation of organic matter, or of the fertiliser, led to growth, ammonia oxidation and N2O production by AOA only, with low N2O yield. High ammonium supply, from free urea within the fertiliser or after urea addition, led to growth of both groups, but AOB-dominated ammonia oxidation was associated with twofold greater N2O yield than that dominated by AOA. This study therefore demonstrates growth of both AOA and AOB at high ammonium concentration, confirms AOA dominance during low ammonium supply and suggests that slow release or organic fertilisers potentially mitigate N2O emissions through differences in niche specialisation and N2O production mechanisms in AOA and AOB.
Subject terms: Microbiology, Ecology
Introduction
Microbes play central roles in global biogeochemical cycles but, despite evidence for niche differentiation, it is often difficult to identify and quantity the consequences of microbial community composition for rates of biogeochemical processes. Microbes are particularly important in the terrestrial nitrogen cycle, where they are solely responsible for many of the key processes, including nitrification, the oxidation of ammonia (NH3), via nitrite (NO2−), to nitrate (NO3−). NH3 oxidation is accompanied by production of nitrous oxide (N2O), an important greenhouse gas, with 300-fold and 20-fold greater global warming potentials than carbon dioxide and methane, respectively [1], while mono-nitrogen oxides, formed from N2O, contribute to depletion of stratospheric ozone [2]. N2O emissions following N-fertilisation of agricultural soils dominate N2O production in terrestrial environments and are predicted to increase with increased fertiliser demands [3]. Nitrification also leads to substantial loss of nitrogen fertilisers, through leaching of NO3− [4] and sequential reduction of NO3− by denitrifiers to NO2−, nitric oxide (NO), N2O and/or N2 [5].
There is some evidence for niche differentiation between archaeal and bacterial NH3 oxidisers (AOA and AOB), which are major players in soil NH3 oxidation [6, 7]. For example, the existence of obligately acidophilic AOA [8, 9], but not AOB, explains the global dominance of AOA in acid soils [10]. There is also evidence for a differential effect of ammonium (NH4+), with AOA rather than AOB being favoured in ‘low NH4+’, acidic or unfertilised soils [11–17, 49] and AOB being favoured in soils treated with high levels of NH4+-based fertiliser [14, 17, 18]. Attempts have been made to explain this by higher affinity for NH3 in AOA, based on reported lower Km values (0.13–0.69 µM total ammonia nitrogen (TAN, NH3 + NH4+) for cultured marine and soil AOA [19–21] than those for AOB, which are 2–4 orders of magnitude higher [22–24]. While this provides a compelling explanation for numerical dominance of AOA in oceans, where TAN concentrations are in the nM range, it is less convincing in soil where bulk TAN concentrations are above the range of Km values for both AOA and AOB. In addition, Hink et al. [25] and Kits et al. [26] report similar Km values for AOB (Nitrosomonas europaea), Nitrosopumilus maritimus and other AOA and differences in substrate affinity do not explain niche differentiation in fertilised soil, where NH3 will be in excess. An alternative explanation is greater sensitivity of AOA to inhibition by high NH4+ concentration, based on studies of relatively few cultured AOA, but the recently isolated and enriched AOA Candidatus Nitrosocosmicus species [27–29] can grow at NH3 concentrations that inhibit other cultured AOA, with Candidatus Nitrosocosmicus franklandus growing at up to 100 mM NH4+, suggesting that NH3 toxicity does not clearly differentiate AOA and AOB. Soil microcosm studies also suggest greater complexity in the relationships between NH3 concentration, supply rate and AOA and AOB abundance and activity. For example, AOA can grow in soil amended with 0, 20 or 200 µg NH4+–N g−1 soil [18], can dominate oxidation of NH3 derived from mineralisation of native or added organic nitrogen and may not be stimulated by addition of inorganic NH4+ [49, 30]. These findings therefore suggest that AOB will dominate NH3 oxidation after addition of N-fertiliser at high concentration, while AOA will dominate when NH4+ is supplied at low rates, through mineralisation of organic or of slow-release fertilisers.
Niche differentiation of AOA and AOB associated with NH4+ supply has the potential to influence, significantly, N2O emissions due to their apparently distinct physiological processes. AOB produce N2O enzymatically via conversion of hydroxylamine (an intermediate in NH3 oxidation) to N2O via NO, rather than NO2− (incomplete hydroxylamine oxidation) [31, 32], and via nitrifier denitrification, the sequential reduction of NO2− to NO and N2O [32, 33]. In contrast, there is no genomic or physiological evidence for enzymatic production of N2O by AOA and NH3 oxidation-associated N2O emission is believed to result from an abiotic reaction between hydroxylamine and NO or NO2− [34, 35]. These physiological differences are consistent with measured N2O yields (N2O–N produced per NO2–N generated from NH3 oxidation), with those from AOA cultures (0.004–0.23%) at the lower end of the range of those from AOB cultures (0.1–1%) [35–40]. This is also consistent with the low N2O yield associated with NH3 oxidation by AOA in an agricultural soil (~0.5‰), approximately half that of AOB [17]. These results therefore suggest that fertilisation strategies stimulating AOA rather than AOB would lead to lower N2O emissions in agricultural soils.
Material and methods
Soil microcosms
Soil microcosms were constructed as described in Hink et al. [17]. Briefly, soil was sampled from the upper 10 cm of a pH 6.5 sandy loam agricultural soil (SRUC, Craibstone, Scotland; grid reference NJ872104) with an organic C content of 5.9–6.4% (for soil characteristics see Kemp et al. [41] and Bartram et al. [42]) before sieving (3.35-mm mesh size) and storage at 4 °C before use. Water content was determined by drying the soil at 100°C for 24 h and microcosms were established in 120-ml serum bottles filled with 13.6 ± 0.02 g fresh soil (10 g dry soil, 27% volumetric water content). Bottles were sealed with butyl rubber stoppers and aluminium caps and pre-incubated at 30 °C in the dark for 7 days. Aerobic conditions were maintained by opening and re-sealing bottles after 4 days.
Following pre-incubation, microcosms were incubated at 30 °C in the dark, in the presence and absence of nitrification inhibitors (see below) with amendments designed to provide a single supply of NH4+ at high concentration or continuous supply of NH4+ at a low concentration during each of two phases of incubation (Fig. S1). NH4+ was supplied through mineralisation of native organic nitrogen and, in some treatments, by additional mineralisation of urea or of a slow-release, urea-based fertiliser (Azolon, Aglukon, Düsseldorf, Germany) that contains 15% free urea and 85% polymethylene urea. Free urea was converted to NH4+ within 8 h by ureolytic soil microorganisms, while urea was released at a low rate by mineralisation of polymethylene urea.
Mineralisation of native organic nitrogen occurred in all treatments and was the sole source of NH4+ in controls (addition of water only). In all fertiliser treatments, NH4+ was also produced by mineralisation, at a similar rate, of polymethylene urea. Both of these processes continued throughout incubation (for 24 days). Fertiliser addition at day 0 additionally led to a high initial concentration of NH4+, through mineralisation of free urea in the fertiliser, which was oxidised to NO3− within ~10 days (phase 1), determined in preliminary experiments. A second, single supply of high NH4+ was achieved by addition of free urea to previously fertilised (and non-inhibited) treatments at the end of phase 1. This was rapidly converted to NH4+ and oxidised within the first 7 days of phase 2 (days 10–24).
Thus, at the beginning of phase 1, microcosms were amended with 0.5 ml water or 0.5 ml 1:100-diluted fertiliser. The final volumetric water content in all microcosms was 29% (equivalent to ~60% water-filled pore space (WFPS)). At the beginning of phase 2, microcosms were amended with 0.3 ml water or 0.3 ml urea solution (50 µg N g−1 soildw) resulting in a volumetric water content of 30%.
Microcosms were also incubated in the presence or absence of the NH3 oxidiser inhibitors acetylene (inhibitory for both AOA and AOB) [12] and 1-octyne (inhibitory for AOB but not AOA) [17, 43]. This enabled differentiation between NH3 oxidation-related and non-NH3 oxidation-related processes, particularly N2O production, and between AOA and AOB activity and associated N2O production. Inhibitors were applied by injection into the headspace, with three treatments: air (no inhibitor), acetylene (0.1% v/v) or 1-octyne (0.03% v/v).
Each treatment was performed in triplicate. At each sampling point (at least twice weekly), gas samples (5 ml) were taken from each microcosm and transferred into evacuated 3-ml glass vials (Labco, Lampeter, UK) for subsequent N2O analysis. Microcosms were destructively sampled and soil was stored immediately after sampling at −20 °C for further chemical (storage ≤ 2 weeks) and molecular analysis (storage ≤ 4 months). Oxic conditions in the remaining microcosms were maintained by opening and re-sealing twice weekly, while re-establishing concentrations of acetylene or 1-octyne where appropriate.
Chemical analysis of soil and gas samples
Due to the rapid conversion of urea to NH4+, nitrification kinetics were determined as temporal changes in concentrations of NH4+, NO2− and NO3−, which were measured colorimetrically in 96-well microplates following KCl (1 M) extraction (1:5 soil:KCl ratio (v/v)). Ammonium was determined using the indophenol method [44] as described previously [8], with a detection limit of 20 µM. The measurement of NO2− and NO3− was modified from Shinn [45], and Doane and Horwath [46] as follows: NO2−was measured in a 50-µl sample by sequentially adding 60 µl of diazotising reagent (2.2 mM sulphanilamide in 3.3 M HCl) and 20 µl coupling reagent (0.12 mM N-(1-naphthyl)-ethylenediamine in 0.12 M HCl). NO2− and combined NO2− and NO3− were determined before and after reduction of NO3− to NO2− by addition of 20 µl vanadium chloride solution (4.5 mM vanadium(III) chloride in 1 M HCl) and incubation in the dark at 35 °C for 90 min, respectively. NO2− was below the detection level (2.5 µM) in all samples and nitrification kinetics were determined as rates of NH3 oxidation or NO3− production. Soil pH was measured in water (1.5 g wet soil + 3 ml deionised water). N2O in the headspace samples was determined with an Agilent 6890 gas chromatograph equipped with a 63Ni electron capture detector (Santa Clara, CA, USA), as described in Hink et al. [17].
Nucleic acid extraction and estimation of ammonia oxidiser abundance
Nucleic acids were extracted from 0.5 g wet soil as described previously by Nicol and Prosser [47]. The abundances of AOA and AOB were assessed by qPCR of the respective ammonia monooxygenase subunit A (amoA) gene in all samples except those treated with acetylene, as previous studies using this soil (e.g. [12, 17]) have demonstrated complete inhibition of AOA and AOB growth and activity using the same or lower concentrations of acetylene. NH3 oxidiser growth was assessed by quantification of AOA and AOB amoA genes as described previously [48] with r2 values > 0.99 and amplification efficiencies of 95–99% and 88–95% for archaeal and bacterial assays, respectively.
Statistical analysis
Statistical analysis was performed using R 3.3.3 (http://www.rproject.org/). Temporal differences in NH4+, NO3− and N2O concentrations were assessed by ANOVA and comparison of the slopes of the linear models with fertiliser and inhibitor treatment as categorical factors and time as a continuous factor. N2O accumulation was analysed by fitting the regression to the cumulative data over time (estimated using all possible combinations of N2O accumulated in 3−4-day intervals of the destructively sampled replicates over the whole incubation period). Differences between N2O yield of AOA, AOB and combined AOA and AOB were determined by ANOVA, followed by a Tukey post hoc test. Differences in amoA gene abundance between treatments were assessed independently within phase 1 and phase 2 (due to an unbalanced design) by investigating the overlapping of 95% confidence intervals of linear regressions fitted to log10 transformed temporal changes in AOA amoA gene abundance (log10 y = ax + b) and quadratic polynomial regressions fitted to temporal changes in AOB amoA gene abundance (y = y0 + ax + bx2).
Results
Nitrification, N2O accumulation and ammonia oxidiser growth at low NH4+ supply
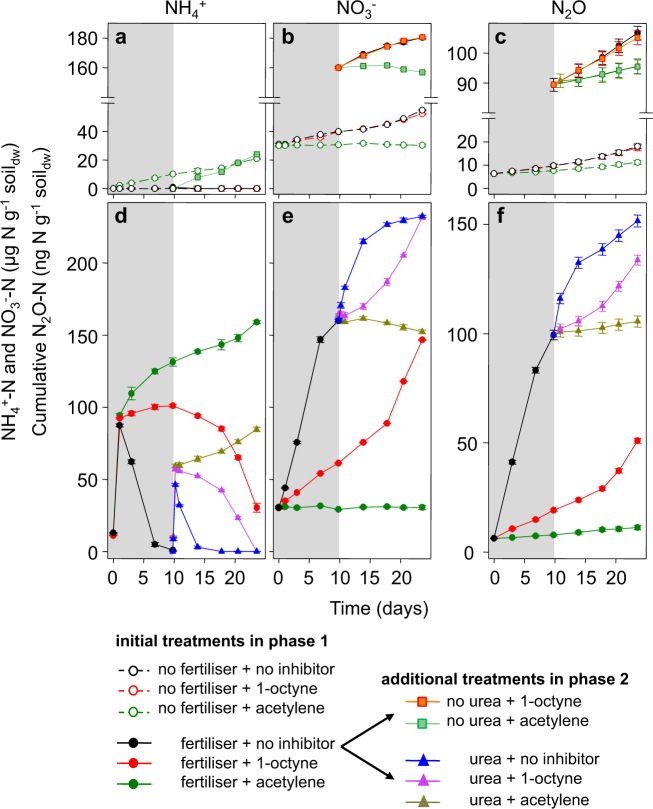
Nitrification in control microcosms was driven in both phases 1 and 2 by mineralisation of native organic nitrogen. Mineralisation rate during phase 1 was ~1 µg NH4+ g−1 soildw d−1, estimated as the increase in NH4+ in acetylene-amended control microcosms, in which both AOA and AOB were inhibited and NO3− production was negligible (Fig. 1a, b). A small increase in N2O (~6 ng N2O-N g−1 soildw after 24 days) was also observed in acetylene-treated microcosms (Fig. 1c).
Fig. 1. Changes in NH4+, NO3− and N2O during incubation of soil microcosms for 24 days.
Microcosms were incubated after amendment with a slow-release, urea-based fertiliser that contained 15% free urea, or with water only (no fertiliser), in combination with 1-octyne, acetylene or no inhibitor. a–c present data in which NH4+ was supplied at a low continuous rate, through slow mineralisation of native organic nitrogen (phases 1 and 2) or of polymethylene urea (phase 2). d–f present data in which NH4+ was supplied at a single high concentration, through rapid mineralisation of free urea within the slow-release fertiliser (phase 1) or through addition of urea (phase 2). Phase 1 (days 0–10) is indicated by a grey background and phase 2 (days 10–24) by a white background. Inhibitor treatments were applied to fertiliser-amended microcosms by additional amendment with urea or water. NH4+ and NO3− concentrations were determined in destructively sampled microcosms and cumulative N2O production was determined following repeated sampling of headspace gas. Data represent mean values and standard errors of three replicate microcosms
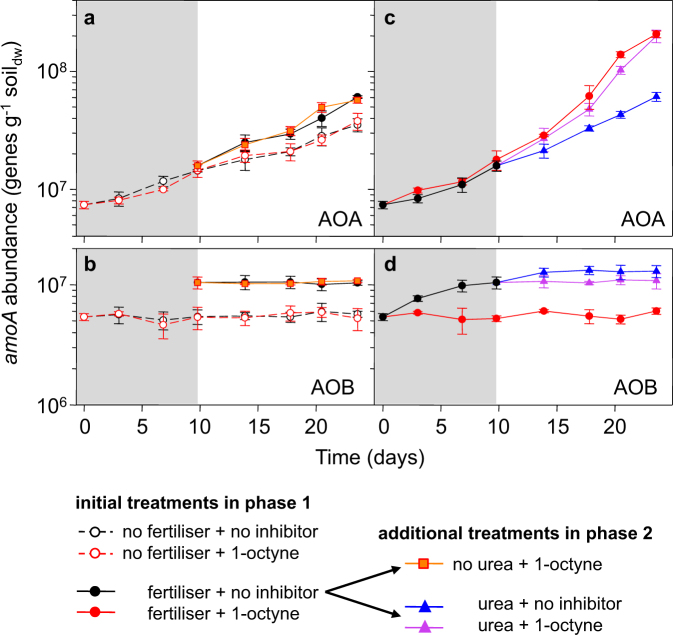
In non-inhibited control microcosms, NO3− was produced at a rate similar to the mineralisation rate (~1 µg NO3− g−1 soildw d−1) and NH4+ was below the minimum detection level throughout incubation (Fig. 1a, b). NH3 oxidation in these microcosms increased N2O production by ~7 ng N2O-N g−1 soildw (Fig. 1c). NO3− and N2O production rates in 1-octyne-inhibited and non-inhibited microcosms were not significantly different, suggesting that oxidation of NH3 derived from mineralisation of native organic nitrogen was performed by AOA and not AOB. This was confirmed by analysis of temporal changes in AOA and AOB abundances. AOA grew in control microcosms throughout incubation for 24 days and specific growth rates were similar in non-inhibited and 1-octyne-treated microcosms (Fig. 2a, Fig. S2a). AOB amoA gene abundance, however, did not change during incubation in the presence or absence of 1-octyne (Fig. 2c; Fig. S3a). Nitrification activity was accompanied in these microcosms by a slight decrease in pH, from 6.6 to 6.5 due to proton release associated with NH3 oxidation, as commonly observed (Fig. S4).
Fig. 2. Changes in abundance of archaeal and bacterial amoA genes during incubation of soil microcosms for 24 days.
Quantification was performed on extracted DNA from destructively sampled soil microcosms that were amended with fertiliser or water only (no fertiliser) in combination with 1-octyne or no inhibitor. a, b present data in which NH4+ was supplied at a low continuous rate, through slow mineralisation of native organic nitrogen (phases 1 and 2) or of polymethylene urea (phase 2). c, d present data in which NH4+ was supplied at a single high concentration, through rapid mineralisation of free urea within the slow-release fertiliser (phase 1) or through addition of urea (phase 2). Representation of phases 1 and 2 and treatments are as described in the legend for Fig. 1. Mean concentrations and standard errors of triplicate microcosms are plotted. Differences in temporal changes were assessed by comparing confidence intervals of regression analysis (see Figs. S2 and S3)
NH4+ was also supplied at low rates in fertilised microcosms during phase 2, after consumption, during phase 1, of NH4+ initially present at high concentration following conversion of free urea. These microcosms were incubated with or without addition of acetylene or 1-octyne in phase 2. Addition of acetylene enabled estimation of the rate of production of NH4+ in phase 2, derived from both slow release of urea and mineralisation of native organic nitrogen. This rate, ~2 µg NH4+ g−1 soildw d−1 (Fig. 1a), was approximately twice the rate of production from mineralisation of native organic nitrogen alone (P < 0.001). NO3− decreased slightly in these microcosms by ~5 µg NO3− g−1 soildw (Fig. 1b) and pH decreased from 6.1 to 6.0 (Fig. S4). The decrease in NO3− may have resulted from immobilisation or reduction of NO3−, although potential N2O emission due to denitrification was negligible, as N2O accumulation in acetylene-treated microcosms was generally very low and not significantly different from accumulation in control microcosms (P > 0.05). The rate of NH4+ supply was confirmed by measurement of NO3− production in non-inhibited microcosms, where NH4+ was stoichiometrically converted to NO3− and differences in NO3− concentration between the acetylene-treated and non-inhibited microcosms reflected the mineralisation rate (~2 µg NO3− g−1 soildw d−1; Fig. 1b; P < 0.001).
AOA growth was greater in fertilised, 1-octyne-inhibited microcosms during phase 2 than in fertilised, non-inhibited microcosms during phase 1 (Fig. 2a) and was greater in both of these treatments than in unfertilised microcosms during phase 2 (Fig. 2a, Fig. S2b). 1-octyne prevented growth of AOB (Fig. 2b) and NO3− production (Fig. 1b), and therefore N2O accumulation (10 ng N2O–N g−1 soildw; Fig. 1c) and N2O production were associated with archaeal, rather than bacterial NH3 oxidation in microcosms in which NH4+ was supplied at a continuous low rate.
Nitrification, N2O accumulation and ammonia oxidiser growth at high NH4+ supply
In fertilised microcosms, NH4+ concentration increased to ~13 µg NH4+ g−1 soildw immediately after amendment at the beginning of phase 1, through rapid conversion of free urea contained in the fertiliser, and reached ~90 µg NH4+ g−1 soildw after 1 day of incubation (Fig. 1d). In microcosms additionally treated with acetylene, NH4+ concentration continued to increase throughout incubation, due to mineralisation of native organic nitrogen and additional supply through slow release of urea, following mineralisation of polymethylene urea. There was no detectable increase in NO3− concentration in the presence of acetylene (Fig. 1e; P < 0.05), but a slight increase in N2O accumulation that was similar to that in acetylene-treated control microcosms (Fig. 1f; P > 0.05). In the absence of inhibitors, NH4+ was quickly and stoichiometrically converted to NO3−, at a rate of ~17 µg N g−1 soildw d−1, estimated during the first week of incubation. This rate then decreased until NH4+ became undetectable, after incubation for 10 days (Fig. 1d and e). N2O accumulation followed similar kinetics, with an initial high rate of ~11 ng N2O-N g−1 soildw d−1 (Fig. 1f).
NH3 oxidation was accompanied by significant growth of AOB, which ceased as NH4+ was depleted (Fig. 2d; Fig. S3a). AOA amoA gene abundance also increased during phase 1, at high NH4+ concentration, at similar rates in the absence and presence of 1-octyne (Fig. 2c), and abundances were significantly greater than those at low NH4+ supply (Fig. S2a). N2O accumulation at high concentrations of NH4+ therefore resulted from the activities of both AOA and AOB. In the presence of 1-octyne, initial nitrification and N2O accumulation rates were reduced by ~80% and 90%, respectively, indicating that AOB dominated NH3 oxidation and associated N2O production. 1-octyne application inhibited AOB growth (Fig. 2d) and NH4+ concentration remained high throughout phase 2 (Fig. 1e), leading to greater stimulation of AOA growth than in microcosms with lower NH4+ concentration (Fig. 1a, b; Fig. S2b). NH3 oxidation in phase 1 decreased soil pH from ~6.9 to ~6.1, which was counterbalanced by an initial increase of ~0.3 units of pH following fertiliser addition, leading to an overall difference between the pH of fertilised and unfertilised soil of only ~0.5 at day 10 (Fig. S4).
NH4+ was also supplied at high concentration following rapid conversion (within 8 h) of urea added at the beginning of phase 2, in the presence or absence of inhibitors. This was followed by a slower increase in NH4+ concentration in acetylene-treated microcosms due to production of NH4+ from native organic nitrogen and the slow-release fertiliser (Fig. 1c). In non-inhibited, urea-amended microcosms, NH4+ was stoichiometrically converted to NO3− within 4 days and initial nitrification rates and N2O kinetics were similar to those in phase 1 in non-inhibited, fertilised microcosms. This implies that AOB were not affected by the decrease in pH during phase 1. The initial high rates were likely associated with AOB growth, but AOB amoA gene abundances were not significantly different from those in the treatment with no urea added (Fig. S3b).
AOA growth was similar to that when NH4+ was derived from slow release of urea, but was stimulated more after addition of 1-octyne (Fig. 2c, Fig. S2b). However, this growth was not significantly different to that estimated when NH4+ concentration was high throughout incubation in microcosms amended with fertiliser in the presence of 1-octyne from the beginning of incubation (Fig. S2b).
Together these data indicate that both AOA and AOB are capable of NH3 oxidation, growth and N2O production at high concentrations of NH4+ but that, in this soil, the contribution of AOB is greater. In addition, growth and activity of AOA dominated when NH4+ became undetectable and when 1-octyne was present at high NH4+ concentrations, which led to greater stimulation of growth, likely due to the removal of competition with AOB for substrate.
N2O yield of AOA and AOB
N2O yield was determined as the amount of N2O produced per NO3− produced and was used to relate nitrification activity and N2O production by AOA and AOB (Fig. 3). To assess N2O production associated with NH3 oxidation only, non-ammonia oxidiser N2O produced in acetylene-treated microcosms was subtracted from that produced in those in which NH3 was oxidised. Only AOA were responsible for the N2O emission associated with the consumption of NH4+ derived from native organic nitrogen, from slow release of the fertiliser in phase 2 and from high NH4+ concentration in the presence of the AOB inhibitor, 1-octyne. In all of these situations, N2O yield was similar at ~0.35‰. Both AOB and AOA were responsible for consumption of NH4+, initially at high concentration, derived from free urea contained in the fertiliser in phase 1, during which N2O yield was ~0.75‰. As AOB were responsible for ~80% of the NH3 oxidation under these conditions, the AOB N2O yield was calculated to be ~0.85‰.
Fig. 3. The yield of N2O associated with ammonia oxidation by AOA and AOB.
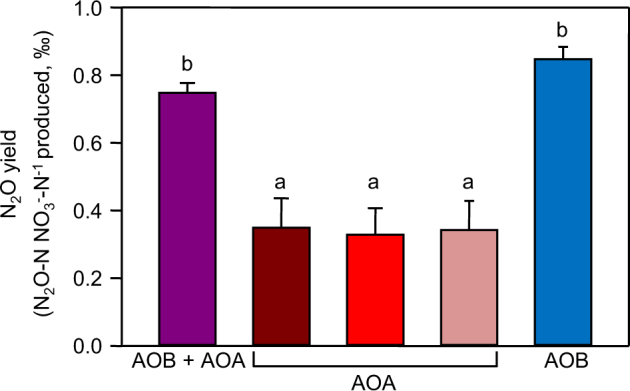
N2O yield associated with activity of both AOA and AOB at high NH4+ concentration in fertilised microcosms during phase 1 (purple bar); AOA only oxidising NH3 derived from mineralisation of native organic nitrogen (dark red bar), from mineralisation of native organic nitrogen and from slowly released urea during phase 2 (medium red bar) or from mineralisation of free fertiliser-urea with inhibition of AOB by 1-octyne during phases 1 and 2 (light red bar); AOB only, calculated based on the known yield of AOA and both AOA and AOB, in addition to the observation that under conditions where both were contributing to NH3 oxidation, ~80% was performed by AOB (blue bar). Mean yields and standard errors are plotted. Significant differences are indicated by different lower case letters
Discussion
Niche specialisation of AOA and AOB associated with NH4+ supply and concentration
A major aim of this study was to test the hypothesis that AOA and AOB dominate NH3 oxidation under conditions of low and high NH4+ supply, respectively. Evidence for this arose from correlations of AOA and AOB relative abundances in soils subjected to different fertiliser regimes [14–17], lack of stimulation of NH3 oxidation by supply of relatively high concentrations of inorganic NH4+, but stimulation by organic nitrogen in AOA-dominated soils [30, 49], and higher relative abundances of AOA where NH4+ is typically derived from mineralisation of native organic nitrogen [11, 30]. Detailed analysis of the effects of NH4+ supply and concentration on competition between AOA and AOB was not possible in previous studies, but was enabled here by the controlled supply of NH4+ through use of a slow-release fertiliser or addition of urea, use of inhibitors of both AOA and AOB and a specific inhibitor of AOB, and simultaneous measurement of NH4+ concentration and AOA and AOB abundances. Competition is used, here, in terms of its effect on cell yield rather than specific growth rate. Both AOA and AOB will be expected to grow at their maximum specific rates when NH3 concentration is high, but cell yield of both AOA and AOB will be reduced when both are utilising NH3.
This study provides strong evidence that AOA successfully outcompete AOB in soil in which NH4+ is continuously supplied at a low rate. This was achieved through mineralisation of native organic nitrogen or through additional supply using a slow-release fertiliser (phase 2), after oxidation of NH4+ derived from free urea that is also present in this fertiliser (phase 1). Mineralisation rate (~1 µg N g soildw d−1) was similar to those observed in previous studies of this soil at near neutral or neutral pH, which also indicated preferential activity of AOA [12, 13, 17]. AOA are also >10-fold more abundant than AOB in other soils with low NH4+ supply from mineralisation [11, 14–16, 18, 49]. AOA abundance increased but AOB abundance did not change significantly and nitrification rates were identical in the presence and absence of 1-octyne, which reduces nitrification strongly in AOB-dominated soils due to inactivation of AOB ammonia monooxygenase, as indicated by inhibition of activity, growth and transcriptional activity of AOB [16, 17, 43, 50]. Slow release of urea in phase 2 of this study doubled the NH4+ supply rate to ~2 µg N g soildw d−1 and increased AOA growth and nitrification rate, again with no detectable AOB growth or activity.
Reduction in NH4+ concentration to undetectable levels demonstrates that potential NH3 oxidation rate is greater than mineralisation rate in this soil and the simplest explanation for growth of AOA and not AOB is that AOA have significantly greater affinity for NH3 than AOB. This is consistent with reported higher Km values for several AOB cultures (27–825 µM TAN) [20, 51, 52] than pure (N. maritimus) and enrichment cultures of AOA (0.13–0.69 µM TAN) [19–21]. Recent determination of similar Km values of N. europaea and N. maritimus [25] and of other AOA [26] questions the strength of this argument. In addition, this proposed explanation does not consider the complexity of the soil environment, where NH4+ is adsorbed to clay minerals and supply of NH4+ produced from mineralisation of organic nitrogen or urea requires diffusion through a spatially heterogeneous environment. Moreover, Km values have not been determined for typical soil AOA or AOB and may not reflect those of natural communities, while soil NH4+ concentrations are generally well above the nM Km values previously reported for AOA.
AOB growth occurred only when NH4+ supply exceeded potential NH3 oxidation rate when NH4+ was formed from free urea, initially present in the fertiliser (phase 1) or urea added separately (phase 2). In both cases, AOB growth ceased when NH4+ became undetectable, whereas AOA growth continued. This finding is consistent with several studies reporting AOB growth or high relative abundance in soils with high NH4+ availability, such as those subjected to high levels of NH4+-based fertiliser [14, 17, 18, 53]. However, AOA also grew under these conditions, despite high NH4+ concentration. In phase 1, AOA growth was significantly greater than that with low NH4+ supply and in phase 2, AOA growth in microcosms with high NH4+ availability was significantly greater when AOB were inhibited by 1-octyne, suggesting direct competition between AOA and AOB for NH3. AOB did, however, dominate NH3 oxidation, contributing 80%, as determined by comparison of initial rates with and without specific inhibition of AOB.
Although dominance of AOB in heavily fertilised soils has been explained through differences in Km values (see above), any effects on growth and activity at NH4+ concentrations significantly higher than Km values will be negligible. The alternative explanation of greater sensitivity of AOA to high NH4+ concentrations is also not supported, as both AOA and AOB grew at moderately high NH4+ concentrations. The recent cultivation of three Nitrosocosmicus strains capable of growth at high NH4+ concentration [27–29] also suggests that this is not a general explanation for AOB dominance.
Changes in the activity and competitive ability of AOA may have resulted from changes in community composition, potentially selecting, for example, for NH3-tolerant phylotypes such as N. franklandus [28]. Hink et al. [17] did not observe changes in the transcriptionally active AOA community in response to high NH4+ supply and removal of competition with AOB (1-octyne application) during incubation of the same soil for 13 days, but detected changes after 20 days of incubation. Verhamme et al. [18] observed AOA growth and changes in community structure in soil that was unamended or repeatedly spiked with ‘intermediate’ and ‘high’ concentrations of NH4+ to maintain concentrations of 20 and 200 NH4+–N g soildw. In contrast, significant growth of AOB was only detected at the highest concentration of added NH4+. Soil pH influences AOB and AOA growth, activity and community composition [6, 7] and NH3 oxidation is accompanied by the release of protons that can reduce soil pH. The largest pH change in the current study was from 6.9 to 6.1 during phase 1 incubation of fertilised microcosms. This, however, did not significantly influence AOB activity, which dominated in these microcosms, as nitrification rate was similar during the consumption of NH4+ in phase 1, derived from free urea in the fertiliser, and urea added in phase 2. There is no evidence, therefore, that reduction in soil pH resulted in dominance of AOA over AOB under conditions of low NH4+ supply.
These results provide evidence for the hypothesis that AOA are responsible for NH3 oxidation when NH4+ is supplied at a low rate. They also provide evidence that AOB outcompete AOA at high supply rate but AOA growth occurred at high NH4+ concentration and accelerated when AOB were inhibited. There is therefore evidence for niche differentiation associated with NH4+ supply rate but the precise underlying mechanism remains unclear.
The consequences of NH4+ supply rate for N2O production and mitigation strategies
AOA and AOB were responsible for N2O production in this aerobic soil. Emissions from heterotrophic denitrifiers were unlikely or negligible, as N2O production was always associated with NH3 oxidation and NO3− production, denitrification was excluded previously under similar experimental conditions [17] and denitrifier activity in this soil is very low at similar moisture content (WFPS ≤60%) [54]. Nevertheless, N2O accumulated slowly in acetylene-treated microcosms, when NH3 oxidiser growth and activity were inhibited. This was not further investigated, but a possible explanation is abiotic production, although such reactions are usually negligible when the intermediates nitrite and hydroxylamine are not provided by NH3 oxidisers [55–57].
Production of N2O and NO3− was generally coordinate, but relative rates of production and, consequently, N2O yield were dependent on the organisms dominating NH3 oxidation. Under conditions of low NH4+ supply, when AOA dominated NH3 oxidation, or at high NH4+ supply, when AOB were inhibited, N2O yield was ~0.35‰, while at high NH4+ supply, when ~80% of NH3 oxidation was performed by AOB, N2O yield was ~0.75‰ and estimated as ~0.85‰ calculated for AOB only. These yields are similar to values of 0.5‰, and 0.9‰, for AOA and AOB, respectively, reported previously by Hink et al. [17]. They are also consistent with current understanding of mechanisms for N2O production in AOA, where production is limited to presumably abiotic hybrid formation [34, 35], and in AOB, which can additionally produce N2O through nitrifier denitrification and as a by-product of hydroxylamine metabolism [34]. Although NH4+ concentration may have led to selection, or selective activity of different AOA phylotypes, N2O yield did not differ significantly when AOA dominated at low NH4+ concentrations or at high NH4+ concentrations when AOB were specifically inhibited. Giguere et al. [50] obtained a relationship of increasing AOA-associated N2O production and accumulated NO2−, which is an intermediate during NH3 oxidation. However, NO2− was below the detection limit in the study and there is no evidence of NO2− accumulation during nitrification in Craibstone soil (e.g. [48]).
These results therefore demonstrate the consequences of niche specialisation in an important group of nitrogen-cycling organisms and consequences for rates of an important biogeochemical process. They also suggest that knowledge of NH3 oxidiser community composition can inform fertiliser strategies to optimise nitrogen fertiliser use efficiency, can minimise fertiliser loss and N2O emissions from terrestrial systems and can improve prediction of N2O emissions in climate change models. Current strategies to increase fertiliser use efficiency include application of nitrification (NH3 oxidiser) inhibitors [58], specific timing of fertiliser application [59, 60] and use of slow-release fertilisers [61–63]. Nitrification rate of inorganic fertiliser, supplied at high concentration, is lower in AOA-dominated soils (e.g. acidic soils) than those dominated by AOB, while nitrification rate in soil dominated by AOB is lower when NH4+ is derived from slow-release fertilisers or slowly degradable organic fertilisers. Coordinate NH3 oxidation and N2O production under aerobic conditions indicate that any strategy to reduce NH3 oxidation will mitigate N2O production, in addition to reducing production of N2O by denitrifiers at lower oxygen concentrations. However, strategies that increase dominance of NH3 oxidation by AOA (e.g. reduction in pH, development and use of specific AOB inhibitors) will decrease the proportion of oxidised NH3 that is converted into N2O. For example, long-term fertilisation with organic, rather than mineral nitrogen has been shown to increase AOA abundance [64] and, in a 3-year experiment, application of composted sludge or dried pellets reduced N2O emissions by >60% and slow-release fertiliser reduced emissions by 85%, in comparison with conventional mineral fertilisation [65]. Obviously, such strategies require consideration of potential effects on crop yield, in addition to feasibility and cost, but the study provides the basis for better informed development of fertilisation strategies and the potential to improve predictions of N2O emissions from terrestrial environments.
Electronic supplementary material
Acknowledgements
The authors are members of the Nitrous Oxide Research Alliance (NORA), a Marie Skłodowska-Curie ITN and research project under the EU’s seventh framework programme (FP7). GN is funded by the AXA Research Fund and CGR by a Royal Society University Research Fellowship (UF150571) and a Natural Environment Research Council (NERC) Standard Grant (NE/K016342/1). We thank Dr Robin Walker and the SRUC Craibstone Estate (Aberdeen) for access to the agricultural plots, Dr Alex Douglas for statistical advice and Philipp Schleusner for assisting microcosm construction and sampling.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
The online version of this article (10.1038/s41396-017-0025-5) contains supplementary material, which is available to authorised users.
References
- 1.Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, et al. IPCC 2013: climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge and New York: Cambridge University Press; 2013.
- 2.Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): the dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- 3.Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 4.Glass ADM. Nitrogen use efficiency of crop plants: physiological constraints upon nitrogen absorption. Crit Rev Plant Sci. 2003;22:453–470. doi: 10.1080/07352680390243512. [DOI] [Google Scholar]
- 5.Zumft WG. Cell biology and molecular basis of denitrification. Microbiol Mol Biol R. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prosser JI, Nicol GW. Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol. 2008;10:2931–2941. doi: 10.1111/j.1462-2920.2008.01775.x. [DOI] [PubMed] [Google Scholar]
- 7.Prosser JI, Nicol GW. Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Tr Microbiol. 2012;20:523–531. doi: 10.1016/j.tim.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Lehtovirta-Morley LE, Ge C, Ross J, Yao H, Nicol GW, Prosser JI. Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds. FEMS Microbiol Ecol. 2014;89:542–552. doi: 10.1111/1574-6941.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proc Natl Acad Sci USA. 2011;108:15892–15897. doi: 10.1073/pnas.1107196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gubry-Rangin C, Hai B, Quince C, Engel M, Thomson BC, James P, et al. Niche specialization of terrestrial archaeal ammonia oxidizers. Proc Natl Acad Sci USA. 2011;108:21206–21211. doi: 10.1073/pnas.1109000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubry‐Rangin C, Nicol GW, Prosser JI. Archaea rather than bacteria control nitrification in two agricultural acidic soils. FEMS Microbiol Ecol. 2010;74:566–574. doi: 10.1111/j.1574-6941.2010.00971.x. [DOI] [PubMed] [Google Scholar]
- 12.Offre P, Prosser JI, Nicol GW. Growth of ammonia‐oxidizing archaea in soil microcosms is inhibited by acetylene. FEMS Microbiol Ecol. 2009;70:99–108. doi: 10.1111/j.1574-6941.2009.00725.x. [DOI] [PubMed] [Google Scholar]
- 13.Tourna M, Freitag TE, Nicol GW, Prosser JI. Growth, activity and temperature responses of ammonia oxidizing archaea and bacteria in soil microcosms. Environ Microbiol. 2008;10:1357–1364. doi: 10.1111/j.1462-2920.2007.01563.x. [DOI] [PubMed] [Google Scholar]
- 14.Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, et al. Ammonia‐oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol. 2010;72:386–394. doi: 10.1111/j.1574-6941.2010.00861.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LM, Hu HW, Shen JP, He JZ. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. ISME J. 2012;6:1032–1045. doi: 10.1038/ismej.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X, Bottomley PJ, Myrold DD. Contributions of ammonia-oxidizing archaea and bacteria to nitrification in Oregon forest soils. Soil Biol Biochem. 2015;85:54–62. doi: 10.1016/j.soilbio.2015.02.034. [DOI] [Google Scholar]
- 17.Hink Linda, Nicol Graeme W., Prosser James I. Archaea produce lower yields of N2 O than bacteria during aerobic ammonia oxidation in soil. Environmental Microbiology. 2016;19(12):4829–4837. doi: 10.1111/1462-2920.13282. [DOI] [PubMed] [Google Scholar]
- 18.Verhamme DT, Prosser JI, Nicol GW. Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J. 2011;5:1067–1071. doi: 10.1038/ismej.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung MY, Park SJ, Min D, Kim JS, Rijpstra WIC, Sinninghe Damsté JS, et al. Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil. Appl Environ Microbiol. 2011;77:8635–8647. doi: 10.1128/AEM.05787-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martens-Habbena W, Berube PM, Urakawa H, de La Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature. 2009;461:976–979. doi: 10.1038/nature08465. [DOI] [PubMed] [Google Scholar]
- 21.Park BJ, Park SJ, Yoon DN, Schouten S, Damsté JSS, Rhee SK. Cultivation of autotrophic ammonia-oxidizing archaea from marine sediments in coculture with sulfur-oxidizing bacteria. Appl Environ Microbiol. 2010;76:7575–7587. doi: 10.1128/AEM.01478-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keen GA, Prosser JI. Steady state and transient growth of autotrophic nitrifying bacteria. Arch Microbiol. 1987;147:73–79. doi: 10.1007/BF00492908. [DOI] [Google Scholar]
- 23.Koper TE, Stark JM, Habteselassie MY, Norton JM. Nitrification exhibits Haldane kinetics in an agricultural soil treated with ammonium sulfate or dairy-waste compost. FEMS Microbiol Ecol. 2010;74:316–322. doi: 10.1111/j.1574-6941.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 24.Prosser JI. Autotrophic nitrification in bacteria. Adv Microb Physiol. 1989;30:125–181. doi: 10.1016/S0065-2911(08)60112-5. [DOI] [PubMed] [Google Scholar]
- 25.Hink Linda, Lycus Pawel, Gubry-Rangin Cécile, Frostegård Åsa, Nicol Graeme W., Prosser James I., Bakken Lars R. Kinetics of NH3 -oxidation, NO-turnover, N2 O-production and electron flow during oxygen depletion in model bacterial and archaeal ammonia oxidisers. Environmental Microbiology. 2017;19(12):4882–4896. doi: 10.1111/1462-2920.13914. [DOI] [PubMed] [Google Scholar]
- 26.Kits KD, Sedlacek CJ, Lebedeva EV, Han P, Bulaev A, Pjevac P, et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature. 2017;549:269–272. doi: 10.1038/nature23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung MY, Kim JG, Sinninghe Damsté JS, Rijpstra WIC, Madsen EL, Kim SJ, et al. A hydrophobic ammonia‐oxidizing archaeon of the Nitrosocosmicus clade isolated from coal tar‐contaminated sediment. Environ Microbiol Rep. 2016;8:983–992. doi: 10.1111/1758-2229.12477. [DOI] [PubMed] [Google Scholar]
- 28.Lehtovirta-Morley LE, Ross J, Hink L, Weber EB, Gubry-Rangin C, Thion C, et al. Isolation of ‘Candidatus Nitrosocosmicus franklandus’, a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration. FEMS Microbiol Ecol. 2016;92:fiw057. doi: 10.1093/femsec/fiw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauder LA, Albertsen M, Engel K, Schwarz J, Nielsen PH, Wagner M, et al. Cultivation and characterization of Candidatus Nitrosocosmicus exaquare, an ammonia-oxidizing archaeon from a municipal wastewater treatment system. ISME J. 2017;11:1142–1157. doi: 10.1038/ismej.2016.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stopnišek N, Gubry-Rangin C, Höfferle Scaron, Nicol GW, Mandič-Mulec I, Prosser JI. Thaumarchaeal ammonia oxidation in an acidic forest peat soil is not influenced by ammonium amendment. Appl Environ Microbiol. 2010;76:7626–7634. doi: 10.1128/AEM.00595-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper AB, Terry KR. Hydroxylamine oxidoreductase of Nitrosomonas: production of nitric oxide from hydroxylamine. BBA-Enzymol. 1979;571:12–20. doi: 10.1016/0005-2744(79)90220-1. [DOI] [PubMed] [Google Scholar]
- 32.Kozlowski JA, Price J, Stein LY. Revision of N2O-producing pathways in the ammonia-oxidizing bacterium Nitrosomonas europaea ATCC 19718. Appl Environ Microbiol. 2014;80:4930–4935. doi: 10.1128/AEM.01061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arp DJ, Stein LY. Metabolism of inorganic N compounds by ammonia-oxidizing bacteria. Crit Rev Biochem Mol Biol. 2003;38:471–495. doi: 10.1080/10409230390267446. [DOI] [PubMed] [Google Scholar]
- 34.Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J. 2016;10:1836–1845. doi: 10.1038/ismej.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stieglmeier M, Mooshammer M, Kitzler B, Wanek W, Zechmeister-Boltenstern S, Richter A, et al. Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J. 2014;8:1135–1146. doi: 10.1038/ismej.2013.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson IC, Poth M, Homstead J, Burdige D. A comparison of NO and N2O production by the autotrophic nitrifier Nitrosomonas europaea and the heterotrophic nitrifier Alcaligenes faecalis. Appl Environ Microbiol. 1993;59:3525–3533. doi: 10.1128/aem.59.11.3525-3533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang QQ, Bakken LR. Nitrous oxide production and methane oxidation by different ammonia-oxidizing bacteria. Appl Environ Microbiol. 1999;65:2679–2684. doi: 10.1128/aem.65.6.2679-2684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung MY, Well R, Min D, Giesemann A, Park SJ, Kim JG, et al. Isotopic signatures of N2O produced by ammonia-oxidizing archaea from soils. ISME J. 2014;8:1115–1125. doi: 10.1038/ismej.2013.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JG, Jung MY, Park SJ, Rijpstra WIC, Sinninghe Damsté JS, Madsen EL, et al. Cultivation of a highly enriched ammonia‐oxidizing archaeon of thaumarchaeotal group I. 1b from an agricultural soil. Environ Microbiol. 2012;14:1528–1543. doi: 10.1111/j.1462-2920.2012.02740.x. [DOI] [PubMed] [Google Scholar]
- 40.Shaw LJ, Nicol GW, Smith Z, Fear J, Prosser JI, Baggs EM. Nitrosospira spp. can produce nitrous oxide via a nitrifier denitrification pathway. Environ Microbiol. 2006;8:214–222. doi: 10.1111/j.1462-2920.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 41.Kemp JS, Paterson E, Gammack SM, Cresser MS, Killham K. Leaching of genetically modified Pseudomonas fluorescens through organic soils: Influence of temperature, soil pH, and roots. Biol Fert Soil. 1992;13:218–224. doi: 10.1007/BF00340579. [DOI] [Google Scholar]
- 42.Bartram AK, Jiang X, Lynch MDJ, Masella AP, Nicol GW, Dushoff J, et al. Exploring links between pH and bacterial community composition in soils from the Craibstone Experimental Farm. FEMS Microbiol Ecol. 2014;87:403–415. doi: 10.1111/1574-6941.12231. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AE, Vajrala N, Giguere AT, Gitelman AI, Arp DJ, Myrold DD, et al. Use of aliphatic n-alkynes to discriminate soil nitrification activities of ammonia-oxidizing thaumarchaea and bacteria. Appl Environ Microbiol. 2013;79:6544–6551. doi: 10.1128/AEM.01928-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandeler E, Gerber H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soil. 1988;6:68–72. doi: 10.1007/BF00257924. [DOI] [Google Scholar]
- 45.Shinn MB. Colorimetric method for determination of nitrate. Ind Eng Chem Anal Ed. 1941;13:33–35. doi: 10.1021/i560089a010. [DOI] [Google Scholar]
- 46.Doane TA, Horwath WR. Spectrophotometric determination of nitrate with a single reagent. Anal Lett. 2003;36:2713–2722. doi: 10.1081/AL-120024647. [DOI] [Google Scholar]
- 47.Nicol GW, Prosser JI. Strategies to determine diversity, growth and activity of ammonia oxidising archaea in soil. Meth Enzymol. 2011;496:3–34. doi: 10.1016/B978-0-12-386489-5.00001-4. [DOI] [PubMed] [Google Scholar]
- 48.Thion C, Prosser JI. Differential response of non-adapted ammonia oxidising archaea and bacteria to drying rewetting stress. FEMS Microbiol Ecol. 2014;90:380–389. doi: 10.1111/1574-6941.12395. [DOI] [PubMed] [Google Scholar]
- 49.Levičnik‐Höfferle Scaron, Nicol GW, Ausec L, Mandić‐Mulec I, Prosser JI. Stimulation of thaumarchaeal ammonia oxidation by ammonia derived from organic nitrogen but not added inorganic nitrogen. FEMS Microbiol Ecol. 2012;80:114–123. doi: 10.1111/j.1574-6941.2011.01275.x. [DOI] [PubMed] [Google Scholar]
- 50.Giguere AT, Taylor AE, Suwa Y, Myrold DD, Bottomley PJ. Uncoupling of ammonia oxidation from nitrite oxidation: Impact upon nitrous oxide production in non-cropped Oregon soils. Soil Biol Biochem. 2017;104:30–38. doi: 10.1016/j.soilbio.2016.10.011. [DOI] [Google Scholar]
- 51.Stark JM, Firestone MK. Kinetic characteristics of ammonium-oxidizer communities in a California oak woodland-annual grassland. Soil Biol Biochem. 1996;28:1307–1317. doi: 10.1016/S0038-0717(96)00133-2. [DOI] [Google Scholar]
- 52.Jiang QQ, Bakken LR. Comparison of Nitrosospira strains isolated from terrestrial environments. FEMS Microbiol Ecol. 1999;30:171–186. doi: 10.1111/j.1574-6941.1999.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhong W, Bian B, Gao N, Min J, Shi W, Lin X, Shen W. Nitrogen fertilization induced changes in ammonia oxidation are attributable mostly to bacteria rather than archaea in greenhouse-based high N input vegetable soil. Soil Biol Biochem. 2016;93:150–159. doi: 10.1016/j.soilbio.2015.11.003. [DOI] [Google Scholar]
- 54.Bateman EJ, Baggs EM. Contributions of nitrification and denitrification to N2O emissions from soils at different water-filled pore space. Biol Fert Soil. 2005;41:379–388. doi: 10.1007/s00374-005-0858-3. [DOI] [Google Scholar]
- 55.Heil J, Liu S, Vereecken H, Brüggemann N. Abiotic nitrous oxide production from hydroxylamine in soils and their dependence on soil properties. Soil Biol Biochem. 2015;84:107–115. doi: 10.1016/j.soilbio.2015.02.022. [DOI] [Google Scholar]
- 56.Mørkved PT, Dörsch P, Bakken LR. The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Biol Biochem. 2007;39:2048–2057. doi: 10.1016/j.soilbio.2007.03.006. [DOI] [Google Scholar]
- 57.Zhu-Barker X, Cavazos AR, Ostrom NE, Horwath WR, Glass JB. The importance of abiotic reactions for nitrous oxide production. Biogeochem. 2015;126:251–267. doi: 10.1007/s10533-015-0166-4. [DOI] [Google Scholar]
- 58.Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, et al. Scope and strategies for regulation of nitrification in agricultural systems - challenges and opportunities. Crit Rev Plant Sci. 2006;25:303–335. doi: 10.1080/07352680600794232. [DOI] [Google Scholar]
- 59.Campbell CA, Myers RJK, Curtin D. Managing nitrogen for sustainable crop production. Fert Res. 1995;42:277–296. doi: 10.1007/BF00750521. [DOI] [Google Scholar]
- 60.Dinnes DL, Karlen DL, Jaynes DB, Kaspar TC, Hatfield JL, Colvin TS, et al. Nitrogen management strategies to reduce nitrate leaching in tile-drained Midwestern soils. Agron J. 2002;94:153–171. doi: 10.2134/agronj2002.1530. [DOI] [Google Scholar]
- 61.Balkcom KS, Blackmer AM, Hansen DJ, Morris TF, Mallarino AP. Testing soils and cornstalks to evaluate nitrogen management on the watershed scale. J Environ Qual. 2003;32:1015–1024. doi: 10.2134/jeq2003.1015. [DOI] [PubMed] [Google Scholar]
- 62.Shoji S, Kanno H. Use of polyolefin-coated fertilizers for increasing fertilizer efficiency and reducing nitrate leaching and nitrous oxide emissions. Nut Cycl Agroecosys. 1994;39:147–152. [Google Scholar]
- 63.Zvomuya F, Rosen CJ, Russell MP, Gupta SC. Nitrate leaching and nitrogen recovery following application of polyolefin-coated urea to potato. J Environ Qual. 2003;32:480–489. doi: 10.2134/jeq2003.4800. [DOI] [PubMed] [Google Scholar]
- 64.Zhou X, Fornara D, Wasson EA, Wang D, Ren G, Christie P, et al. Effects of 44 years of chronic nitrogen fertilization on the soil nitrifying community of permanent grassland. Soil Biol Biochem. 2015;91:76–83. doi: 10.1016/j.soilbio.2015.08.031. [DOI] [Google Scholar]
- 65.Ball BC, McTaggart IP, Scott A. Mitigation of greenhouse gas emissions from soil under silage production by use of organic manures or slow-release fertilizer. Soil Use Manag. 2004;20:287–295. doi: 10.1079/SUM2004257. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.