Abstract
Background and aims
Coronary artery calcification (CAC) is common among patients with chronic kidney disease (CKD) and predicts the risk for cardiovascular disease (CVD). We examined the associations of novel risk factors with CAC progression among patients with CKD.
Methods
Among 1,123 CKD patients in the Chronic Renal Insufficiency Cohort (CRIC) Study, CAC was measured in Agatston units at baseline and a follow-up visit using electron beam computed tomography or multidetector computed tomography.
Results
Over an average 3.3-year follow-up, 109 (25.1%) participants without CAC at baseline had incident CAC and 124 (18.0%) participants with CAC at baseline had CAC progression, defined as an annual increase of ≥100 Agatston units. After adjustment for established atherosclerotic risk factors, several novel risk factors were associated with changes in CAC over follow-up. Changes in square root transformed CAC score associated with 1 SD greater level of risk factors were −0.20 (95% confidence interval, −0.31 to −0.10; p <0.001) for estimated glomerular filtration rate, 0.14 (0.02 to 0.25; p=0.02) for 24-hour urine albumin, 0.25 (0.15 to 0.34; p <0.001) for cystatin C, −0.17 (−0.27 to −0.07; p <0.001) for serum calcium, 0.14 (0.03 to 0.24; p=0.009) for serum phosphate, 0.24 (0.14 to 0.33; p <0.001) for fibroblast growth factor-23, 0.13 (0.04 to 0.23; p=0.007) for total parathyroid hormone, 0.17 (0.07 to 0.27; p <0.001) for interleukin-6, and 0.12 (0.02 to 0.22; p=0.02) for tumor necrosis factor-α.
Conclusions
Reduced kidney function, calcium and phosphate metabolism disorders, and inflammation, independent of established CVD risk factors, may progress CAC among CKD patients.
Keywords: coronary artery disease, chronic kidney disease, risk factors, epidemiology
1. Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in patients with chronic kidney disease (CKD) [1,2]. Prospective cohort studies have shown that the presence [3,4] and progression [5,6] of coronary artery calcification (CAC) identify coronary atherosclerosis and predict the risk of CVD and all-cause mortality in the general population, beyond established CVD risk factors. In CKD patients, presence of CAC is more common and more severe[7,8] and shows a stronger association with risk of CVD and mortality [4,9,10]. Studies have documented a dose-response relationship between declining estimated glomerular filtration rate (eGFR) and increasing CAC score [11] and suggest high incidence and rapid progression of CAC in CKD patients [7].
Longitudinal analyses identified several traditional risk factors for CAC progression in the general population, including older age, male sex, white race/ethnicity, hypertension, obesity, diabetes, and family history of CVD [12]. These established risk factors are common in CKD patients and may partially explain the greater burden of CAC in CKD patients. However, risk factors for CAC progression have not been well-studied in CKD patients and novel risk factors have primarily been assessed in cross-sectional analyses [13].
The Chronic Renal Insufficiency Cohort (CRIC) Study provides a unique opportunity to examine novel risk factors for the progression of CKD and CVD among patients with pre-dialysis CKD. In this analysis, we investigated the associations between novel risk factors and CAC progression among patients with CKD.
2. Materials and methods
2.1 Study population
The CRIC Study includes a racially- and ethnically-diverse group of men and women aged 21 to 74 years with mild-to-moderate CKD based on an eGFR entry criteria 20 to 70 mL/min/1.73m2. A total of 3,939 participants were enrolled from 7 clinical centers in the United States between May 2003 and August 2008 [14]. Patients with severe heart failure, cirrhosis, HIV infection, polycystic kidney disease, renal cell carcinoma, those who received chronic dialysis or a kidney transplant, and those taking immunosuppressant drugs were excluded. Of the entire cohort, 1,142 were randomly selected, stratified by age, sex, race/ethnicity, diabetes status, and eGFR, for electron beam computed tomography (CT) or multidetector CT to measure CAC at baseline. In addition, electron beam or multidetector CT was performed in all eligible participants from 3 clinical centers for an ancillary study. Participants with a history of coronary artery revascularization were excluded from electron beam CT/multidetector CT examination. A repeated CAC measurement was obtained among 1,123 participants an average of 3.3 years (SD 0.7) later, which was included in the present analyses (Supplementary Fig. 1). The study was approved by the institutional review boards from each of the participating clinical centers and all participants provided written informed consent.
2.2 Data collection
All CRIC Study data were collected by trained study staff at baseline and annual clinical visits. Data collection procedures were standardized across study sites. A baseline medical history questionnaire collected information on demographic characteristics, lifestyle risk factors, previous history of CVD, and medication use. Physical activity was calculated as total metabolic equivalents per week. Body weight, height, waist circumference, and blood pressure (BP) were measured using standard protocols [14]. Hypertension was defined as systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg and/or current use of antihypertensive medications.
2.3 Laboratory measurements
Glucose, cholesterol, calcium, phosphate, alkaline phosphatase, total parathyroid hormone (PTH), glycated hemoglobin, and uric acid were measured using standard laboratory methods. High-sensitive C-reactive protein (hsCRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), homocysteine, and cystatin C were measured using the particle enhanced immunonephelometry method. Fibrinogen was measured using the immunochemical reaction method. Urinary albumin was measured by radioimmunoassay. Fibroblast growth factor-23 (FGF23) was measured by a second generation C-terminal assay (Immutopics). Diabetes was defined as a fasting glucose of ≥126 mg/dL, nonfasting glucose of ≥200 mg/dL, or the use of antidiabetic medications. Estimated glomerular filtration rate (eGFR) was calculated using the estimated equation derived from the CRIC cohort [15]. Homeostasis model of assessment (HOMA) was calculated to evaluate insulin resistance using the following formula: (fasting serum insulin [μU/mL] X fasting plasma glucose [mmol/L])/22.5. All laboratory measurements were performed in a centralized laboratory at the University of Pennsylvania.
2.4 Coronary artery calcification measurement
Trained and certified technologists scanned all participants twice using phantoms of known physical calcium concentrations. A cardiologist read all CT scans at a central reading center (Los Angeles Biomedical Research Institute, Harbor-UCLA Medical Center, Torrance, California). The total Agatston score was computed according to standard methods [16]. We used the average Agatston score from the 2 scans in all analyses. We evaluated the continuous change in CAC score according to the square root transformed method, which accounts for interscan variability [17]. Among those with no baseline CAC, incidence was defined as any increase in CAC score over follow-up. Among those with baseline CAC, progression was defined as an annual increase in CAC score ≥100 Agatston units, which significantly increases risk for coronary heart disease [6]. In addition, due to uncertainty regarding the best definition of CAC progression, we conducted 2 sensitivity analyses among those with baseline CAC: the first defining CAC progression as an increase in the square root transformed CAC score >2.5 mm3 over follow-up and the second defining CAC progression as an increase in CAC score ≥300 Agatston units over follow-up. We also repeated analyses using the total volume score instead of the Agatston score.
2.5 Statistical analysis
Baseline characteristics of the study participants were summarized as the mean (standard deviation [SD]) for continuous variables and percentages for categorical variables, by baseline presence of CAC. Statistical significance was tested using analysis of variance for continuous variables and the chi-square test for categorical variables. Continuous variables with skewed distributions are summarized as the median (interquartile range [IQR]) and were log-transformed to stabilize variances and approximate a normal distribution. Correlation between risk factors was assessed with Pearson’s correlation coefficient.
Multivariable linear regression was used to evaluate the annual square root transformed changes in CAC score and 95% confidence intervals (CIs) from baseline to follow-up associated with 1 SD greater level of risk factors. Adjusted odds ratios (ORs) and 95% CIs of CAC progression associated with 1 SD greater level of risk factors were estimated using multivariable logistic regression. Additionally, multivariable linear regression was used to evaluate the mean annual square root transformed changes in CAC score and 95% CIs by tertiles of risk factors.
Covariates included in regression models were selected based on prior knowledge and the backward elimination method. Two regression models were used in the analysis of each risk factor: 1) multivariable model 1 including age, sex, race/ethnicity, and clinical site; and 2) multivariable model 2 including variables in model 1 plus history of CVD, use of statin medications, physical activity, and established ACC/AHA atherosclerotic CVD risk factors (total cholesterol, high-density lipoprotein [HDL] cholesterol, systolic BP, use of antihypertensive medications, diabetes, and current smoking). The baseline CAC score was included as a covariate in analyses of participants with CAC at baseline. The follow-up time between CT scans was included as a covariate in models other than those using annualized differences in CAC score. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina). All p-values were 2-sided and statistical significance was defined as p <0.05.
3. Results
3.1 Baseline characteristics
Of the 1,123 CRIC participants, 689 (61.4%) had baseline CAC and, compared to those with no baseline CAC, were more likely to be older, male, white race/ethnicity, have a history of clinical CVD, hypertension, diabetes, use of antihypertensive medication or statin medications, and were less physically active (Table 1). On average, participants with baseline CAC had higher systolic BP and lower levels of HDL cholesterol and LDL cholesterol. In addition, participants with baseline CAC had lower eGFR and higher levels of glycated hemoglobin, HOMA-insulin resistance (HOMA-IR), uric acid, total plasma homocysteine, fibrinogen, IL-6, TNF-α.
Table 1.
Baseline characteristics of CRIC study participants according to baseline CAC score.
| Baseline
CAC=0 (n=434) |
Baseline CAC
>0 (n=689) |
p value | |
|---|---|---|---|
| Age (years) | 50.9 ± 12.2 | 60.6 ± 9.3 | <0.001 |
|
| |||
| Female | 247 (56.9%) | 274 (39.8%) | <0.001 |
|
| |||
| Race/ethnicity | |||
| Non-Hispanic White | 167 (38.5%) | 309 (44.8%) | 0.03 |
| Non-Hispanic Black | 176 (40.6%) | 219 (31.8%) | |
| Hispanic | 73 (16.8%) | 126 (18.3%) | |
| Other | 18 (4.1%) | 35 (5.1%) | |
|
| |||
| High school education | 361 (83.2%) | 551 (80.1%) | 0.22 |
|
| |||
| Physical activity (total MET) | 249.1 ± 191.2 | 202.5 ± 132.0 | <0.001 |
|
| |||
| Current cigarette smoking | 37 (8.5%) | 67 (9.7%) | 0.57 |
|
| |||
| Alcohol consumption | 289 (66.6%) | 437 (63.4%) | 0.31 |
|
| |||
| History of CVD | 50 (11.5%) | 177 (25.7%) | <0.001 |
|
| |||
| 10-year atherosclerotic CVD riska | |||
| <5% | 207 (53.9%) | 97 (19.0%) | <0.001 |
| 5–15% | 117 (30.5%) | 208 (40.8%) | |
| >15% | 60 (15.6%) | 205 (40.2%) | |
|
| |||
| Hypertension | 329 (75.8%) | 636 (92.3%) | <0.001 |
|
| |||
| Use of antihypertensive medications | 352 (81.1%) | 651 (94.6%) | <0.001 |
|
| |||
| Diabetes mellitus | 127 (29.3%) | 357 (51.8%) | <0.001 |
|
| |||
| Use of statin medications | 172 (39.6%) | 433 (62.9%) | <0.001 |
|
| |||
| Systolic blood pressure (mm Hg) | 122.0 ± 20.1 | 126.6 ± 20.0 | <0.001 |
|
| |||
| Body mass index (kg/m2) | 30.5 ± 6.8 | 31.1 ± 6.2 | 0.19 |
|
| |||
| High-density lipoprotein (mg/dL) | 51.7 ± 17.4 | 47.4 ± 14.5 | <0.001 |
|
| |||
| Low-density lipoprotein (mg/dL) | 109.1 ± 36.2 | 100.5 ± 32.3 | <0.001 |
|
| |||
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 48.6 ± 19.3 | 44.4 ± 15.9 | <0.001 |
|
| |||
| 24-hour urine albumin (g/24 hours) | 0.05 (0.01–0.61) | 0.04 (0.01–0.39) | 0.86 |
|
| |||
| Cystatin C (mg/L) | 1.46 ± 0.74 | 1.54 ± 0.61 | 0.06 |
|
| |||
| Calcium (mg/dL) | 9.29 ± 0.52 | 9.33 ± 0.52 | 0.13 |
|
| |||
| Phosphate (mg/dL) | 3.62 ± 0.65 | 3.69 ± 0.70 | 0.06 |
|
| |||
| Fibroblast growth factor-23 (RU/mL) | 120.0 (79.2–223.3) | 131.2 (91.6–210.5) | 0.10 |
|
| |||
| Total parathyroid hormone (pg/mL) | 54.0 (37.0–86.4) | 53.0 (36.9–82.0) | 0.35 |
|
| |||
| Alkaline phosphatase (U/L) | 89.5 ± 32.9 | 89.4 ± 30.1 | 0.94 |
|
| |||
| Hemoglobin A1c (%) | 6.05 ± 1.28 | 6.50 ± 1.40 | <0.001 |
|
| |||
| HOMA-insulin resistanceb | 3.18 (2.11–5.22) | 3.98 (2.61–6.72) | <0.001 |
|
| |||
| Uric acid (mg/dL) | 6.96 ± 1.89 | 7.19 ± 1.80 | 0.04 |
|
| |||
| Homocysteine (μmol/L) | 13.1 ± 4.6 | 14.5 ± 5.1 | <0.001 |
|
| |||
| Fibrinogen (mg/dL) | 3.84 ± 1.11 | 4.01 ± 1.09 | 0.01 |
|
| |||
| High sensitive C-reactive protein (mg/L) | 2.21 (0.91–4.98) | 2.04 (0.92–4.60) | 0.74 |
|
| |||
| Interleukin-6 (pg/mL) | 1.45 (0.92–2.32) | 1.70 (1.09–2.58) | 0.002 |
|
| |||
| Tumor necrosis factor-α (mg/dL) | 2.00 (1.30–3.00) | 2.20 (1.40–3.30) | 0.007 |
Data are expressed as mean ± standard deviation or median (interquartile range) and n (%). CAC, coronary artery calcification; IQR, interquartile range; SD, standard deviation.
Among those with no history of cardiovascular disease.
HOMA–insulin resistance = (fasting serum insulin [μU/mL] × fasting plasma glucose [mmol/L])/22.
3.2 Annual increases in CAC score by novel risk factors
Figure 1 shows adjusted mean annual increases in square root transformed CAC score by tertiles of novel risk factors. The mean annual change in square root transformed CAC score among all participants was 0.96. Decreased kidney function, denoted by lower eGFR (p=0.003) and higher cystatin C (p=0.004), resulted in greater annual CAC increases compared with other tertiles. A positive, dose-response relationship was observed for increasing tertiles of FGF23 (p=0.006). J-shaped relationships were seen for tertiles of total PTH (p=0.002), IL-6 (p=0.02), and TNF-α (p=0.008), characterized by relatively smaller annual increases in CAC score for the second tertiles compared with other tertiles.
Figure 1.
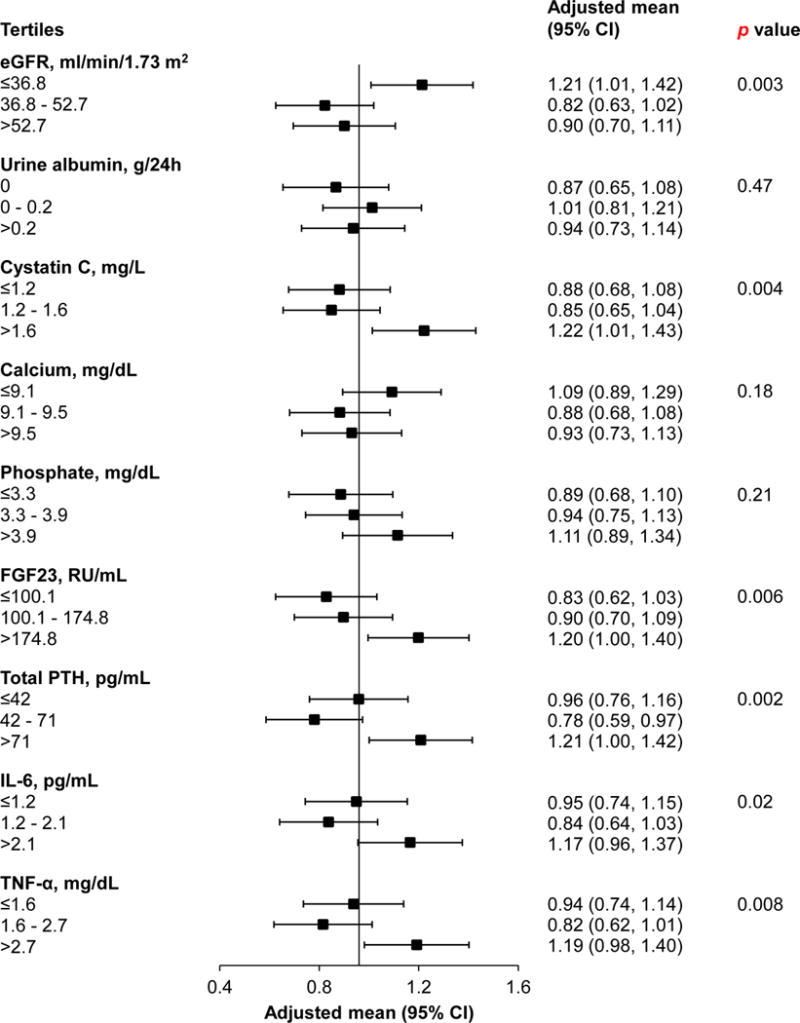
Adjusted mean annual change in square root transformed CAC score by tertiles of novel risk factors.
The vertical line indicates the mean annual change in square root transformed CAC score among all participants, equal to 0.96. All analyses were adjusted for age, sex, race/ethnicity, clinical site, baseline CAC score, total cholesterol, HDL cholesterol, systolic BP, use of antihypertensive medications, diabetes, current smoking, history of CVD, use of statin medications, and physical activity.
CAC, coronary artery calcification; eGFR, estimated glomerular filtration rate; FGF23, fibroblast growth factor-23; PTH, parathyroid hormone; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α.
3.3 Risk factors for continuous CAC progression
After adjusting for age, sex, race/ethnicity, clinical site, and baseline CAC score, lower eGFR and higher 24-hour urine albumin and cystatin C were associated with increases in square root transformed CAC score (Table 2). In addition, several other novel risk factors were associated with increased CAC score, including serum phosphate, FGF23, total PTH, glycated hemoglobin, HOMA-IR, fibrinogen, IL-6, and TNF-α, while serum calcium was associated with decreased CAC score. After further adjustment for total cholesterol, HDL cholesterol, systolic BP, use of antihypertensive medications, diabetes, current smoking, history of CVD, use of statin medications, and physical activity, 24-hour urine albumin and cystatin C remained significantly associated with increased CAC score, and eGFR remained significantly associated with decreased CAC score. Additionally, serum phosphate, FGF23, total PTH, IL-6, and TNF-α remained significantly associated with increased CAC score while serum calcium remained significantly associated with decreased CAC score.
Table 2.
Annual square root transformed change in CAC associated with novel risk factors among 1,123 CRIC study participants
| Variablesa | Multivariable model 1b | Multivariable model 2c | ||
|---|---|---|---|---|
| Square root transformed change in Agatston units/year (95% CI) | p value | Square root transformed change in Agatston units/year (95% CI) | p value | |
| Estimated glomerular filtration rate (17.4 ml/min/1.73 m2) | −0.26 (−0.36, −0.16) | <0.001 | −0.20 (−0.31, −0.10) | <0.001 |
| Log (24-hour urine albumin, 0.53 g/24 hours) | 0.23 (0.12, 0.33) | <0.001 | 0.14 (0.02, 0.25) | 0.02 |
| Cystatin C (0.66 mg/L) | 0.29 (0.19, 0.39) | <0.001 | 0.25 (0.15, 0.34) | <0.001 |
| Calcium (0.52 mg/dL) | −0.20 (−0.30, −0.10) | <0.001 | −0.17 (−0.27, −0.07) | <0.001 |
| Phosphate (0.68 mg/dL) | 0.21 (0.11, 0.31) | <0.001 | 0.14 (0.03, 0.24) | 0.009 |
| Log (fibroblast growth factor-23, 0.82 RU/mL) | 0.28 (0.19, 0.38) | <0.001 | 0.24 (0.14, 0.33) | <0.001 |
| Log (total parathyroid hormone, 0.67 pg/mL) | 0.18 (0.08, 0.27) | <0.001 | 0.13 (0.04, 0.23) | 0.007 |
| Alkaline phosphatase (31.2 U/L) | −0.02 (−0.12, 0.07) | 0.63 | −0.07 (−0.17, 0.02) | 0.14 |
| Hemoglobin A1c (1.37%) | 0.20 (0.11, 0.30) | <0.001 | 0.10 (−0.03, 0.22) | 0.12 |
| Log (HOMA-insulin resistance, 0.62) | 0.13 (0.03, 0.23) | 0.01 | 0.03 (−0.08, 0.14) | 0.59 |
| Uric acid (1.84 mg/dL) | 0.07 (−0.03, 0.17) | 0.19 | 0.03 (−0.07, 0.14) | 0.51 |
| Homocysteine (4.93 μmol/L) | 0.08 (−0.02, 0.19) | 0.13 | 0.02 (−0.08, 0.13) | 0.66 |
| Fibrinogen (1.10 mg/dL) | 0.15 (0.05, 0.25) | 0.002 | 0.08 (−0.02, 0.18) | 0.10 |
| Log (high sensitive C-reactive protein, 0.80 mg/L) | 0.02 (−0.08, 0.12) | 0.66 | 0.04 (−0.06, 0.13) | 0.45 |
| Log (interleukin-6, 0.57 pg/mL) | 0.18 (0.08, 0.28) | <0.001 | 0.17 (0.07, 0.27) | <0.001 |
| Log (tumor necrosis factor-α, 0.52 mg/dL) | 0.15 (0.05, 0.25) | 0.004 | 0.12 (0.02, 0.22) | 0.02 |
One standard deviation increase.
Adjusted for age, sex, race/ethnicity, clinical site, and baseline CAC score.
Adjusted for age, sex, race/ethnicity, clinical site, baseline CAC score, total cholesterol, HDL cholesterol, systolic BP, use of antihypertensive medications, diabetes, current smoking, history of CVD, use of statin medications, and physical activity.
3.4 Risk factors for categorical CAC progression
Among 434 participants with baseline CAC=0, 109 (25.1%) had incident CAC. After adjusting for age, sex, race/ethnicity, clinical sites, and follow-up time between CT scans, lower eGFR and higher 24-hour urine albumin and cystatin C were associated with increased odds of incident CAC (Table 3). Higher serum phosphate, FGF23, total PTH, glycated hemoglobin, fibrinogen, IL-6, and TNF-α were also significantly associated with increased odds of incident CAC. After further adjustment for established CVD risk factors, higher serum phosphate, FGF23, total PTH, fibrinogen, and IL-6 remained significantly associated with increased odds of incident CAC.
Table 3.
Odds Ratios for Incident Coronary Artery Calcification Associated with Novel Risk Factors among Participants without CAC at Baselinea
| Variablesb | Multivariable-adjustedc | Multivariable-adjustedd | ||
|---|---|---|---|---|
| Odds ratios (95% CI) | p value | Odds ratios (95% CI) | p value | |
| Estimated glomerular filtration rate (17.4 ml/min/1.73 m2) | 0.73 (0.57–0.93) | 0.01 | 0.80 (0.61–1.04) | 0.10 |
| Log (24-hour urine albumin, 0.53 g/24 hours) | 1.44 (1.11–1.87) | 0.006 | 1.21 (0.90–1.63) | 0.21 |
| Cystatin C (0.66 mg/L) | 1.24 (1.01–1.53) | 0.04 | 1.16 (0.92–1.45) | 0.21 |
| Calcium (0.52 mg/dL) | 0.82 (0.63–1.06) | 0.13 | 0.89 (0.67–1.18) | 0.43 |
| Phosphate (0.68 mg/dL) | 1.59 (1.22–2.08) | <0.001 | 1.51 (1.13–2.01) | 0.005 |
| Log (fibroblast growth factor-23, 0.82 RU/mL) | 1.38 (1.12–1.71) | 0.003 | 1.32 (1.05–1.67) | 0.02 |
| Log (total parathyroid hormone, 0.67 pg/mL) | 1.44 (1.13–1.84) | 0.003 | 1.34 (1.03–1.74) | 0.03 |
| Alkaline phosphatase (31.2 U/L) | 0.99 (0.79–1.24) | 0.93 | 0.85 (0.67–1.09) | 0.20 |
| Hemoglobin A1c (1.37%) | 1.39 (1.09–1.78) | 0.009 | 1.22 (0.87–1.72) | 0.25 |
| Log (HOMA-insulin resistance, 0.62) | 1.24 (0.98–1.58) | 0.08 | 1.06 (0.79–1.43) | 0.70 |
| Uric acid (1.84 mg/dL) | 1.22 (0.95–1.57) | 0.11 | 1.10 (0.84–1.44) | 0.49 |
| Homocysteine (4.93 μmol/L) | 1.27 (0.97–1.66) | 0.09 | 1.24 (0.93–1.65) | 0.15 |
| Fibrinogen (1.10 mg/dL) | 1.46 (1.14–1.87) | 0.003 | 1.32 (1.01–1.73) | 0.04 |
| Log (high sensitive C-reactive protein, 0.80 mg/L) | 1.16 (0.91–1.48) | 0.23 | 1.04 (0.80–1.36) | 0.75 |
| Log (interleukin-6, 0.57 pg/mL) | 1.63 (1.22–2.17) | <0.001 | 1.46 (1.08–1.99) | 0.02 |
| Log (tumor necrosis factor-α, 0.52 mg/dL) | 1.39 (1.09–1.77) | 0.009 | 1.28 (0.99–1.66) | 0.06 |
Incident coronary artery calcification was defined as any increase in CAC from the baseline to follow-up visit.
One standard deviation increase.
Adjusted for age, sex, race/ethnicity, clinical site, and follow-up time between CT scans.
Adjusted for age, sex, race/ethnicity, clinical site, follow-up time between CT scans, total cholesterol, HDL cholesterol, systolic BP, use of antihypertensive medications, diabetes, current smoking, history of CVD, use of statin medications, and physical activity.
Among 689 participants with baseline CAC >0, 124 (18.0%) had CAC progression. After adjusting for age, sex, race/ethnicity, clinical site, and baseline CAC score, lower eGFR and higher 24-hour urine albumin, cystatin C, serum phosphate, FGF23, total PTH, glycated hemoglobin, HOMA-IR, and TNF-α were associated with increased odds of CAC progression. After further adjustment for established CVD risk factors, lower eGFR and serum calcium and higher 24-hour urine albumin, cystatin C, serum phosphate, FGF23, and TNF-α remained significantly associated with increased odds of CAC progression.
3.5 Correlation among risk factors
There were several significant correlations among novel risk factors (Supplementary Table 1). For example, kidney function, denoted by eGFR, was positively correlated with serum calcium and negatively correlated with serum phosphate, FGF23, total PTH, alkaline phosphatase, fibrinogen, hsCRP, IL-6, and TNF-α. Significant correlations were observed within other etiologic groups, including calcium and phosphate metabolism (serum calcium, serum phosphate, FGF23, total PTH, and alkaline phosphatase; absolute values of r range from 0.11 to 0.36) and inflammation (fibrinogen, hsCRP, IL-6, and TNF-α; absolute values of r range from 0.13 to 0.48).
3.6 Sensitivity analyses
In a sensitivity analysis defining CAC progression as an increase in the square root transformed CAC score >2.5 mm3 over follow-up, 453 (65.7%) of those with baseline CAC had CAC progression (Supplementary Table 2). Multivariable-adjusted analyses indicated higher cystatin C and total PTH were associated with CAC progression. In another sensitivity analysis defining CAC progression as an increase of ≥300 Agatston units over follow-up, 137 (19.9%) of those with baseline CAC had CAC progression. Multivariable-adjusted analyses indicated lower eGFR and serum calcium and higher 24-hour urine albumin, cystatin C, serum phosphate, FGF23, and TNF-α were significantly associated with CAC progression (Supplementary Table 3). Sensitivity analyses repeated using total volume scores instead of Agatston scores yielded similar results (Supplemental Tables 4 and 5).
4. Discussion
In this prospective analysis of 1,123 CKD patients, we documented associations of CAC progression with several novel risk factors involved in kidney function, calcium-phosphate metabolism, and inflammation. Lower eGFR and serum calcium, and higher 24-hour urine albumin, cystatin C, serum phosphate, FGF23, total PTH, IL-6, and TNF-α were significantly associated with increases in CAC. These results provide valuable insights into the etiology of CAC progression among CKD patients. Furthermore, identification of novel risk factors implicated in both CAC progression and other pathologies, including reduced kidney function and calcium-phosphate metabolism, may reveal shared biological mechanisms and potential points of surveillance or intervention in CKD patients, beyond established risk factors.
Cardiovascular disease and mortality are closely linked with CKD [2]. The presence and progression of CAC predict the risk of CVD and mortality in the general population and in patients with comorbidities, including CKD [3–10]. Few longitudinal studies of risk factors for CAC progression have been conducted in CKD patients. An analysis of 562 CKD patients without CVD in the Multi-Ethnic Study of Atherosclerosis (MESA) identified only male sex and diabetes as significant predictors of incidence and progression of CAC, respectively [7]. Another analysis in 421 adults without CKD or CVD found no association between kidney function markers and CAC progression [18]. However, other studies conducted in adults without CKD reported contradictory results, finding associations between CAC progression and reduced eGFR [19] and microalbuminuria [20]. We documented several significant associations between increased CAC and kidney function markers, including lower eGFR and higher 24-hour urine albumin and cystatin C. These findings remained statistically significant after adjustment for established risk factors common to both CKD and CVD, suggesting an independent association between reduced kidney function and CAC progression in CKD patients.
We also documented significant associations between CAC progression and novel risk factors uniquely important to CKD patients. Impaired renal phosphate excretion is common in CKD patients, resulting in hyperphosphatemia, hypocalcemia, vascular calcification, and increased risk of mortality [21,22]. We found that higher serum phosphate was associated with increases in CAC score, supporting a previously-identified positive dose-response relationship between serum phosphate and prevalence of CAC [23]. Furthermore, we found that increases in FGF23 and total PTH, both important regulators of calcium-phosphate metabolism, were significantly associated with increased CAC score. FGF23 is associated with CVD in CKD patients [24] and is associated with CAC progression in dialysis patients [25]. However, a previous cross-sectional analysis of CRIC participants did not find an association with CAC prevalence or severity, suggesting a limited role of FGF23 in CAC in CKD patients [26]. In the present analysis, higher FGF23 was significantly associated with CAC progression after adjustment for established CVD risk factors, in both those with and without baseline CAC. Additionally, we identified novel evidence that lower serum calcium levels are associated with CAC progression. Conversely, previous studies conducted in dialysis patients suggested higher serum calcium levels are associated with CAC [27,28], potentially due to worsening secondary hyperparathyroidism and calcium supplementation, which are common in end-stage renal disease. Previous studies and the present analysis support a link between CKD and CAC progression due, in part, to abnormal calcium-phosphate biochemistry, which may be the most important pathogenic factor in vascular calcification among CKD patients [13,21].
Previous research suggested that other biomarkers are not predictive of CVD and mortality beyond established risk factors in CKD patients [29]. Similarly, an analysis of MESA participants at low risk for CVD suggested novel risk factors were not predictive of CAC progression [30]. However, in our analysis of CKD patients at high risk for CVD, we documented several novel risk factors and biomarkers associated with increases in CAC, including FGF23, total PTH, glycated hemoglobin, HOMA-IR, fibrinogen, IL-6, and TNF-α, which have previously been observed in cross-sectional analyses of patients with CKD [13]. After adjustment for established risk factors, many novel biomarkers remained associated with CAC progression, including IL-6 and TNF-α, which are both components of the inflammatory response and have been implicated as independent predictors of CVD risk [31] and have been associated with CAC progression in patients with diabetes and microalbuminuria [32].
The present study has several strengths. This is the first analysis of novel risk factors for CAC progression conducted in CKD patients, who share an excess burden of CVD and mortality. The CRIC study employs standardized methods, including standardized CAC readings in a centralized reading center, which minimizes bias. In addition, we analyzed many novel risk factors, including those uniquely important to CKD patients. However, the present study has limitations. Findings are based on one followup CAC measurement which prohibits analysis of CAC score trends, although we were still able to establish temporality between baseline risk factor measurements and changes in CAC. Another complication is disagreement regarding definitions of CAC progression. We assessed several common approaches that are predictive of clinical outcomes, especially coronary heart disease, and sensitivity analyses did not substantively impact our conclusions. Additionally, we could not distinguish between intimal and medial calcification, owing to limitations in CAC measurement. Increased CAC in CKD patients may be partly due to medial calcification, a separate process from atherosclerotic intimal calcification that can result in hypertension and left ventricular hypertrophy. While the 2 types of calcification may represent somewhat different pathologies, both are associated with increased risk of CVD and all-cause mortality in patients with advanced CKD [28]. Finally, the CRIC study only included patients with pre-existing CKD. Future studies should evaluate associations of the identified novel risk factors with CAC progression in patients without CKD.
Our study indicates that reduced kidney function, calcium and phosphate metabolism disorders, and inflammation, independent of established CVD risk factors, might play a role in CAC progression among CKD patients. Further studies are warranted to confirm these findings and to develop novel treatments to slow the progression of CAC among patients with CKD.
Supplementary Material
Table 4.
Odds Ratios for Coronary Artery Calcification Progression Associated with Novel Risk Factors among Participants with CAC >0 at Baselinea
| Variablesb | Multivariable-adjustedc | Multivariable-adjustedd | ||
|---|---|---|---|---|
| Odds ratios (95% CI) | p value | Odds ratios (95% CI) | p value | |
| Estimated glomerular filtration rate (17.4 ml/min/1.73 m2) | 0.52 (0.38–0.70) | <0.001 | 0.55 (0.40–0.76) | <0.001 |
| Log (24-hour urine albumin, 0.53 g/24 hours) | 1.44 (1.14–1.82) | 0.002 | 1.36 (1.05–1.77) | 0.02 |
| Cystatin C (0.66 mg/L) | 1.67 (1.32–2.13) | <0.001 | 1.68 (1.29–2.18) | <0.001 |
| Calcium (0.52 mg/dL) | 0.70 (0.55–0.89) | 0.003 | 0.72 (0.56–0.92) | 0.01 |
| Phosphate (0.68 mg/dL) | 1.71 (1.34–2.19) | <0.001 | 1.55 (1.21–2.00) | <0.001 |
| Log(fibroblast growth factor-23, 0.82 RU/mL) | 1.66 (1.30–2.11) | <0.001 | 1.66 (1.28–2.15) | <0.001 |
| Log(total parathyroid hormone, 0.67 pg/mL) | 1.30 (1.02–1.65) | 0.03 | 1.24 (0.97–1.60) | 0.09 |
| Alkaline phosphatase (31.2 U/L) | 1.04 (0.82–1.31) | 0.77 | 1.03 (0.80–1.33) | 0.81 |
| Hemoglobin A1c (1.37%) | 1.39 (1.13–1.71) | 0.002 | 1.11 (0.85–1.44) | 0.44 |
| Log (HOMA-insulin resistance, 0.62) | 1.32 (1.06–1.66) | 0.02 | 1.09 (0.84–1.40) | 0.53 |
| Uric acid (1.84 mg/dL) | 0.96 (0.76–1.23) | 0.77 | 0.91 (0.70–1.17) | 0.44 |
| Homocysteine (4.93 μmol/L) | 1.20 (0.95–1.52) | 0.12 | 1.08 (0.84–1.39) | 0.55 |
| Fibrinogen (1.10 mg/dL) | 1.04 (0.83–1.32) | 0.72 | 1.01 (0.79–1.28) | 0.95 |
| Log (high sensitive C-reactive protein, 0.80 mg/L) | 0.89 (0.70–1.13) | 0.35 | 1.00 (0.78–1.28) | 0.99 |
| Log (interleukin-6, 0.57 pg/mL) | 0.91 (0.71–1.16) | 0.44 | 0.93 (0.72–1.21) | 0.60 |
| Log (tumor necrosis factor-α, 0.52 mg/dL) | 1.36 (1.08–1.72) | 0.008 | 1.44 (1.12–1.86) | 0.004 |
Coronary artery calcification progression was defined as an annual increase of ≥100 Agatston units from the baseline to follow-up visit.
One standard deviation increase.
Adjusted for age, sex, race/ethnicity, clinical site, and baseline CAC score.
Adjusted for age, sex, race/ethnicity, clinical site, baseline CAC score, total cholesterol, HDL cholesterol, systolic BP, use of antihypertensive medications, diabetes, current smoking, history of CVD, use of statin medications, and physical activity.
Highlights.
Low estimated-glomerular filtration rate and high albuminuria and serum cystatin C increased CAC progression in CKD patients
Calcium and phosphate metabolism disorders (low serum calcium, and high serum phosphate and fibroblast growth factor-23) increased CAC progression in CKD patients
Elevated inflammatory biomarkers (interleukin-6 and tumor necrosis factor-α) increased CAC progression among CKD patients
Associations of novel risk factors with CAC progression were independent of traditional CVD factors
Acknowledgments
The authors thank the participants, investigators, and staff of the CRIC study for their time and commitment. The CRIC Principal Investigators are Lawrence J. Appel, MD, MPH, Harold I. Feldman, MD, MSCE, Alan S. Go, MD, Jiang He, MD, PhD, John W. Kusek, PhD, James P. Lash, MD, Akinlolu Ojo, MD, PhD, Mahboob Rahman, MD, Raymond R. Townsend, MD.
Financial support
Funding for the CRIC Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this study was supported in part by the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, the Johns Hopkins Institute for Clinical and Translational Research (ICTR) UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, and Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
JDB and JH designed the study and drafted the manuscript. JDB, WY, XZ, and JH acquired and analyzed the data. JDB, JC, WY, MB, ASG, JEG, RRK, WSP, MPR, ACR, SER, XZ, and JH interpreted the data and revised the manuscript.
References
- 1.Go A, Chertow G, Fan D, Mcculloch CE, Hsu C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJL, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 3.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 4.Rennenberg RJMW, Kessels AGH, Schurgers LJ, Van Engelshoven JMA, De Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: A meta-analysis, Vasc Heal. Risk Manag. 2009;5:185–197. doi: 10.2147/VHRM.S4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Young R, Lopez VA, Kronmal RA, Nasir K, Blumenthal RS, Detrano RC, Bild DE, Guerci AD, Liu K, Shea S, Szklo M, Post W, Lima J, Bertoni A, Wong ND. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61:1231–1239. doi: 10.1016/j.jacc.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestenbaum BR, Adeney KL, De Boer IH, Ix JH, Shlipak MG, Siscovick DS, de Boer IH. Incidence and progression of coronary calcification in chronic kidney disease: the Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009;76:991–8. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shantouf RS, Budoff MJ, Ahmadi N, Ghaffari A, Flores F, Gopal A, Noori N, Jing J, Kovesdy CP, Kalantar-Zadeh K. Total and individual coronary artery calcium scores as independent predictors of mortality in hemodialysis patients. Am J Nephrol. 2010;31:419–425. doi: 10.1159/000294405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roe P, Wolfe M, Joffe M, Rosas SE. Inflammation, coronary artery calcification and cardiovascular events in incident renal transplant recipients. Atherosclerosis. 2010;212:589–594. doi: 10.1016/j.atherosclerosis.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L, Ford V, Raj D, Porter AC, Soliman EZ, Wright JT, Jr, Wolf M, He J. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol. 2017 doi: 10.1001/jamacardio.2017. [Epub ahead of print] doi:10.1001/jamacardio.2017.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McEvoy JW, Blaha MJ, DeFilippis AP, Budoff MJ, Nasir K, Blumenthal RS, Jones SR. Coronary artery calcium progression: An important clinical measurement? J Am Coll Cardiol. 2010;56:1613–1622. doi: 10.1016/j.jacc.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 13.He J, Reilly M, Yang W, Chen J, Go AS, Lash JP, Rahman M, DeFilippi C, Gadegbeku C, Kanthety R, Tao K, Hamm LL, Ojo A, Townsend R, Budoff M. Risk factors for coronary artery calcium among patients with chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study) Am J Cardiol. 2012;110:1735–41. doi: 10.1016/j.amjcard.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic renal insufficiency cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 17.Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, Eckel RH, Rewers M. Evaluating Changes in Coronary Artery Calcium: An Analytic Method That Accounts for Interscan Variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 18.Jassal SK, Chonchol M, Laughlin GA, Cummins KM, Smits G, Kramer CK, Ix JH, Barrett-Connor E. Kidney function and progression of coronary artery calcium in community-dwelling older adults (from the Rancho Bernardo Study) Am J Cardiol. 2012;110:1425–1433. doi: 10.1016/j.amjcard.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuttle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clin J Am Soc Nephrol. 2009;4:1968–1973. doi: 10.2215/CJN.01250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Defilippis AP, Kramer HJ, Katz R, Wong ND, Bertoni AG, Carr J, Budoff MJ, Blumenthal RS, Nasir K. Association between coronary artery calcification progression and microalbuminuria: The MESA study. JACC Cardiovasc Imaging. 2010;3:595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covic A, Kanbay M, Voroneanu L, Turgut F, Serban DN, Serban IL, Goldsmith DJ. Vascular calcification in chronic kidney disease. Clin Sci. 2010;119:111–121. doi: 10.1042/CS20090631. http://dx.doi.org/10.1042/CS20090631. [DOI] [PubMed] [Google Scholar]
- 22.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL. Serum Phosphate Levels and Mortality Risk among People with Chronic Kidney Disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/asn.2004070602. [DOI] [PubMed] [Google Scholar]
- 23.Adeney KL, Siscovick DS, Ix JH, Seliger SL, Shlipak MG, Jenny NS, Kestenbaum BR. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–60. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan AM, Chirinos JA, Litt H, Yang W, Rosas SE. FGF-23 and the progression of coronary arterial calcification in patients new to dialysis. Clin J Am Soc Nephrol. 2012;7:2017–2022. doi: 10.2215/CJN.02160212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scialla JJ, Lau WL, Reilly MP, Isakova T, Yang HY, Crouthamel MH, Chavkin NW, Rahman M, Wahl P, Amaral AP, Hamano T, Master SR, Nessel L, Chai B, Xie D, Kallem RR, Chen J, Lash JP, Kusek JW, Budoff MJ, Giachelli CM, Wolf M. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–68. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman W, Goldin J, Kuizon B, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff R, Salusky I. Coronary-artery Calcification in Young Adults with End-stage Renal Disease Who Are Undergoing Dialysis. N Engl J Med. 2000;342:1478–83. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 28.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 29.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–45. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 30.Okwuosa TM, Greenland P, Burke GL, Eng J, Cushman M, Michos ED, Ning H, Lloyd-Jones DM. Prediction of coronary artery calcium progression in individuals with low Framingham Risk Score: The multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. 2012;5:144–153. doi: 10.1016/j.jcmg.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cesari M, Penninx BWJH, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory Markers and Onset of Cardiovascular Events: Results from the Health ABC Study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 32.von Scholten BJ, Reinhard H, Hansen TW, Schalkwijk CG, Stehouwer C, Parving HH, Jacobsen PK, Rossing P. Markers of inflammation and endothelial dysfunction are associated with incident cardiovascular disease, all-cause mortality, and progression of coronary calcification in type 2 diabetic patients with microalbuminuria. J Diabetes Complicat. 2015;30:248–255. doi: 10.1016/j.jdiacomp.2015.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


