Abstract
Objective
Measures of HDL function are associated with cardiovascular disease. However, the effects of regular exercise on these measures is largely unknown. Thus, we examined the effects of different doses of exercise on three measures of HDL function in two randomized clinical exercise trials.
Approach and Results
Radiolabeled and BODIPY-labeled cholesterol efflux capacity and HDL-apoA-I exchange were assessed before and after six months of exercise training in two cohorts: STRRIDE-PD (N=106) and E-MECHANIC (N=90). STRRIDE-PD participants completed one of four exercise interventions differing in amount and intensity. E-MECHANIC participants were randomized into one of two exercise groups (8 or 20 kcal/kg/week (KKW)) or a control group. HDL-C significantly increased in the High-amount/Vigorous-intensity group (3±5 mg/dL, p=0.02) of STRRIDE-PD, while no changes in HDL-C were observed in E-MECHANIC. In STRRIDE-PD, global radiolabeled efflux capacity significantly increased 6.2% (SEM 0.06) in the High-amount/Vigorous-intensity group compared to all other STRRIDE-PD groups (range: −2.4 to −8.4%, SEM 0.06). In E-MECHANIC, non-ABCA1 radiolabeled efflux significantly increased 5.7% (95% CI 1.2–10.2%) in the 20 KKW group compared to the control group, with no change in the 8 KKW group (2.6%, 95% CI −1.4 to 6.7%). This association was attenuated when adjusting for change in HDL-C. Exercise training did not affect BODIPY-labeled cholesterol efflux capacity or HDL-apoA-I exchange in either study.
Conclusions
Regular prolonged vigorous exercise improves some but not all measures of HDL function. Future studies are warranted to investigate whether the effects of exercise on cardiovascular disease are mediated in part by improving HDL function.
Keywords: exercise training, cholesterol, HDL, efflux, apolipoprotein A-I
Subject codes: Lipids and cholesterol, Exercise, Risk Factors
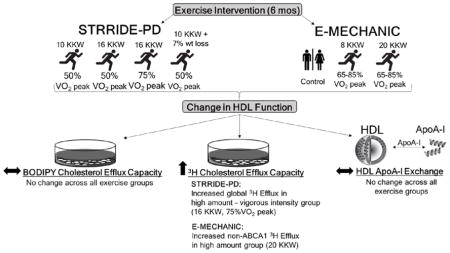
Visual Overview
Introduction
High-density lipoproteins (HDL) consist of a wide spectrum of heterogeneous particles that differ in size, density, composition, and biological function. HDL particles have multiple atheroprotective activities, with reverse cholesterol transport thought to be one of the most important.1 A critical first step in reverse cholesterol transport is the efflux of cholesterol from macrophage foam cells in the artery to HDL. This critical first step has been interrogated in multiple observational human studies with an ex vivo macrophage-specific cholesterol efflux assay. Cholesterol efflux capacity has been inversely associated with both prevalent and incident cardiovascular disease (CVD), independent of traditional risk factors including HDL-cholesterol (HDL-C) levels.2–5 Furthermore, the rate of HDL-apolipoprotein A-I (apoA-I) exchange (HAE), an indirect measure of cholesterol efflux capacity that measures the ability of apoA-I to exchange on and off HDL6–8, has been shown to be reduced in patients with metabolic syndrome or acute coronary syndrome (ACS).7, 9 These results along with the inability of recent randomized, controlled drug trials and Mendelian randomization studies to demonstrate a causal relationship between HDL-C and CVD has led to a focus on identifying therapies that improve HDL function (i.e., quality) rather than HDL-C quantity.10
Although regular exercise increases HDL-C levels11, 12 and alters HDL particle subclass concentrations13, 14 in a dose-response manner, its effects on HDL functionality are less well known. Several exercise training studies have demonstrated the beneficial effects of regular exercise on various HDL functions, including endothelial15, anti-oxidative16, 17, and anti-inflammatory properties18, 19 in adults with CVD, diabetes, and metabolic syndrome. However, these studies only included one exercise dose and thus the dose-response relationship of exercise and HDL function is currently unknown. Furthermore, few studies have examined the effects of regular exercise on HDL-mediated cholesterol efflux, with mixed results.19–23 These studies were limited by small sample sizes and differing intervention durations, study populations, efflux assays, and cell lines. To date, only two studies in patients with CVD20, 21 have examined the effects of regular exercise on cholesterol efflux capacity measured using the validated assays from recent longitudinal studies2, 5, namely the efflux of 3H-labeled cholesterol from J774 macrophages to apolipoprotein B–depleted plasma or serum. To our knowledge, no study has examined the effects of exercise training on efflux capacity using the fluorescence-labeled (BODIPY) cholesterol4 or HAE assays7, nor has any study examined these associations in healthy adults. Therefore, the purpose of the present study was to examine the effects of different intensities and doses of exercise on cholesterol efflux capacity and HAE in two randomized clinical exercise trials of healthy and pre-diabetic adults.
Materials and Methods
The effects of standardized, regular endurance exercise on cholesterol efflux capacity were examined across two exercise training studies (STRRIDE-PD and E-MECHANIC) with five distinct exercise interventions, including a lifestyle intervention, which are described below.
STRRIDE-PD study
The STRRIDE-PD (Studies of Targeted Risk Reduction Interventions through Defined Exercise, in individuals with Pre-Diabetes) study compared three six-month exercise-only groups differing in amount and/or intensity to a lifestyle intervention group (diet plus exercise group similar to the first six months of the Diabetes Prevention Program). Specifically, sedentary, moderately overweight/obese (body mass index (BMI) 25–35 kg/m2), non-smoking adults (N=237) aged 45–75 years old with pre-diabetes (i.e., impaired fasting glucose 100–125 mg/dL; or in up to 20% of the subject population, high normal fasting glucose 95–99), but no history of diabetes or CVD, were randomly assigned to one of four groups: 1) low-amount/moderate-intensity exercise (10 kcal/kg/wk (KKW) at 50% V̇O2reserve); 2) high-amount/moderate-intensity exercise (16 KKW at 50% V̇O2reserve); 3) high-amount/vigorous-intensity exercise (16 KKW at 75% V̇O2reserve); and 4) clinical lifestyle intervention (diet + exercise): 10 KKW at 50% V̇O2reserve (same as group 1) plus a diet designed to reduce body weight by 7%.24 Overall, 175 participants completed the intervention. The present study included 106 participants that completed the exercise training program and had data for all three measures of HDL function. There were no differences in baseline characteristics between subjects with and without HDL function data (data not shown).
E-MECHANIC study
The E-MECHANIC (Examination of Mechanisms of exercise-induced weight compensation) study was a three-arm, six-month randomized (1:1:1) controlled trial. Sedentary, overweight/obese (BMI 25–45 kg/m2), non-diabetic (fasting plasma glucose 76–106 mg/dL), non-smoking adults with no history of diabetes or CVD aged 18 to 65 years (N=198) were randomly assigned to a control group or one of two exercise groups that reflect the current recommendations for 1) general health (8 KKW, or approximately 800 to 1,000 kcal/wk) or 2) weight loss (20 KKW, or approximately 2,000 to 2,500 kcal/wk). In the two exercise groups, the exercise intensity for each participant was set at a target heart rate associated with 65% to 85% of peak oxygen uptake (V̇O2peak).25 The present study included 90 participants that completed the study and had data for all three measures of HDL function.
Blood draws and lipids
In STRRIDE-PD, blood samples were taken after a 10-hr fast at baseline and between 16–24 hours after the last exercise training session. In E-MECHANIC, blood was collected after a 12-hr fast at baseline and during the last week of exercise (24–72 hrs after last exercise session). In both studies, measurement of total cholesterol, HDL-C, and triglycerides (TG) was performed on a Beckman Coulter DXC 600 Pro system (Brea, CA) using standardized methods. In STRRIDE-PD comprehensive lipoprotein subfraction analysis was also performed before and after exercise training via NMR spectroscopy using the LipoProfile-3 algorithm (LipoScience, Inc, now LabCorp, Morrisville, N.C.) as previously described.26 GlycA concentration was also assessed via NMR spectroscopy as previously described.27
Assessment of HDL function
All assays were performed on stored (−80°C) baseline and post-training plasma (STRRIDE-PD) or serum (E-MECHANIC) samples. Change in each individual HDL function was calculated as the absolute difference between the post-training and baseline values.
General cholesterol efflux capacity assay procedures
The following procedures were common to both the radiolabeled (3H) and fluorescence-labeled cholesterol efflux capacity assays. Global and ATP-binding cassette transporter A1 (ABCA1)-mediated cholesterol efflux were measured using J774 mouse macrophage cells in the presence and absence of cAMP. Cells were incubated with 3H- or fluorescence-labeled cholesterol and 2 μg/mL acyl–coenzyme A:cholesterol acyltransferase inhibitor inhibitor (Santos, Sigma-Aldrich). Cells were then incubated overnight in 0.2% BSA with or without 8-(4-chlorophenylthio)-cyclic AMP. After washing, the cholesterol labeled cells were incubated with 2.8% apolipoprotein B (apoB)-depleted plasma/serum for 4 hours. The amount of 3H- or fluorescence-labeled cholesterol released was measured through liquid scintillation counting or spectrophotometer, respectively. Cholesterol efflux capacity is calculated as the amount of effluxes cholesterol expressed as a fraction (%) of the initial cell content of cholesterol. Results were normalized to the measured efflux by a pooled reference depleted-depleted sample evaluated on every plate. All samples were run in duplicate and the average value is reported.
3H cholesterol efflux capacity assay
Measurement of the efflux of radiolabeled cholesterol from J774 macrophages to apoB-depleted plasma/serum was performed with the use of a previously described method.2 This assay quantifies total efflux mediated by known pathways of cholesterol efflux from macrophages, including ATP-binding cassette transporter A1 (ABCA1) and G1 (ABCG1), scavenger receptor B1, and aqueous diffusion.28 Due to timing issues, the radiolabeled cholesterol efflux capacity assay was performed in different laboratories, with Dr. Anand Rohatgi’s laboratory at the University of Texas Southwestern Medical Center analyzing plasma samples from STRRIDE-PD and Vascular Strategies (Plymouth Meeting, PA) analyzing serum samples from E-MECHANIC. As such, the methods slightly differed between the two laboratories. The assays in STRRIDE-PD were performed with stimulation by cAMP, which up regulates ABCA1 and thus represents global efflux. The assays in E-MECHANIC were performed with and without stimulation by cAMP, thus providing values for global efflux, as well as non-ABCA1 dependent efflux.29 ABCA1-dependent efflux was calculated as the difference between global (+cAMP) and non-ABCA1 (-cAMP) efflux.
BODIPY cholesterol efflux capacity assay
The efflux of boron dipyrromethene difluoride (BODIPY)-labeled cholesterol from J774 macrophages to apoB-depleted plasma (STRRIDE-PD) or serum (E-MECHANIC) was measured using a high throughput cell-based assay performed in 96-well plates as previously described.4 The Rohatgi lab performed the BODIPY efflux assays for both cohorts (STRRIDE-PD and E-MECHANIC). The BODIPY efflux assays were performed with stimulation by cAMP, thus providing values for global cholesterol efflux only.
HDL-apoA-I exchange (HAE) assay
HAE measures the ability of spin labeled apolipoprotein A-I (apoA-I) to exchange on and off of HDL, thus representing HDL remodeling dynamics as well as potential cholesterol efflux capacity. The HAE assay was performed in Dr. Michael Oda’s laboratory at Children’s Hospital Oakland Research Institute, as previously described.6, 7 Briefly, apoB-depleted plasma or serum was prepared through polyethylene glycol precipitation and mixed with 3 mg/mol spin-labeled apoA-I30 in a 3:1 ratio. After incubation for 15 minutes at 37°C, electron paramagnetic resonance measurements were performed with Broker scan (STRRIDE-PD) or EMX Nan (E-MECHANIC) spectrometers. HAE activity represents the sample:internal standard signal ratio at 37 °C. All samples were read in duplicate and averaged.
Assessment of cardio metabolic traits
The E-MECHANIC study included measures of body composition via dual-energy X-ray absorptiometry, fasting blood glucose, resting metabolic rate, and energy intake and expenditure through food intake tests and doubly labeled water.25 The STRRIDE-PD study measured body composition using air plethysmography (BodPod) and insulin and glucose traits through a two-hour oral glucose tolerance test using standard protocols.24
Statistical analysis
Paired t-tests were used to examine the effects of exercise training on each of the measured HDL functions in all exercise groups combined, as well as within each exercise group. In E-MECHANIC, two sample t-tests were used to compare changes in HDL function between 6-month follow-up and baseline between each exercise group and the control group. Normality and equal variance tests were performed on baseline and post-training measures of HDL function and revealed all but baseline HAE were normally distributed. Results were unchanged using non-parametric tests for HAE, thus all results for change in HAE with exercise are for parametric tests.
General linear models adjusted for age, sex, race, baseline BMI, and baseline value (of dependent variable) were used to examine the effects of training on measures of HDL function across exercise intervention groups. Pearson correlations were used to examine the relationships between all three measures of HDL function. Multivariable stepwise regression models were used to examine baseline predictors of exercise induced changes in each measure of HDL function. Baseline predictors in STRRIDE-PD included baseline trait value, body composition, NMR-measured HDL subfraction concentrations, standard lipid panel, cardiorespiratory fitness, and measures of insulin and glucose homeostasis. Baseline predictors in E-MECHANIC included baseline value, BMI, standard lipid panel, blood pressure, glucose, and energy intake and expenditure. All analyses were performed separately within each study cohort using SAS 9.4 (Cary, NC), with statistical significance set at a two-tailed p < 0.05.
Results
The basic characteristics of the participants from STRRIDE-PD and E-MECHANIC at baseline and in response to the interventions can be found in Tables 1 and 2, respectively. At baseline there were no differences between groups within each study for any of the variables presented. The use of cholesterol lowering medications was low and did not show much change during the intervention across both studies, as 21% and 17% of STRRIDE-PD subjects (Table 1) and 12% of E-MECHANIC subjects (Table 2) were taking cholesterol lowering medications at baseline and six months, respectively. Aside from one subject at baseline in STRRIDE-PD, at both time points in both studies all subjects listed as taking cholesterol lowering medications were taking a form of statin. Across both studies, women had higher baseline levels of HAE and HDL-C compared to men, while in E-MECHANIC women had higher baseline levels of radiolabeled non-ABCA1 efflux and lower triglycerides compared to men (data not shown).
Table 1.
Basic characteristics of STRRIDE-PD participants.
| Variable | STRRIDE-PD Group (N=106 total) | ||||
|---|---|---|---|---|---|
| Low-Mod N=29 |
High-Mod N=27 |
High-Vig N=24 |
Clinical Lifestyle N=26 |
||
| Male/Female (N) | 11/18 | 11/16 | 8/16 | 9/17 | |
| White (%) | 79 | 81 | 71 | 77 | |
| Age (yrs) | 56.8 ± 8.1 | 61.0 ± 6.5 | 58.6 ± 6.7 | 57.0 ± 7.9 | |
| Cholesterol medication use, N (%) | Baseline | 6 (21%) | 3 (11%) | 6 (25%) | 7 (27%) |
| Post-training | 6 (21%) | 3 (11%) | 5 (21%) | 4 (15%) | |
| Weight (kg) | Baseline | 87.4 ± 11.5 | 86.6 ± 11.9 | 84.9 ± 9.9 | 88.4 ± 16.0 |
| Delta* | −0.5 ± 3.0 | −1.5 ± 2.8‡ | −1.5 ± 3.2‡ | −6.5 ± 5.5§ | |
| BMI (kg/m2) | Baseline | 31.3 ± 1.1 | 31.6 ± 5.1 | 30.4 ± 4.4 | 31.6 ± 6.8 |
| Delta* | −0.2 ± 1.0 | −0.6 ± 1.1‡ | −0.6 ± 1.1‡ | −2.3 ± 1.9§ | |
| HDL-C (mg/dl) | Baseline | 48 ± 24 | 46 ± 13 | 45 ± 12 | 43 ± 12 |
| Delta | −2 ± 14 | 0 ± 5 | 3 ± 5‡ | 2 ± 7 | |
| TG (mg/dl) | Baseline | 125 ± 57 | 116 ± 36 | 136 ± 77 | 119 ± 58 |
| Delta† | 1 ± 46 | 14 ± 45 | −14 ± 46 | −23 ± 36‡ | |
| Total Cholesterol (mg/dl) | Baseline | 188 ± 40 | 190 ± 27 | 184 ± 25 | 177 ± 33 |
| Delta | 1 ± 23 | 0 ± 18 | −2 ± 16 | −6 ± 22 | |
| 3H efflux (global) | Baseline | 0.7 ±0.2 | 0.8 ± 0.3 | 0.9 ± 0.2 | 0.9 ± 0.2 |
| Delta | −0.03 ± 0.1 | −0.04 ± 0.2 | 0.05 ± 0.2 | −0.04 ± 0.1 | |
| BODIPY efflux | Baseline | 0.6 ±0.2 | 0.71± 0.2 | 0.7 ± 0.2 | 0.7 ± 0.2 |
| Delta | 0.04 ± 0.2 | −0.02 ± 0.1 | −0.02 ± 0.2 | −0.06 ± 0.2 | |
| HAE | Baseline | 49.8 ± 11.8 | 47.2 ± 9.7 | 48.8 ± 10.2 | 49.4 ± 7.5 |
| Delta | −1.5 ± 7.9 | 1.3 ± 5.5 | 1.6 ± 4.6 | 0.2 ± 6.5 | |
Data represented as mean ± SD. Delta = post-training value – baseline value.
p<0.0001 and
p=0.04 for differences in mean values between groups.
Clinical lifestyle mean value significantly different from all other groups.
High-Mod group different from Low-Mod and Clinical Lifestyle groups.
p<0.05 and
p<0.0001 for within group training response (paired t-test of difference from baseline).
BMI, body mass index; TG, triglycerides.
Table 2.
Basic characteristics of E-MECHANIC participants.
| Variable | E-MECHANIC Group (N=90 total) | |||
|---|---|---|---|---|
| Control N=29 |
8 KKW N=33 |
20 KKW N=28 |
||
| Male/Female (N) | 9/20 | 6/27 | 8/20 | |
| White (%) | 72 | 67 | 71 | |
| Age (yrs) | 50.2 ± 11.0 | 48.9 ± 10.7 | 45.7 ± 13.9 | |
| Cholesterol medication use, N (%) | Baseline | 5 (17%) | 3 (9%) | 3 (11%) |
| Post-training | 6 (21%) | 3 (9%) | 3 (11%) | |
| Weight (kg) | Baseline | 90.3 ± 15.4 | 90.7 ± 17.4 | 83.4 ± 13.8 |
| Delta | 0.4 ± 3.5 | 0.3 ± 2.9 | −1.4 ± 3.1* | |
| BMI (kg/m2) | Baseline | 32.4 ± 4.6 | 32.2 ± 4.9 | 29.9 ± 4.4 |
| Delta† | 0.2 ± 1.2 | 0.07 ± 1.0 | −0.5 ± 1.1* | |
| HDL-C (mg/dl) | Baseline | 58 ± 15 | 60 ± 15 | 57 ± 20 |
| Delta | −2 ± 7 | −1 ± 8 | 1 ± 6 | |
| TG (mg/dl) | Baseline | 117 ± 57 | 106 ± 45 | 98± 50 |
| Delta | −3 ± 49 | −10 ± 39 | −1 ± 30 | |
| Total Cholesterol (mg/dl) | Baseline | 209 ± 27 | 199 ± 35 | 189 ± 40 |
| Delta | −16 ± 31 | −2 ± 17 | 2 ± 20 | |
| 3H efflux (Global) | Baseline | 0.87 ± 0.2 | 0.82 ± 0.2 | 0.83 ± 0.2 |
| Delta | −0.03 ± 0.1 | 0.01 ± 0.2 | −0.01 ± 0.1 | |
| BODIPY efflux | Baseline | 0.94 ± 0.3 | 0.87 ± 0.3 | 0.86 ± 0.3 |
| Delta | −0.08 ± 0.2 | −0.06 ± 0.2 | −0.03 ± 0.3 | |
| HAE | Baseline | 63.3 ± 12.3 | 62.4 ± 8.6 | 60.7 ± 12.7 |
| Delta | −2.4 ± 9.0 | 1.4 ± 8.8 | 0.9 ± 11.1 | |
Data represented as mean ± SD. Delta = post-training value – baseline value.
p<0.05 for within group training response (paired t-test of difference from baseline).
p=0.04 for differences in mean values between groups. Mean value for 20 KKW group significantly different from control and 8 KKW groups.
BMI, body mass index; TG, triglycerides.
Exercise training and HDL-C
In STRRIDE-PD, exercise training resulted in a significant increase in HDL-C in the High-amount/Vigorous group only (Table 1), while no significant changes in HDL-C were observed within exercise groups or between the exercise groups and the control group after exercise training in E-MECHANIC (Table 2).
Exercise training and 3H-labeled cholesterol efflux
STRRIDE-PD
There was a significant effect of intervention group (p=0.04) on change in global radiolabeled efflux capacity, with the high amount/vigorous intensity group showing significantly larger exercise-induced increases in global radiolabeled efflux compared to the other three groups (Figure 1). Specifically, the high amount/vigorous intensity group increased radiolabeled efflux by an average of 6.2% (standard error of mean (SEM) 6.1) in adjusted models, while the adjusted mean change was −8.4% (SEM 6.6) in the low amount/moderate intensity group, −4.2% (SEM 6.5) in the high amount/moderate intensity group, and −2.4% (SEM 6.0) in the Clinical lifestyle group, respectively. The overall group difference was attenuated when also adjusting for change in HDL-C (p=0.09), but the differences between the high amount/vigorous intensity group and the other groups remained (p<0.05).
Figure 1.
Adjusted mean (SEM) percent change of radiolabeled global efflux (A) and BODIPY-labeled global efflux (B) in response to exercise training in STRRIDE-PD. Values adjusted for age, sex, race, baseline BMI, and baseline value. ap=0.005 and bp<0.05 for difference from High-Vig.
E-MECHANIC
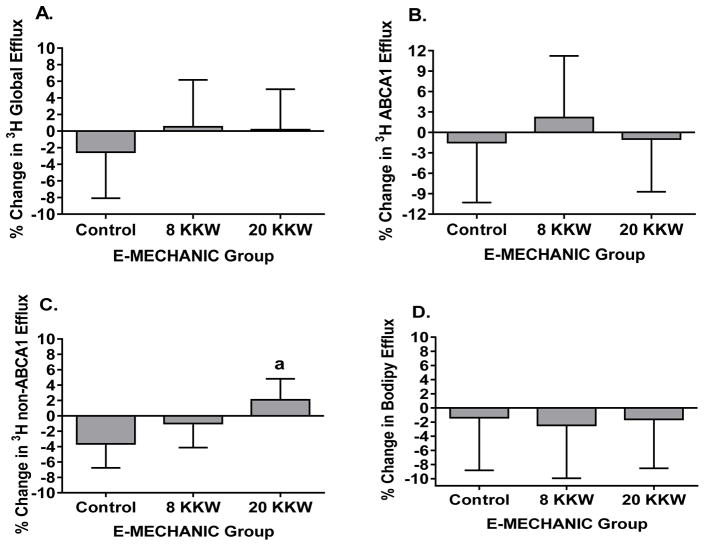
No significant changes in radiolabeled efflux capacity were observed after 6-months of exercise training compared to baseline in the individual exercise groups. However, there was a significant effect of group (p=0.046) on percent change in non-ABCA1 dependent efflux, as the 20 KKW group showed a 5.7% (95% CI 1.2–10.2%) greater increase in non-ABCA1 efflux compared to the control group (Figure 2). This association was attenuated when also adjusting for change in HDL-C (p=0.07). No significant differences compared to the control group were found in either exercise group for radiolabeled global or ABCA1 efflux capacity.
Figure 2.
Adjusted mean (SEM) percent change of radiolabeled global (A), ABCA1 (B), and non-ABCA1 (C) efflux, and BODIPY-labeled global efflux (D) in response to exercise training in E-MECHANIC. Values adjusted for age, sex, race, baseline BMI, and baseline value. ap=0.01 for difference from control group.
Exercise training and BODIPY-labeled cholesterol efflux
STRRIDE-PD
In STRRIDE-PD, there was no effect of exercise training on BODIPY efflux capacity in any of the four groups, nor were there any group differences in BODIPY-efflux responses to training (Figure 1).
E-MECHANIC
No significant changes in BODIPY-labeled efflux capacity were observed after 6-months of exercise training in E-MECHANIC, with both exercise groups experiencing an average decrease (Figure 2). Similarly, there were no differences between the individual exercise groups and the control group for changes in BODIPY efflux capacity in either unadjusted or adjusted models.
Exercise training and HDL-apoA-I-exchange
STRRIDE-PD and E-MECHANIC
No significant changes in HAE were observed after 6-months of exercise training in STRRIDE-PD or E-MECHANIC (Supplemental Figure I). In general, all exercise groups experienced a (non-significant) mean increase in HAE, except the low amount/moderate intensity group of STRRIDE-PD.
Baseline predictors of exercise-induced changes in HDL function
STRRIDE-PD
In multivariable regression models in the combined exercise groups of STRRIDE-PD, baseline value was the strongest predictor of exercise-induced changes in all three measures of HDL function (Supplemental Table SI). Specifically, baseline levels of radiolabeled cholesterol efflux, BODIPY-labeled cholesterol efflux, and HAE explained 8.5% (p=0.005), 19.6% (p<0.0001), and 17.4% (p<0.0001) of the variance in the exercise-induced change of each respective measure. The correlations between baseline and change values in the combined groups of STRRIDE-PD were −0.28 (p=0.004) for radiolabeled efflux, −0.44 (p<0.0001) for BODIPY efflux, and −0.39 (p<0.0001) for HAE.
Other significant baseline predictors in the models included concentration of medium HDL particles (HDL-P) and total cholesterol for change in radiolabeled efflux; total cholesterol, GlycA (NMR marker of inflammation), and insulin area under the curve for change in BODIPY efflux; and total HDL-P, VO2max, and exercise group for change in HAE (Supplemental Table I).
E-MECHANIC
In multivariable regression models in the combined exercise groups of E-MECHANIC, baseline levels of BODIPY-labeled cholesterol efflux and HAE explained 31.5% and 24.3% of the variance in the exercise-induced change of each respective measure (both p<0.0001) (Supplemental Table II). However, baseline triglycerides level was the strongest and lone predictor of exercise-induced change in radiolabeled efflux, explaining 12.3% (p=0.006), 10.5% (p=0.01), and 12.0% (p=0.006) of the variance in change in global, ABCA1, and non-ABCA1 efflux capacity, respectively (Supplemental Table II). Baseline cholesterol medication use explained 4.2% (p=0.02) of the variance in change in BODIPY-labeled efflux.
In the combined exercise groups, the correlation between baseline and change values was only significant for BODIPY-labeled efflux at r = −0.64 (p<0.0001), with the correlation much stronger in the 8 KKW group (r = −0.81, p<0.0001) compared to the 20 KKW group (r = −0.39, p=0.01).
Correlation of three measures of HDL function
There was no correlation between baseline radiolabeled and BODIPY-labeled cholesterol efflux capacity in either study, while baseline global radiolabeled efflux capacity was significantly correlated with HAE in both studies (E-MECHANIC: r=0.50, p<0.0001; STRRIDE-PD: r=0.25, p=0.01). In E-MECHANIC the correlation between baseline HAE and ABCA1 and non-ABCA1 radiolabeled efflux was 0.48 (p<0.0001) and 0.36 (p=0.0005), respectively. In STRRIDE-PD, exercise training-induced change in global radiolabeled efflux capacity was positively correlated with change in HAE (r=0.25, p=0.009). In E-MECHANIC, there were no correlations between exercise-induced changes in any of the measures of HDL function.
There was large inter-individual variation in the responsiveness of all three measures of HDL function to exercise training, as shown in Supplemental Figure II. Heat maps displaying the direction of exercise response (positive or negative) across the three HDL function traits, as well as HDL-C, are shown for each participant of STRRIDE-PD and E-MECHANIC in Supplemental Figure III. In both studies, there did not appear to be any clear patterns of exercise response across the three measures of HDL function, as 13–14% of participants were non-responders across all three measures (i.e., value did not change or decreased with training), 35–38% were non-responders for 2 of 3 measures, 35–37% were responders (i.e., value increased with training) for 2 of 3, and 12–14% were responders for all 3 measures (Supplemental Figure IV).
Correlation of HDL function with cardio metabolic traits
The correlation of HDL function traits with cardio metabolic traits, including NMR-measured lipoprotein subfractions, body composition, cardiorespiratory fitness, and insulin- and glucose-related traits, at baseline and in response to exercise training for both studies are shown by sex in Supplemental Tables III–VI.
STRRIDE-PD
Changes with training (Supplemental Table IV)
In men, changes in radiolabeled global efflux were positively correlated with change in body weight (r=0.41, p=0.01) and waist circumference (r=0.44, p=0.01), while change in BODIPY-labeled efflux was positively correlated with change in hip circumference (r=0.41, p=0.02). In women, change in radiolabeled global efflux was negatively correlated with change in the Matsuda Index of insulin sensitivity (r = −0.32, p=0.01) and positively with change in total HDL-P, HDL-P size, and HDL-C (range: r = 0.25–035, p<0.05).
E-MECHANIC
Changes with training (exercise groups only; Supplemental Table VI)
Change in HAE was correlated with change in percent body fat (r = −0.41, p=0.04) and change in total daily energy expenditure (r=0.50, p=0.009) in men. Change in radiolabeled global, ABCA1, and non-ABCA1 (range: r = −0.57 to −0.63, p≤0.03) efflux were negatively correlated with change in proportion of calories from fat, while non-ABCA1 efflux was negatively correlated with change in total caloric intake (r = −0.57, p=0.03) in men. No significant correlations between change variables were found in women.
Discussion
The present study is the first to examine the effects of exercise training on radiolabeled efflux, BODIPY-labeled efflux, and HAE in the same individuals. The major finding was that across two randomized trials comprising six different exercise interventions, regular endurance exercise improved radiolabeled cholesterol efflux capacity only when a high amount of vigorous exercise was performed. Specifically, global radiolabeled cholesterol efflux capacity increased with exercise training in the high amount, vigorous intensity group of STRRIDE-PD, while non-ABCA1 radiolabeled cholesterol efflux capacity increased in the 20 KKW group of E-MECHANIC. Alternatively, regular endurance exercise nor regular endurance exercise plus weight loss (i.e., Clinical Lifestyle group of STRRIDE-PD) resulted in significant improvements in ABCA1 radiolabeled efflux, BODIPY-labeled efflux, or HAE.
Our results are in general agreement with those from two recent investigations of the effects of exercise training on radiolabeled cholesterol efflux capacity from J774 macrophage cells in subjects with peripheral artery disease20 and ACS.21 A six-month treadmill exercise program in 33 patients and 26 controls with peripheral artery disease found no effects of training on radiolabeled cholesterol efflux capacity.20 However, it is possible that the peripheral artery disease patients did not reach a sufficient intensity of exercise to induce changes in efflux, as the program began with walking at 2.0 mph or lower. Conversely, Koba et al.15 found that six months of cardiac rehabilitation (intensity of 40–60% of heart rate reserve) significantly increased radiolabeled cholesterol efflux capacity in 57 patients with ACS compared to baseline levels, but not in comparison to 11 controls who did not undergo the rehabilitation program. Further subgroup analysis found that the significant increases in cholesterol efflux capacity were only observed in ACS patients (N=10) with high exercise tolerance and complete risk factor control.21
Taken together with the present results, these findings suggest that an exercise intensity and/or dose threshold may need to be exceeded in order to improve radiolabeled cholesterol efflux capacity. The existing literature on the effects of exercise training on HDL-C and HDL-P subclass traits indicates that exercise volume, rather than intensity, has the greatest influence on the HDL profile. Specifically, it is well-established that a dose-response relationship exists between exercise training volume and changes in HDL-C, with the literature suggesting an exercise threshold of 1200–2200 kcal/week to elicit favorable changes in HDL-C.11 Furthermore, the STRRIDE I study found that increases in the mean size of HDL-P and concentration of large HDL-P were related to the amount of exercise and not the intensity of exercise.13 The present study suggests that exercise intensity may be more important than volume in terms of improving cholesterol efflux capacity, however further dose-response studies are needed to verify these results across diverse exercise interventions and populations.
Both of the previously mentioned studies differ from the present study in terms of their inclusion of CVD patients and their high rate of statin usage. In the study of peripheral artery disease patients, 75% were on statins during the trial, although statin use was controlled for in the analyses.20 In the cardiac rehabilitation study, 70% of the ACS patients started or strengthened their statin treatment over the course of the intervention and the authors found increases in cholesterol efflux capacity were limited to this subgroup.21 In the present study statin use was low, with only 11 (5 controls, 6 exercise group) of 90 subjects (12%) in E-MECHANIC and 22 of 106 subjects in STRRIDE-PD (21%) on cholesterol lowering medications (mostly statins) at baseline. This small sample size of subjects taking cholesterol medications precluded us from performing stratified analyses. Overall, while statins cause modest increases in HDL-C and apoA-I, the impact of statin therapy on cholesterol efflux capacity is likely insignificant as studies have shown both small positive31–34 and null2, 35–38 associations of statins on efflux capacity.
Our findings of increases in global and non-ABCA1 radiolabeled cholesterol efflux capacity, but no changes in ABCA1 radiolabeled efflux, BODIPY-labeled efflux, and HAE agree with the known effects of exercise on HDL metabolism and remodeling. Namely, on average exercise increases HDL particle size, the concentration of large HDL particles, HDL-C, and apoA-I.11, 14 These findings also reflect the inherent differences in the radiolabeled and BODIPY-labeled efflux assays. The efflux of cholesterol from macrophages is mediated by four known pathways: ABCA1, ABCG1, scavenger receptor B1, and aqueous diffusion. In J774 macrophage cells stimulated with cAMP and labeled with 3H-cholesterol, global cholesterol efflux represents all four pathways (ABCA1: 34%, scavenger receptor B1: 20%, aqueous diffusion: 46%, ABCG1: negligible).39 In contrast, J774 macrophage cells stimulated with cAMP and labeled with BODIPY-cholesterol primarily measures ABCA1-mediated cholesterol efflux.40 ABCA1-mediated efflux is associated with lipid poor apoA-I acceptor particles (pre-β HDL), while ABCG1 mediates cholesterol efflux to mature HDL particles.41 HAE provides a measure of the ability of HDL to remodel and release lipid-poor apoA-I.7, 8 A recent study found HAE was a significant contributor to global- and ABCA1-specific radiolabeled efflux, independent of HDL-C, explaining about 25% of the variance in both measures.6
Given that exercise is known to increase HDL size and/or the concentration of large HDL particles, it is more likely that the global and non-ABCA1 efflux pathways would be increased with exercise as compared to ABCA1-mediated efflux, as found in our study. It is also thought that pre-β HDL rapidly remodels to α-HDL with exercise.42 This may also explain why we only observed changes in radiolabeled efflux measures and not BODIPY efflux or HAE with exercise training. In STRRIDE-PD, the concentration of large HDL-P significantly increased and small HDL-P significantly decreased in response to exercise training, which was reflected by a significant increase in mean HDL-P size (data not shown). Given that the concentration of total HDL-P did not change with training, the exercise-induced changes in NMR-based HDL subfractions likely reflect a shift of pre-beta-1 HDL to larger HDL particles (as opposed to de novo large HDL-P synthesis) and thus may correspond to the absence of changes in ABCA1-mediated radiolabeled and BODIPY-labeled efflux and HAE observed in the present study.
Previous studies have used other cellular models to examine the effects of exercise on cholesterol efflux capacity with mostly null results. One study found that a 4-month aerobic exercise training program did not alter HDL3-mediated 14C-labeled cholesterol efflux from Swiss mouse peritoneal macrophages in 11 healthy and 11 diabetic subjects.23 A 10-week walk/run interval program in 27 subjects with metabolic syndrome found no changes in the efflux of 3H-labeled cholesterol from RAW264.7 macrophages to HDL3.19 Lastly, a study of 15 obese women found that a 9-week lifestyle intervention significantly increased cholesterol efflux of 14C-labeled cholesterol from macrophages (derived from THP1 monocytes) to whole serum in only the 13 women that exhibited significant weight loss.22 Although the confluence of results from the existing literature, including the present study, suggest little to no effects of regular endurance exercise on cholesterol efflux capacity, differences in efflux assay, study populations, study duration, and exercise programs make it difficult to directly compare results across studies. As described by Ronsein and Vaisar39, existing assays of efflux capacity differ by cell type and cholesterol acceptor and can vary widely between different laboratories. The absence of standards and controls (e.g., normalizing efflux values relative to a pooled control sample in each assay) may affect the accuracy and comparability of the assays. To date, only the radio- and BODIPY-labeled efflux assays using J774 macrophages have been validated in clinical populations.2–5 Despite both efflux capacity assays showing agreement in their association with CVD outcomes, we found no correlation between the two measures in either training cohort. However, we did replicate the strong to moderate correlations between radiolabeled efflux and HAE as previously shown by Borja et al.6
The present study represents the largest examination to date of the effects of exercise interventions on validated measures of HDL function and is further aided by the inclusion of different doses of exercise and high adherence rates in both cohorts. However, our study also has several limitations. Radiolabeled cholesterol efflux capacity was measured in two different labs with slightly different methods (+/− cAMP stimulation). Furthermore, both studies were lacking measures of apoA-I, while the E-MECHANIC study lacked NMR measures of lipoprotein subfractions, for correlation with the HDL function measures.
In conclusion, HDL dysfunction likely manifests before the diagnosis of CVD. Thus, treatments that can attenuate or prevent HDL dysfunction are highly desirable. Although exercise represents a promising therapeutic option in combating HDL dysfunction (more so than weight loss based on our results), the jury is still out on whether exercise produces beneficial effects on clinically relevant measures of HDL functionality. We found little effect of six-months of exercise training on cholesterol efflux capacity and HAE, with only the highest doses of exercise improving radiolabeled efflux capacity. Future studies using standardized methodologies in diverse populations are needed to determine the effects of exercise on the atheroprotective properties of HDL and the mechanisms mediating these effects.
Supplementary Material
Highlights.
A high dose of vigorous intensity exercise maintained over six months increased radiolabeled cholesterol efflux capacity in previously sedentary adults.
Baseline levels were the strongest predictors of exercise-induced changes in HDL function.
Future dose-response studies are needed to verify these results and examine whether exercise-induced improvements in HDL function reduce the risk of cardiovascular disease.
Acknowledgments
Sources of funding
This work was supported by multiple grants from the NIH and other programs. The E-MECHANIC study (Clinical Trials registration NCT01264406) was funded by NHLBI R01 HL102166 and a NIDDK NORC Center Grant P30DK072476. The STRRIDE-PD study was funded by NIDDK R01 DK081559 (Clinical Trials registration NCT00962962). JWA and MAS were supported in part by U54 GM104940 from the NIGMS, which funds the Louisiana Clinical and Translational Science Center. MAS was supported by NIGMS COBRE center grant 8P20 GM-1033528. MNO was supported by California Tobacco Related Disease Research Program 21RT-0125. AR was supported by NIH/NHLBI R01HL136724, NIH/NHLBI K08HL118131, and AHA 15CVGPSD27030013.
Nonstandard Abbreviations and Acronyms
- ABCA1
ATP-binding cassette transporter A1
- ABCG1
ATP-binding cassette transporter G1
- BMI
body mass index
- BODIPY
boron dipyrromethene difluoride
- CVD
cardiovascular disease
- HDL
high-density lipoprotein
- HDL-C
HDL cholesterol
- HDL-P
HDL particle
- HAE
HDL-apoA-I exchange
- KKW
kilocalories per kilogram per week
Footnotes
Disclosures
MAS is a consultant for Genetic Direction, LLC. MNO is a founder of and owns a significant stake in Seer Biologics, Inc. AR has a research grant from Merck and is a consultant for Merck, CSL Limited, HDL Diagnostics, and Cleveland HeartLabs. The other authors declare no conflicts.
References
- 1.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritsch A, Scharnagl H, Marz W. Hdl cholesterol efflux capacity and cardiovascular events. N Engl J Med. 2015;372:1870–1871. doi: 10.1056/NEJMc1503139. [DOI] [PubMed] [Google Scholar]
- 4.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: A prospective case-control study. Lancet Diabetes Endocrinol. 2015;3:507–513. doi: 10.1016/S2213-8587(15)00126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borja MS, Ng KF, Irwin A, Hong J, Wu X, Isquith D, Zhao XQ, Prazen B, Gildengorin V, Oda MN, Vaisar T. HDL-apolipoprotein A-I exchange is independently associated with cholesterol efflux capacity. J Lipid Res. 2015;56:2002–2009. doi: 10.1194/jlr.M059865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borja MS, Zhao L, Hammerson B, Tang C, Yang R, Carson N, Fernando G, Liu X, Budamagunta MS, Genest J, Shearer GC, Duclos F, Oda MN. HDL-apoA-I exchange: Rapid detection and association with atherosclerosis. PLoS One. 2013;8:e71541. doi: 10.1371/journal.pone.0071541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavigiolio G, Geier EG, Shao B, Heinecke JW, Oda MN. Exchange of apolipoprotein A-I between lipid-associated and lipid-free states: A potential target for oxidative generation of dysfunctional high density lipoproteins. J Biol Chem. 2010;285:18847–18857. doi: 10.1074/jbc.M109.098434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borja MS, Hammerson B, Tang C, Savinova OV, Shearer GC, Oda MN. Apolipoprotein A-I exchange is impaired in metabolic syndrome patients asymptomatic for diabetes and cardiovascular disease. PLoS One. 2017;12:e0182217. doi: 10.1371/journal.pone.0182217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D’Agostino RB, Sr, Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ. High-density lipoproteins: A consensus statement from the national lipid association. Journal of clinical lipidology. 2013;7:484–525. doi: 10.1016/j.jacl.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Durstine JL, Grandjean PW, Davis PG, Ferguson MA, Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: A quantitative analysis. Sports Medicine. 2001;31:1033–1062. doi: 10.2165/00007256-200131150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Kodama S, Tanaka S, Saito K, Shu M, Sone Y, Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, Ohashi Y, Yamada N, Sone H. Effect of aerobic exercise training on serum levels of high-density lipoprotein cholesterol: A meta-analysis. Arch Intern Med. 2007;167:999–1008. doi: 10.1001/archinte.167.10.999. [DOI] [PubMed] [Google Scholar]
- 13.Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med. 2002;347:1483–1492. doi: 10.1056/NEJMoa020194. [DOI] [PubMed] [Google Scholar]
- 14.Sarzynski MA, Burton J, Rankinen T, Blair SN, Church TS, Despres JP, Hagberg JM, Landers-Ramos R, Leon AS, Mikus CR, Rao DC, Seip RL, Skinner JS, Slentz CA, Thompson PD, Wilund KR, Kraus WE, Bouchard C. The effects of exercise on the lipoprotein subclass profile: A meta-analysis of 10 interventions. Atherosclerosis. 2015;243:364–372. doi: 10.1016/j.atherosclerosis.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams V, Besler C, Fischer T, Riwanto M, Noack F, Hollriegel R, Oberbach A, Jehmlich N, Volker U, Winzer EB, Lenk K, Hambrecht R, Schuler G, Linke A, Landmesser U, Erbs S. Exercise training in patients with chronic heart failure promotes restoration of high-density lipoprotein functional properties. Circ Res. 2013;113:1345–1355. doi: 10.1161/CIRCRESAHA.113.301684. [DOI] [PubMed] [Google Scholar]
- 16.Casella-Filho A, Chagas AC, Maranhao RC, Trombetta IC, Cesena FH, Silva VM, Tanus-Santos JE, Negrao CE, da Luz PL. Effect of exercise training on plasma levels and functional properties of high-density lipoprotein cholesterol in the metabolic syndrome. Am J Cardiol. 2011;107:1168–1172. doi: 10.1016/j.amjcard.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Iborra RT, Ribeiro IC, Neves MQ, Charf AM, Lottenberg SA, Negrao CE, Nakandakare ER, Passarelli M. Aerobic exercise training improves the role of high-density lipoprotein antioxidant and reduces plasma lipid peroxidation in type 2 diabetes mellitus. Scand J Med Sci Sports. 2008;18:742–750. doi: 10.1111/j.1600-0838.2007.00748.x. [DOI] [PubMed] [Google Scholar]
- 18.Roberts CK, Ng C, Hama S, Eliseo AJ, Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of hdl in overweight/obese men with cardiovascular risk factors. J Appl Physiol (1985) 2006;101:1727–1732. doi: 10.1152/japplphysiol.00345.2006. [DOI] [PubMed] [Google Scholar]
- 19.Sang H, Yao S, Zhang L, Li X, Yang N, Zhao J, Zhao L, Si Y, Zhang Y, Lv X, Xue Y, Qin S. Walk-run training improves the anti-inflammation properties of high-density lipoprotein in patients with metabolic syndrome. J Clin Endocrinol Metab. 2015;100:870–879. doi: 10.1210/jc.2014-2979. [DOI] [PubMed] [Google Scholar]
- 20.Albaghdadi MS, Wang Z, Gao Y, Mutharasan RK, Wilkins J. High-density lipoprotein subfractions and cholesterol efflux capacity are not affected by supervised exercise but are associated with baseline interleukin-6 in patients with peripheral artery disease. Front Cardiovasc Med. 2017;4:9. doi: 10.3389/fcvm.2017.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koba S, Ayaori M, Uto-Kondo H, Furuyama F, Yokota Y, Tsunoda F, Shoji M, Ikewaki K, Kobayashi Y. Beneficial effects of exercise-based cardiac rehabilitation on high-density lipoprotein-mediated cholesterol efflux capacity in patients with acute coronary syndrome. J Atheroscler Thromb. 2016;23:865–877. doi: 10.5551/jat.34454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kralova Lesna I, Suchanek P, Kovar J, Poledne R. Life style change and reverse cholesterol transport in obese women. Physiol Res. 2009;58(Suppl 1):S33–38. doi: 10.33549/physiolres.931856. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro IC, Iborra RT, Neves MQ, Lottenberg SA, Charf AM, Nunes VS, Negrao CE, Nakandakare ER, Quintao EC, Passarelli M. HDL atheroprotection by aerobic exercise training in type 2 diabetes mellitus. Med Sci Sports Exerc. 2008;40:779–786. doi: 10.1249/MSS.0b013e3181632d2d. [DOI] [PubMed] [Google Scholar]
- 24.Slentz CA, Bateman LA, Willis LH, Granville EO, Piner LW, Samsa GP, Setji TL, Muehlbauer MJ, Huffman KM, Bales CW, Kraus WE. Effects of exercise training alone vs a combined exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: A randomised controlled trial. Diabetologia. 2016;59:2088–2098. doi: 10.1007/s00125-016-4051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers CA, Johnson WD, Earnest CP, Rood JC, Tudor-Locke C, Johannsen NM, Cocreham S, Harris M, Church TS, Martin CK. Examination of mechanisms (e-mechanic) of exercise-induced weight compensation: Study protocol for a randomized controlled trial. Trials. 2014;15:212. doi: 10.1186/1745-6215-15-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Otvos JD, Shalaurova I, Wolak-Dinsmore J, Connelly MA, Mackey RH, Stein JH, Tracy RP. Glyca: A composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61:714–723. doi: 10.1373/clinchem.2014.232918. [DOI] [PubMed] [Google Scholar]
- 28.de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. doi: 10.1161/ATVBAHA.109.199158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kempen HJ, Gomaraschi M, Bellibas SE, Plassmann S, Zerler B, Collins HL, Adelman SJ, Calabresi L, Wijngaard PL. Effect of repeated apoa-imilano/popc infusion on lipids, (apo)lipoproteins, and serum cholesterol efflux capacity in cynomolgus monkeys. J Lipid Res. 2013;54:2341–2353. doi: 10.1194/jlr.M033779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oda MN, Forte TM, Ryan RO, Voss JC. The c-terminal domain of apolipoprotein a-i contains a lipid-sensitive conformational trigger. Nat Struct Biol. 2003;10:455–460. doi: 10.1038/nsb931. [DOI] [PubMed] [Google Scholar]
- 31.Franceschini G, Calabresi L, Colombo C, Favari E, Bernini F, Sirtori CR. Effects of fenofibrate and simvastatin on hdl-related biomarkers in low-hdl patients. Atherosclerosis. 2007;195:385–391. doi: 10.1016/j.atherosclerosis.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Guerin M, Egger P, Soudant C, Le Goff W, van Tol A, Dupuis R, Chapman MJ. Dose-dependent action of atorvastatin in type iib hyperlipidemia: Preferential and progressive reduction of atherogenic apob-containing lipoprotein subclasses (VLDL-2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis. 2002;163:287–296. doi: 10.1016/s0021-9150(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto-Sasaki M, Yasuda T, Monguchi T, Nakajima H, Mori K, Toh R, Ishida T, Hirata K. Pitavastatin increases hdl particles functionally preserved with cholesterol efflux capacity and antioxidative actions in dyslipidemic patients. J Atheroscler Thromb. 2013;20:708–716. doi: 10.5551/jat.17210. [DOI] [PubMed] [Google Scholar]
- 34.Triolo M, Annema W, de Boer JF, Tietge UJ, Dullaart RP. Simvastatin and bezafibrate increase cholesterol efflux in men with type 2 diabetes. Eur J Clin Invest. 2014;44:240–248. doi: 10.1111/eci.12226. [DOI] [PubMed] [Google Scholar]
- 35.de Vries R, Kerstens MN, Sluiter WJ, Groen AK, van Tol A, Dullaart RP. Cellular cholesterol efflux to plasma from moderately hypercholesterolaemic type 1 diabetic patients is enhanced, and is unaffected by simvastatin treatment. Diabetologia. 2005;48:1105–1113. doi: 10.1007/s00125-005-1760-0. [DOI] [PubMed] [Google Scholar]
- 36.Khera AV, Demler OV, Adelman SJ, Collins HL, Glynn RJ, Ridker PM, Rader DJ, Mora S. Cholesterol efflux capacity, high-density lipoprotein particle number, and incident cardiovascular events: An analysis from the JUPITER trial (justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin) Circulation. 2017;135:2494–2504. doi: 10.1161/CIRCULATIONAHA.116.025678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronsein GE, Hutchins PM, Isquith D, Vaisar T, Zhao XQ, Heinecke JW. Niacin therapy increases high-density lipoprotein particles and total cholesterol efflux capacity but not abca1-specific cholesterol efflux in statin-treated subjects. Arterioscler Thromb Vasc Biol. 2016;36:404–411. doi: 10.1161/ATVBAHA.115.306268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sviridov D, Hoang A, Ooi E, Watts G, Barrett PH, Nestel P. Indices of reverse cholesterol transport in subjects with metabolic syndrome after treatment with rosuvastatin. Atherosclerosis. 2008;197:732–739. doi: 10.1016/j.atherosclerosis.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Ronsein GE, Vaisar T. Inflammation, remodeling, and other factors affecting HDL cholesterol efflux. Curr Opin Lipidol. 2017;28:52–59. doi: 10.1097/MOL.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sankaranarayanan S, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Asztalos BF, Bittman R, Rothblat GH. A sensitive assay for ABCA1-mediated cholesterol efflux using bodipy-cholesterol. J Lipid Res. 2011;52:2332–2340. doi: 10.1194/jlr.D018051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jessup W, Gelissen IC, Gaus K, Kritharides L. Roles of ATP binding cassette transporters A1 and G1, scavenger receptor BI and membrane lipid domains in cholesterol export from macrophages. Curr Opin Lipidol. 2006;17:247–257. doi: 10.1097/01.mol.0000226116.35555.eb. [DOI] [PubMed] [Google Scholar]
- 42.Trejo-Gutierrez JF, Fletcher G. Impact of exercise on blood lipids and lipoproteins. Journal of clinical lipidology. 2007;1:175–181. doi: 10.1016/j.jacl.2007.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.