Abstract
The androgen receptor (AR) is a promising therapeutic target for a subset of triple-negative breast cancers (TNBCs) in which AR is expressed. However, the mechanistic action of AR and the degree to which primary and metastatic tumors depend on AR, both before and after conventional treatment, remain to be defined. We discuss preclinical and clinical data for AR+ TNBC, the difficulties in monitoring AR protein levels, new methods for determining AR status, the influence of AR on “stemness” in the context of TNBC, the role of combined inhibition of sex steroid production and AR, and the role of AR in regulation of the immune system. Although the exact role of AR in subsets of TNBC is still being characterized, new therapies that target AR and the production of androgens may provide additional options for patients with TNBC for whom chemotherapy is currently the sole treatment option.
Keywords: Triple-negative Breast Cancer (TNBC), Androgen Receptor (AR), Enzalutamide, TNBC Subtype, TNBC Cell Lines
Introduction
The androgen receptor (AR) is widely expressed in breast cancer (BC) [1]. It is expressed in up to half of triple-negative BC (TNBC) tumors [2], which, by definition, lack estrogen and progesterone receptors (ER and PR) as well as amplification of the human epidermal growth factor receptor 2 (HER2). Preclinical and clinical data suggest that AR is a promising therapeutic target for a subset of BC and perhaps should be the fourth receptor to be routinely examined. In this review, we discuss the roles of AR in TNBC, methods for detecting AR status, the influence of AR on “stemness” in the context of TNBC, inhibition of AR and/or sex steroid production, and AR in the immune system. Our goal is to describe the current state of research that underlies the development of novel-targeted therapy for the treatment of AR-expressing (AR+) TNBC.
The Role of Androgen Receptor Signaling in Breast Cancer
Targeted treatment for BC has historically focused on ER and HER2; however, AR is emerging as another promising therapeutic target since it is even more widely expressed in BC than ER and PR [3]. In a study of 2171 invasive BCs in women, AR protein was expressed in 77% overall, but expression varied by BC subtype, with 88% of ER+ BC, 59% of HER2+ BC, and 32% of TNBC positive for AR protein by immunohistochemistry (IHC) [1, 4]. In a study of 32 men with breast cancer, AR was expressed in 65% of all BCs and in 85% of ER+ tumors [5]. However, the mechanistic action of AR in the clinically defined BC subtypes and the manner and degree to which primary and metastatic tumors of different subtypes rely on AR, both prior to treatment and after conventional therapy, are still actively being determined in preclinical models and clinical trials.
As with PR, AR expression has been associated with a more favorable prognosis and prolonged survival in patients with ER+ breast cancer [6–9]. The prognostic value of AR is less clear for BC tumor types that are not ER+ [10]. Although AR positivity is indicative of a more well-differentiated tumor and therefore has a better prognosis [11–13], among the TNBC subtypes, the luminal AR TNBC subtype that expresses high AR levels had a lower pathological complete response (pCR) to neoadjuvant chemotherapy than other TNBC subtypes [14]. Recent preclinical studies suggest that AR can drive growth and survival in ER+, HER2+, and TNBC cell lines [4, 15–22]. In ER+ BC, AR becomes particularly important in the context of resistance to anti-estrogen or aromatase inhibitor therapy, where tumor cells can evolve to become growth-dependent on androgens and AR under conditions of ER inhibition or estrogen deprivation [4, 17, 23–25]. A high ratio of AR:ER protein is associated with an increased risk of recurrence while on tamoxifen and decreased likelihood of disease-specific and overall survival [4]. In addition, the AR inhibitor enzalutamide blocked both androgen- and estrogen-stimulated tumor growth in AR+/ER+ BC xenograft and PDX preclinical models [4, 17]. In ER+ BC cell lines, complex interactions between AR and ER lead to transcriptional activity that affects the expression of genes involved in BC growth and survival [17].
AR is also significantly associated with HER2 amplification and promotes cell proliferation following treatment with the androgen dihydrotestosterone (DHT) or other synthetic androgens [20, 26–28]. Based on studies in the HER2-enriched BC cell line MDA-MB-453 (ER−/HER2+/AR+), AR induces an increase in HER3 via the Wnt signaling pathway to promote HER2 signaling [10, 20]. Another positive feedback loop that may feed into HER2-mediated cell proliferation exists in the AR and ERK pathways [29]. Synergistic inhibition of proliferation was observed in vitro and in vivo with combined mTOR inhibition and anti-androgens in multiple HER2+ and TNBC cell lines containing activating PIK3CA mutations [21]. Depending on the cell line, DHT induced an increase in either phosphorylated HER2 (pHER2), phosphorylated HER3 (pHER3), or both, that was attenuated by AR inhibition. Conversely, inhibition of the mTOR pathway caused an increase in total AR, pHER2, and pHER3, and these effects were abrogated by enzalutamide and seviteronel [21].
Interestingly, in a rat model of obesity-associated postmenopausal mammary carcinoma, nuclear AR was higher in tumors that progressed after ovariectomy compared to tumors that regressed. Administration of enzalutamide blocked tumor progression in rats after ovariectomy and prevented new tumor formation [30]. IL-6, which was higher in plasma of obese versus lean rats, sensitized BC cells to low levels of testosterone [30], providing an example of how obesity-associated cytokines and growth factors can affect how tumors respond to steroid hormones and hormonal therapy in all subtypes of BC.
AR regulates growth factors such as the EGFR ligand amphiregulin (AREG) in TNBC cell lines in vitro and in vivo, and AR activation and inhibition significantly affected levels of AREG [16] and other factors such as JAG1 (a ligand for Notch receptors and target of the canonical Wnt signaling pathway in progenitor cells), chitinase (CHI3L1/YKL40), and growth/differentiation factor (GDF)-15 [22]. Perhaps future studies will identify the key AR-regulated proteins most indicative of AR dependence, but these may differ with BC subtype, disease progression, and prior treatment.
Androgen Receptor Inhibition in Triple-Negative Breast Cancer
TNBC comprises approximately 15 to 20% of newly diagnosed BCs [31]. TNBC is an aggressive BC subtype with a risk of recurrence that peaks around 3 years after diagnosis [31, 32]. Because TNBC lacks the most common therapeutic targets ER, PR, and HER2, chemotherapy is the only available therapeutic option [15]. There are, as yet, no U.S. Food and Drug Administration (FDA)-approved targeted therapies available for chemoresistant TNBC disease (although the PARP [poly ADP ribose polymerase] inhibitor olaparib is anticipated to be approved for germline BRCA-mutated BC in the near future), but one avenue of current research is focused on the therapeutic inhibition of AR (Fig. 1).
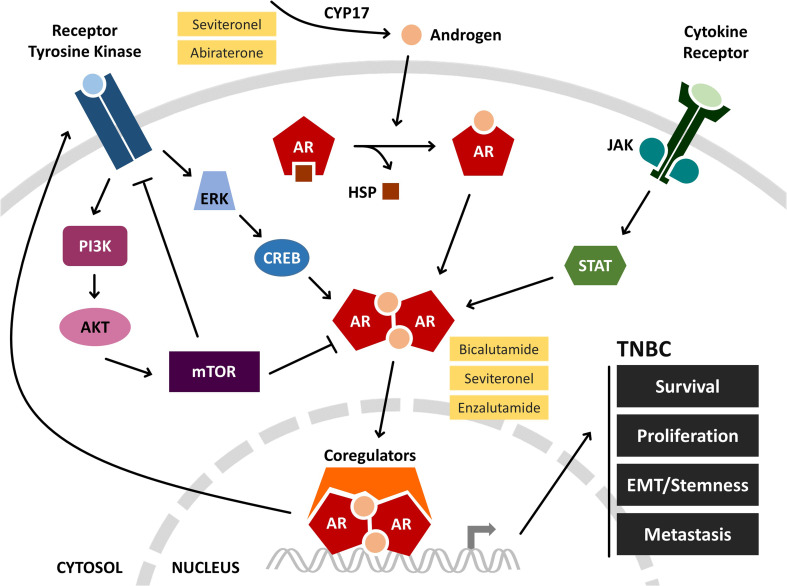
Fig. 1.
AR signaling and therapeutic interventions in TNBC. Androgen synthesis is catalyzed by the enzyme CYP17. Binding of androgens to AR causes dissociation from HSP and AR dimerization. AR dimers translocate to the nucleus, associate with coregulatory proteins, and initiate transcription. In TNBC, AR signaling promotes cancer progression, and this can be blocked at various stages with pharmacological inhibitors (yellow). Cross-talk between AR and other signaling pathways is being used to establish rational drug combinations that are currently being explored for their effectiveness. Interconnecting lines are indicative of overall effects, positive or negative, on pathway activation. AKT protein kinase B, AR androgen receptor, CREB cAMP response element-binding protein, CYP17 cytochrome P450 17, EMT epithelial-to-mesenchymal transition, ERK extracellular signal-regulated kinase, HSP heat shock protein, JAK Janus kinase, PI3K phosphatidylinositide 3-kinase, mTOR mechanistic target of rapamycin, STAT signal transducer and activator of transcription, TNBC triple-negative breast cancer
Gene expression profiling has revealed four subtypes of TNBC: basal-like 1 (BL1), basal-like 2 (BL2), mesenchymal-like (ML), and luminal androgen receptor (LAR), each with distinct gene signatures [19, 33]. The LAR subtype, which expresses high AR, is of particular interest because it closely resembles the previously described molecular apocrine tumors [26, 34] and has a gene expression profile and chromatin-binding pattern similar to luminal, ER+ BC despite being ER-negative [19, 26, 34–36]. Recent studies found up to half of TNBC to be AR+ (defined as > 10% of tumor cells staining positive for AR), irrespective of TNBC subtype [37]. While the function of AR in TNBC is not well known, preclinical data suggest that AR drives tumor growth and survival, even in cells that express relatively low levels of AR. Consequently, AR is currently under investigation as a potential therapeutic target for TNBC tumors [4, 16, 19, 22, 37].
High AR and its regulation of classically ER-controlled genes make the LAR TNBC subtype more luminal (hence the designation) [19] and slower growing. A comparison of the clinical relevance of the subtype classification of TNBC reported that the BL2 and LAR subtypes had the lowest pathological complete response rates following neoadjuvant chemotherapy (0 and 10%, respectively) [14]. While meta-analysis of 13 studies (N = 2826 TNBC patients) indicated that AR+ TNBC patients had lower grade tumors (P < 0.001) and prolonged disease-free survival (hazard ratio [HR], 0.809; 95% confidence interval [CI], 0.659–0.995, P < 0.05), these patients also had a higher incidence of lymph node metastases (P < 0.01) [6]. Thus, while LAR may be more indolent than the other TNBC molecular subtypes, it may benefit less from chemotherapy (likely because it is less proliferative). Consequently, LAR represents a subtype for which targeted therapy is feasible, given the many drugs either already approved or in development for targeting AR or androgen synthesis in prostate cancer.
Preclinical research on AR in TNBC initially focused on the LAR subtype and high expression of downstream targets of AR signaling [19, 38]. MDA-MB-453 and other LAR cell lines are AR-driven and are sensitive to the early-generation AR antagonist bicalutamide [4, 19]. Additional studies from the Richer Laboratory at the University of Colorado have demonstrated that AR inhibition with enzalutamide in four TNBC cell lines representing three non-LAR TNBC subtypes (BL2, ML, and MSL [mesenchymal stem-like, a TNBC subtype later reclassified [19, 33]]) increased apoptosis and decreased baseline tumor cell proliferation, migration, invasion, and anchorage-independent growth [16]. Furthermore, the predominately nuclear expression of AR in TNBC primary tumors suggests that AR is transcriptionally active [16, 37]. These results suggest that even TNBC subtypes with low AR expression can still critically depend on AR and that it may be the less proliferative AR+ cells that persist and recur. Indeed, in a preclinical model, AR inhibition (enzalutamide) combined with paclitaxel was strikingly more effective at preventing recurrence than paclitaxel alone [22].
While detailed analyses are not yet available, preclinical and clinical results indicate that even TNBC with low AR expression may benefit from AR inhibition. AR pathway activation is likely critical, and in the enzalutamide trial in TNBC, a 35% clinical benefit rate (CBR; the proportion of patients who experienced a complete response, partial response, or stable disease at 16 weeks of therapy) was observed in patients who had a certain, presumably androgen-driven, gene signature [39, 40] more predictive of response than degree of AR positivity by IHC. This is not terribly surprising, however, since the same is true of ER, where even patients whose tumors express down to 1% ER positivity can receive benefit from anti-estrogen and estrogen deprivation therapies, and ER activity readouts such as PR status, MammaPrint, and Oncotype DX are indicators of the degree of ER dependence. To date, the precise AR-regulated genes essential to TNBC biology remain to be determined and validated as predictive biomarkers of therapeutic benefit.
Androgen Receptor Mutations and Splice Variants in Triple-Negative Breast Cancer
Very few mutations have been found in AR in TNBC compared to castration-resistant prostate cancer (CRPC). Sequencing data in The Cancer Genome Atlas (TCGA) from 93 analyzed TNBCs identified two patients with single missense mutation (described by Barton et al. [15]). The AR mutational status may increase under selective pressure if AR-targeted agents become more commonplace in BC treatment.
AR splice variants that affect AR function are relatively more common in BC than AR mutations [41–44]. One splice variant (Δ3AR) has a deletion of exon 3 and was predicted to lack the second zinc finger within the DNA-binding domain and have reduced or no ability to bind to androgen response elements and activate transcription [41]. In some BC tissues, this AR variant had relatively high expression compared to the full-length protein, indicating a potential role in regulating the growth of these tumors. Another splice variant, AR45, has low expression levels in normal breast tissue [42]. This splice variant lacks exon 1 and is preceded by a novel 7–amino acid long N-terminal extension that inhibits AR function [43]. AR45 and another AR splice variant, AR-V7 (formerly known as AR3), are found in the TNBC cell lines MDA-MB-453 and MDA-MB-231 [44]. Although AR splice variants have been identified in BC cell lines, further studies are needed to characterize AR splice variant expression in TNBC specimens, particularly after the selective pressure of anti-androgen therapy.
The AR-V7 splice variant is of particular interest since it is associated with resistance to anti-androgen therapy in CRPC [45, 46]. This isoform of AR produces a protein product that lacks the C-terminal ligand-binding domain but retains the transcriptionally active N-terminal domain. Although it is unable to bind ligand, AR-V7 is constitutively active in a ligand-independent manner and is capable of promoting activation of target genes. Research on the AR-V7 splice variant in CRPC using circulating tumor cells (CTCs) demonstrates that the presence of AR-V7 is associated with poorer outcomes (prostate-specific antigen [PSA] response, PSA progression-free survival [PFS], clinical or radiographic PFS, and overall survival [OS]) [45–47]. In addition, AR-V7 is associated with better OS with chemotherapy (taxane) than anti-androgen therapy (abiratone, enzalutamide, or apalutamide) [48]. Whether AR-V7 has similar effects in TNBC remains to be seen.
Measurement of Androgen Receptor Protein Expression by Immunohistochemistry
All staining procedures must be standardized and validated clinically and Clinical Laboratory Improvement Amendments (CLIA)-certified in order to be utilized for clinical treatment decisions. IHC methods are well established for clinical use for certain proteins such as ER and HER2 in breast cancer [49], both of which are used as predictive biomarkers when evaluating targeted treatment. The current pathological characterization of AR expression levels in BC is largely based on IHC results using formalin-fixed, paraffin-embedded (FFPE) tissue samples obtained from primary or metastatic tumor biopsies [28] and is not routine or standardized. The detection of AR has improved as more sensitive and specific antibodies to AR have been developed; over time, this has resulted in an increased percentage of TNBC study samples reported as AR+.
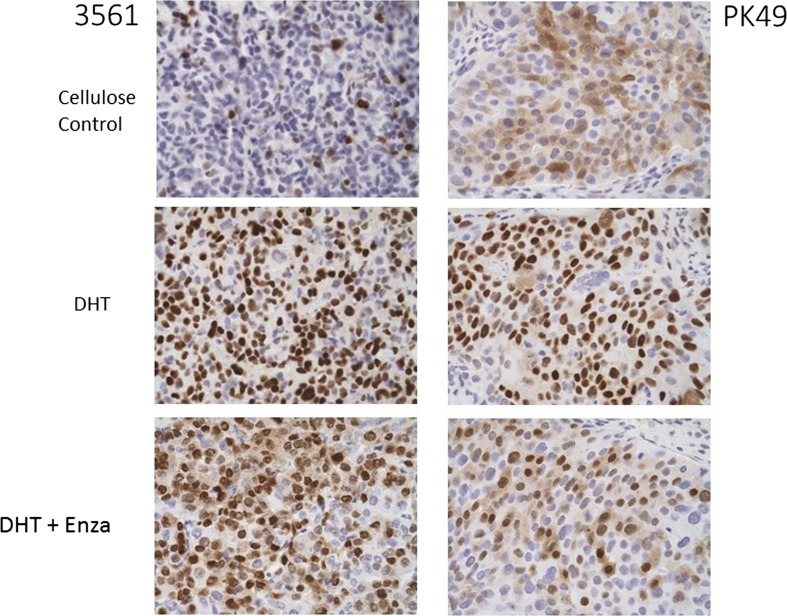
In addition to considerations regarding the technical aspects of AR IHC, including the validation and standardization currently underway for BC, tumor biology must also be considered for appropriate interpretation of IHC results. Characterizing AR expression in TNBC accurately can be difficult, in part due to tumor heterogeneity. While tumor heterogeneity is not unique to TNBC, the extent of heterogeneity in TNBC between molecular subtypes and even intratumoral genomic heterogeneity makes defining common features of this type of BC particularly challenging [37]. Another important consideration is whether the patient received prior treatment, and if so, what type of treatment. Certain therapies, such as aromatase inhibitors, increase circulating androgen levels [50–55]. AR protein is stabilized and translocated to the nucleus in breast cancer upon binding to androgens [56, 57], and this effect is abrogated by the new-generation anti-androgen enzalutamide (Fig. 2). The effect was observed in ER+ MCF7 cells and the LAR BC cell line MDA-MB-453 grown as xenografts in mice treated with estradiol versus DHT [4]. Likewise, in the non-LAR but AR+ SUM159PT TNBC cell line grown in cycling mice, AR was nuclear and decreased with anti-androgen [16]. In two patient-derived xenografts (PDX) originally low for nuclear AR by IHC, AR increased in mice given DHT versus cellulose control (Fig. 2), indicating that even TNBCs that have low AR have the capacity to respond to androgen agonists and antagonists. Currently, there are insufficient data to ascertain how well AR IHC results will correlate with measures of AR dependency and clinical outcome, as well as the minimum percentage of cells positive for nuclear AR that should be considered as AR+, and predictors of response to anti-androgen therapy.
Fig. 2.
Two TNBC patient-derived xenografts grown in mice had low AR, but nuclear AR increased upon exposure of mice to DHT and this effect was reduced by subsequent treatment with the anti-androgen enzalutamide. Mice were implanted with silastic tubing containing either cellulose only (10 mg) or a mixture of cellulose and DHT (2 and 8 mg, respectively) at the time of placement of TNBC PDX tumor pieces into the mammary glands. When tumors reached an average of 55 mm3 in size, mice were either continued on DHT alone or given DHT + Enza in chow fed ad libitum for a target dose of 50 mg/kg/day for 3.5 weeks. FFPE tumors (PDX 3561 and PDX PK49) were stained for AR (SP107, CellMarque, ×40 magnification). AR androgen receptor, DHT dihydrotestosterone, Enza enzalutamide, PDX patient-derived xenografts, TNBC triple-negative breast cancer, FFPE formalin-fixed paraffin-embedded
Finally, regarding AR status, it is also important to consider the influence of AR splice variants on staining results. In an overview of AR splice variants found in prostate cancer, the authors noted that several of these variants lack the C-terminal ligand-binding domain [58]. AR signaling is constitutively active in such variants, potentially contributing to resistance to androgen deprivation therapy (ADT) [58–61]. Additionally, AR has many phosphorylation sites, although the contribution of these sites to transcriptional activity is not yet as clear as it is for ER, where there clearly are sites phosphorylated upon addition of ligand that affect receptor activity and turnover.
New Methods for Determining Androgen Receptor Status
Alternative approaches to clinical characterization of AR+ BC are currently in the early stages of development. Given the long-term understanding of the role of androgens in prostate cancer pathogenesis, several novel approaches are being investigated, including blood-based methods using CTCs, circulating tumor DNA (ctDNA), and exosomes [62]. These blood-based approaches allow for more frequent and less invasive assessments, which are helpful for diseases like CRPC and TNBC, where AR status can change over the course of disease progression [45, 46]. The less invasive approaches of liquid biopsies are especially useful for difficult to biopsy lesions, as described below in a case study.
In prostate cancer, CTCs can be used to identify mutations in AR, AR expression and function, and response to therapy [63–66]. In one study of progressive metastatic CRPC, evaluation of CTCs revealed that AR expression and nuclear localization varied both within and between patients, suggesting that a molecularly diverse, AR-centric pathobiology underlies castration resistance [66]. In another study of CRPC, CTC-based assays were used to track AR expression in real time in patients treated with enzalutamide and abiraterone, another inhibitor of androgen synthesis [67]. For BC, a presentation at the 2016 annual meeting of the American Association of Cancer Research reported characterization of AR expression and heterogeneity in CTCs of patients with metastatic BC [68].
Similarly, ctDNA has been used to identify AR mutations in CRPC. Two studies have examined a missense mutation in the ligand-binding domain of AR that conferred resistance to the second-generation anti-androgens enzalutamide and apalutamide (ARN-509) [69, 70]. There is growing use of ctDNA techniques in BC in place of traditional tumor biopsies [71], and similar to CRPC, it could be a valuable tool to determine AR status.
In addition, research on prostate cancer suggests that exosomes may serve as potential biomarkers of AR status as well as predictors of therapeutic response. Exosomes act as mediators of cell-to-cell communication in the local tumor microenvironment and play a role in cancer progression and metastasis [72, 73]. One study demonstrated that AR is present in prostate cancer-related exosomes [74]. Furthermore, patients with aggressive prostate cancer exhibited higher levels of prostate cancer-related exosomes in the blood than prostate cancer patients without metastases or healthy volunteers [74]. In another study, plasma-derived exosomal RNA was used to detect the presence of an AR splice variant and predict resistance to hormonal therapy in metastatic CRPC patients [75]. Future experiments will determine if similar results are observed in BC patients.
As an example, a case study in which a blood-based method was used to determine a breast cancer patient’s AR status is presented below.
Case Study of Liquid Biopsy Analysis to Determine Current AR Status in a TNBC Patient with an Inaccessible Lesion
AR expression in TNBCs is likely to be indicative of a tumor that may respond to AR-targeted therapy. However, for a variety of reasons, it is likely that archival FFPE tumor samples may not be indicative of the current AR expression level, and patients with metastatic disease may not have accessible tumor for reassessment. It is crucial to identify biomarkers predictive of response to anti-androgens in TNBC. This patient vignette illustrates how peripheral blood, which is easily accessible, can be used to quantify AR utilizing a digital assay that counts copies of AR mRNA. Not only can this assay provide a potential predictive biomarker for AR-targeted therapy, it can also dynamically follow the level of AR in a patient during the course of their therapy.
The subject was a 63-year-old female initially diagnosed with TNBC in June 2011 who underwent a left breast mastectomy. She was disease-free until 2014; upon disease recurrence, she was treated with capecitabine from May to December 2014, followed by nab-paclitaxel plus carboplatin combination therapy from December 2014 to May 2015 and then nab-paclitaxel as a single agent from June 2015 to January 2016. The subject had progressive visceral (lung) disease and was enrolled into the phase 1 portion of CLARITY-01, the phase 1/2 study of seviteronel, in February 2016. She was considered AR− at study entry based on IHC of an archival FFPE sample from a metastatic lymph node biopsy obtained in 2014. Eastern Cooperative Oncology Group (ECOG) performance status at screening was 1.
The subject began seviteronel dosing at 450 mg once daily in 28-day continuous dosing cycles and responded to treatment with radiographic stable disease for almost six 28-day cycles. To better understand the AR status of her current disease state, the subject was scheduled to have a lung metastasis biopsy, but the procedure was considered unfeasible due to the tumor location. Instead, peripheral blood was collected in a PAXgene tube for RNA stabilization, and a sensitive, digital assay for AR mRNA using the droplet digital PCR (ddPCR) platform demonstrated a high level of AR positivity. This provided a real-time, minimally invasive assessment of the cancer cell AR status in a patient who met the criteria for clinical activity (complete response, partial response, or stable disease at 16 weeks of therapy) in the clinical study.
To establish the assay, AR full-length and AR-V7 splice-variant mRNA copy numbers were determined by ddPCR analysis of peripheral blood drawn into PAXgene blood RNA tubes. Cutoffs were established by comparison with healthy controls and patients with localized prostate cancer, stage D0 prostate cancer, and metastatic prostate cancer. These studies allowed development of an AR-V7 cutoff that correlated with metastatic disease. Previous studies have utilized mononuclear cells isolated from peripheral blood and ddPCR analysis [76] or whole blood collected in PAXgene blood RNA tubes and quantitative PCR analysis [77].
Androgen Receptor and Epithelial-to-Mesenchymal Transition
Epithelial-to-mesenchymal transition (EMT) is a process through which normal or carcinoma cells can lose cell-cell junctions/polarity and develop a more migratory, stem cell-like phenotype [78]. While this process is critical during embryonic development, it has also been implicated at certain steps in the metastatic cascade [79]. In BC, AR contributes to EMT and metastasis in several ways. Loss of E-cadherin, an epithelial marker, is a common EMT event that helps promote metastasis [80]. In ER+ BC, AR activation causes a decrease in E-cadherin and an increase in stem cell-like properties and EMT-related gene expression as well as an increase in metastasis [81, 82]. These results are supported by recent evidence linking activated AR to acquisition of a stem-like phenotype in TNBC cells and increased MDA-MB-231 xenograft growth [83]. In TNBC, AR promotes survival in anchorage-independent conditions and maintains a CSC-like tumor-initiating population [22]. Correspondingly, in TNBC PDX models, AR mRNA was among the transcripts upregulated in CTCs and micrometastases as compared to primary tumors [18]. Furthermore, in a mouse mammary tumor virus-polyoma middle tumor antigen (MMTV-PyMT) model, ER and PR proteins are absent but AR protein is abundant in lung metastases and AR inhibition significantly decreased cancer cell invasion and anchorage-independent growth in vitro [84]. Together, these data suggest that AR may facilitate BC metastasis by protecting against apoptosis in an anchorage-independent tumor cell population with EMT or stem cell-like properties.
The association between AR and EMT has important clinical implications. While a large percentage of TNBC patients responds favorably to chemotherapy, many will relapse with chemoresistant disease since chemotherapy often fails to target the slower growing population of cells (that are either CSC-like or more epithelial, as in the case of LAR TNBC). If AR promotes EMT and stemness, then the combination of AR inhibitors and chemotherapy may be a rational and impactful drug combination for patients. Preclinical data in a TNBC xenograft model support this hypothesis, demonstrating that the combination of paclitaxel and enzalutamide given simultaneously significantly decreased tumor growth and recurrence when compared to paclitaxel alone [22].
The Influence of Androgen Receptor on Immune Oncology
AR expression in cells within the tumor microenvironment could have significant effects on tumor growth and progression. AR is expressed in a number of immune cells, both innate and adaptive, and knockout of AR can have profound effects on immune cell maturation and function [85–88]. In particular, AR activation alters T cell immunity by suppressing T cell (CD4 and CD8) proliferation and inhibiting CD4 T-helper differentiation [89, 90]. Given that T cells play a prominent role in anti-tumor immunity and that T cell infiltration is a predictive marker in TNBC [91–93], the systemic use of anti-androgens (or androgen agonists) could have significant effects on anti-tumor immune activity. It was previously reported that androgen deprivation in prostate cancer patients leads to an increase in T cell infiltration into the prostate [94]. More recent studies investigated the effects of anti-androgens on CD8 T cell anti-tumor activity in both prostate cancer and BC [59, 95–97]. To date, however, it remains unclear how long-term AR targeting therapies will affect the immune system and whether they will boost or be detrimental to anti-tumor immunity. It would be particularly beneficial if endocrine therapy proved useful in combination with targeted immunotherapies such as PD-L1 (programmed death ligand 1) interfering antibodies [98, 99].
Targeted Agent Activity Alone and in Combination for Treatment of AR+ Triple-Negative Breast Cancer
With AR possibly playing a central role in AR+ TNBC tumorigenesis, AR-targeted agents, such as androgen biosynthesis inhibitors (eg, cytochrome P450 C17a [CYP17] inhibitors) and inhibitors of AR activation (eg, AR antagonists), are being examined in clinical trials [100] (Table 1). To date, data are available from studies of bicalutamide, enzalutamide, seviteronel, and abiraterone acetate, and a study of orteronel (TAK-700) is in progress [101].
Table 1.
Clinical trials of AR-targeted therapies in TNBC
| NCT no. | Phase | Patient population | Treatment | Treatment type | Start date | Status note |
|---|---|---|---|---|---|---|
| NCT00468715 | II | Metastatic AR+ TNBC | Bicalutamide | AR antagonist | Mar 2007 | |
| NCT02348281 | II | Advanced AR+ TNBC; PM | Bicalutamide | AR antagonist | Jan 2015 | Terminated |
| NCT03055312 | III | Metastatic AR+ TNBC | Bicalutamide | AR antagonist | Dec 2016 | |
| NCT02605486 | I/II | Metastatic AR+ BC | Bicalutamide + palbociclib | AR antagonist + CDK4/6 inhibitor | Nov 2015 | |
| NCT03090165 | I/II | Advanced AR+ TNBC | Bicalutamide + ribociclib | AR antagonist + CDK4/6 inhibitor | Mar 2017 | |
| NCT01889238 | II | Advanced AR+ TNBC | Enzalutamide | AR antagonist | June 2013 | |
| NCT02750358 | II | Early stage AR+ TNBC; adjuvant | Enzalutamide | AR antagonist | May 2016 | |
| NCT02676986 | II | AR+ BC; neoadjuvant | Enzalutamide (± exemestane) | AR antagonist (±AI) | Aug 2015 | |
| NCT02689427 | IIB | AR+ TNBC; neoadjuvant | Enzalutamide ± paclitaxel | AR antagonist ± chemotherapy | Sep 2016 | |
| NCT02929576 | III | Advanced AR+ TNBC | Enzalutamide ± paclitaxel | AR antagonist ± chemotherapy | Sep 2016 | Withdrawn |
| NCT02457910 | I/II | Advanced AR+ TNBC; PM | Enzalutamide ± taselisib | AR antagonist ± PI3K inhibitor | June 2015 | |
| NCT03207529 | I | Metastatic AR+ BC; PTEN+ | Enzalutamide ± alpelisib | AR antagonist ± PI3K inhibitor | Dec 2017 | |
| NCT01842321 | II | Advanced AR+ TNBC | Abiraterone + prednisone | CYP17 inhibitor + corticosteroid | July 2013 | |
| NCT01990209 | II | Metastatic AR+ BC | Orteronel | CYP17 inhibitor | Mar 2014 | |
| NCT02580448 | I/II | Advanced AR+ BC; ER+/HER2− and TNBC | Seviteronel | CYP17 inhibitor/AR antagonist | Aug 2015 | |
| NCT02067741 | II | Metastatic AR+ BC; ER+/HER2− and TNBC | CR1447 (4-OH-testosterone) | AR agonist | May 2016 | |
| NCT02000375 | II | Metastatic AR+ BC; ER+ and TNBC, PM | DHEA | AR agonist | Mar 2013 | Terminated |
| NCT02368691 | II | Advanced AR+ TNBC | GTx-024 | Selective AR modulator | June 2015 | Terminated |
| NCT02971761 | II | Advanced AR+ TNBC | GTx-024 + pembrolizumab | Selective AR modulator + anti-PD-1 | June 2017 |
AI aromatase inhibitor, AR androgen receptor, BC breast cancer, CDK cyclin-dependent kinase, CYP17 cytochrome P450 17, DHEA dehydroepiandrosterone, ER estrogen receptor, HER2 human epidermal growth factor receptor 2, NCT national clinical trial, PD-1 programmed cell death protein 1, PI3K phosphoinositide 3-kinase, PM postmenopausal, PTEN phosphatase and tensin homolog, TNBC triple-negative breast cancer
The first trial of an anti-androgen in BC was a phase 2 trial of bicalutamide in AR+/ER− metastatic BC. Bicalutamide, long used to treat prostate cancer, is a competitive antagonist that permits AR nuclear translocation and binding to DNA but in an inactive form [102]. In a phase 2 trial, 5 of 26 patients with AR+/ER−/PR− BC treated with bicalutamide had stable disease for at least 6 months, resulting in a 24-week CBR of 19% [103]. AR expression in the 5 patients was varied: 10−20% (1 patient), > 50% (1 patient), > 80% (2 patients), and > 90% (1 patient). Median PFS was 12 weeks (95% CI, 11–22 weeks), and the most common drug-related adverse events (AEs) were fatigue, hot flashes, limb edema, and aspartate aminotransferase or alkaline aminotransferase elevations. This was the first clinical trial to establish the activity of anti-AR therapy in advanced BC and the potential of targeting AR in AR-dependent, ER-independent BC [103]. However, while disease stabilization in 5 patients with AR+/ER– metastatic BC is indicative of AR inhibition, it is also possible that these 5 patients had more indolent disease since LAR TNBCs are less proliferative than other subtypes of TNBC.
The next trial of an AR inhibitor in TNBC was with enzalutamide, a newer-generation AR competitive inhibitor approved by the FDA to treat men with metastatic CRPC [57]. Enzalutamide, like bicalutamide, directly binds to AR and is a competitive antagonist but has a > fivefold higher binding affinity than bicalutamide and, in contrast to bicalutamide, impairs AR nuclear translocation, inhibits AR-DNA binding and gene regulation, and consequently has no partial agonist activity [56, 104, 105]. In a phase 2 study of enzalutamide in advanced AR+ TNBC, 26 (35%) of 75 evaluable patients demonstrated a 16-week CBR, and 22 (29%) demonstrated a 24-week CBR [39]. Of the 26 patients who had CBR at 16 weeks, 2 had a complete response and 5 patients had a partial response. Median PFS was 14 weeks (95% CI, 8–19 weeks). The most common therapy-related AEs were fatigue, nausea, decreased appetite, diarrhea, and hot flush.
The non-steroidal CYP17 inhibitor orteronel (TAK-700) was initially being developed for the treatment of CRPC but failed in phase 3. It is currently being investigated in women with AR+ TNBC [101].
A phase 2 trial investigated the efficacy of abiraterone acetate—an irreversible and potent inhibitor of CYP17—with prednisone in women with metastatic or inoperable locally advanced AR+ TNBC [106]. Of 30 patients who were eligible and evaluable for the primary endpoint, 6 (20%) had a CBR at 6 months. Of these 6 patients, 1 had a complete response and 5 had stable disease. However, this proportion of patients achieving clinical benefit was insufficient to meet predefined criteria to reject the null hypothesis. The most common drug-related AEs were fatigue, hypertension, hypokalemia, and nausea, with the majority being grade 1 or 2.
In addition, the clinical benefit of seviteronel, a non-steroidal selective CYP17 17,20 lyase and AR inhibitor that blocks both testosterone and estradiol production and inhibits AR activation, was recently reported from an ongoing phase 1/2 study that includes a separate cohort of women with unresectable locally advanced or metastatic AR+ TNBC in addition to ER+/HER2− BC [107]. The 16-week CBR for AR+ TNBC was 2 of 6 patients (33%), allowing full stage 2 accrual. Declines in CTCs were observed in 7 of 10 evaluable patients (AR+ TNBC and ER+ BC patients), including all patients who met clinical benefit criteria across both cohorts. The most common AEs were fatigue, dizziness, nausea, and decreased appetite, all grade 1 or 2.
The first-generation AR antagonist bicalutamide and the next-generation AR antagonist enzalutamide have both demonstrated clinical activity in patients with AR+ TNBC, as discussed above. Seviteronel, with a dual mechanism of action of selective CYP17 lyase inhibition and AR antagonism, also has demonstrated initial clinical activity and full phase 2 clinical development is ongoing in women with AR+ TNBC in addition to other types of male and female BC [108]. Thus, a strategy of combined targeting of androgen biosynthesis and AR inhibition appears promising for the treatment of AR+ TNBC.
Other combination strategies utilizing AR-targeted agents with non-endocrine agents are also under clinical investigation in AR+ TNBC. For example, AR antagonism in AR+ TNBC is being explored in combination with a cell cycle cyclin-dependent kinase CDK4 and CDK6 inhibitor (bicalutamide + palbociclib) [109] and with a phosphoinositide 3-kinase (PI3K) inhibitor (enzalutamide + taselisib) [110]. The combination of AR antagonists and taxanes is also being investigated [111] since taxanes have been shown to inhibit the translocation of the AR from the cytoplasm to the nucleus in prostate cancer [112] and were effective together in preventing recurrent disease in a preclinical model of TNBC [22]. Further work is needed to develop rational combinations that utilize AR-targeted agents in AR+ TNBC.
Conclusion
Most types of BC, including TNBC, can be driven in part by activated, ligand-bound AR. While IHC using FFPE samples is the traditional means of measuring AR expression in tumors, new blood-based approaches may provide improved real-time AR assessment. Several novel drugs are currently in development that target AR and/or androgen production that may provide additional options for patients with AR+ TNBC for whom chemotherapy is the only current treatment option.
As preclinical research strives to better model the clinical situation, varied approaches are being taken, such as utilization of patient-derived xenografts and mouse mammary carcinoma models with intact immune systems derived from genetically engineered transgenic models, spontaneous arising tumors, or chemically induced tumor models to examine the effects of AR inhibition on both the anti-tumor immune response and the immune system in general. There remains much to be learned regarding how to leverage the impact of endocrine therapy (even in TNBC) on host anti-tumor immunity and develop optimal combination regimens with other therapies for TNBC. There is also evidence that anti-androgens may have off-target effects [97], and research into these alternative mechanisms of action is ongoing. In conclusion, modeling various clinically relevant physiological states such as pre- or postmenopause, postpartum pregnancy-associated BC, and obesity in immune-intact animals is an important direction for future research and will address contemporary questions.
Acknowledgments
The authors thank Elise Eller, PhD, and Kelly Kilibarda, PhD, of Whitsell Innovations, Inc., Chapel Hill, NC, USA, for providing medical writing support, which was funded by Innocrin Pharmaceuticals Inc., Durham, North Carolina, USA, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). We also acknowledge Beatrice Babbs in the Richer Laboratory at the University of Colorado for immunohistochemistry.
Compliance with Ethical Standards
Conflict of Interest
JRE and ESBB declare that they are employed by and have stock ownership in Innocrin Pharmaceuticals, Inc. All other authors declare that they have no potential conflict of interest.
Contributor Information
Anthony D. Elias, Phone: (720) 848-0347, Email: anthony.elias@ucdenver.edu
Jennifer K. Richer, Phone: (303) 724-3725, Email: jennifer.richer@ucdenver.edu
References
- 1.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24(7):924–931. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi JP, Yang YL, Zhu H, Wang J, Jia Y, Liu N, Song YJ, Zan LK, Zhang X, Zhou M, Gu YH, Liu T, Hicks DG, Tang P. Expression of the androgen receptor and its correlation with molecular subtypes in 980 Chinese breast cancer patients. Breast Cancer (Auckl) 2012;6:1–8. doi: 10.4137/BCBCR.S8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guedj M, Marisa L, de Reynies A, Orsetti B, Schiappa R, Bibeau F, MacGrogan G, Lerebours F, Finetti P, Longy M, Bertheau P, Bertrand F, Bonnet F, Martin AL, Feugeas JP, Bièche I, Lehmann-Che J, Lidereau R, Birnbaum D, Bertucci F, de Thé H, Theillet C. A refined molecular taxonomy of breast cancer. Oncogene. 2012;31(9):1196–1206. doi: 10.1038/onc.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D'Amato NC, Spoelstra NS, Edgerton SM, Jean A, Guerrero J, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16(1):R7. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sas-Korczynska B, Adamczyk A, Niemiec J, Harazin-Lechowska A, Ambicka A, Jakubowicz J. Androgen receptor in male breast cancer. Pol J Pathol. 2015;66(4):347–352. doi: 10.5114/pjp.2015.57065. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Pan B, Zhu H, Zhou Y, Mao F, Lin Y, Xu Q, Sun Q. Prognostic value of androgen receptor in triple negative breast cancer: a meta-analysis. Oncotarget. 2016;7(29):46482–46491. doi: 10.18632/oncotarget.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vera-Badillo FE, Templeton AJ, de Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, Seruga B, Tannock IF, Ocana A, Amir E. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(1):djt319. doi: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- 8.Qu Q, Mao Y, Fei XC, Shen KW. The impact of androgen receptor expression on breast cancer survival: a retrospective study and meta-analysis. PLoS One. 2013;8(12):e82650. doi: 10.1371/journal.pone.0082650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, Kim SI, Park BW, Lee KS. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22(8):1755–1762. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- 10.Lim E, Ni M, Hazra A, Tamini R, Brown M. Elucidating the role of androgen receptor in breast cancer. Clin Invest. 2012;2(10):1003–1011. doi: 10.4155/cli.12.88. [DOI] [Google Scholar]
- 11.Asano Y, Kashiwagi S, Goto W, Tanaka S, Morisaki T, Takashima T, Noda S, Onoda N, Ohsawa M, Hirakawa K, Ohira M. Expression and clinical significance of androgen receptor in triple-negative breast cancer. Cancers (Basel) 2017;9:1. doi: 10.3390/cancers9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang D, Xu S, Zhang Q, Zhao W. The expression and clinical significance of the androgen receptor and E-cadherin in triple-negative breast cancer. Med Oncol. 2012;29(2):526–533. doi: 10.1007/s12032-011-9948-2. [DOI] [PubMed] [Google Scholar]
- 13.Hickey TE, Robinson JL, Carroll JS, Tilley WD. Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol. 2012;26(8):1252–1267. doi: 10.1210/me.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, Symmans WF, Ueno NT. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19(19):5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton VN, D'Amato NC, Gordon MA, Christenson JL, Elias A, Richer JK. Androgen receptor biology in triple negative breast cancer: a case for classification as AR+ or quadruple negative disease. Horm Cancer. 2015;6(5-6):206–213. doi: 10.1007/s12672-015-0232-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton VN, D'Amato NC, Gordon MA, Lind HT, Spoelstra NS, Babbs BL, Heinz RE, Elias A, Jedlicka P, Jacobsen BM, Richer JK. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol Cancer Ther. 2015;14(3):769–778. doi: 10.1158/1535-7163.MCT-14-0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Amato NC, Gordon MA, Babbs B, Spoelstra NS, Carson Butterfield KT, Torkko KC, Phan VT, Barton VN, Rogers TJ, Sartorius CA, Elias A, Gertz J, Jacobsen BM, Richer JK. Cooperative dynamics of AR and ER activity in breast cancer. Mol Cancer Res. 2016;14(11):1054–1067. doi: 10.1158/1541-7786.MCR-16-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, Zhou A, Eyob H, Balakrishnan S, Wang CY, Yaswen P, Goga A, Werb Z. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526(7571):131–135. doi: 10.1038/nature15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, Rimm DL, Liu XS, Brown M. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20(1):119–131. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon MA, D’Amato NC, Gu H, Babbs B, Wulfkuhle JD, Petricoin EF, Gallagher I, Dong T, Torkko K, Liu B, et al. Synergy between androgen receptor antagonism and inhibition of mTOR and HER2 in breast cancer. Mol Cancer Ther. 2017;16(7):1389–1400. doi: 10.1158/1535-7163.MCT-17-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton VN, Christenson JL, Gordon MA, Greene LI, Rogers TJ, Butterfield K, Babbs B, Spoelstra NS, D'Amato NC, Elias A, Richer JK. Androgen receptor supports an anchorage-independent, cancer stem cell-like population in triple-negative breast cancer. Cancer Res. 2017;77(13):3455–3466. doi: 10.1158/0008-5472.CAN-16-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Amicis F, Thirugnansampanthan J, Cui Y, Selever J, Beyer A, Parra I, Weigel NL, Herynk MH, Tsimelzon A, Lewis MT, Chamness GC, Hilsenbeck SG, Andò S, Fuqua SAW. Androgen receptor overexpression induces tamoxifen resistance in human breast cancer cells. Breast Cancer Res Treat. 2010;121(1):1–11. doi: 10.1007/s10549-009-0436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvell DM, Richer JK, Singh M, Spoelstra N, Finlayson C, Borges VF, Elias AD, Horwitz KB. Estrogen regulated gene expression in response to neoadjuvant endocrine therapy of breast cancers: tamoxifen agonist effects dominate in the presence of an aromatase inhibitor. Breast Cancer Res Treat. 2008;112(3):489–501. doi: 10.1007/s10549-008-9923-6. [DOI] [PubMed] [Google Scholar]
- 25.Harvell DM, Spoelstra NS, Singh M, McManaman JL, Finlayson C, Phang T, Trapp S, Hunter L, Dye WW, Borges VF, et al. Molecular signatures of neoadjuvant endocrine therapy for breast cancer: characteristics of response or intrinsic resistance. Breast Cancer Res Treat. 2008;112(3):475–488. doi: 10.1007/s10549-008-9897-4. [DOI] [PubMed] [Google Scholar]
- 26.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 27.Agoff SN, Swanson PE, Linden H, Hawes SE, Lawton TJ. Androgen receptor expression in estrogen receptor-negative breast cancer. Immunohistochemical, clinical, and prognostic associations. Am J Clin Pathol. 2003;120(5):725–731. doi: 10.1309/42F00D0DJD0J5EDT. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, Park BW, Lee KS. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21(3):488–492. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 29.Chia KM, Liu J, Francis GD, Naderi A. A feedback loop between androgen receptor and ERK signaling in estrogen receptor-negative breast cancer. Neoplasia. 2011;13(2):154–166. doi: 10.1593/neo.101324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wellberg EA, Checkley LA, Giles ED, Johnson SJ, Oljira R, Wahdan-Alaswad R, Foright RM, Dooley G, Edgerton SM, Jindal S, Johnson GC, Richer JK, Kabos P, Thor AD, Schedin P, MacLean PS, Anderson SM. The androgen receptor supports tumor progression after the loss of ovarian function in a preclinical model of obesity and breast cancer. Horm Cancer. 2017;8(5-6):269–285. doi: 10.1007/s12672-017-0302-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, Cronin KA. 2014. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 106 [DOI] [PMC free article] [PubMed]
- 32.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann BD, Jovanovic B, Chen X, Estrada MV, Johnson KN, Shyr Y, Moses HL, Sanders ME, Pietenpol JA. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11(6):e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, Macgrogan G, Bergh J, Cameron D, Goldstein D, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24(29):4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 35.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8(8):R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson JL, Macarthur S, Ross-Innes CS, Tilley WD, Neal DE, Mills IG, Carroll JS. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011;30(15):3019–3027. doi: 10.1038/emboj.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNamara KM, Yoda T, Takagi K, Miki Y, Suzuki T, Sasano H. Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol. 2013;133:66–76. doi: 10.1016/j.jsbmb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Aleskandarany MA, Abduljabbar R, Ashankyty I, Elmouna A, Jerjees D, Ali S, Buluwela L, Diez-Rodriguez M, Caldas C, Green AR, Ellis IO, Rakha EA. Prognostic significance of androgen receptor expression in invasive breast cancer: transcriptomic and protein expression analysis. Breast Cancer Res Treat. 2016;159(2):215–227. doi: 10.1007/s10549-016-3934-5. [DOI] [PubMed] [Google Scholar]
- 39.Traina TA, Miller K, Yardley DA, O'Shaughnessy J, Cortes J, Awada A, Kelly CM, Trudeau ME, Schmid P, Gianni L et al. 2015. Results from a phase 2 study of enzalutamide (ENZA), an androgen receptor (AR) inhibitor, in advanced AR+ triple-negative breast cancer (TNBC). J Clin Oncol 33
- 40.Gucalp A, Traina TA. Androgen receptor-positive, triple-negative breast cancer. Cancer. 2017;123(10):1686–1688. doi: 10.1002/cncr.30683. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Daffada AA, Chan CM, Dowsett M. Identification of an exon 3 deletion splice variant androgen receptor mRNA in human breast cancer. Int J Cancer. 1997;72(4):574–580. doi: 10.1002/(SICI)1097-0215(19970807)72:4<574::AID-IJC4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 42.Hu DG, Hickey TE, Irvine C, Wijayakumara DD, Lu L, Tilley WD, Selth LA, Mackenzie PI. Identification of androgen receptor splice variant transcripts in breast cancer cell lines and human tissues. Horm Cancer. 2014;5(2):61–71. doi: 10.1007/s12672-014-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahrens-Fath I, Politz O, Geserick C, Haendler B. Androgen receptor function is modulated by the tissue-specific AR45 variant. FEBS J. 2005;272(1):74–84. doi: 10.1111/j.1432-1033.2004.04395.x. [DOI] [PubMed] [Google Scholar]
- 44.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AMH, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, de Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, Silberstein JL, Taylor MN, Maughan BL, Denmeade SR, Pienta KJ, Paller CJ, Carducci MA, Eisenberger MA, Luo J. Clinical significance of androgen receptor splice variant-7 mRNA detection in circulating tumor cells of men with metastatic castration-resistant prostate cancer treated with first- and second-line abiraterone and enzalutamide. J Clin Oncol. 2017;35(19):2149–2156. doi: 10.1200/JCO.2016.70.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinestel J, Luedeke M, Arndt A, Schnoeller TJ, Lennerz JK, Wurm C, Maier C, Cronauer MV, Steinestel K, Schrader AJ. (2015) Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget [DOI] [PMC free article] [PubMed]
- 48.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, Johnson A, Jendrisak A, Bambury R, Danila D, McLaughlin B, Wahl J, Greene SB, Heller G, Marrinucci D, Fleisher M, Dittamore R. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2(11):1441–1449. doi: 10.1001/jamaoncol.2016.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol. 2014;5(3):382–392. doi: 10.5306/wjco.v5.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dimitrakakis C, Bondy C. Androgens and the breast. Breast Cancer Res. 2009;11(5):212. doi: 10.1186/bcr2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gallicchio L, Macdonald R, Wood B, Rushovich E, Helzlsouer KJ. Androgens and musculoskeletal symptoms among breast cancer patients on aromatase inhibitor therapy. Breast Cancer Res Treat. 2011;130(2):569–577. doi: 10.1007/s10549-011-1611-2. [DOI] [PubMed] [Google Scholar]
- 52.Morris KT, Toth-Fejel S, Schmidt J, Fletcher WS, Pommier RF. High dehydroepiandrosterone-sulfate predicts breast cancer progression during new aromatase inhibitor therapy and stimulates breast cancer cell growth in tissue culture: a renewed role for adrenalectomy. Surgery. 2001;130(6):947–953. doi: 10.1067/msy.2001.118378. [DOI] [PubMed] [Google Scholar]
- 53.Ingle JN, Kalari KR, Buzdar AU, Robson ME, Goetz MP, Desta Z, Barman P, Dudenkov TT, Northfelt DW, Perez EA, Flockhart DA, Williard CV, Wang L, Weinshilboum RM. Estrogens and their precursors in postmenopausal women with early breast cancer receiving anastrozole. Steroids. 2015;99(Pt A):32–38. doi: 10.1016/j.steroids.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossi E, Morabito A, Di Rella F, Esposito G, Gravina A, Labonia V, Landi G, Nuzzo F, Pacilio C, De Maio E, di Maio M, Piccirillo MC, de Feo G, D’Aiuto G, Botti G, Chiodini P, Gallo C, Perrone F, de Matteis A. Endocrine effects of adjuvant letrozole compared with tamoxifen in hormone-responsive postmenopausal patients with early breast cancer: the HOBOE trial. J Clin Oncol. 2009;27(19):3192–3197. doi: 10.1200/JCO.2008.18.6213. [DOI] [PubMed] [Google Scholar]
- 55.Hadji P, Kauka A, Bauer T, Tams J, Hasenburg A, Kieback DG. Effects of exemestane and tamoxifen on hormone levels within the Tamoxifen Exemestane Adjuvant Multicentre (TEAM) trial: results of a German substudy. Climacteric. 2012;15(5):460–466. doi: 10.3109/13697137.2011.647839. [DOI] [PubMed] [Google Scholar]
- 56.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS, AFFIRM Investigators Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 58.Lu J, Van der Steen T, Tindall DJ. Are androgen receptor variants a substitute for the full-length receptor? Nat Rev Urol. 2015;12(3):137–144. doi: 10.1038/nrurol.2015.13. [DOI] [PubMed] [Google Scholar]
- 59.Ardiani A, Gameiro SR, Kwilas AR, Donahue RN, Hodge JW. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget. 2014;5(19):9335–9348. doi: 10.18632/oncotarget.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan SC, Dehm SM. Constitutive activity of the androgen receptor. Adv Pharmacol. 2014;70:327–366. doi: 10.1016/B978-0-12-417197-8.00011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hickey TE, Irvine CM, Dvinge H, Tarulli GA, Hanson AR, Ryan NK, Pickering MA, Birrell SN, Hu DG, Mackenzie PI, Russell R, Caldas C, Raj GV, Dehm SM, Plymate SR, Bradley RK, Tilley WD, Selth LA. Expression of androgen receptor splice variants in clinical breast cancers. Oncotarget. 2015;6(42):44728–44744. doi: 10.18632/oncotarget.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 63.Jiang Y, Palma JF, Agus DB, Wang Y, Gross ME. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem. 2010;56(9):1492–1495. doi: 10.1373/clinchem.2010.143297. [DOI] [PubMed] [Google Scholar]
- 64.Lilja H, Scher HI. Detection of androgen receptor mutations in circulating tumor cells: highlights of the long road to clinical qualification. Clin Chem. 2010;56(9):1375–1377. doi: 10.1373/clinchem.2010.150896. [DOI] [PubMed] [Google Scholar]
- 65.Miyamoto DT, Lee RJ, Stott SL, Ting DT, Wittner BS, Ulman M, Smas ME, Lord JB, Brannigan BW, Trautwein J, Bander NH, Wu CL, Sequist LV, Smith MR, Ramaswamy S, Toner M, Maheswaran S, Haber DA. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012;2(11):995–1003. doi: 10.1158/2159-8290.CD-12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reyes EE, VanderWeele DJ, Isikbay M, Duggan R, Campanile A, Stadler WM, Vander Griend DJ, Szmulewitz RZ. Quantitative characterization of androgen receptor protein expression and cellular localization in circulating tumor cells from patients with metastatic castration-resistant prostate cancer. J Transl Med. 2014;12(1):313. doi: 10.1186/s12967-014-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crespo M, van Dalum G, Ferraldeschi R, Zafeiriou Z, Sideris S, Lorente D, Bianchini D, Rodrigues DN, Riisnaes R, Miranda S, Figueiredo I, Flohr P, Nowakowska K, de Bono JS, Terstappen LWMM, Attard G. Androgen receptor expression in circulating tumour cells from castration-resistant prostate cancer patients treated with novel endocrine agents. Br J Cancer. 2015;112(7):1166–1174. doi: 10.1038/bjc.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujii T, Reuben JM, Krupa R, Graf R, Johnson C, Dugan L, Louw J, Lim B, Barcenas CH, Marx AN, et al. Androgen receptor expression on circulating tumor cells (CTCs) in metastatic breast cancer. Cancer Res. 2016;76(14 Supplement):496–496. doi: 10.1158/1538-7445.AM2016-496. [DOI] [Google Scholar]
- 69.Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, Moon M, Maneval EC, Chen I, Darimont B, Hager JH. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020–1029. doi: 10.1158/2159-8290.CD-13-0226. [DOI] [PubMed] [Google Scholar]
- 70.Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, Monahan JE, Stegmeier F, Roberts TM, Sellers WR, Zhou W, Zhu P. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide) Cancer Discov. 2013;3(9):1030–1043. doi: 10.1158/2159-8290.CD-13-0142. [DOI] [PubMed] [Google Scholar]
- 71.Canzoniero JV, Park BH. Use of cell free DNA in breast oncology. Biochim Biophys Acta. 2016;1865(2):266–274. doi: 10.1016/j.bbcan.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 72.Soekmadji C, Russell PJ, Nelson CC. Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers (Basel) 2013;5(4):1522–1544. doi: 10.3390/cancers5041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hosseini-Beheshti E, Choi W, Weiswald LB, Kharmate G, Ghaffari M, Roshan-Moniri M, Hassona MD, Chan L, Chin MY, Tai IT, Rennie PS, Fazli L, Tomlinson Guns ES. Exosomes confer pro-survival signals to alter the phenotype of prostate cells in their surrounding environment. Oncotarget. 2016;7(12):14639–14658. doi: 10.18632/oncotarget.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mizutani K, Terazawa R, Kameyama K, Kato T, Horie K, Tsuchiya T, Seike K, Ehara H, Fujita Y, Kawakami K, Ito M, Deguchi T. Isolation of prostate cancer-related exosomes. Anticancer Res. 2014;34(7):3419–3423. [PubMed] [Google Scholar]
- 75.Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, van Schaik RH, Danesi R. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur Urol. 2016;71(4):680–687. doi: 10.1016/j.eururo.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 76.Qu F, Xie W, Nakabayashi M, Zhang H, Jeong SH, Wang X, Komura K, Sweeney CJ, Sartor O, Lee GM, et al. Association of AR-V7 and prostate-specific antigen RNA levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin Cancer Res. 2017;23(3):726–734. doi: 10.1158/1078-0432.CCR-16-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu X, Ledet E, Li D, Dotiwala A, Steinberger A, Feibus A, Li J, Qi Y, Silberstein J, Lee B, Dong Y, Sartor O, Zhang H. A whole blood assay for AR-V7 and ARv567es in patients with prostate cancer. J Urol. 2016;196(6):1758–1763. doi: 10.1016/j.juro.2016.06.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marcucci F, Stassi G, De Maria R. Epithelial-mesenchymal transition: a new target in anticancer drug discovery. Nat Rev Drug Discov. 2016;15(5):311–325. doi: 10.1038/nrd.2015.13. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, Zhou BP. Epithelial-mesenchymal transition—a hallmark of breast cancer metastasis. Cancer Hallm. 2013;1(1):38–49. doi: 10.1166/ch.2013.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu YN, Liu Y, Lee HJ, Hsu YH, Chen JH. Activated androgen receptor downregulates E-cadherin gene expression and promotes tumor metastasis. Mol Cell Biol. 2008;28(23):7096–7108. doi: 10.1128/MCB.00449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng J, Li L, Zhang N, Liu J, Zhang L, Gao H, Wang G, Li Y, Zhang Y, Li X, Liu D, Lu J, Huang B. Androgen and AR contribute to breast cancer development and metastasis: an insight of mechanisms. Oncogene. 2016;36(20):2775–2790. doi: 10.1038/onc.2016.432. [DOI] [PubMed] [Google Scholar]
- 83.Zhu A, Li Y, Song W, Xu Y, Yang F, Zhang W, Yin Y, Guan X. Antiproliferative effect of androgen receptor inhibition in mesenchymal stem-like triple-negative breast cancer. Cell Physiol Biochem. 2016;38(3):1003–1014. doi: 10.1159/000443052. [DOI] [PubMed] [Google Scholar]
- 84.Christenson JL, Butterfield KT, Spoelstra NS, Norris JD, Josan JS, Pollock JA, McDonnell DP, Katzenellenbogen BS, Katzenellenbogen JA, Richer JK. MMTV-PyMT and derived Met-1 mouse mammary tumor cells as models for studying the role of the androgen receptor in triple-negative breast cancer progression. Horm Cancer. 2017;8(2):69–77. doi: 10.1007/s12672-017-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lai JJ, Lai KP, Zeng W, Chuang KH, Altuwaijri S, Chang C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: lessons from conditional AR knockout mice. Am J Pathol. 2012;181(5):1504–1512. doi: 10.1016/j.ajpath.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chuang KH, Altuwaijri S, Li G, Lai JJ, Chu CY, Lai KP, Lin HY, Hsu JW, Keng P, Wu MC, Chang C. Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J Exp Med. 2009;206(5):1181–1199. doi: 10.1084/jem.20082521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viselli SM, Reese KR, Fan J, Kovacs WJ, Olsen NJ. Androgens alter B cell development in normal male mice. Cell Immunol. 1997;182(2):99–104. doi: 10.1006/cimm.1997.1227. [DOI] [PubMed] [Google Scholar]
- 88.Olsen NJ, Kovacs WJ. Effects of androgens on T and B lymphocyte development. Immunol Res. 2001;23(2-3):281–288. doi: 10.1385/IR:23:2-3:281. [DOI] [PubMed] [Google Scholar]
- 89.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, Hurwitz AA, McKean DJ, Celis E, Leibovich BC, Allison JP, Kwon ED. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173(10):6098–6108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 90.Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A. 2014;111(27):9887–9892. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, Rugo HS, Hwang ES, Jirström K, West BL, Coussens LM. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 93.West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13(6):R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–14570. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ardiani A, Farsaci B, Rogers CJ, Protter A, Guo Z, King TH, Apelian D, Hodge JW. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res. 2013;19(22):6205–6218. doi: 10.1158/1078-0432.CCR-13-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kwilas AR, Ardiani A, Gameiro SR, Richards J, Hall AB, Hodge JW. Androgen deprivation therapy sensitizes triple negative breast cancer cells to immune-mediated lysis through androgen receptor independent modulation of osteoprotegerin. Oncotarget. 2016;7(17):23498–23511. doi: 10.18632/oncotarget.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pu Y, Xu M, Liang Y, Yang K, Guo Y, Yang X, Fu YX. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumor relapse. Sci Transl Med. 2016;8(333):333ra347. doi: 10.1126/scitranslmed.aad5659. [DOI] [PubMed] [Google Scholar]
- 98.Emens LA, Braiteh FS, Cassier P, Delord J-P, Eder JP, Fasso M, Xiao Y, Wang Y, Molinero L, Chen DS et al. (2015) Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC). AACR Annual Meeting
- 99.Adams S, Diamond JR, Hamilton EP, Pohlmann PR, Tolaney SM, Molinero L, He X, Waterkamp D, Funke RP, Powderly JD (2016) Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). ASCO Annu Meet
- 100.Elias A, Kabos P (2017) Triple negative breast cancer: a review. Int Med Rev 3(4). 10.18103/imr.v3i4.394
- 101.Orteronel as monotherapy in patients with metastatic breast cancer (MBC) that expresses the androgen receptor (AR). Available at: [https://clinicaltrials.gov/ct2/show/NCT01990209]. Accessed May 5, 2017
- 102.Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002;277(29):26321–26326. doi: 10.1074/jbc.M203310200. [DOI] [PubMed] [Google Scholar]
- 103.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, Blackwell K, Rugo H, Nabell L, Forero A, Stearns V, Doane AS, Danso M, Moynahan ME, Momen LF, Gonzalez JM, Akhtar A, Giri DD, Patil S, Feigin KN, Hudis CA, Traina TA, on behalf of the Translational Breast Cancer Research Consortium (TBCRC 011) Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19(19):5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jung ME, Ouk S, Yoo D, Sawyers CL, Chen C, Tran C, Wongvipat J. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC) J Med Chem. 2010;53(7):2779–2796. doi: 10.1021/jm901488g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Makkonen H, Kauhanen M, Jaaskelainen T, Palvimo JJ. Androgen receptor amplification is reflected in the transcriptional responses of vertebral-cancer of the prostate cells. Mol Cell Endocrinol. 2011;331(1):57–65. doi: 10.1016/j.mce.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 106.Bonnefoi H, Grellety T, Tredan O, Saghatchian M, Dalenc F, Mailliez A, L'Haridon T, Cottu P, Abadie-Lacourtoisie S, You B, Mousseau M, Dauba J, del Piano F, Desmoulins I, Coussy F, Madranges N, Grenier J, Bidard FC, Proudhon C, MacGrogan G, Orsini C, Pulido M, Gonçalves A. A phase II trial of abiraterone acetate plus prednisone in patients with triple-negative androgen receptor positive locally advanced or metastatic breast cancer (UCBG 12-1) Ann Oncol. 2016;27(5):812–818. doi: 10.1093/annonc/mdw067. [DOI] [PubMed] [Google Scholar]
- 107.Gucalp A, Danso MA, Elias AD, Bardia A, Ali HY, Potter D, Gabrail NY, Haley BB, Khong HT, Riley EC et al (2017) Phase (Ph) 2 stage 1 clinical activity of seviteronel, a selective CYP17-lyase and androgen receptor (AR) inihibitor, in women with advanced AR+ triple-negative breast cancer (TNBC) or estrogen receptor (ER)+ BC: CLARITY-01. J Clin Oncol 35: abstr 1102
- 108.CYP17 lyase and androgen receptor inhibitor treatment with seviteronel trial (INO-VT-464-006; NCT02580048) (CLARITY-01). Available at: [https://clinicaltrials.gov/ct2/show/NCT02580448]. Accessed March 1, 2017
- 109.Palbociclib in combination with bicalutamide for the treatment of AR(+) metastatic breast cancer (MBC). Available at: [https://clinicaltrials.gov/ct2/show/NCT02605486]. Accessed March 1, 2017
- 110.Taselisib and enzalutamide in treating patients with androgen receptor positive triple-negative metastatic breast cancer. Available at: [https://clinicaltrials.gov/ct2/show/NCT02457910]. Accessed March 1, 2017
- 111.Phase IIB neoadjuvant enzalutamide (ZT) plus taxol for androgen receptor (AR)-positive triple-negative breast cancer (AR+ TNBC). Available at: [https://clinicaltrials.gov/ct2/show/NCT02689427]. Accessed June 6, 2017
- 112.Zhu ML, Horbinski CM, Garzotto M, Qian DZ, Beer TM, Kyprianou N. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 2010;70(20):7992–8002. doi: 10.1158/0008-5472.CAN-10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]