Abstract
Objective
Arterial calcification and stiffening increase the risk of reconstruction failure, amputation, and mortality in patients with peripheral arterial disease, but underlying mechanisms and prevalence are unclear.
Approach and Results
Fresh human femoropopliteal arteries (FPA) were obtained from n=431 tissue donors 13–82 years old (mean age 53±16 years) recording the in situ longitudinal pre-stretch. Arterial diameter, wall thickness, and opening angles were measured optically, and stiffness was assessed using planar biaxial extension and constitutive modeling. Histological features were determined using transverse and longitudinal Verhoeff–Van Gieson and Alizarin stains. Medial calcification was quantified using a 7-stage grading scale and was correlated with structural and mechanical properties and clinical characteristics. Almost half (46%) of the FPAs had identifiable medial calcification. Older arteries were more calcified, but small calcium deposits were observed in arteries as young as 18 years old. After controlling for age, positive correlations were observed between calcification, diabetes, dyslipidemia, and body mass index. Tobacco use demonstrated a negative correlation. Calcified arteries were larger in diameter, but had smaller circumferential opening angles. They were also stiffer longitudinally and circumferentially, had thinner tunica media and external elastic lamina with more discontinuous elastic fibers.
Conclusions
Although aging is the dominant risk factor for FPA calcification and stiffening, these processes appear to be linked and can begin at a young age. Calcification is associated with the presence of certain risk factors and with elastic fiber degradation, suggesting overlapping molecular pathways that require further investigation.
Keywords: femoropopliteal artery, calcification, stiffening, risk factors
INTRODUCTION
Peripheral arterial disease (PAD) often manifests as atherosclerotic occlusive disease of the femoropopliteal artery (FPA) obstructing blood flow to the lower limb. It is associated with high morbidity, mortality, and impairment in the quality of life. Arterial calcification in the lower extremity, particularly in the setting of diabetes mellitus, doubles cardiovascular mortality and quadruples the risk of amputation1,2. However, the etiology of arterial calcification and its prevalence are not well understood.
Historically, calcification was viewed as a passive degenerative dystrophic process associated with aging and end-stage atherosclerosis; however recent studies have demonstrated that it is a complex regulated biological process2 that is associated with crystallization of hydroxyapatite in the extracellular matrix3,4, resulting in arterial stiffening and occurring either in conjunction or independently of occlusive lesions5–7. Since heavily calcified arteries cannot be compressed by the blood pressure cuff, it was first suggested to use ankle brachial index (ABI) as a surrogate for calcification burden8; however this technique, while simple, only allows detection of advanced calcifications and is not suitable for younger healthier arteries in their initial stages of calcification. The same limitation applies to fluoroscopy9 or Computed Tomography10 (CT) assessments of FPA calcium. A more accurate measure of arterial calcification requires histological assessment, but those are usually limited to old and diseased arteries stemming from amputation specimens that make determination of etiology and contributing factors challenging. One exception is a recent study performed by Vasuri et al.11 using 143 femoral arteries from 14–59 year-old donors, but they have detected calcifications in only 25% of their specimens, which made correlations with risk factors challenging. In addition, they have not performed mechanical characterization of their specimens, leaving the association between stiffness and arterial calcification burden unexplored.
Our team has collected a large database of interlinked mechanical and structural characteristics from 431 human subjects in a wide range of ages12. This database was used to assess prevalence of FPA calcification using cross-sectional and longitudinal histology, develop a comprehensive 7-stage calcification scale, and study associations between calcification burden, demographics, risk factors and arterial stiffness.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Data Supplement. Briefly, histological and mechanical analyses of femoropopliteal arteries from 431 tissue donors 13–82 years old were used to determine prevalence of calcification, and its associations with arterial morphometry, stiffness, and risk factors.
RESULTS
Prevalence and calcification scale
Although VVG does not specifically stain for calcification, as opposed to Alizarin Red S, it allows studying tissue artifacts related to calcification. Since calcified arteries had to be decalcified for adequate sectioning, either stain could be used to assess gross calcification burden using these artifacts. Comparison of Alizarin Red S and Verhoeff-Van Gieson stains in a subsample of 35 arteries demonstrated that the two stains were equivalent for determining location, amount, and overall calcification burden (Figure 1); therefore further quantification and analysis were performed using VVG-stained sections as they provided additional information on elastic fiber characteristics.
Figure 1.
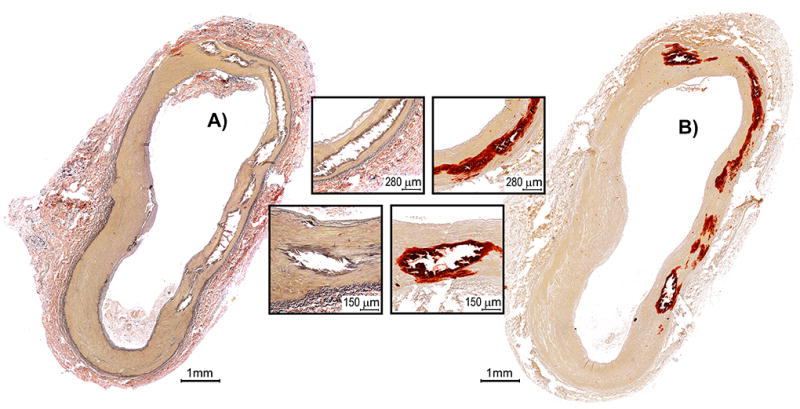
A) Verhoeff–Van Gieson and B) Alizarin Red S stains of a transverse section of the FPA demonstrating multiple areas of medial calcification (inserts) in the absence of intimal disease. Note that location, amount, and overall calcification burden can be determined using either stain, even though VVG does not specifically strain for calcium and determination relies on tissue artifacts related to calcification. VVG-stained section also provides information on elastic fiber characteristics (strained black).
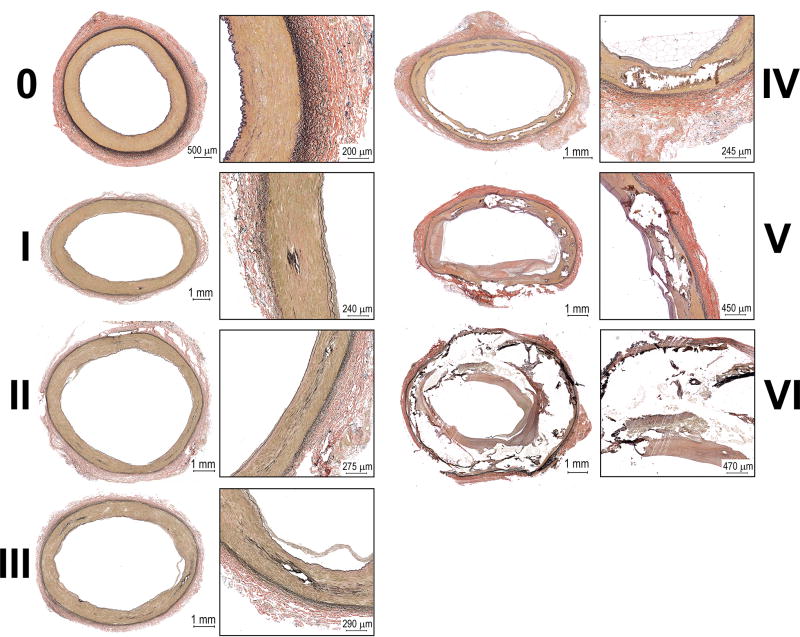
A seven-stage classification system was developed to assess calcium burden (Figure 2). Arteries at Stage 0 had no identifiable calcification. Stage I was associated with a single small calcified area less than a couple hundred micron in diameter. Stage II could include two or more of these areas, but not covering the entire sample. Arteries at Stage III had calcification affecting most of the sample, but severity of calcification was not sufficient to affect tissue integrity. Stage IV was associated with local loss of tissue integrity due to advanced calcification, but this process did not affect the entire sample. Arteries at Stage V included more severe calcification that affected most of the sample and jeopardized its integrity. Finally, Stage VI included entirely calcified FPAs with completely jeopardized tissue integrity.
Figure 2.
Calcification burden of the FPA demonstrating progression from a healthy artery with no calcification (0) to advanced calcific deposits spread throughout tunica media. Note that presence of medial calcification can be independent of intimal disease and can originate in the middle of the wall or closer to internal or external elastic laminae.
In many samples medial calcification was independent of intimal disease, as many samples had severe atherosclerosis without calcification, or significant calcification without atherosclerosis. There was no co-localization of calcification and elastic fibers, and calcified areas were frequently observed away from both internal and external elastic laminae. Scaling performed by different operators was consistent (r>0.9), and average calcification score that incorporated all transverse and longitudinal sections was used for analysis. Almost half (46%) of the FPAs had identifiable medial calcification. Age positively correlated with calcification burden (r=0.41, p < 0.01), but small calcium deposits were observed in arteries as young as 18 years old. Prevalence of calcification by age group was as follows: ≤20 years old (7%), 21–30 years old (17%), 31–40 years old (16%), 41–50 years old (48%), 51–60 years old (46%), 61–70 years old (64%), and in subjects older than 71 years (61%). Gender had no effect on the calcification burden (p=0.58).
Associations with demographics and risk factors
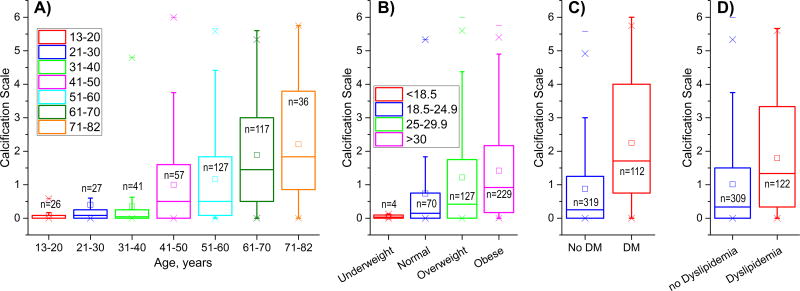
Positive correlations were observed between calcification burden and BMI (r=0.15), HTN (r=0.24), DM (r=0.32), dyslipidemia (r=0.23), and CAD (r=0.16) (p < 0.01 for all), while drug abuse correlated negatively (r=−0.15, p < 0.01). Tobacco use assessed on a scale of “Never, Former Light, Former Heavy, Current Light, Current Heavy” did not correlate with calcification burden (r=−0.07, p=0.18). After controlling for age, correlations with BMI (r=0.10, p=0.04), DM (r=0.32, p < 0.01), and dyslipidemia (r=0.12, p=0.01) remained statistically significant, but correlations with HTN (p=0.06), CAD (p=0.10), and drug abuse (p=0.11) lost statistical significance. Interestingly, correlation with tobacco use gained statistical significance after controlling for age and correlation was negative (r=−0.18, p < 0.01). Furthermore, correlation remained statistically significant (and negative) when the scale was reduced to “Never, Former, Current” (r=−0.179, p < 0.01). Statistically significant associations of calcification burden with demographics and risk factors are presented in Figure 3. Note that Age and BMI were analyzed as continuous variables rather than groups.
Figure 3.
Associations of calcification burden with demographics and risk factors: A) age, B) Body Mass Index (BMI), C) Diabetes (DM), and D) Dyslipidemia. All associations were statistically significant before and after controlling for age. Note that age and BMI are continuous variables separated into groups for better visualization. Here boxes bound 25th and 75th percentiles, whiskers extend to 5th and 95th percentiles, 99th percentile is marked with a cross (x), and maximum values are represented by a minus sign (−). Additionally, mean values are marked with a hollow square, and median is represented by a horizontal line within each box.
Calcification versus structural and morphometric characteristics
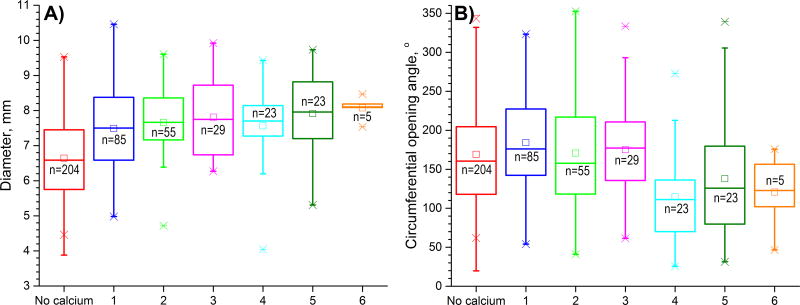
More severely calcified arteries were larger in diameter (r=0.36, p < 0.01, Figure 4A) and had thicker wall (r=0.10, p=0.03). After controlling for age, correlation with wall thickness lost statistical significance, while correlation with diameter weakened to r=0.17 but remained statistically significant (p < 0.01). More severe calcification was associated with smaller circumferential opening angles (r=−0.17 before controlling for age [Figure 4B], and r=−0.26 after, p < 0.01), but larger longitudinal opening angles (i.e. flatter axial strips) (r=0.34, p < 0.01 before controlling for age, no statistical significance after). More calcified arteries had less longitudinal pre-stretch when excised from the body (r=−0.33, p < 0.01), but this effect was likely associated with aging as no correlation was observed after controlling for age. Calcified arteries were more diseased (r=0.74 before and r=0.68, p < 0.01 after controlling for age), and FPA disease stage was assessed using a scale presented in Figure 5A and described in Kamenskiy et al13. Briefly, Stage I arteries had no intimal thickening, no lipid pools, and no calcification. Stage II FPAs had minor intimal thickening and some smooth muscle cell proliferation, but no protruding plaques. Stage III arteries had plaques with minor protrusion into the lumen, and could have had small pools of extracellular lipid, but no calcium. Stage IV arteries had minor to moderate calcification, protruding plaque with <30% stenosis, and moderate pools of extracellular lipid. Stage V arteries had severe medial calcification, a stenosis >75%, or external elastic lamina degradation and enlarged diameter consistent with early aneurysm formation. These arteries could also have large pools of extracellular lipid. Stage VI arteries had extremely calcified medial layers, total vessel occlusions, or fully developed aneurysms that represent the most severely damaged artery walls. Non-parametric Levene’s test demonstrated that variances between disease stage groups were different (p < 0.01), therefore Games-Howell post-hoc test was performed to compare between groups and statistically significant results are marked by brackets in Figure 5B.
Figure 4.
A) FPA diameters (mm) and B) circumferential opening angles (°) in FPAs with different calcification burdens. Note that both diameter and circumferential opening angle are continuous variables separated into groups for better visualization. Here boxes bound 25th and 75th percentiles, whiskers extend to 5th and 95th percentiles, 99th percentile is marked with a cross (x), and maximum values are represented by a minus sign (−). Additionally, mean values are marked with a hollow square, and median is represented by a horizontal line within each box.
Figure 5.
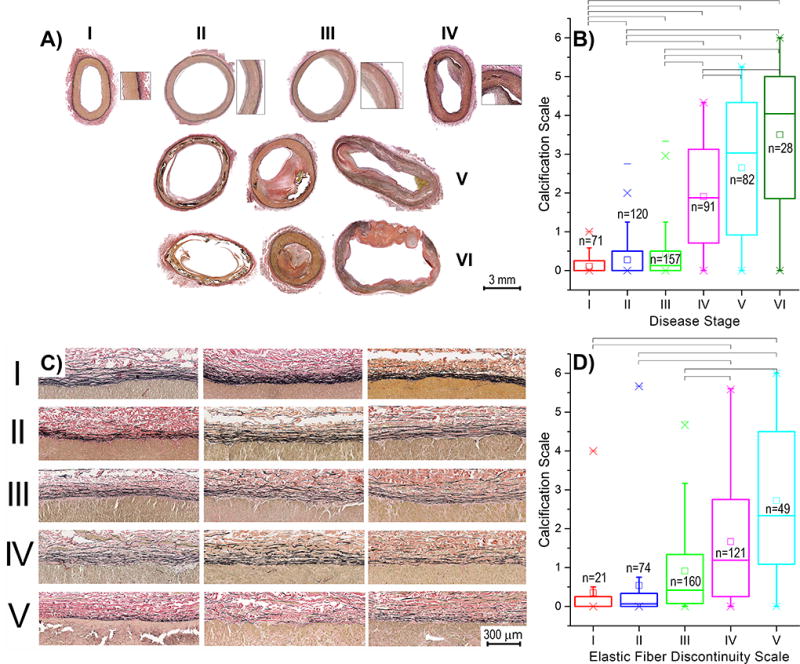
A) FPA disease stage classification and association between disease stage and calcification burden (B). Disease stages V and VI may include severe medial calcification, occlusive disease, or aneurysm13. C) Elastic fiber discontinuity scale developed using longitudinal sections of the FPAs, and association between elastic fiber discontinuity and calcification burden (D). Three samples in panel (C) are provided for each level of discontinuity to demonstrate variability. Longitudinal elastic fibers presented in these images are located in the external elastic lamina of the FPA. Brackets in panels (B) and (D) indicate statistically significant differences between groups obtained using post-hoc ANOVA. Boxes in panels B) and D) bound 25th and 75th percentiles, whiskers extend to 5th and 95th percentiles, 99th percentile is marked with a cross (x), and maximum values are represented by a minus sign (−). Additionally, mean values are marked with a hollow square, and median is represented by a horizontal line within each box.
Calcified arteries had thinner tunica media (r=−0.26, p < 0.01), and this result was statistically significant after controlling for age (r=−0.34, p < 0.01). Negative correlations with calcification burden were also observed for external elastic lamina thickness (r=−0.29, p < 0.01), and elastic fiber density (r=−0.20, p < 0.01), but only the former remained significant after controlling for age (r=−0.24, p < 0.01). Calcified arteries had more discontinuous elastic fibers (r=0.44, p < 0.01), and this result was not a consequence of aging (r=0.31, p < 0.01 after controlling for age). Elastic fiber discontinuity scale is presented in Figure 5C and associations between fiber discontinuity and calcification are plotted in Figure 5D. Note that elastic fiber discontinuity can only be quantified using longitudinal sections of the artery because elastic fibers in the FPA are oriented longitudinally. Non-parametric Levene’s test demonstrated equal variances between the groups (p=0.55), and Hochberg’s GT2 test was used to perform post-hoc analysis with statistically significant results marked by brackets in Figure 5D.
Effects on stiffness
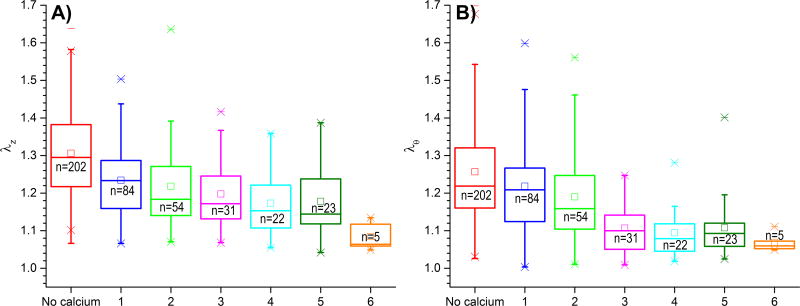
Calcification was associated with high arterial stiffness in both longitudinal (r=−0.42, p < 0.01) and circumferential (r=−0.44, p < 0.01) directions as demonstrated by decrease in longitudinal and circumferential stretches (Figure 6A and B, respectively). This effect was not a consequence of aging as results maintained their statistical significance after controlling for age (r=−0.20, r=−0.32, p < 0.01). Interestingly, though correlations for both directions were similar before controlling for age (r=−0.42 and r=−0.44), correlation in longitudinal direction became weaker (although still significant) after age was taken into account.
Figure 6.
Associations between calcification burden and FPA stretches λ at 100 kPa Cauchy stress level describing longitudinal (A, λz) and circumferential (B, λθ) compliance of the artery. Note that more calcification is associated with less compliance (higher stiffness) in both longitudinal and circumferential directions. Note that stretches are continuous variables separated into groups for better visualization. Here boxes bound 25th and 75th percentiles, whiskers extend to 5th and 95th percentiles, 99th percentile is marked with a cross (x), and maximum values are represented by a minus sign (−). Additionally, mean values are marked with a hollow square, and median is represented by a horizontal line within each box.
DISCUSSION
Calcification of the FPA is a relatively common condition13 that is known to increase the risk of reconstruction failure, amputation, and mortality in patients with PAD2. While correlations between vascular calcification and age, chronic kidney disease, and type II diabetes mellitus were previously reported14–16, the underlying mechanisms and prevalence of FPA calcification in general population are not clear due to lack of data from younger and healthier arteries and inability to longitudinally study this process in humans. Lack of consensus on the definition of calcification and classification of its burden11 further complicates research in this relatively unexplored area. At the same time, data from initial states of calcification can help better understand the etiology and pathophysiology of calcification, help define the contributing risk factors, and point to potential mechanisms that may be involved in this process. The goal of this study was to understand prevalence of FPA calcification in a large sample of 431 human subjects 13–82 years old, to determine contributions of risk factors to FPA calcification, and study effects of calcification on arterial stiffness.
Our data demonstrate that almost half (46%) of the FPAs in our sample had identifiable medial calcification. Older arteries were more calcified, but small calcium deposits were observed in arteries as young as 18 years old with no signs of intimal disease. In general, FPA calcification increased from 10–15% in subjects younger than 40 years old, to almost 50% in 41–60-year-old subjects, and over 60% in subjects older than 61 years. This percentage is significantly higher than the 25% overall prevalence found previously examining 143 femoral arteries from 14–59-year-old donors11 (average age 38±13 years), which may be attributed to both younger age, and lower prevalence of risk factors in previously analyzed sample compared to current data (DM 3% vs 25%, HTN 9% vs 51%, dyslipidemia 4% vs 29%). Our analysis also shows higher percentage of calcification than the 33% reported for subjects older than 45 years17,18 using CT, but differences are likely because calcification that can be detected with CT is already at a fairly advanced stage. Note too, that previously proposed peripheral calcification scale that is based on fluoroscopic evaluation of calcification length9 is also primarily targeted for relatively advanced calcification burdens compared to histological assessments.
Positive correlations were observed between calcification, diabetes, dyslipidemia, and body mass index. Interestingly, tobacco use demonstrated negative correlation after controlling for age. Latter in particular requires additional investigation as it does not yet have a clear explanation. Currently, there is no consensus in the literature regarding association of arterial calcification and risk factors. Kroger et al.8 did not find correlations between arterial calcification and Framingham risk factors (age, diabetes, systolic blood pressure, LDL-C, HDL-C, smoking, and renal function), but their subjects were 45–75 years old and an ABI of 1.3-1.5 was used as a surrogate for calcification19. Since crude ABI assessment is only able to detect advanced-stage disease, these results are likely applicable to only a subset of subjects with severe calcification burden. In a different study, Vasuri et al.11 have used arterial histology and subjects from a wider range of ages. While they also have not found correlations between calcification and any risk factors except hypertension, their sample size included only 36 arteries with histologically identified calcification.
Similar to our results, other studies reported correlations between risk factors and calcification, particularly between diabetes and chronic kidney disease14–16. While latter was not known for our study population, association between diabetes and calcification burden was strongest among all risk factors. Contribution of diabetes to arterial calcification has been demonstrated in both cell culture and animal models. In culture, high glucose was shown to increase the expression of osteogenic or chondrogenic markers, such as osteochondrogenic transcription factors Cbfa1 and bone morphogenic protein 2 by SMCs20–22. In mice, high glucose produced an inflammatory response associated with chronic metabolic, osmotic, and oxidative stresses23. These oxidative and hyperlipidemic signals were shown to induce the secretion of inflammatory mediators from macrophages24,25 and contribute to calcification.
In terms of morphometry, calcified arteries in our study were larger in diameter, but had smaller circumferential opening angles. Circumferential opening angle is an indicator of arterial remodeling26. Arteries adapt to the changing mechanical and biological conditions attempting to maintain homeostatic stress levels, which results in residual stresses that can be assessed by cutting the artery radially and measuring the resulting opening angle27–29. Altered ability of a calcified artery to accommodate the changing loading conditions through the process of growth, may stimulate attempts to strengthen (i.e. stiffen) the arterial wall through changing its mechanical properties, perhaps by inducing calcification. While this speculation requires further investigation, the effects of mechanical loading certainly have profound influence on arterial adaptation, and may trigger a range of vascular pathologies, which may also include calcification. These results are in agreement with findings of Lanzer et al.3 who suggested that ring-like calcifications probably interfere with positive arterial remodeling. They also agree with our recent findings that diabetic arteries (which are usually more calcified) do not increase their circumferential opening angle with age as opposed to their age-matched controls30.
While it is difficult to reconcile detailed descriptions of molecular mechanisms with coarse-grained mechanical quantities like stress, strain, and stiffness31, these characteristics nonetheless are informative to understand the consequences of these molecular changes. Our experimental data demonstrate that calcification was associated with higher stiffness of the FPA tissue in both longitudinal and circumferential directions. In our previous study we have demonstrated higher arterial stiffness in diabetic arteries, while present results show an association between diabetes and calcification. Elevated glucose levels play an important role in transforming SMCs into osteoblast-like cells32. PAD patients with DM are significantly more likely to present with arterial calcification than PAD patients without DM33. Our recent study demonstrated significant circumferential stiffening of diabetic arteries compared to age-matched controls30. We speculated that since SMCs that transform into osteoblast-like cells are oriented primarily circumferentially in the FPA12, glucose-induced calcification was likely to affect the artery in the circumferential direction as well – both in terms of stiffness and in terms of remodeling ability, i.e. the ability to change diameter and opening angle with age. Correlations between diabetes, calcification, and high circumferential stiffness, have indeed been reported here. Interestingly, in our previous study, diabetic arteries had longitudinal stiffness of the age-matched controls30, suggesting that intramural elastin was affected to a lesser degree than circumferential SMCs. Our current results also demonstrate that though correlation between calcification burden and longitudinal stiffness remained statistically significant after controlling for age, correlation for the circumferential direction was significantly stronger.
While the molecular cues underpinning the calcification process remain elusive3,34, multiple stimulators and inhibitors emanating from cells in the arterial wall and in the surrounding tissues, as well as the effects of extracellular matrix have been discovered that contribute to this process2. In the arterial wall, the SMC-rich media layer is thought to contain a sub-population of SMCs that in the setting of diminished inhibitors or increased stimulators of calcification are capable of losing their ability to express SMC-specific markers and express more osteogenic or chondrogenic markers, such as the osteochondrogenic transcription factors Cbfa1, Msx2, and Sox935,36. Interestingly, our results demonstrate that calcified arteries had thinner tunica media, which can be a consequence of phenotypical switch, or apoptosis that was shown to occur next to areas of recent calcification37. In addition, this compaction of the medial layer suggests a possible increase in intramural stresses acting on the cells, which may trigger mechanobiological responses such as increased expression of osteoptotegrin38, a member of the tumor necrosis factor ligand and receptor-signaling family, which has been shown to be increased in patients with arterial calcification2. Further research is obviously needed to explore these important mechanisms.
In the adventitia, a subset of cells expressing a bone morphogenic protein 2 (BMP-2)-Msx2 signaling program can also influence the phenotype of medial cells and may be responsible for inducing calcification2. In addition, collateral vessels that originate from the adventitia, may also allow the migration of multipotent adventitial myofibroblasts or pericytes than can differentiate into osteoblast-like cells through the effects of bone signaling proteins such as BMPs or osteoprotegrins2.
The extracellular matrix proteins, particularly elastin precursors and elastin degradation products, are also thought to play a significant role in arterial calcification39,40. Elastolytic matrix metalloproteinases (MMPs) are secreted by inflammatory cells in the media and adventitia, which leads to release of elastin degradation products that in turn act as chemokines to recruit other inflammatory cells that contribute to calcification41. In rats, administration of the antibiotic doxycycline which inhibits MMP activity, was shown to reduce vitamin D–induced and calcium chloride-induced arterial calcification42,43. Initiation and spread of calcification is therefore thought to occur along the elastic fibers44,45, ultimately leading to destruction of these fibers and spreading to the surrounding medial SMCs41. These findings agree with our results demonstrating correlation between calcification burden and elastic fiber discontinuity and thickness of the EEL. However, this mechanism also suggests that calcification should first occur at either internal or external elastic laminae yet this was not the case for a large number of younger FPAs that started developing calcification in the middle of tunica media, away from elastic fibers that appear intact and continuous. In addition, in a large number of samples calcification observed in young arteries was not associated with any signs of intimal thickening, which suggests that calcification may not be related to advanced atherosclerosis as previously thought46.
Like with any other study, results reported here should be independently validated and viewed in the context of study limitations. Risk factors we used were reported by the next of kin, and therefore may not contain perfectly accurate information. In addition, many risk factors, like diabetes and hypertension should be considered in terms of their duration, severity, and medication control rather than binary variables. Another limitation is related to the composition of our sample that primarily included Caucasian males. While we have not observed sizable effects of gender on FPA calcification, a number of studies have demonstrated effects of estrogen47 on SMC calcification, as well as ethnic differences in calcification presence and quantity18, which warrants further investigation. Use of histology, though significantly more precise for detecting early calcification than CTA or ABI, is limited to the choice of a particular arterial section. Use of multiple transverse and longitudinal sections for each subject reduced the chances of under or over-estimating the overall calcification burden for each artery, but this limitation must be taken into consideration when interpreting the results. In addition, use of Alizarin Red S stain as opposed to VVG, may allow for detection of even more subtle calcifications on these histological sections which may even further increase prevalence of FPA calcium in younger subjects. Finally, additional studies are required to determine the underlying pathophysiological pathways leading to formation and progression of FPA calcification. While these limitations are being addressed, presented work provides a basic understanding of the incidence of arterial calcification in human femoropopliteal arteries in a wide range of ages, defines risk factors that contribute to higher calcification burden, and determines associations between arterial calcium and arterial stiffness. This information can help formulate further studies targeting the underlying pathophysiological mechanisms.
Supplementary Material
HIGHLIGHTS.
Calcification of the femoropopliteal artery is common and can occur in young subjects that do not have intimal disease. Prevalence dramatically increases after 40 years of age.
More calcification is associated with older age, diabetes, dyslipidemia, and high body mass index. Tobacco use has negative association with calcification.
Calcified arteries are stiffer, larger in diameter, more diseased, and have more discontinuous elastic fibers.
Acknowledgments
Authors wish to acknowledge the Nebraska Organ Recovery System (NORS), and the Charles and Mary Heider Fund for Excellence in Vascular Surgery for their help and support.
Sources of Funding: Research reported in this publication was supported in part by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01 HL125736.
Footnotes
Disclosures: Authors declare that they have no conflict of interest in relation to this submission.
References
- 1.Lehto S, Niskanen L, Suhonen M, Rönnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 1996;16(8):978–83. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 2.Guzman RJ. Clinical, cellular, and molecular aspects of arterial calcification. J. Vasc. Surg. 2007;45(6 SUPPL):57–63. doi: 10.1016/j.jvs.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanzer P, Boehm M, Sorribas V, et al. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014;35(23):1515–1525. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Mönckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100(21):2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 5.Block GA, Spiegel DM, Ehrlich J, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 6.Budoff MJ, Yu D, Nasir K, et al. Diabetes and progression of coronary calcium under the influence of statin therapy. Am. Heart J. 2005;149(4):695–700. doi: 10.1016/j.ahj.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 7.Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification: Lessons learned from the aorta. Arterioscler. Thromb. Vasc. Biol. 2006;26(7):1423–1430. doi: 10.1161/01.ATV.0000220441.42041.20. [DOI] [PubMed] [Google Scholar]
- 8.Kröger K, Stang A, Kondratieva J, et al. Prevalence of peripheral arterial disease - Results of the Heinz Nixdorf Recall Study. Eur. J. Epidemiol. 2006;21(4):279–285. doi: 10.1007/s10654-006-0015-9. [DOI] [PubMed] [Google Scholar]
- 9.Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: Prevalence, mechanism, detection, and clinical implications. Catheter. Cardiovasc. Interv. 2014;83(6):212–220. doi: 10.1002/ccd.25387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C-L, Wu I-H, Wu Y-W, et al. Association of lower extremity arterial calcification with amputation and mortality in patients with symptomatic peripheral artery disease. PLoS One. 2014;9(2):e90201. doi: 10.1371/journal.pone.0090201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasuri F, Fittipaldi S, Pacilli A, Buzzi M, Pasquinelli G. The incidence and morphology of Monckeberg’s medial calcification in banked vascular segments from a monocentric donor population. Cell Tissue Bank. 2016;17(2):219–223. doi: 10.1007/s10561-016-9543-z. [DOI] [PubMed] [Google Scholar]
- 12.Kamenskiy A, Seas A, Deegan P, et al. Constitutive description of human femoropopliteal artery aging. Biomech. Model. Mechanobiol. 2017;16(2):681–692. doi: 10.1007/s10237-016-0845-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamenskiy A, Seas A, Bowen G, et al. In situ longitudinal pre-stretch in the human femoropopliteal artery. Acta Biomater. 2016;32:231–237. doi: 10.1016/j.actbio.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ. Res. 2004;95(6):560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 15.Shao J-S, Cheng S-L, Sadhu J, Towler DA. Inflammation and the Osteogenic Regulation of Vascular Calcification: A Review and Perspective. Hypertension. 2010;55(3):579–592. doi: 10.1161/HYPERTENSIONAHA.109.134205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deas DSJ, Marshall AP, Bian A, Shintani A, Guzman RJ. Association of cardiovascular and biochemical risk factors with tibial artery calcification. Vasc. Med. 2015;20(4):326–331. doi: 10.1177/1358863X15581448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jain T, Peshock R, McGuire DK, et al. African Americans and Caucasians have a similar prevalence of coronary calcium in the Dallas Heart Study. J. Am. Coll. Cardiol. 2004;44(5):1011–1017. doi: 10.1016/j.jacc.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: The Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111(10):1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 19.Hiatt WR, Hoag S, Hamman RF. Effect of Diagnostic Criteria on the Prevalence of Peripheral Arterial Disease: The San Luis Valley Diabetes Study. Circulation. 1995;91(5):1472–1479. doi: 10.1161/01.cir.91.5.1472. [DOI] [PubMed] [Google Scholar]
- 20.Shioi A, Nishizawa Y, Jono S, Koyama H, Hosoi M, Morii H. β-Glycerophosphate Accelerates Calcification in Cultured Bovine Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 1995;15(11) doi: 10.1161/01.atv.15.11.2003. [DOI] [PubMed] [Google Scholar]
- 21.Chen NX, Duan D, O’Neill KD, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol. Dial. Transplant. 2006;21(12):3435–3442. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]
- 22.Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000;87(7):E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 23.Towler DA, Bidder M, Latifi T, Coleman T, Semenkovich CF. Diet-induced diabetes activates an osteogenic gene regulatory program in the aortas of low density lipoprotein receptor-deficient mice. J. Biol. Chem. 1998;273(46):30427–30434. doi: 10.1074/jbc.273.46.30427. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Zalewski A, Liu Y, et al. Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation. 2003;108(4):472–478. doi: 10.1161/01.CIR.0000080378.96063.23. [DOI] [PubMed] [Google Scholar]
- 25.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105(5):650–5. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 26.Humphrey JD. Cardiovascular Solid Mechanics: Cells, Tissues, and Organs. Springer; 2002. [Google Scholar]
- 27.Kamenskiy AV, Pipinos II, Dzenis Ya, et al. Effects of age on the physiological and mechanical characteristics of human femoropopliteal arteries. Acta Biomater. 2015;11:304–313. doi: 10.1016/j.actbio.2014.09.050. [DOI] [PubMed] [Google Scholar]
- 28.Humphrey JD, Eberth JF, Dye WW, Gleason RL. Fundamental role of axial stress in compensatory adaptations by arteries. J. Biomech. 2009;42(1):1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphrey JD. Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 2008;50(2):53–78. doi: 10.1007/s12013-007-9002-3. [DOI] [PubMed] [Google Scholar]
- 30.Desyatova A, MacTaggart J, Kamenskiy A. Constitutive Modeling of Human Femoropopliteal Artery Biaxial Stiffening Due to Aging and Diabetes. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.09.042. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphrey JD, Dufresne ER, Schwartz M a. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tavridou A, Petridis I, Vasileiadis M, et al. Association of VKORC1 -1639 G>A polymorphism with carotid intima-media thickness in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011;94(2):236–241. doi: 10.1016/j.diabres.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Jude EB, Oyibo SO, Chalmers N, Boulton aJ. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24(8):1433–1437. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- 34.Avogaro A, Fadini GP. Mechanisms of ectopic calcification: implications for diabetic vasculopathy. Cardiovasc. Diagn. Ther. 2015;5(5):343–52. doi: 10.3978/j.issn.2223-3652.2015.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tintut Y, Parhami F, Boström K, Jackson SM, Demer LL. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J. Biol. Chem. 1998;273(13):7547–7553. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 36.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler. Thromb. Vasc. Biol. 2003;23(3):489–494. doi: 10.1161/01.ATV.0000059406.92165.31. [DOI] [PubMed] [Google Scholar]
- 37.Schoppet M, Al-Fakhri N, Franke FE, et al. Localization of osteoprotegerin, tumor necrosis factor-related apoptosis-inducing ligand, and receptor activator of nuclear factor-kappaB ligand in Mönckeberg’s sclerosis and atherosclerosis. J. Clin. Endocrinol. Metab. 2004;89(8):4104–4112. doi: 10.1210/jc.2003-031432. [DOI] [PubMed] [Google Scholar]
- 38.Yu HC, Wu TC, Chen MR, Liu SW, Chen JH, Lin KMC. Mechanical stretching induces osteoprotegerin in differentiating C2C12 precursor cells through noncanonical Wnt pathways. J. Bone Miner. Res. 2010;25(5):1128–1137. doi: 10.1002/jbmr.9. [DOI] [PubMed] [Google Scholar]
- 39.Fischer JW, Steitz SA, Johnson PY, et al. Decorin promotes aortic smooth muscle cell calcification and colocalizes to calcified regions in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2004;24(12):2391–2396. doi: 10.1161/01.ATV.0000147029.63303.28. [DOI] [PubMed] [Google Scholar]
- 40.Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-β1 induce osteogenic responses in smooth muscle cells. Biochem. Biophys. Res. Commun. 2005;334(2):524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 41.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: Role of matrix metalloproteinases. Circulation. 2004;110(22):3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler. Thromb. Vasc. Biol. 2000;20(2):317–27. doi: 10.1161/01.atv.20.2.317. [DOI] [PubMed] [Google Scholar]
- 43.Qin X, Corriere MA, Matrisian LM, Guzman RJ. Matrix metalloproteinase inhibition attenuates aortic calcification. Arterioscler. Thromb. Vasc. Biol. 2006;26(7):1510–1516. doi: 10.1161/01.ATV.0000225807.76419.a7. [DOI] [PubMed] [Google Scholar]
- 44.Tanimura A, McGregor DH, Anderson HC. Matrix vesicles in atherosclerotic calcification. Proc. Soc. Exp. Biol. Med. 1983;172(2):173–7. doi: 10.3181/00379727-172-41542. [DOI] [PubMed] [Google Scholar]
- 45.Bobryshev Y V. Calcification of elastic fibers in human atherosclerotic plaque. Atherosclerosis. 2005;180(2):293–303. doi: 10.1016/j.atherosclerosis.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 46.McCullough P a, Agrawal V, Danielewicz E, Abela GS. Accelerated atherosclerotic calcification and mönckeberg’s sclerosis: A continuum of advanced vascular pathology in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008;3(6):1585–1598. doi: 10.2215/CJN.01930408. [DOI] [PubMed] [Google Scholar]
- 47.Balica M, Boström K, Shin V, Tillisch K, Demer LL. Calcifying Subpopulation of Bovine Aortic Smooth Muscle Cells Is Responsive to 17β-Estradiol. Circulation. 1997;95(7) doi: 10.1161/01.cir.95.7.1954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.