Abstract
Oxidative stress has been implicated in a number of neurodegenerative diseases spanning various fields of research. Reactive oxygen species can be beneficial or harmful, depending on their concentration. High levels of reactive oxygen species can lead to oxidative stress, which is an imbalance between free radicals and antioxidants. Increased oxidative stress can result in cell loss. Interestingly, sex differences have been observed in oxidative stress generation, which may underlie sex differences observed in neurodegenerative disorders. An enhanced knowledge of the role of sex hormones on oxidative stress signaling and cell loss can yield valuable information, leading to sex-based mechanistic approaches to neurodegeneration.
Keywords: Alzheimer’s disease, Parkinson’s disease, testosterone, estrogen, menopause, aging
Oxidative Stress Generation
Oxygen is required by many living organisms for survival. Inefficient oxygen metabolism can be damaging and lead to oxidative stress [1]. Oxidative stress occurs when a biological system is overwhelmed by reactive oxygen species (ROS) due to its inability to counteract these free radicals [2]. Free radicals include peroxides, superoxides, hydroxyl radicals, and singlet oxygen [3, 4]. They are extremely unstable, reacting quickly with many biological products. Under normal homeostatic conditions, ROS plays a role in immunity, homeostasis, and signal transduction pathways [5, 6]. However, when antioxidants are inadequate to balance free radicals and ROS, oxidative stress occurs. These free radicals can trigger lipid peroxidation reactions [7], as well oxidative stress DNA mutations [8]. As the imbalance continues, oxidative stress can induce apoptosis, programmed cell death [9, 10].
Sex differences in oxidative stress have been observed in numerous basic and clinical studies, wherein males exhibit higher oxidative stress than females [11–17]. Interestingly, sex differences have been observed in the enzyme NADPH oxidase (NOX) [11, 13, 18–20], which is a major oxidative stress generator in cells [21]. NOX is a multi-subunit enzyme, consisting of membrane bound catalytic subunits and cytosolic regulatory subunits. The catalytic subunits are gp91phox and p22phox, while the regulatory subunits are p40phox, p47phox, p67phox, and Rac. The regulatory complex translocates to the membrane upon p47phox phosphorylation, leading to subsequent activation of the enzyme [22]. The activated NOX complex catalyzes the transfer of electrons from NADPH to an oxygen molecule, resulting in the formation of reactive intermediates [23]. In different cell types, increased NOX has been observed in males compared to females. For example, using mesenteric arterioles microvessels, Dantas et.al found increased NOX subunits (p47phox, p22phox, p67phox, and gp91phox) in young adult male rats compared to females [11]. Similarly, young healthy male rats have increased oxidative stress and NOX expression in aortic and cerebral arterial cells compared to young healthy female rats [19, 20].
Oxidative stress can be influenced by homocysteine. Homocysteine, a non-protein α-amino acid, is an indicator of low folate and B-12 status [24], and has been used as a marker for oxidative stress [25, 26]. Studies have found homocysteine can increase NOX-mediated superoxide production, and this increase in superoxide production can be blocked by the NOX inhibitor, apocynin [18]. Similar to NOX, sex differences in homocysteine levels have been observed. Homocysteine levels are higher in men than women [14, 26–32]. Sex hormones can also influence homocysteine levels. In young healthy transsexuals homocysteine levels were influenced sex hormones. Specifically, male to female transsexuals receiving estrogen hormone therapy experienced decreased homocysteine, whereas female to male transsexuals receiving androgen hormone therapy had increased homocysteine levels [33]. These studies indicate sex hormones may underlie the observed sex differences in oxidative stress generation.
Although males generally have higher oxidative stress than females, oxidative stress is not always damaging. ROS can play a role in homeostasis, such as preconditioning. Preconditioning is a protective process, wherein exposure to a small insult allows the cells to better withstand a subsequent larger insult. This process has been observed in several types of cells (e.g. astrocytes, neurons, fibroblasts, muscle) [34–40]. In our basic science studies, physiological levels of testosterone can increase oxidative stress and be neuroprotective by preconditioning the cell against damage from subsequent exposures to oxidative stress [41, 42]. However, there appears to be a limit in testosterone’s preconditioning capabilities. If oxidative stress is too high, then testosterone can be damaging.
Oxidative Stress and Aging
Oxidative stress has been linked with aging [43, 44]. Indeed, one of the major theories of aging is the Free Radical Theory of Aging. This theory proposes oxidative damage to cells, due to a buildup of free radicals in a biological system over a time period, results in aging and aging-associated diseases [45–47]. Sex differences in oxidative stress persist and worsen with aging in both animal and clinical samples, wherein males continue to have higher oxidative stress than females [48–50]. Aging, specifically menopause, plays a significant role in oxidative stress status in females. Menopause is a period characterized by a dramatic decline of estrogens, which normally occurs around 50–52 years of age in Caucasian women and 48–50 years of age in African-American and Hispanic women [51–57].
As expected, homocysteine increases with age [14, 26–32, 58], indicating elevated oxidative stress [25, 26]. Homocysteine levels further increase with menopause [59–63]. An elegant study by Hak et.al. used aged-matched post- and pre-menopausal women (46–55 years of age) and found homocysteine levels were significantly elevated after menopause [64]. Although other studies have not find this association [65, 66], it could be due a lack of adjusting for age. Similar findings have been observed in animal models, in which downstream targets of homocysteine, such as NOX [18], increased with aging, in general, and in female rats that have undergone ovariectomy, an experimental animal model for menopause [67, 68].
Our laboratory examined the role of aging and homocysteine levels using plasma samples from healthy Caucasian men (n = 700) and women (n = 1,061) over the age of 50 from the Texas Alzheimer’s Research Care and Consortium (TARCC) funded by the state of Texas (Table 1). Our data showed homocysteine, used as a marker for oxidative stress, significantly increased with age in both men and women. No differences in homocysteine levels were found between the men and women (Figure 1), which is consistent with prior studies indicating menopause increases homocysteine levels in healthy women to levels observed in healthy men [59–63]. Although hormone manipulations were not examined in the TARCC cohort, estrogen hormone replacement can decrease homocysteine levels in post-menopausal women [69–72]. Interestingly, estrogen hormone replacement therapy in post-menopausal women is associated with decreased testosterone, along with the expected increased estradiol [71, 73, 74]. Generally, menopause is associated with the loss of estrogen, but testosterone levels are maintained [75–78]. The effects of testosterone on menopause are understudied. Since estrogen hormone replacement therapy decreased testosterone levels in post-menopausal women and our prior studies showed that testosterone increased oxidative stress [41, 42], it is possible testosterone may mediate the elevation of homocysteine in post-menopausal women.
TABLE 1.
Sample population characteristics. Plasma samples were obtained from Caucasian men and women enrolled in the Texas Alzheimer’s Research Care and Consortium (TARCC). Cognitively intact controls performed within normal limits on all cognitive testing. AD patients met consensus-based diagnosis for probable AD based on NINCDS-ADRDA criteria [182]. Institutional Review Board approval was obtained at each TARCC site and written informed consent was obtained from participants and/or caregivers.
| Men | Women | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | N | Mean | St. Dev. | N | Mean | St. Dev. |
| Age (years) | 700 | 72.71 | 8.93 | 1061 | 72.21 | 9.74 |
| min. age | max. age | min. age | max. age | |||
| 51.00 | 94.00 | 50.00 | 102.00 | |||
|
|
||||||
| N | % | N | % | |||
| hyperlipidemia | 442 | 63.14 | 578 | 54.48 | ||
| hypertension | 442 | 63.14 | 637 | 60.04 | ||
| obese | 149 | 21.29 | 264 | 24.88 | ||
| Alzheimer’s disease | 405 | 57.86 | 516 | 48.63 | ||
| cognitively intact | 295 | 42.14 | 545 | 51.37 | ||
| < high school diploma | 82 | 11.71 | 168 | 15.83 | ||
| high school diploma | 98 | 14.00 | 290 | 27.33 | ||
| ≤ 4 yrs. college | 315 | 45.00 | 432 | 40.72 | ||
| > college | 205 | 29.29 | 171 | 16.12 | ||
Figure 1.
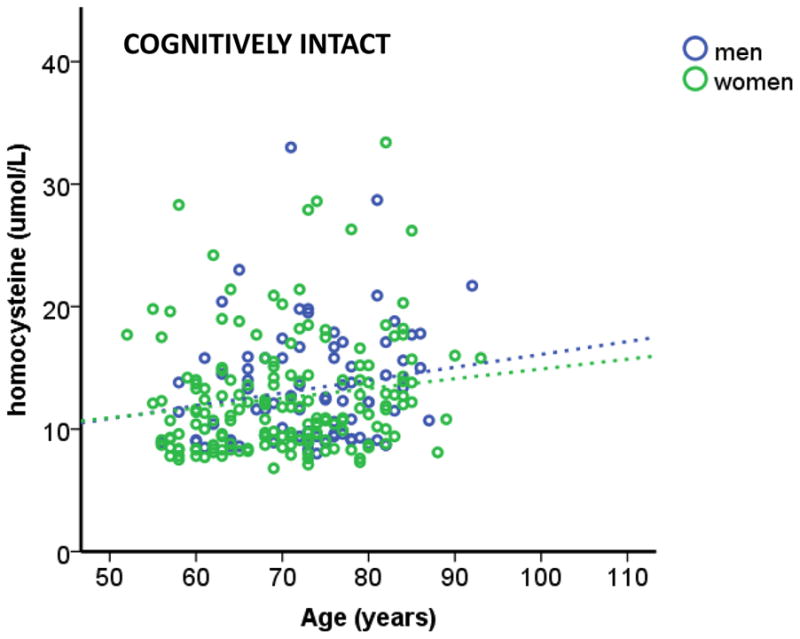
Homocysteine levels (oxidative stress) increased with age in cognitively intact participants, regardless of sex. A significant regression equation was found (F (2, 248) = 3.821, p < 0.05), with an R2 of 0.030. TARCC participants’ homocysteine levels are equal to 7.455 + 0.086 (age) − 0.495 (sex), where age is measured in years and sex is coded as men and women. Cognitively intact participants’ homocysteine levels increased 0.086 umol/L for every year and men had higher (0.495 umol/L) homocysteine (non-significant) than women. Only age was a significant predictor of homocysteine levels. Specific methods for sample collection are available in our prior publication [26]. Serum total homocysteine was assayed in the Atherosclerosis Clinical Research Laboratory at Baylor College of Medicine.
In 2001, approximately 40% of post-menopausal women were on some form of hormone replacement therapy [79]. Since there were not any conclusive trial data about the risks and benefits of hormone replacement therapy in post-menopausal women, the National Institutes Health (NIH) sponsored a large randomized, placebo controlled, double blind clinical trial called the Women’s Health Initiative (WHI). Participants were post-menopausal women between the age of 50–79 years old at intake. This trial consisted of two arms: estrogen only replacement for post-menopausal women with a prior hysterectomy (n = 10,739) and estrogen + progesterone replacement for post-menopausal women with intact uteri (n = 16,608). Outcomes examined included stroke, venous thromboembolism, cancer, osteoporosis, and coronary heart disease [80]. Results from both arms found no protective effects of either estrogen only or estrogen + progesterone hormone replacement therapy, whereas adverse effects on cardiovascular disease (e.g. pulmonary embolism, deep vein thrombosis, and ischemic stroke) were observed [81]. Due to these negative effects, the WHI study was terminated early [82]. Based on these results, use of hormone replacement therapy in post-menopausal women drastically declined [83].
Within the past few years, data from the WHI study have been reassessed. Researchers found 83% of the WHI participants were women several years from menopause. Furthermore, the average of age of participants was 63 years old, which is 12–13 years past menopause [84]. When the study outcomes were stratified by age of the participants, the results showed decreased cardiovascular risk and total mortality in younger women with hormone replacement therapy compared to the older women [85, 86]. No evidence of increased stroke was found in women 50–59 years of age in the estrogen only hormone replacement therapy group [87]. Thus, both a women’s age and years from menopause are important factors for determining the impact of hormone replacement therapy. These factors are used to determine the “window of opportunity” for hormone replacement therapy in women, wherein the benefit/risk ratio is more protective in women less than 10 years from menopause [88].
Oxidative stress has been proposed as one mechanism underlying the “window of opportunity” for hormone replacement therapy. Several studies found estrogen can decrease oxidative stress [89–91]. However, estrogen’s protective effects were found to be conditional [90, 92]. Based on these conditional effects of estrogen, Dr. Roberta Brinton coined the term “Healthy Cell Bias of Estrogen Action.” Estrogen exposure to healthy cells results in estrogen being protective against subsequent insults, such as oxidative stress. However, this protective effect is not observed with estrogen exposure in unhealthy cells [90]. Indeed, we have found similar effects with testosterone in our in vitro studies [41], indicating the “Healthy Cell Bias” theory applies to all sex hormones in oxidative stress environments. Similarly, clinical studies examining the impact of testosterone replacement therapy found conditional effects of testosterone, in which testosterone replacement therapy was associated with adverse effects in aged men (mean age is 74 years old) with chronic diseases [93, 94].
Oxidative Stress and Neurodegenerative Diseases
Oxidative stress has been implicated in several age-associated neurodegenerative diseases, including Alzheimer’s disease [95], Parkinson’s disease [96], and non-neurodegenerative diseases (e.g. cancers, sickle cell disease, cardiovascular diseases, and diabetes) [97–100]. Age is one of the greatest risk factors for both Alzheimer’s and Parkinson’s diseases. Furthermore, oxidative stress is a key feature in these progressive neurodegenerative disorders [96, 101]. Increased oxidative stress has been shown to be involved in cell loss in key brain regions (e.g. substantia nigra, cortex, and hippocampus) involved in the clinical manifestations of Alzheimer’s and Parkinson’s diseases [96, 102]. Interestingly, sex differences have been observed in both disorders.
Sex Differences in Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, which affects millions of people universally. It has been recorded as the second most common neurological disease [103]. One characteristic feature of PD is neuronal death in the substantia nigra of the brain, specifically dopaminergic neurons. Mechanisms underlying this cell death include oxidative stress and inflammation [41, 104]. This results in the established symptoms of PD relating to the motor system, which include tremor, rigidity, bradykinesia and postural instability [105]. Generally, these symptoms manifest when 80% of the dopaminergic cells within the substantia nigra are lost [106]. The etiology of PD still remains elusive [107]. Aging is one of the principal risk factors for the development of idiopathic Parkinson’s disease [108]. Along with aging, sex-related differences in PD have also been recognized [109]. Therefore, it is probable sex hormones play a vital role in this phenomenon, especially as men are 1.5–2 times more at risk of developing PD than women [110–112].
Several studies propose estrogen underlies this sex bias in PD. Women displayed a less severe PD phenotype than men at presentation. Indeed, studies found PD symptoms worsen for pre-menopausal women during menstruation, when estrogen levels are low [113]. Unsurprisingly, severity of PD increases in post-menopausal women compared to pre-menopausal women, due to the loss of estrogen during menopause. Clinical studies found estrogen hormone replacement can diminish the severity of early PD manifestations [114–116]. Therefore, estrogen was proposed to be neuroprotective for dopaminergic neurons in the substantia nigra, and can help mitigate PD progression [117–119].
Contrary to these reports, some studies were unable to find estrogen neuroprotection [120, 121], indicating another mechanism may be mediating this sex difference in PD. One possibility could be testosterone. Currently, the role of testosterone in neurodegeneration is understudied. Few studies have examined the impact of testosterone on PD, compared to studies on estrogen protection. Only one clinical study has been conducted on aged men with PD and treated with L-DOPA, and the results showed testosterone replacement therapy did not impact motor or non-motor PD features [122]. Further, this group observed no interactions between PD medications and testosterone levels [123]. Although this is an understudied area, basic science studies have yielded more information. Increased oxidative stress, via NOX, in the substantia nigral dopaminergic neurons has been reported in male rats compared to female rats [124]. Studies from our lab found testosterone is an oxidative stressor in dopaminergic neurons, and its actions may be involved in this oxidative stress sex difference [41, 42]. In other studies using a 6-OHDA rat model, we observed testosterone can exacerbate oxidative stress damage, resulting in motor impairments [125]. It is possible testosterone may play a role in the increased PD incidence in post-menopausal women compared to pre-menopausal women [112], especially as post-menopausal women are more androgenic than estrogenic [126, 127]. Further research needs to be conducted on testosterone and PD.
Sex Differences in Alzheimer’s disease
Oxidative stress plays a key role in the pathogenesis of Alzheimer’s disease (AD) [128–130]. Indeed, increased NOX activity has been linked with AD progression and individuals converting from cognitively intact to dementia status [130–132]. Furthermore, associations between homocysteine and AD have been reported. Elevated homocysteine has been shown to contribute to dementia and AD progression [133–141]. Homocysteine can increase oxidative stress and cell loss in the hippocampus (one of the major brain regions affected in AD) [142]. AD risk is doubled in patients that have greater than 14 umol/L homocysteine, and thus homocysteine has been indicated as a potential AD risk factor [134, 140].
Sex differences have been reported in AD, wherein AD disproportionally affects women more than men in both prevalence and severity [121]. Based on this sex difference, several studies have examined the influence of estrogen on AD. In both in vivo and in vitro models estrogen protected cells from AD-associated insults [143–149], such as β-amyloid and APP oxidative stress insults [150–152]. Furthermore, estrogen had a positive impact on cognition in surgically menopausal women [153, 154] and post-menopausal women [155–158]. Supporting the role of estrogen in neuroprotection, AD risk increases in post-menopausal women [152]. These studies indicate estrogen can act as a neuroprotectant in AD.
Interestingly, estrogen hormone replacement therapy on cognition in post-menopausal women is equivocal. Subsequent studies based on the WHI included the Women’s Health Initiative Memory Study (WHIMS). The effects of hormone replacement therapy on cognition in post-menopausal women were assessed. Unlike the WHI study, the WHIMS study participants were at least 65 years old [92, 159]. Initial results indicated that hormone therapy had a negative impact on cognition. Specifically, estrogen + progesterone was linked with increased dementia and decreased verbal memory [92, 159–161]. Decline in verbal memory is one of the earliest predictors of AD [162, 163]. However, subsequent clinical studies that used peri-menopausal women, instead of post-menopausal women, found estrogen hormone therapy decreased dementia and AD risk [164, 165]. These studies indicate the beneficial effects of estrogen are conditional and may be biased toward protection of healthy cells [90].
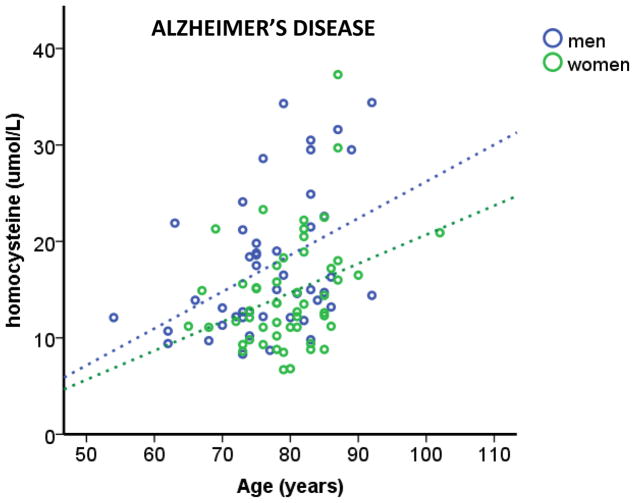
Similar to estrogen, studies have indicated androgens can have protective and negative effects on AD. However, it appears testosterone effects are dependent on the cellular environment. One such variable that can result in androgens negatively impacting cells is oxidative stress [41, 166]. Using plasma samples from TARCC participants diagnosed with AD, we found oxidative stress increased with age in both men and women (Figure 2), consistent with other studies [167]. Interestingly, we observed men with AD have higher levels of homocysteine, used as a marker for oxidative stress, than women with AD (Figure 2). In this cohort, hypertension and hyperlipidemia were more prevalent in men with AD than women with AD compared to cognitively intact men and women, respectively. Specifically, 61% (chi-squared p < 0.05) of men with AD had hyperlipidemia, unlike women with AD (50%; chi-squared p = 0.967). In addition, 58% (chi-squared p < 0.05) of men with AD had hypertension, whereas hypertension was present in 51% (chi-squared p = 0.579) of women with AD. Both hypertension and hyperlipidemia can increase oxidative stress [11, 168–171], which may increase the oxidative load enough to switch testosterone from a protective hormone to a damaging hormone. Indeed, our prior studies using the TARCC cohort showed endogenous testosterone levels were only associated with cognitive impairment under high oxidative stress (homocysteine levels >12 μmol/L) [172]. This effect of testosterone on cognition was lost when the cohort was not stratified based on oxidative stress, similar to findings in a recent study showing no effects of testosterone replacement therapy on age-associated memory impairment. This study by Resnick et.al. was a large, multi-site, clinical study, in which participants were men over 65 years of age (n = 788) and exposed to testosterone replacement therapy for one year. Regardless if the men were cognitively intact or impaired prior to hormone replacement therapy, no effects of testosterone were found [173]. No measures of oxidative stress were assayed in this study. Interestingly, majority of the participants in this study were on antihypertensives and phosphodiesterase inhibitors, which decrease oxidative stress [174–180]. Therefore, it is quite plausible testosterone replacement therapy affects cognition in men with elevated levels of oxidative stress.
Figure 2.
Homocysteine levels increased with age in both men and women with AD, with men have significantly higher homocysteine levels (oxidative stress) than women. A linear regression was calculated to homocysteine levels based on age and sex. A significant regression equation was found (F (2, 95) = 11.220, p < 0.05), with an R2 of 0.191. TARCC participants’ homocysteine levels are equal to -5.525 + 0.348 (age) − 3.788 (sex), where age is measured in years and sex is coded as men and women. AD participants’ homocysteine levels increased 0.348 umol/L for every year and men had higher (3.788 umol/L) homocysteine than women. Both age and sex were significant predictors of homocysteine levels.
Conclusion
Sex differences have been observed in oxidative stress and its related diseases. Estrogen and testosterone have been reported to contribute to sex differences in neurodegenerative diseases [181]. Elucidating sex hormone pathways in neurons may provide therapeutic targets to slow down the progression of neurodegenerative disorders by providing sex-based mechanistic approaches. Not only do studies indicate that sex is an important variable in oxidative stress and neurodegeneration, oxidative stress may be a key factor in determining how sex hormones impact neuronal function as either a neuroprotective or neurodamaging agent.
Acknowledgments
This study was supported by the Texas Alzheimer’s Research and Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer’s Disease and Related Disorders. Research was supported by the National Institutes of Health (NIH) R01 NS088514 to RLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wood PM. The potential diagram for oxygen at pH 7. The Biochemical journal. 1988;253(1):287–9. doi: 10.1042/bj2530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative Stress in Atherosclerosis. Current atherosclerosis reports. 2017;19(11):42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM, Woodman RC. Chronic granulomatous disease. Seminars in hematology. 1990;27(3):247–59. [PubMed] [Google Scholar]
- 4.Hayyan M, Hashim MA, AlNashef IM. Superoxide Ion: Generation and Chemical Implications. Chemical reviews. 2016;116(5):3029–85. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- 5.Franchini AM, Hunt D, Melendez JA, Drake JR. FcgammaR-driven release of IL-6 by macrophages requires NOX2-dependent production of reactive oxygen species. The Journal of biological chemistry. 2013;288(35):25098–108. doi: 10.1074/jbc.M113.474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free radical biology & medicine. 1997;22(1–2):269–85. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 7.Das UN. Essential fatty acids, lipid peroxidation and apoptosis, Prostaglandins, leukotrienes, and essential fatty acids. 1999;61(3):157–63. doi: 10.1054/plef.1999.0085. [DOI] [PubMed] [Google Scholar]
- 8.Kujoth GC, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations and apoptosis in mammalian aging. Cancer research. 2006;66(15):7386–9. doi: 10.1158/0008-5472.CAN-05-4670. [DOI] [PubMed] [Google Scholar]
- 9.Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annual review of cell and developmental biology. 1999;15:269–90. doi: 10.1146/annurev.cellbio.15.1.269. [DOI] [PubMed] [Google Scholar]
- 10.Samhan-Arias AK, Martin-Romero FJ, Gutierrez-Merino C. Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosis. Free radical biology & medicine. 2004;37(1):48–61. doi: 10.1016/j.freeradbiomed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Dantas AP, do Franco MC, Silva-Antonialli MM, Tostes RC, Fortes ZB, Nigro D, Carvalho MH. Gender differences in superoxide generation in microvessels of hypertensive rats: role of NAD(P)H-oxidase. Cardiovasc Res. 2004;61(1):22–9. doi: 10.1016/j.cardiores.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Jung O, Schreiber JG, Geiger H, Pedrazzini T, Busse R, Brandes RP. gp91phox-containing NADPH oxidase mediates endothelial dysfunction in renovascular hypertension. Circulation. 2004;109(14):1795–801. doi: 10.1161/01.CIR.0000124223.00113.A4. [DOI] [PubMed] [Google Scholar]
- 13.Wong PS, Randall MD, Roberts RE. Sex differences in the role of NADPH oxidases in endothelium-dependent vasorelaxation in porcine isolated coronary arteries. Vascul Pharmacol. 2015;72:83–92. doi: 10.1016/j.vph.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Pitla S, Nagalla B. Gender-related differences in the relationship between plasma homocysteine, anthropometric and conventional biochemical coronary heart disease risk factors in middle-aged Indians. Ann Nutr Metab. 2009;54(1):1–6. doi: 10.1159/000199452. [DOI] [PubMed] [Google Scholar]
- 15.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, Tamai H, Takeshita A. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22(3):438–42. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 16.Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34(5):546–52. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 17.Khalifa AR, Abdel-Rahman EA, Mahmoud AM, Ali MH, Noureldin M, Saber SH, Mohsen M, Ali SS. Sex-specific differences in mitochondria biogenesis, morphology, respiratory function, and ROS homeostasis in young mouse heart and brain. Physiol Rep. 2017;5(6) doi: 10.14814/phy2.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edirimanne VE, Woo CW, Siow YL, Pierce GN, Xie JY, KO Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can J Physiol Pharmacol. 2007;85(12):1236–47. doi: 10.1139/Y07-112. [DOI] [PubMed] [Google Scholar]
- 19.Miller AA, Drummond GR, Mast AE, Schmidt HH, Sobey CG. Effect of gender on NADPH-oxidase activity, expression, and function in the cerebral circulation: role of estrogen. Stroke. 2007;38(7):2142–9. doi: 10.1161/STROKEAHA.106.477406. [DOI] [PubMed] [Google Scholar]
- 20.Brandes RP, Mugge A. Gender differences in the generation of superoxide anions in the rat aorta. Life Sci. 1997;60(6):391–6. doi: 10.1016/s0024-3205(96)00663-7. [DOI] [PubMed] [Google Scholar]
- 21.Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nature Reviews Drug Discovery. 2011;10(6):453–71. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Babior BM. NADPH Oxidase: An Update. Blood. 1999;93(5):1464. [PubMed] [Google Scholar]
- 23.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochimica et biophysica acta. 2004;1657(1):1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, Scott JM. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50(1):3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 25.Marlatt MW, Lucassen PJ, Perry G, Smith MA, Zhu X. Alzheimer’s disease: cerebrovascular dysfunction, oxidative stress, and advanced clinical therapies. J Alzheimers Dis. 2008;15(2):199–210. doi: 10.3233/jad-2008-15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham RL, Singh M, O’Bryant SE, Hall JR, Barber RC. Oxidative Stress, Testosterone, and Cognition among Caucasian and Mexican-American Men with and without Alzheimer’s Disease. J Alzheimers Dis. 2014;40:563–573. doi: 10.3233/JAD-131994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobsen DW, Gatautis VJ, Green R, Robinson K, Savon SR, Secic M, Ji J, Otto JM, Taylor LM., Jr Rapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate concentrations in healthy subjects. Clin Chem. 1994;40(6):873–81. [PubMed] [Google Scholar]
- 28.Wu LL, Wu J, Hunt SC, James BC, Vincent GM, Williams RR, Hopkins PN. Plasma homocyst(e)ine as a risk factor for early familial coronary artery disease. Clin Chem. 1994;40(4):552–61. [PubMed] [Google Scholar]
- 29.Brattstrom L, Lindgren A, Israelsson B, Andersson A, Hultberg B. Homocysteine and cysteine: determinants of plasma levels in middle-aged and elderly subjects. J Intern Med. 1994;236(6):633–41. doi: 10.1111/j.1365-2796.1994.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 30.Powers RW, Majors AK, Lykins DL, Sims CJ, Lain KY, Roberts JM. Plasma homocysteine and malondialdehyde are correlated in an age- and gender-specific manner. Metabolism. 2002;51(11):1433–8. doi: 10.1053/meta.2002.35587. [DOI] [PubMed] [Google Scholar]
- 31.Nygard O, Vollset SE, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, Ueland M, Kvale G. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. Jama. 1995;274(19):1526–33. doi: 10.1001/jama.1995.03530190040032. [DOI] [PubMed] [Google Scholar]
- 32.Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, Tverdal A, Tell GS, Nygard O, Vollset SE. The Hordaland Homocysteine Study: a community-based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136(6 Suppl):1731S–1740S. doi: 10.1093/jn/136.6.1731S. [DOI] [PubMed] [Google Scholar]
- 33.Giltay EJ, Hoogeveen EK, Elbers JM, Gooren LJ, Asscheman H, Stehouwer CD. Effects of sex steroids on plasma total homocysteine levels: a study in transsexual males and females. J Clin Endocrinol Metab. 1998;83(2):550–3. doi: 10.1210/jcem.83.2.4574. [DOI] [PubMed] [Google Scholar]
- 34.Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, Tsujita T, Chowdhry S, Patani R, Chandran S, Horsburgh K, Hayes JD, Hardingham GE. Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A. 2011;108(1):E1–2. doi: 10.1073/pnas.1015229108. author reply E3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci. 2011;14(11):1363–8. doi: 10.1038/nn.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segev-Amzaleg N, Trudler D, Frenkel D. Preconditioning to mild oxidative stress mediates astroglial neuroprotection in an IL-10-dependent manner. Brain Behav Immun. 2013;30:176–85. doi: 10.1016/j.bbi.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 37.del Zoppo GJ, Becker KJ, Hallenbeck JM. Inflammation after stroke: is it harmful? Arch Neurol. 2001;58(4):669–72. doi: 10.1001/archneur.58.4.669. [DOI] [PubMed] [Google Scholar]
- 38.Han H, Wang H, Long H, Nattel S, Wang Z. Oxidative preconditioning and apoptosis in L-cells. Roles of protein kinase B and mitogen-activated protein kinases. J Biol Chem. 2001;276(28):26357–64. doi: 10.1074/jbc.M011136200. [DOI] [PubMed] [Google Scholar]
- 39.Pang CY, Yang RZ, Zhong A, Xu N, Boyd B, Forrest CR. Acute ischaemic preconditioning protects against skeletal muscle infarction in the pig. Cardiovasc Res. 1995;29(6):782–8. [PubMed] [Google Scholar]
- 40.DeFily DV, Chilian WM. Preconditioning protects coronary arteriolar endothelium from ischemia-reperfusion injury. Am J Physiol. 1993;265(2 Pt2):H700–6. doi: 10.1152/ajpheart.1993.265.2.H700. [DOI] [PubMed] [Google Scholar]
- 41.Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology. 2013;154(11):4281–92. doi: 10.1210/en.2013-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes S, Singh M, Su C, Cunningham RL. Effects of Oxidative Stress and Testosterone on Pro-Inflammatory Signaling in a Female Rat Dopaminergic Neuronal Cell Line. Endocrinology. 2016;157(7):2824–35. doi: 10.1210/en.2015-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romano AD, Serviddio G, de Matthaeis A, Bellanti F, Vendemiale G. Oxidative stress and aging. Journal of nephrology. 2010;23(Suppl 15):S29–36. [PubMed] [Google Scholar]
- 44.Fannin J, Rice KM, Thulluri S, Arvapalli RK, Wehner P, Blough ER. The Effects of Aging on Indices of Oxidative Stress and Apoptosis in the Female Fischer 344/Nnia X Brown Norway/BiNia Rat Heart. The Open Cardiovascular Medicine Journal. 2013;7:113–21. doi: 10.2174/1874192401307010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hekimi S, Lapointe J, Wen Y. Taking a “good” look at free radicals in the aging process. Trends in cell biology. 2011;21(10):569–76. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 47.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 48.Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL, Behrens MM. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One. 2009;4(5):e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali SS, Marcondes MC, Bajova H, Dugan LL, Conti B. Metabolic depression and increased reactive oxygen species production by isolated mitochondria at moderately lower temperatures. J Biol Chem. 2010;285(42):32522–8. doi: 10.1074/jbc.M110.155432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kayali R, Cakatay U, Tekeli F. Male rats exhibit higher oxidative protein damage than females of the same chronological age. Mech Ageing Dev. 2007;128(5–6):365–9. doi: 10.1016/j.mad.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 51.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 1992;14(2):103–15. doi: 10.1016/0378-5122(92)90003-m. [DOI] [PubMed] [Google Scholar]
- 52.Luoto R, Kaprio J, Uutela A. Age at natural menopause and sociodemographic status in Finland. Am J Epidemiol. 1994;139(1):64–76. doi: 10.1093/oxfordjournals.aje.a116936. [DOI] [PubMed] [Google Scholar]
- 53.Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R. Factors influencing the age at natural menopause. J Chronic Dis. 1987;40(11):995–1002. doi: 10.1016/0021-9681(87)90113-5. [DOI] [PubMed] [Google Scholar]
- 54.Magursky V, Mesko M, Sokolik L. Age at the menopause and onset of the climacteric in women of Martin District, Czechoslovkia. Statistical survey and some biological and social correlations. Int J Fertil. 1975;20(1):17–23. [PubMed] [Google Scholar]
- 55.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38(3):425–40. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gold EB, Bromberger J, Crawford S, Samuels S, Greendale GA, Harlow SD, Skurnick J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–74. doi: 10.1093/aje/153.9.865. [DOI] [PubMed] [Google Scholar]
- 57.Alvarado G, Rivera R, Ruiz R, Arenas M, Rueda D. The characteristics of the menstrual bleeding pattern in a group of normal women from Durango. Ginecol Obstet Mex. 1988;56:127–31. [PubMed] [Google Scholar]
- 58.Henry OR, Benghuzzi H, Taylor HA, Jr, Tucci M, Butler K, Jones L. Suppression of homocysteine levels by vitamin B12 and folates: age and gender dependency in the Jackson Heart Study. Am J Med Sci. 2012;344(2):110–5. doi: 10.1097/MAJ.0b013e31823782a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boers GH, Smals AG, Trijbels FJ, Leermakers AI, Kloppenborg PW. Unique efficiency of methionine metabolism in premenopausal women may protect against vascular disease in the reproductive years. J Clin Invest. 1983;72(6):1971–6. doi: 10.1172/JCI111161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brattstrom LE, Hultberg BL, Hardebo JE. Folic acid responsive postmenopausal homocysteinemia. Metabolism. 1985;34(11):1073–7. doi: 10.1016/0026-0495(85)90082-4. [DOI] [PubMed] [Google Scholar]
- 61.Wouters MG, Moorrees MT, van der Mooren MJ, Blom HJ, Boers GH, Schellekens LA, Thomas CM, Eskes TK. Plasma homocysteine and menopausal status. Eur J Clin Invest. 1995;25(11):801–5. doi: 10.1111/j.1365-2362.1995.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 62.Chillemi R, Simpore J, Persichilli S, Minucci A, D’Agata A, Musumeci S. Elevated levels of plasma homocysteine in postmenopausal women in Burkina Faso. Clin Chem Lab Med. 2005;43(7):765–71. doi: 10.1515/CCLM.2005.131. [DOI] [PubMed] [Google Scholar]
- 63.Kang SS, Wong PW, Cook HY, Norusis M, Messer JV. Protein-bound homocyst(e)ine. A possible risk factor for coronary artery disease. J Clin Invest. 1986;77(5):1482–6. doi: 10.1172/JCI112461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hak AE, Polderman KH, Westendorp IC, Jakobs C, Hofman A, Witteman JC, Stehouwer CD. Increased plasma homocysteine after menopause. Atherosclerosis. 2000;149(1):163–8. doi: 10.1016/s0021-9150(99)00321-4. [DOI] [PubMed] [Google Scholar]
- 65.Andersson A, Brattstrom L, Israelsson B, Isaksson A, Hamfelt A, Hultberg B. Plasma homocysteine before and after methionine loading with regard to age, gender, and menopausal status. Eur J Clin Invest. 1992;22(2):79–87. doi: 10.1111/j.1365-2362.1992.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 66.Bruschi F, Daguati R, Parazzini F, Dal Pino D, Fiore V, Di Pace R, Melotti D, Moroni S, Maffioletti C, Rossi M, Crosignani PG. Age, menopausal status and homocysteine levels in women around menopause. Eur J Obstet Gynecol Reprod Biol. 2005;120(2):195–7. doi: 10.1016/j.ejogrb.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 67.Kan H, Hu W, Wang Y, Wu W, Yin Y, Liang Y, Wang C, Huang D, Li W. NADPH oxidase-derived production of reactive oxygen species is involved in learning and memory impairments in 16-month-old female rats. Mol Med Rep. 2015;12(3):4546–53. doi: 10.3892/mmr.2015.3894. [DOI] [PubMed] [Google Scholar]
- 68.Gortan Cappellari G, Losurdo P, Mazzucco S, Panizon E, Jevnicar M, Macaluso L, Fabris B, Barazzoni R, Biolo G, Carretta R, Zanetti M. Treatment with n-3 polyunsaturated fatty acids reverses endothelial dysfunction and oxidative stress in experimental menopause. J Nutr Biochem. 2013;24(1):371–9. doi: 10.1016/j.jnutbio.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 69.van der Mooren MJ, Demacker PN, Blom HJ, de Rijke YB, Rolland R. The effect of sequential three-monthly hormone replacement therapy on several cardiovascular risk estimators in postmenopausal women. Fertil Steril. 1997;67(1):67–73. doi: 10.1016/s0015-0282(97)81858-4. [DOI] [PubMed] [Google Scholar]
- 70.van der Mooren MJ, Wouters MG, Blom HJ, Schellekens LA, Eskes TK, Rolland R. Hormone replacement therapy may reduce high serum homocysteine in postmenopausal women. Eur J Clin Invest. 1994;24(11):733–6. doi: 10.1111/j.1365-2362.1994.tb01069.x. [DOI] [PubMed] [Google Scholar]
- 71.Williams MS, Cushman M, Ouyang P, Heckbert SR, Kalyani RR, Vaidya D. Association of Serum Sex Hormones with Hemostatic Factors in Women On and Off Hormone Therapy: The Multiethnic Study of Atherosclerosis. J Womens Health (Larchmt) 2016;25(2):166–72. doi: 10.1089/jwh.2015.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morris MS, Jacques PF, Selhub J, Rosenberg IH. Total homocysteine and estrogen status indicators in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2000;152(2):140–8. doi: 10.1093/aje/152.2.140. [DOI] [PubMed] [Google Scholar]
- 73.Stomati M, Hartmann B, Spinetti A, Mailand D, Rubino S, Albrecht A, Huber J, Petraglia F, Genazzani AR. Effects of hormonal replacement therapy on plasma sex hormone-binding globulin, androgen and insulin-like growth factor-1 levels in postmenopausal women. J Endocrinol Invest. 1996;19(8):535–41. doi: 10.1007/BF03349013. [DOI] [PubMed] [Google Scholar]
- 74.Waaseth M, Bakken K, Dumeaux V, Olsen KS, Rylander C, Figenschau Y, Lund E. Hormone replacement therapy use and plasma levels of sex hormones in the Norwegian Women and Cancer postgenome cohort - a cross-sectional analysis. BMC Womens Health. 2008;8:1. doi: 10.1186/1472-6874-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Handelsman DJ, Sikaris K, Ly LP. Estimating age-specific trends in circulating testosterone and sex hormone-binding globulin in males and females across the lifespan. Ann Clin Biochem. 2016;53(Pt 3):377–84. doi: 10.1177/0004563215610589. [DOI] [PubMed] [Google Scholar]
- 76.Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von Muhlen D. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85(2):645–51. doi: 10.1210/jcem.85.2.6405. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Ding J, Bush TL, Longenecker JC, Nieto FJ, Golden SH, Szklo M. Relative androgen excess and increased cardiovascular risk after menopause: a hypothesized relation. Am J Epidemiol. 2001;154(6):489–94. doi: 10.1093/aje/154.6.489. [DOI] [PubMed] [Google Scholar]
- 78.Vermeulen A. The hormonal activity of the postmenopausal ovary. J Clin Endocrinol Metab. 1976;42(2):247–53. doi: 10.1210/jcem-42-2-247. [DOI] [PubMed] [Google Scholar]
- 79.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. Jama. 2004;291(1):47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 80.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13(9 Suppl):S78–86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 81.Howard BV, Rossouw JE. Estrogens and cardiovascular disease risk revisited: the Women’s Health Initiative. Curr Opin Lipidol. 2013;24(6):493–9. doi: 10.1097/MOL.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J I. Writing Group for the Women’s Health Initiative. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 83.Ettinger B, Wang SM, Leslie RS, Patel BV, Boulware MJ, Mann ME, McBride M. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause. 2012;19(6):610–5. doi: 10.1097/gme.0b013e31823a3e5d. [DOI] [PubMed] [Google Scholar]
- 84.Clarkson TB. The new conundrum: do estrogens have any cardiovascular benefits? Int J Fertil Womens Med. 2002;47(2):61–8. [PubMed] [Google Scholar]
- 85.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P, Prentice R I. Women’s Health Initiative. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166(3):357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 86.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. Jama. 2007;297(13):1465–77. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 87.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J Investigators WHI. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. Jama. 2011;305(13):1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sood R, Faubion SS, Kuhle CL, Thielen JM, Shuster LT. Prescribing menopausal hormone therapy: an evidence-based approach. Int J Womens Health. 2014;6:47–57. doi: 10.2147/IJWH.S38342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- 90.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31(10):529–37. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Borras C, Gambini J, Vina J. Mitochondrial oxidant generation is involved in determining why females live longer than males. Front Biosci. 2007;12:1008–13. doi: 10.2741/2120. [DOI] [PubMed] [Google Scholar]
- 92.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH S. Women’s Health Initiative Memory. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 93.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363(2):109–22. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basaria S, Davda MN, Travison TG, Ulloor J, Singh R, Bhasin S. Risk factors associated with cardiovascular events during testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2013;68(2):153–60. doi: 10.1093/gerona/gls138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Hwang O. Role of oxidative stress in Parkinson’s disease. Experimental neurobiology. 2013;22(1):11–7. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Halliwell B. Oxidative stress and cancer: have we moved forward? The Biochemical journal. 2007;401(1):1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 98.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. British journal of haematology. 2006;132(1):108–13. doi: 10.1111/j.1365-2141.2005.05834.x. [DOI] [PubMed] [Google Scholar]
- 99.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. Journal of hypertension. 2000;18(6):655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 100.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circulation research. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang W, Zhang X, Chen W. Role of oxidative stress in Alzheimer’s disease. Biomedical Reports. 2016;4(5):519–22. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, Medrano WA, Calzavara MB, Registro S, Andersen ML, Machado RB, Carvalho RC, de Ribeiro RA, Tufik S, Frussa-Filho R. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology. 2004;46(6):895–903. doi: 10.1016/j.neuropharm.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 103.Sherer TB, Chowdhury S, Peabody K, Brooks DW. Overcoming obstacles in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2012;27(13):1606–11. doi: 10.1002/mds.25260. [DOI] [PubMed] [Google Scholar]
- 104.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free radical biology & medicine. 2010;49(11):1603–16. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stoessl AJ, Lehericy S, Strafella AP. Imaging insights into basal ganglia function, Parkinson’s disease, and dystonia. Lancet. 2014;384(9942):532–44. doi: 10.1016/S0140-6736(14)60041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR. Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Annals of Neurology. 2004;56(4):532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- 107.Schlossmacher MG, Tomlinson JJ, Santos G, Shutinoski B, Brown EG, Manuel D, Mestre T. Modelling idiopathic Parkinson disease as a complex illness can inform incidence rate in healthy adults: the P(R)EDIGT score. The European Journal of Neuroscience. 2017;45(1):175–91. doi: 10.1111/ejn.13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing research reviews. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lubomski M, Louise Rushworth R, Lee W, Bertram KL, Williams DR. Sex differences in Parkinson’s disease. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2014;21(9):1503–6. doi: 10.1016/j.jocn.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 110.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? Journal of neurology, neurosurgery and psychiatry. 2004;75(4):637–9. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Risk tables for parkinsonism and Parkinson’s disease. Journal of Clinical Epidemiology. 2002;55(1):25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 112.Van Den Eeden S, Tanner C, Bernstein A, Fross R, Leimpeter A, Bloch D, Nelson L. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157(11):1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 113.Quinn NP, Marsden CD. Menstrual-related fluctuations in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 1986;1(1):85–7. doi: 10.1002/mds.870010112. [DOI] [PubMed] [Google Scholar]
- 114.Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early Parkinson’s disease. Neurology. 1999;52(7):1417–21. doi: 10.1212/wnl.52.7.1417. [DOI] [PubMed] [Google Scholar]
- 115.Miller DB, Ali SF, O’Callaghan JP, Laws SC. The Impact of Gender and Estrogen on Striatal Dopaminergic Neurotoxicity. Annals of the New York Academy of Sciences. 1998;844(1):153–165. doi: 10.1111/j.1749-6632.1998.tb08230.x. [DOI] [PubMed] [Google Scholar]
- 116.Sandyk R. Estrogens and the pathophysiology of Parkinson’s disease. The International journal of neuroscience. 1989;45(1–2):119–22. doi: 10.3109/00207458908986223. [DOI] [PubMed] [Google Scholar]
- 117.Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson’s disease. Clinical and experimental pharmacology & physiology. 2007;34(7):555–65. doi: 10.1111/j.1440-1681.2007.04616.x. [DOI] [PubMed] [Google Scholar]
- 118.Mozley LH, Gur RC, Mozley PD, Gur RE. Striatal dopamine transporters and cognitive functioning in healthy men and women. The American journal of psychiatry. 2001;158(9):1492–9. doi: 10.1176/appi.ajp.158.9.1492. [DOI] [PubMed] [Google Scholar]
- 119.Dluzen DE. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. Journal of Neurocytology. 2000;29(5):387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- 120.Popat RA, Van Den Eeden SK, Tanner CM, McGuire V, Bernstein AL, Bloch DA, Leimpeter A, Nelson LM. Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology. 2005;65(3):383–90. doi: 10.1212/01.wnl.0000171344.87802.94. [DOI] [PubMed] [Google Scholar]
- 121.Carter CL, Resnick EM, Mallampalli M, Kalbarczyk A. Sex and gender differences in Alzheimer’s disease: recommendations for future research. Journal of women’s health (2002) 2012;21(10):1018–23. doi: 10.1089/jwh.2012.3789. [DOI] [PubMed] [Google Scholar]
- 122.Okun MS, Fernandez HH, Rodriguez RL, Romrell J, Suelter M, Munson S, Louis ED, Mulligan T, Foster PS, Shenal BV, Armaghani SJ, Jacobson C, Wu S, Crucian G. Testosterone therapy in men with Parkinson disease: results of the TEST-PD Study. Arch Neurol. 2006;63(5):729–35. doi: 10.1001/archneur.63.5.729. [DOI] [PubMed] [Google Scholar]
- 123.Okun MS, Wu SS, Jennings D, Marek K, Rodriguez RL, Fernandez HH. Testosterone level and the effect of levodopa and agonists in early Parkinson disease: results from the INSPECT cohort. J Clin Mov Disord. 2014;1:8. doi: 10.1186/2054-7072-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rodriguez-Perez AI, Valenzuela R, Joglar B, Garrido-Gil P, Guerra MJ, Labandeira-Garcia JL. Renin angiotensin system and gender differences in dopaminergic degeneration. Mol Neurodegener. 2011;6(1):58. doi: 10.1186/1750-1326-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cunningham RL, Macheda T, Watts LT, Poteet E, Singh M, Roberts JL, Giuffrida A. Androgens exacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion. Horm Behav. 2011;60(5):617–24. doi: 10.1016/j.yhbeh.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian Androgen Production in Postmenopausal Women. The Journal of Clinical Endocrinology & Metabolism. 2007;92(8):3040–3043. doi: 10.1210/jc.2007-0581. [DOI] [PubMed] [Google Scholar]
- 127.Havelock JC, Rainey WE, Bradshaw KD, Carr BR. The post-menopausal ovary displays a unique pattern of steroidogenic enzyme expression. Human Reproduction. 2006;21(1):309–317. doi: 10.1093/humrep/dei373. [DOI] [PubMed] [Google Scholar]
- 128.Sultan A, Nesslany F, Violet M, Begard S, Loyens A, Talahari S, Mansuroglu Z, Marzin D, Sergeant N, Humez S, Colin M, Bonnefoy E, Buee L, Galas MC. Nuclear tau, a key player in neuronal DNA protection. The Journal of biological chemistry. 2011;286(6):4566–75. doi: 10.1074/jbc.M110.199976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ibanez-Salazar A, Banuelos-Hernandez B, Rodriguez-Leyva I, Chi-Ahumada E, Monreal-Escalante E, Jimenez-Capdeville ME, Rosales-Mendoza S. Oxidative Stress Modifies the Levels and Phosphorylation State of Tau Protein in Human Fibroblasts. Frontiers in Neuroscience. 2017;11:495. doi: 10.3389/fnins.2017.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ansari MA, Scheff SW. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011;51(1):171–8. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bruce-Keller AJ, Gupta S, Parrino TE, Knight AG, Ebenezer PJ, Weidner AM, LeVine H, 3rd, Keller JN, Markesbery WR. NOX activity is increased in mild cognitive impairment. Antioxid Redox Signal. 2010;12(12):1371–82. doi: 10.1089/ars.2009.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimers Dis. 2006;9(2):167–81. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 133.Loscalzo J. Homocysteine and dementias. N Engl J Med. 2002;346(7):466–8. doi: 10.1056/NEJM200202143460702. [DOI] [PubMed] [Google Scholar]
- 134.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–83. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 135.Kim J, Park MH, Kim E, Han C, Jo SA, Jo I. Plasma homocysteine is associated with the risk of mild cognitive impairment in an elderly Korean population. J Nutr. 2007;137(9):2093–7. doi: 10.1093/jn/137.9.2093. [DOI] [PubMed] [Google Scholar]
- 136.Selley ML, Close DR, Stern SE. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer’s disease. Neurobiol Aging. 2002;23(3):383–8. doi: 10.1016/s0197-4580(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 137.Seshadri S. Elevated plasma homocysteine levels: risk factor or risk marker for the development of dementia and Alzheimer’s disease? J Alzheimers Dis. 2006;9(4):393–8. doi: 10.3233/jad-2006-9404. [DOI] [PubMed] [Google Scholar]
- 138.Haan MN, Miller JW, Aiello AE, Whitmer RA, Jagust WJ, Mungas DM, Allen LH, Green R. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85(2):511–7. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim JM, Stewart R, Kim SW, Shin IS, Yang SJ, Shin HY, Yoon JS. Changes in folate, vitamin B12 and homocysteine associated with incident dementia. J Neurol Neurosurg Psychiatry. 2008;79(8):864–8. doi: 10.1136/jnnp.2007.131482. [DOI] [PubMed] [Google Scholar]
- 140.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005;82(3):636–43. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 141.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55(11):1449–55. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 142.Kruman, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20(18):6920–6. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Brinton RD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer’s disease: recent insights and remaining challenges. Learn Mem. 2001;8(3):121–33. doi: 10.1101/lm.39601. [DOI] [PubMed] [Google Scholar]
- 144.Brinton RD. Impact of estrogen therapy on Alzheimer’s disease: a fork in the road? CNS Drugs. 2004;18(7):405–22. doi: 10.2165/00023210-200418070-00001. [DOI] [PubMed] [Google Scholar]
- 145.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 146.Toran-Allerand CD. Estrogen as a treatment for Alzheimer disease. Jama. 2000;284(3):307–8. [PubMed] [Google Scholar]
- 147.Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9(3):349–54. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- 148.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21(1):107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 149.Simpkins JW, Green PS, Gridley KE, Singh M, de Fiebre NC, Rajakumar G. Role of estrogen replacement therapy in memory enhancement and the prevention of neuronal loss associated with Alzheimer’s disease. Am J Med. 1997;103(3A):19S–25S. doi: 10.1016/s0002-9343(97)00260-x. [DOI] [PubMed] [Google Scholar]
- 150.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer [beta]-amyloid peptides. Nat Med. 1998;4(4):447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 151.Xu H, Wang R, Zhang YW, Zhang X. Estrogen, beta-amyloid metabolism/trafficking, and Alzheimer’s disease. Ann N Y Acad Sci. 2006;1089:324–42. doi: 10.1196/annals.1386.036. [DOI] [PubMed] [Google Scholar]
- 152.Jamshed N, Ozair FF, Aggarwal P, Ekka M. Alzheimer disease in post-menopausal women: Intervene in the critical window period. Journal of Mid-Life Health. 2014;5(1):38–40. doi: 10.4103/0976-7800.127791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13(4):345–57. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 154.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17(5):485–95. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 155.Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. Jama. 1998;279(9):688–95. doi: 10.1001/jama.279.9.688. [DOI] [PubMed] [Google Scholar]
- 156.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48(6):1517–21. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 157.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348(9025):429–32. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 158.LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. Jama. 2001;285(11):1489–99. doi: 10.1001/jama.285.11.1489. [DOI] [PubMed] [Google Scholar]
- 159.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J Investigators W. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 160.Grady D, Yaffe K, Kristof M, Lin F, Richards C, Barrett-Connor E. Effect of postmenopausal hormone therapy on cognitive function: the Heart and Estrogen/progestin Replacement Study. Am J Med. 2002;113(7):543–8. doi: 10.1016/s0002-9343(02)01270-6. [DOI] [PubMed] [Google Scholar]
- 161.Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. Jama. 2000;283(8):1007–15. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 162.Linn RT, Wolf PA, Bachman DL, Knoefel JE, Cobb JL, Belanger AJ, Kaplan EF, D’Agostino RB. The ‘preclinical phase’ of probable Alzheimer’s disease. A 13-year prospective study of the Framingham cohort. Arch Neurol. 1995;52(5):485–90. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 163.Tierney MC, Yao C, Kiss A, McDowell I. Neuropsychological tests accurately predict incident Alzheimer disease after 5 and 10 years. Neurology. 2005;64(11):1853–9. doi: 10.1212/01.WNL.0000163773.21794.0B. [DOI] [PubMed] [Google Scholar]
- 164.Maki PM. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause. 2013;20(6):695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Henderson VW, Benke KS, Green RC, Cupples LA, Farrer LA, Group MS. Postmenopausal hormone therapy and Alzheimer’s disease risk: interaction with age. J Neurol Neurosurg Psychiatry. 2005;76(1):103–5. doi: 10.1136/jnnp.2003.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gatson J, Singh M. Activation of a Membrane-Associated Androgen Receptor Promotes Cell Death in Primary Cortical Astrocytes. 2007 doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- 167.Brunelli E, Domanico F, La Russa D, Pellegrino D. Sex differences in oxidative stress biomarkers. Current drug targets. 2014;15(8):811–5. doi: 10.2174/1389450115666140624112317. [DOI] [PubMed] [Google Scholar]
- 168.Yang RL, Shi YH, Hao G, Li W, Le GW. Increasing Oxidative Stress with Progressive Hyperlipidemia in Human: Relation between Malondialdehyde and Atherogenic Index. J Clin Biochem Nutr. 2008;43(3):154–8. doi: 10.3164/jcbn.2008044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stojiljkovic MP, Lopes HF, Zhang D, Morrow JD, Goodfriend TL, Egan BM. Increasing plasma fatty acids elevates F2-isoprostanes in humans: implications for the cardiovascular risk factor cluster. J Hypertens. 2002;20(6):1215–21. doi: 10.1097/00004872-200206000-00036. [DOI] [PubMed] [Google Scholar]
- 170.Oskarsson HJ, Heistad DD. Oxidative stress produced by angiotensin too. Implications for hypertension and vascular injury. Circulation. 1997;95(3):557–9. doi: 10.1161/01.cir.95.3.557. [DOI] [PubMed] [Google Scholar]
- 171.Vaziri ND, Wang XQ, Oveisi F, Rad B. Induction of oxidative stress by glutathione depletion causes severe hypertension in normal rats. Hypertension. 2000;36(1):142–6. doi: 10.1161/01.hyp.36.1.142. [DOI] [PubMed] [Google Scholar]
- 172.Cunningham RL, Singh M, O’Bryant SE, Hall JR, Barber RC. Oxidative stress, testosterone, and cognition among Caucasian and Mexican-American men with and without Alzheimer’s disease. Journal of Alzheimer’s disease: JAD. 2014;40(3):563–73. doi: 10.3233/JAD-131994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, Shumaker SA, Pleasants DD, Barrett-Connor E, Bhasin S, Cauley JA, Cella D, Crandall JP, Cunningham GR, Ensrud KE, Farrar JT, Lewis CE, Molitch ME, Pahor M, Swerdloff RS, Cifelli D, Anton S, Basaria S, Diem SJ, Wang C, Hou X, Snyder PJ. Testosterone Treatment and Cognitive Function in Older Men With Low Testosterone and Age-Associated Memory Impairment. Jama. 2017;317(7):717–727. doi: 10.1001/jama.2016.21044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sheweita S, Salama B, Hassan M. Erectile dysfunction drugs and oxidative stress in the liver of male rats. Toxicol Rep. 2015;2:933–938. doi: 10.1016/j.toxrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Fan YF, Zhang R, Jiang X, Wen L, Wu DC, Liu D, Yuan P, Wang YL, Jing ZC. The phosphodiesterase-5 inhibitor vardenafil reduces oxidative stress while reversing pulmonary arterial hypertension. Cardiovasc Res. 2013;99(3):395–403. doi: 10.1093/cvr/cvt109. [DOI] [PubMed] [Google Scholar]
- 176.Garcia LA, Hlaing SM, Gutierrez RA, Sanchez MD, Kovanecz I, Artaza JN, Ferrini MG. Sildenafil attenuates inflammation and oxidative stress in pelvic ganglia neurons after bilateral cavernosal nerve damage. Int J Mol Sci. 2014;15(10):17204–20. doi: 10.3390/ijms151017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Khanna HD, Sinha MK, Khanna S, Tandon R. Oxidative stress in hypertension: association with antihypertensive treatment. Indian J Physiol Pharmacol. 2008;52(3):283–7. [PubMed] [Google Scholar]
- 178.Mihalj M, Tadzic R, Vcev A, Rucevic S, Drenjancevic I. Blood Pressure Reduction is Associated With the Changes in Oxidative Stress and Endothelial Activation in Hypertension, Regardless of Antihypertensive Therapy. Kidney Blood Press Res. 2016;41(6):721–735. doi: 10.1159/000450562. [DOI] [PubMed] [Google Scholar]
- 179.Dandona P, Kumar V, Aljada A, Ghanim H, Syed T, Hofmayer D, Mohanty P, Tripathy D, Garg R. Angiotensin II receptor blocker valsartan suppresses reactive oxygen species generation in leukocytes, nuclear factor-kappa B, in mononuclear cells of normal subjects: evidence of an antiinflammatory action. J Clin Endocrinol Metab. 2003;88(9):4496–501. doi: 10.1210/jc.2002-021836. [DOI] [PubMed] [Google Scholar]
- 180.Saez GT, Tormos C, Giner V, Chaves J, Lozano JV, Iradi A, Redon J. Factors related to the impact of antihypertensive treatment in antioxidant activities and oxidative stress by-products in human hypertension. Am J Hypertens. 2004;17(9):809–16. doi: 10.1016/j.amjhyper.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 181.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 2007;78(8):819–24. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]