Abstract
The nucleus accumbens (NAc) and ventral pallidum (VP) are reciprocally connected, and activity within this circuit is thought to promote reward learning. Inconsistent with this notion, we find that disconnecting NAc medial shell and VP greatly enhances the attribution of value to a cue that is paired with reward. This result suggests that medial NAc shell and VP are both needed for attributing value to cues yet can also oppose one-another’s functional contribution.
Keywords: Sign-tracking, nucleus accumbens, ventral pallidum
1. Introduction
Encounters with cues associated with rewards can exert powerful effects on behavior resulting in reward-seeking. Reward-paired cues can also become desirable and sought out in their own right, acquiring incentive salience similar to the reward itself (Berridge, 2004). Cue-directed motivation of this sort can be isolated by measuring sign-tracking behavior. Presentation of a lever conditioned stimulus (CS) that is followed by reward can cause animals to interact with the CS in a manner reflective of the CS’s motivational draw (Hearst and Jenkins, 1974; Flagel and Robinson, 2017). The reciprocally connected ventral pallidum (VP) and nucleus accumbens (NAc) participate in reward-seeking behaviors (Chang et al., 2015; Ahrens et al., 2016; Leung and Balleine, 2013, 2015; Richard et al., 2016; Prasad and McNally, 2016; Smith et al., 2009; Root et al., 2015; Chang et al., 2012; Mahler et al., 2014) as well as in cue attraction reflected in sign-tracking (Flagel et al., 2011; Chang et al., 2015; Ahrens et al., 2016; Chow et al., 2016). NAc-VP interaction is necessary for reward-seeking (Leung and Balleine, 2013; Stefanik et al., 2013; Wang et al., 2014; Creed et al., 2016; Heinsbroek et al., 2017), and both areas can be engaged by rewards and their cues (Richard et al., 2016; Root et al., 2015; Leung and Balleine 2013; Smith et al., 2009). These findings bolster the long-held notion that activation of the NAc and VP promotes motivated behavior (Mogenson et al., 1980). On the other hand, anatomical studies show that NAc and VP are set up to potentially inhibit one another through bidirectionally GABAergic projections (Fonnum et al., 1978; Zahm et al., 1985; Kuo and Chang, 1992; Churchill and Kalivas, 1994). Thus, inhibition of one region would excite the other. In line with this idea, other findings have shown that motivated behaviors can be increased by inhibition of the NAc (particularly the medial shell) or by stimulation of the VP (Pecina and Berridge, 2005; Smith and Berridge, 2005).
The end result is a puzzle for how NAc and VP interaction might support the motivational attraction to reward cues. The present study addressed this question by examining the effects of disconnecting VP and medial NAc shell on the acquisition of sign-tracking using chemogenetics (i.e., DREADDs; Armbruster et al., 2007; Smith et al., 2016). Remarkably, this procedure dramatically accentuated sign-tracking. This result supports the possibility of a mutually inhibitory interaction between the NAc and VP, highlights a stark difference in how this circuit supports goal-directed versus cue-directed behaviors, and presents a paradoxical scenario whereby the attraction to reward cues results from a competitive interaction between the NAc and VP in a manner that cannot be explained by the operations of either site alone.
2. Materials and methods
Sign-tracking
2.1. Animals
The subjects were Long-Evans rats (n = 41; Harlan Laboratories), which weighed 250–300 g on arrival. Rats were housed in a climate-controlled vivarium that was illuminated from 7:00 AM to 7:00 PM. Prior to surgery, rats were pair-housed and then individually housed after surgery for the duration of the experiment. Rats were given ad libitum access to food and water prior to and 2 weeks following surgery. Rats were then placed on food restriction and maintained at 85% of their ad libitum weights throughout the rest of the experiment. Experiments were carried out in accordance with the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and approved by the Dartmouth College Animal Care and Use Committee.
2.2. Surgery
Surgery was performed under aseptic conditions with isoflurane anesthesia. Infusions were made using a 33-gauge beveled needle (World Precision Instruments) and stereotaxic injector (Stoelting). For rats in the NAc shell-VP disconnection experiments, rats received unilateral infusions of an inhibitory DREADD (AAV8-hSyn-Gi-hM4Di-mCitrine; UNC vector core) into the NAc shell (1.0 μl) and VP (0.8 μl) in either opposite hemispheres (Group Contra; n = 14) or the same hemisphere (Group Ipsi; n =12). Coordinates for NAc shell infusions were 1.4 mm anterior of bregma, 0.8 mm from the midline, and 7.2 mm ventral from the skull surface. Coordinates for VP infusions were 0.12 mm anterior of bregma, 2.4 mm from the midline, and 8.2 mm ventral from the skull surface. The location of infusions (i.e., left or right hemisphere) was counterbalanced for each group. For the NAc shell experiment, rats received bilateral infusions (1.0 μl) of either an inhibitory DREADD (same as above; Group Gi; n = 7) or a green fluorescent protein (AAV8-hSyn-GFP; UNC vector core). The coordinates for NAc shell infusions were the same as above. All infusions were made at a rate of 0.15 μl/min. Transgene expression was allowed to take place for 3 weeks before the start of behavioral training.
2.3. Apparatus
Behavioral training was conducted using eight identical standard conditioning chambers (24 x 30.5 x 29 cm; Med Associates) enclosed in sound-attenuating chambers (62 x 56 x 56 cm), each with an exhaust fan to provide airflow and background noise (~ 68 dB). Chambers were made of aluminum front and back walls, clear acrylic sides and top, and grid floors. The front wall of each chamber was outfitted with a recessed food cup. To the left and right of the food cup were retractable levers that were 4.8 cm long and positioned 6.2 cm above the floor. Levers protruded 1.9 cm from the wall when extended. Chambers were illuminated by a house light mounted 15 cm above the floor on the back wall of the chamber. The US was two 45 mg grain-based rodent food pellets (Bioserv). Task events were controlled by a computer located in an adjacent room.
2.4. Behavioral training
Rats received a 30-min magazine training session in which food pellets were delivered freely on a random time 30 s (RT 30 s) schedule, resulting in approximately 60 pellets being delivered.
Following magazine training, rats underwent training in the sign-tracking procedure. Prior to each session, rats received an injection of clozapine N-oxide (CNO; 1 mg/mL/kg in sterile water, i.p.; National Institute of Mental Health’s Chemical Synthesis and Drug Supply Program or Sigma Aldrich). Following injections, rats were left in their transport cages for 30 min to allow for CNO to activate hM4Di receptors. Rats were then placed into the conditioning chambers for the start of each training session (12 days). For each 60-min session, rats were presented 25 CS+ and 25 CS− trials (average intertrial interval: 60 s). Trials were ordered so that no more than two of the same trial type occurred in a sequence. On CS+ trials, one lever was extended for 10 s and was paired with US delivery immediately upon retraction. On CS− trials, the opposite lever was extended for 10 s without US delivery. Assignment of CS+/CS− levers (i.e., left or right) was counterbalanced across all groups and remained the same for each rat throughout training.
2.5. Data analysis
The primary measures analyzed were lever presses/min and the percentage of time spent in the food cup during CS presentations. These measures were subjected to repeated measures ANOVAs with a between-subjects factor of group (Contra vs. Ipsi or Gi vs. GFP) and within-subjects factors of Cue (CS+ vs. CS−) and Session (12 days). For the NAc shell-VP disconnection experiment, the number of days it took for rats to reach peak levels of sign-tracking (i.e., lever presses/min) was also analyzed with a 2 sample t-test. All analyses had a rejection criterion of p < 0.05.
2.6. Histological procedures
Following behavioral training, rats were deeply anesthetized with euthasol (Virbac; 1 ml) and perfused intracardially with 0.9% saline and 10% formalin. Brains were removed, stored overnight in 20% sucrose, and sectioned in 40 μm sections that were mounted on microscope slides and coverslipped with a DAPI-containing hardset mounting medium (Vectashield). To determine hM4D(Gi) expression, slides were viewed under a fluorescent microscope (Olympus) and areas of expression were mapped onto the corresponding boundaries of the Paxinos & Watson atlas (Paxinos and Watson, 2009).
Electrophysiological recordings
2.7. Animals
Twenty-six medial NAc shell units and twenty VP units were recorded from 3 male Long-Evans rats (Harlan Laboratories) weighing 250–300 g on arrival (animal 1 (NAc: 16, VP: 11), animal 2 (NAc: 5, VP: 6), animal 3 (NAc: 5, VP: 3)). Housing was the same as above, but recording rats were given ad libitum access to food and water.
2.8. Surgery
Rats first received hM4Di vector infusions in an identical manner as rats in Group Contra that underwent behavioral testing. After 3 weeks to allow for hM4Di expression, rats were implanted with a head-stage made of 12 individually drivable tetrodes (four 12.5-μm nichrome wires at 150–200 kΩ impedance) that were positioned above the “online” NAc shell and VP sites that did not receive hM4Di infusions. The head-stage was secured with cranial screws and cement, and tetrodes were gradually lowered to the NAc shell and VP over the ensuing week.
2.9. Apparatus and recording procedures
Recordings were conducted in a conditioning chamber (31 x 33 x 34.5 cm; Med Associates). Rats were allowed to freely explore the chamber while electrophysiological activity was recorded using a 96-channel digital Neuralynx system and Cheetah acquisition software. A computer recorded electrical signals that were amplified at 100–1000, sampled at 32 kHz, and filtered for 600–6000 Hz. On each day, rats were habituated to the recording chamber for at least 20 min while recording parameters were configured. Rats then underwent a 20-min baseline recording session, were then injected with CNO (i.p.; 1 mg/kg), and were then returned to the chamber for another 90-min of additional recording. Units that did not maintain detectable spiking activity for the recording duration were discarded. Tetrodes were lowered ~40 μm prior to each session, thus minimizing the probability of repeated sampling. Rats underwent 3–4 recording sessions each until unit recording quality was insufficient. The rats were always still and quiet during recording sessions. Recorded units were assessed online and offline to confirm distinguishing waveform and firing rate characteristics between recorded units from the same tetrode.
2.10. Data analysis
Recorded waveforms from the NAc and VP were separated into units using Plexon Offline Sorter. Mean differences in firing rate for each unit was analyzed using Neuroexplorer, Microsoft Excel, and SPSS. For each unit, activity was binned into 1-min intervals. A 99% confidence interval was created based on the pre-CNO baseline period of activity. Then, for each post-CNO bin, we compared it to this confidence interval. A responsive unit was one that exhibited a change in activity that went beyond the baseline confidence interval for at least 5 consecutive bins. For population comparisons, units of each response type (excited, inhibited, unaffected compared to baseline) were assessed as percentage of total units from the recorded structure and population sizes were compared using t-tests. Three units that met criteria for both inhibition and excitation were allocated to the response type exhibited most by time. For plots of firing rate in responsive populations, activity for each unit per 1-min bin was normalized into z-scores. Then, the average baseline z-score was subtracted from the post-CNO z-scores (per bin) in order to represent firing changes that diverged from baseline.
2.11. Histological procedures
After recording sessions were completed, current (25 μA, 10 s) was passed through each tetrode to create a small lesion for localization. Brains were removed, sliced, and analyzed for placement as with rats that underwent behavioral testing.
3. Results and Discussion
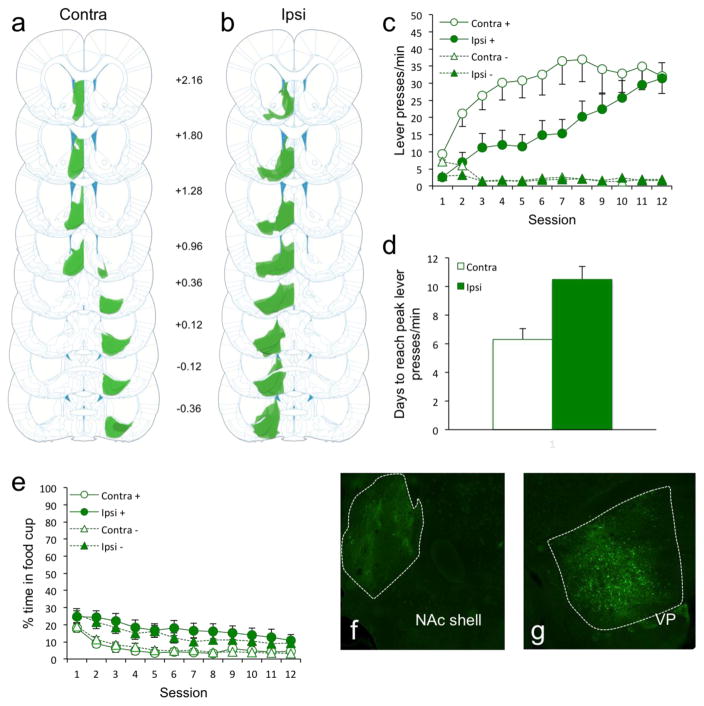
We chemogenetically disconnected the medial NAc shell from the VP and assessed discriminative sign-tracking behavior. The disconnection was achieved by a unilateral infusion of an inhibitory DREADD (AAV8-hSyn-hM4Di-mCitrine) into the NAc and contralateral VP (Group Contra; Fig. 1a). Control rats received identical ipsilateral infusions (Group Ipsi; Fig. 1b), thus disrupting an equivalent amount of NAc and VP activity but leaving communication between the NAc shell and VP intact in one hemisphere. Opposite to the reduction in sign-tracking that occurs with chemogenetic VP disruption (Chang et al., 2015), we found that disconnecting the NAc shell and VP enhanced the acquisition of sign-tracking (lever presses/min; Fig. 1c). Rats in Group Contra showed an accelerated acquisition of sign-tracking compared to rats in Group Ipsi (which acquired sign-tracking at comparable rates as control groups, with and without CNO, in our prior study (Chang et al., 2015)), with terminal levels ultimately being comparable. An ANOVA confirmed main effects of Cue (CS+ vs. CS−; F1,24 = 51.8, p < 0.01), Session (12 days; F11,264 = 13.2, p < 0.01), and Group (Contra vs. Ipsi; F1,24 = 4.3, p = 0.048), and a Cue/Group interaction (F1,24 = 4.3, p = 0.048). In addition, there were Session/Group (F11,264 = 2.9, p < 0.01), Cue/Session (F11,264 = 23.3, p < 0.01), and Cue/Session/Group (F11,264 = 3.6, p < 0.01) interactions. Further confirming faster acquisition, Group Contra rats reached peak levels of sign-tracking 40% (4 days) faster compared to Group Ipsi rats (Fig. 1d; p < 0.01).
Figure 1.
Figure 1(a–b) AAV8-hSyn-hM4Di-mCitrine cell expression in all rats of Group Contra (a; n = 14) and Group Ipsi (b; n =12). Darker areas indicate common areas of overlap in terms of expression between rats. (c) Disconnection of NAc shell and VP dramatically enhanced the acquisition of sign-tracking. (d) Group Contra rats reached peak levels of sign-tracking 40% (4 days) faster compared to Group Ipsi rats. (e) Group Contra rats spent less time in the food cup overall compared to Group Ipsi rats. (f–g) Representative brain slice showing the area of AAV8-hSyn-hM4Di-mCitrine cell expression in the NAc shell (f) and VP (g).
The sign-tracking enhancement was accompanied by reduced food cup-directed behavior during the CSs in Group Contra (Fig. 1e). Neither group showed what would be considered a goal-tracking response throughout the duration of training. An ANOVA confirmed main effects of Session (F11,264 = 17.2, p < 0.01) and Group (F1,24 = 10.4, p < 0.01), but no effect of Cue (F1,24 = 1.4, p = 0.24). There were no significant interactions (p ≥ 0.11). The disconnection thus biased almost all behavior during the CS+ to the lever and away from the reward source.
These results conflict with reduced reward-directed motivation during NAc or VP disruption (Chang et al., 2015; Leung and Balleine, 2015; Prasad and McNally, 2016; Chang et al., 2012; Stefanik et al., 2013; Wang et al., 2014), or NAc/VP circuit disruption (Leung and Balleine, 2013; Smith and Berridge, 2007). In particular, Leung and Balleine (2013) showed that disconnecting NAc shell and VP impaired outcome selective Pavlovian-instrumental transfer (PIT). Methodological differences with the current study aside, the contradictory results suggest that NAc-VP roles in cue-directed and reward-directed behaviors are differently, perhaps oppositely, regulated by the NAc-VP. An interesting future question concerns whether this difference could also relate to plasticity caused by our repeated CNO injections, or whether effects were more acute per-day as expected of the inactivation used by Leung and Balleine (2013).
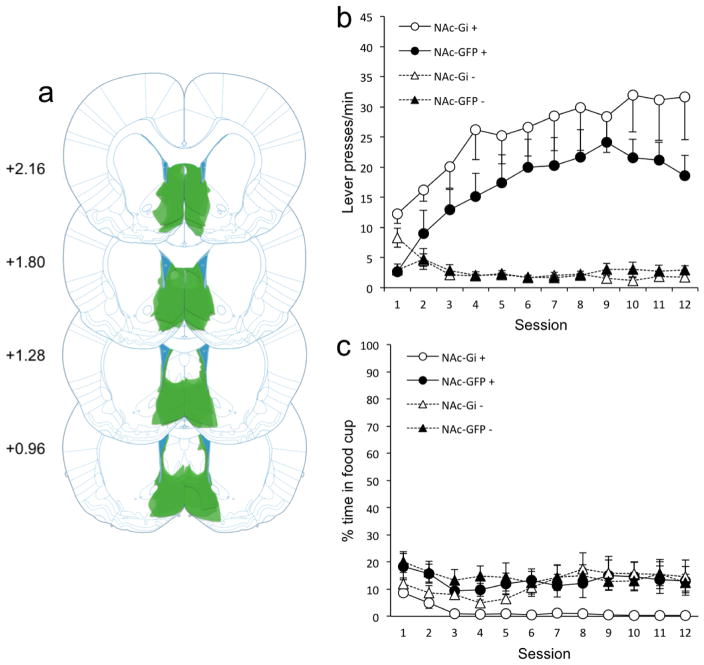
The enhancement in sign-tracking observed with disconnecting NAc shell and VP is also surprising given previous work showing that bilateral NAc shell lesions do not impair sign-tracking acquisition (Chang and Holland, 2013). However, in that study, it is possible that compensatory adaptations from other brain regions may have shadowed any role the NAc shell may have in sign-tracking acquisition. Thus, we next investigated the effects of inhibiting NAc shell activity on sign-tracking to confirm that result. The outcome was the same: bilateral NAc shell inhibition (Group NAc-Gi) had little effect on sign-tracking compared to controls (Group NAc-GFP; Fig. 2b). Although inhibition of the NAc shell produced a modest numerical increase in sign-tracking, this increase was not statistically reliable. An ANOVA confirmed main effects of Cue (F1,13 = 51.5, p < 0.01) and Session (F11,143 = 5.6, p < 0.01), but no effect of Group (F1,13 = 2.8, p = 0.12). There was a Cue/Session interaction (F11,143 = 12.2, p < 0.01). Other interactions were nonsignificant (p ≥ 0.12). This pattern of results is nearly identical to prior work investigating the effects of NAc shell lesions on sign-tracking with a similar number of rats (n = 8; Chang and Holland, 2013). As with the NAc-VP disconnection, bilateral NAc shell inhibition lowered food cup behavior non-discriminately. An ANOVA confirmed main effects of Session (F11,143 = 2.1, p = 0.02) and Group (F1,13 = 6.3, p = 0.03), but no effect of Cue (F1,13 = 4.0, p = 0.07). Interactions were nonsignificant (p ≥ 0.052).
Figure 2.
Figure 2(a) AAV8-hSyn-hM4Di-mCitrine cell expression in all Group NAc-Gi rats (n = 7). Darker areas indicate common areas of overlap in terms of expression between rats. (b) Bilateral NAc shell inhibition had no effect on sign-tracking. (c) Bilateral NAc shell inhibition lowered food cup behavior non-discriminately.
Despite hM4Di spread into the septum, bed nucleus of the stria termanalis (BNST), preoptic area (POA; Ipsi rats only), and diagonal band, we believe the enhancement in sign-tracking we observed was specific to disconnecting NAc shell and VP. To our knowledge, BNST and diagonal band have no projections to NAc or VP. The septum has a one-way projection to NAc only (Sheehan et al. 2004; Swanson & Cowan, 1979). However, the lack of effect on sign-tracking with bilateral NAc shell inhibition suggests that septum-NAc connections are not involved and would therefore have no bearing on our NAc-VP disconnection effect. The POA has one-way projections to both NAc and VP (Chiba & Murata, 1985), and there was some hM4Di expression in the POA of Group Ipsi rats. However, there was no appreciable hM4Di expression in the POA in our group Contra rats, suggesting it was not involved. Together, we believe that the enhancement in sign-tracking that we observed was specific to disrupting connections between NAc and VP, but recognize the possibility that wider circuit influences with septum, POA, and other areas interacting with NAc and VP may have influenced our results.
We also acknowledge that potentially non-specific effects of CNO, or metabolized clozapine, could have occurred. One is generalized locomotor reduction (Gomez et al., 2017). However, in contrast, Group Contra rats in our NAc-VP disconnection study showed enhancements in CS+ responding and exhibited similar low rates of CS− responding compared to Group Ipsi rats, which is contrary to what one would expect if CNO decreased locomotor activity generally. Our current findings are also in line with our previous work showing that CNO at this dose does not have an effect on the expression of sign-tracking once it is acquired, nor can it account for changes in sign-tracking that result from VP inhibition through the hM4Di receptor (Chang et al., 2015).
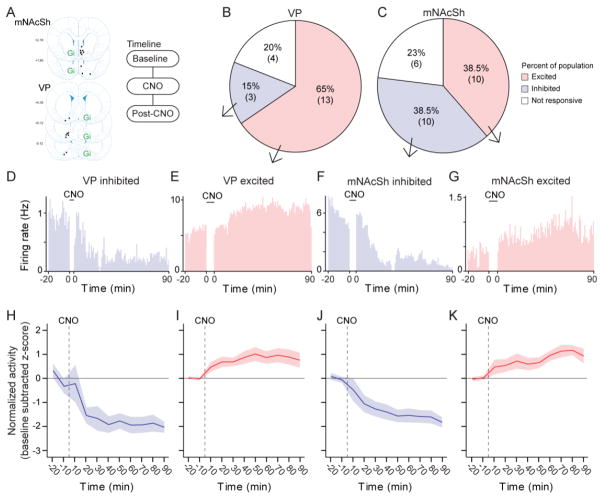
Finally, we sought to confirm that the disconnection manipulation resulted in a perturbation of NAc-VP circuit activity. To do this, we performed dual-site unit recordings of the NAc shell and VP hemispheres that remained “online’ in freely behaving animals outside of the sign-tracking procedure (Fig. 3a). There was a dominant excitation of online VP activity with ipsilateral NAc inhibition (Fig. 3b, de), while online NAc activity with ipsilateral VP DREADD inhibition exhibited both increases and decreases (Fig 3c, f–g). Changes in firing rates of inhibited and excited ensembles were similar between sites (Fig. 3 h–k). Although we caution that non-task activity being measured here is difficult to compare to in-task activity changes, these results are notably consistent with evidence that excitatory VP responses emerge to cues that evoke sign-tracking, while NAc responses exhibit mixed inhibition and excitation changes (Ahrens et al., 2016; Day et al., 2006). Concerning mechanism, the increase in VP activity may be due to larger circuit changes following the disconnection, or possibly through interneurons within the VP that are engaged or inhibited by the disconnection. On this point, it is not possible to know whether the VP units we recorded from are interneurons or projection neurons, nor can we be certain about changes being due to inhibition of NAc input or broader circuit-level alterations.
Figure 3.
Figure 3(a) Map of tetrode placements contralateral to hM4D(Gi) infusions, and recording timeline. (b–c) Populations of units that were excited, inhibited, or unaffected by CNO from recordings in the VP (t6=2.5, p = 0.047 population size comparison) and NAc (t6=0, p = 1). Number of units is shown below percentages (n = 3 rats; n = 26 NAc units, n = 20 VP units). (d–g) Real-time histograms (1-min bins; time zero = CNO) showing single units that were excited or inhibited in the VP and NAc. (h–k) Normalized firing rates (baseline-subtracted z-score) of pooled units with inhibition or excitation in NAc and VP.
While CNO by itself is not known to affect neural activity of the sort achieved here with hM4Di-CNO, we cannot rule out that CNO itself may have influenced NAc/VP activity. Our prior work recording VP activity following Gi activation with 1 mg/kg CNO showed that ~60% of cells were affected (Chang et al. 2015), which accords with the expected penetrance of the hM4Di viral construct (Smith et al., 2016). More chemogenetic studies focusing on the effects of CNO itself versus the effects of CNO on Gi-expressing neurons needs to be done (Gomez et al., 2017), but we believe that CNO alone cannot account for the behavioral effects observed in this study or the prior study that served as the precedent for this work (which showed an opposite sign-tracking reduction).
We conclude that the NAc shell and VP, when they are active, may oppose one another for attribution of value to reward paired cues given the paradoxical enhancement in sign-tracking acquisition here despite previous observations that each alone is important for cue-triggered reward seeking. Our results are particularly surprising given that disconnection of these two regions has been found to impair cue-triggered reward seeking behavior (e.g, PIT; Leung and Balleine, 2013). The disconnection of NAc shell and VP may have achieved a ‘functionally maximal’ state of NAc and VP activity to enhance reward cue attraction. By extension, in the intact brain, reward cue attraction could be ‘submaximal’ due to NAc-VP competition. Despite bilateral medial NAc shell inhibition having no effect on sign-tracking acquisition, we believe that disconnecting the medial NAc shell and VP creates this maximal situation with one VP side active and one medial NAc shell side inactive, resulting in rapidly-peaking sign-tracking levels. Thus, it is not either area alone but their interaction that is critical in regulating reward cue attraction. Future studies investigating the effects of inhibiting direct projections from NAc to VP and vice versa will provide more insight into the nature of NAc-VP interactions that lead to excessive motivation.
Highlights.
Chemogenetic disconnection of medial NAc shell and VP enhances sign-tracking.
Bilateral inhibition of NAc shell activity has no effect on sign-tracking.
The disconnection results in VP disinhibition.
Acknowledgments
This work was supported by funding from NIH Grant F32MH106178 (SEC), NIH Grant F32MH105125 (TPT), and a Whitehall Foundation research grant 2014-05-77 (KSS).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Author contributions: SEC, TPT, and KSS designed research; SEC and TPT performed research; SEC, TPT, and KSS analyzed data; SEC, TPT, and KSS wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrens AM, Meyer PJ, Ferguson LM, Robinson TE, Aldridge JW. Neural activity in the ventral pallidum encodes variation in the incentive value of a reward cue. Journal of Neuroscience. 2016;36:7957–7970. doi: 10.1523/JNEUROSCI.0736-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proceedings of the National Academy of Sciences. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. Motivation concepts in behavioral neuroscience. Physiology & Behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Chang SE, Holland PC. Effects of nucleus accumbens core and shell lesions on autoshaped lever-pressing. Behavioural Brain Research. 2013;256:36–42. doi: 10.1016/j.bbr.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Todd TP, Bucci DJ, Smith KS. Chemogenetic manipulation of ventral pallidal neurons impairs acquisition of sign-tracking in rats. European Journal of Neuroscience. 2015;42:3105–3116. doi: 10.1111/ejn.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Wheeler DS, Holland PC. Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiology of Learning and Memory. 2012;97:441–451. doi: 10.1016/j.nlm.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Murata Y. Afferent and efferent connections of the medial preoptic area in the rat: A WGA-HRP study. Brain Research Bulletin. 1985;14:261–272. doi: 10.1016/0361-9230(85)90091-7. [DOI] [PubMed] [Google Scholar]
- Chow JJ, Nickell JR, Darna M, Beckmann JS. Toward isolating the role of dopamine in the acquisition of incentive salience attribution. Neuropharmacology. 2016;109:320–331. doi: 10.1016/j.neuropharm.2016.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Kalivas PW. A topographically organized gamma-aminobutyric acid projection from the ventral pallidum to the nucleus accumbens in the rat. Journal of Comparative Neurology. 1994;345:579–595. doi: 10.1002/cne.903450408. [DOI] [PubMed] [Google Scholar]
- Creed M, Ntamati NR, Chandra R, Lobo MK, Lüscher C. Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron. 2016;92:214–226. doi: 10.1016/j.neuron.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, Carelli RM. Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. European Journal of Neuroscience. 2006;23:1341–1351. doi: 10.1111/j.1460-9568.2006.04654.x. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE. Neurobiological basis of individual variation in stimulus-reward learning. Current Opinion in Behavioral Sciences. 2017;13:178–185. doi: 10.1016/j.cobeha.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Gottesfeld Z, Grofova I. Distribution of glutatmate decarboxylase, choline acetyl-transferase and aromatic amino acid decarboxylase in the basal ganglia of normal and operated rats. Evidence for striatopallidal, striatoentopeduncular and striatonigral GABAergic fibres. Brain Research. 143:125–138. doi: 10.1016/0006-8993(78)90756-4. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science. 2017;357:503–507. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E, Jenkins H. Monograph of the Psychonomic Society. Austin: 1974. Sign-tracking: the stimulus-reinforcer relation and directed action. [Google Scholar]
- Heinsbroek JA, Neuhofer DN, Griffin WC, Siegel GS, Bobadilla AC, Kupchik YM, Kalivas PW. Loss of plasticity in the D2-accumbens pallidal pathway promotes cocaine seeking. Journal of Neuroscience. 2017;37:757–767. doi: 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo H, Chang HT. Ventral pallido-striatal pathway in the rat brain: a light and electron microscopic study. Journal of Comparative Neurology. 321:626–636. doi: 10.1002/cne.903210409. [DOI] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. The ventral striato-pallidal pathway mediates the effect of predictive learning on choice between goal-directed actions. Journal of Neuroscience. 2013;33:13848–13860. doi: 10.1523/JNEUROSCI.1697-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung BK, Balleine BW. Ventral pallidal projections to mediodorsal thalamus and ventral tegmental area play distinct roles in outcome-specific Pavlovian-instrumental transfer. Journal of Neuroscience. 2015;35:4953–4964. doi: 10.1523/JNEUROSCI.4837-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, Wilson SP, Deisseroth K, Woodward JJ, Aston-Jones G. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature Neuroscience. 2014;17:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Progress in Neurobiology. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 2009. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? Journal of Neuroscience. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad AA, McNally GP. Ventral pallidum output pathways in context-induced reinstatement of alcohol seeking. Journal of Neuroscience. 2016;36:11716–11726. doi: 10.1523/JNEUROSCI.2580-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JM, Ambroggi F, Janak PH, Fields HL. Ventral pallidum neurons encode incentive value and promote cue-elicited instrumental actions. Neuron. 2016;90:1165–1173. doi: 10.1016/j.neuron.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Melendez RI, Zaborszky L, Napier TC. The vental pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Progress in Neurobiology. 2015;130:29–70. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Research Reviews. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. Journal of Neuroscience. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. Journal of Neuroscience. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV. DREADDS: Use and application in behavioral neuroscience. Behavioral Neuroscience. 2016;130:137–155. doi: 10.1037/bne0000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behavioural Brain Research. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. Journal of Neuroscience. 2013;33:13654–13662. doi: 10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region in the rat. Journal of Comparative Neurology. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Wang L, Shen M, Yu Y, Tao Y, Zheng P, Wang F, Ma L. Optogenetic activation of GABAergic neurons in the nucleus accumbens decreases the activity of the ventral pallidum and the expression of cocaine-context-associated memory. The International Journal of Neuropsychopharmacology. 2014;17:753–763. doi: 10.1017/S1461145713001570. [DOI] [PubMed] [Google Scholar]
- Zahm DS, Zaborszky L, Alones VE, Heimer L. Evidence for the coexistence of glutatmate decarboxylase and Met-enkephalin immunoreactivities in axon terminals of rat ventral pallidum. Brain Research. 325:317–321. doi: 10.1016/0006-8993(85)90331-2. [DOI] [PubMed] [Google Scholar]