Abstract
Objective
We aimed to assess racial differences in air pollution exposures to ambient fine particulate (PM2.5) and black carbon (BC) and their association with cardiovascular disease (CVD) risk factors, arterial endothelial function, incident CVD events and all-cause mortality.
Approach and Results
Data from the Heart Strategies Concentrating on Risk Evaluation (HeartSCORE) study were used to estimate one-year average air pollution exposure to PM2.5 and BC using land-use regression models. Correlates of PM2.5 and BC were assessed using linear regression models. Associations with outcomes were determined using Cox proportional hazards models, adjusting for traditional CVD risk factors. Data were available on 1,717 participants (66% female, 45% Blacks, 59±8 years). Blacks had significantly higher exposure to PM2.5 (mean 16.1±0.75 vs. 15.7±0.73μg/m3, p=0.001) and BC (1.19±0.11 vs. 1.16±0.13abs, p=0.001) compared to Whites. Exposure to PM2.5, but not BC, was independently associated with higher blood glucose and worse arterial endothelial function. PM2.5 was associated with a higher risk of incident CVD events and all-cause mortality combined over median follow-up of 8.3 years. Blacks had 1.45 (95% CI:1.00,2.09) higher risk of combined CVD events and all-cause mortality than Whites in models adjusted for relevant covariates. This association was modestly attenuated with adjustment for PM2.5.
Conclusion
PM2.5 exposure was associated with elevated blood glucose, worse endothelial function, and incident CVD events and all-cause mortality. Blacks had a higher rate of incident CVD events and all-cause mortality than Whites that was only partly explained by higher exposure to PM2.5.
Introduction
Racial differences in mortality and cardiovascular disease (CVD) morbidity pose challenges for health care in the United States and worldwide.1–4 Understanding the role of environmental pollution in race-related differences in CVD risk factors and clinical CVD outcome may elucidate a pathophysiologic mechanism for such differences and guide preventive strategies. Epidemiological studies have shown that chronic exposure to airborne fine particulate matter (particles with median aerodynamic diameter <2.5 μm [PM2.5]) is associated with increased CVD risk and mortality.5–8 Although the pathophysiological underpinnings of these associations are not fully understood, potential mechanisms identified in animal and human studies include increased oxidative stress and inflammation leading to endothelial dysfunction, atherosclerotic plaque progression and thrombosis.5, 9–11 Studies of black carbon (BC), a component of ultrafine particulate matter used as a tracer for diesel related emissions, have yielded less consistent results12, 13
Epidemiological data also indicate that racial/ethnic minorities are more likely to reside in areas close to environmental pollution sources, including point sources and heavy roadway traffic areas.14–17 However, racial differences in the exposure to environmental air pollution and their role in disparities in CVD risk and mortality have not been fully elucidated. Therefore, we assessed racial differences in urban air pollution exposures to PM2.5 and BC and their association with CVD risk factors and incident CVD events and all-cause mortality in the Heart Strategies Concentrating on Risk Evaluation (HeartSCORE) study, a prospective community-based cohort in Western Pennsylvania that is prospectively examining racial differences in CVD risk and outcomes since 2003.
Participants and Methods
Study population
HeartSCORE is an ongoing community-based prospective cohort study of 2000 participants with approximately equal representation of Blacks (44%) and Whites (56%) assessing racial and socioeconomic disparities in cardiovascular risk. The methods of HeartSCORE have been described previously.18, 19 Eligibility criteria included age 45 to 75 years at study entry, residence in the greater Pittsburgh, PA, metropolitan area, ability to undergo baseline and annual follow-up visits, and absence of known co-morbidities expected to limit life expectancy to less than 5 years. The Institutional Review Board at the University of Pittsburgh approved the study protocol and all study participants provided written informed consent. The present study included 1717 participants who had available data on air pollution exposures to ambient fine particulate (PM2.5) and black carbon (BC). Data are available from authors upon request, for the purposes of replicating the study.
Exposure determination
We estimated chronic exposures to urban PM2.5 and BC for the year prior to each individual’s baseline clinical date, using adapted versions of previously published land use regression (LUR) methods, incorporating AERMOD dispersion models to better account for the influence of local point sources, as reported in the National Emissions Inventory (NEI) (https://www.epa.gov/air-emissions-inventories/2014-national-emissions-inventory-nei-data)20, 21 Hybrid LUR models (including AERMOD dispersion model terms) were derived from a spatial monitoring campaign including 37 sampling sites distributed across metropolitan Pittsburgh during summer (June 5 to July 26, 2012) and winter (January 8 to March 10, 2013). Geographic information system (GIS)-based covariates were calculated to capture variability in a range of pollution source indicators (e.g., traffic density, industrial emissions, population).20 Hybrid LUR models were developed as mixed models adjusted for repeated measures at each site by season, predicting spatial variation in PM2.5 and BC as a function of the GIS-based source density indicators. We geocoded participant addresses using a composite address locator in ArcGIS to generate point features of residential locations. We used the LUR models to estimate the mean concentrations of PM2.5 and BC for the 300 m surrounding each participant’s residential address, for the 12 months prior to the month of each participant’s baseline clinical date, using daily regulatory data from a centrally-located U.S. EPA Air Quality System (AQS) monitor.20, 21
Covariates and dependent variables
Demographic and medical histories were collected at the baseline visit (2001–2004). Race was self-reported. Participants completed demographic questionnaire including information on marital/co-habiting status, education, and income. Highest education level was categorized as less than high school, beyond high school, and beyond Bachelor’s degree. Annual income was collected in categories < $10K, $20–40K, $40–80K and > $80K. Physical measurements included measurement of vital signs and body fat distribution. Hypertension was defined as blood pressure >140/90 or use of anti-hypertensive medications. Body mass index was calculated as weight/height2 (kg/m2). Laboratory assessments of cholesterol levels were performed on venous blood drawn in the fasting state using the commercially available vertical auto profile technique (VAP, Atherotech, Birmingham, AL). Fasting blood glucose was measured using the glucose oxidase method. Measurement of high-sensitivity C-reactive protein (hsCRP) was performed using an immunoturbidimetric assay on the Roche P Modular system (Roche Diagnostics, Indianapolis, IN), using reagents and calibrators from DiaSorin (Stillwater, MN). Serum interleukin-6 (IL-6) concentrations were measured using commercially available ELISA assay kits (R&D Systems, Minneapolis, MN).
Endothelial function was measured using an Endo-PAT2000 device (Itamar Medical, Caesarea, Israel) adapted from the protocol used by the Framingham Heart Study as previously reported.18, 19, 22 In brief, digital pulse amplitude was measured using the PAT device placed on the tip of each index finger. Baseline PAT signal was measured for 5 minutes on both fingers. Arterial flow was then interrupted on one finger by applying occlusive arm pressure. After 5 minutes the cuff-pressure was abruptly deflated and PAT signal was measured on both fingers for the subsequent 5 minutes. The data were recorded electronically and analyzed using a computerized, automated algorithm. Pulse amplitude response to hyperemia was calculated from the hyperemic fingertip as the ratio of the post-occlusion pulse amplitude to the baseline-pulse amplitude. The result was divided by the corresponding ratio in the control hand to give the PAT ratio (also known as reactive hyperemia index [RHI]). The Framingham reactive hyperemia index (fRHI) was calculated as the natural log-transformation of the RHI.19, 22
Clinical outcomes (all-cause mortality and incident CVD events)
Participants were assessed for incident hospitalization and CVD events by semi-annual questionnaires and during annual follow-up study visits. Incident CVD events were pre-defined as non-fatal myocardial infarction, acute coronary syndrome, stroke, coronary revascularization, or cardiac death. The primary outcome of interest was a combination of CVD events and all-cause mortality. Medical records were obtained for each reported hospitalization. A research nurse and study physician adjudicated incident events independently. Cause of death as cardiac or non-cardiac was ascertained by review of the death certificate obtained from the Commonwealth of Pennsylvania.
Statistical methods
Baseline variables are presented by tertiles of PM2.5 and BC. We also presented baseline variables by race in complementary analyses. Continuous variables are expressed as means (SD) and categorical variables are expressed as proportions. Associations of PM2.5 and BC with CVD risk factors were assessed using linear regression models, adjusted for age, sex, smoking status, and race. Further adjustment was made for income and education status to assess the impact of socioeconomic status. Potential effect modification of the associations of PM2.5 or BC with CVD risk factors by race or sex was investigated by fitting interactions terms between race or sex and the pollutants.
The associations of PM2.5 and BC with incident CVD outcome and all-cause mortality were examined using multivariable-adjusted Cox proportional hazards models. The assumptions of the proportionality of hazards were evaluated using Schoenfeld residuals. Follow-up time was determined by calculating the duration (in years) from the date of initial visit to the date of event, date of last follow-up or the date of censoring, which was on August 7,2014. Adjustment was made for income and education status, as in the linear regression models.
We performed a mediation analyses to assess the potential role of air pollution in explaining the association between Black race and clinical outcomes by adding PM2.5 or BC to Cox proportional hazards models relating race and CVD outcomes, in a model adjusted for CVD risk factors, namely, age, sex, smoking, systolic blood pressure, diabetes, body mas index, total cholesterol, and HDL-cholesterol. The analyses were conducted using the methods described by Ananth and VanderWeele, based on the estimated direct and indirect effects estimated for Black race, as computed on the risk difference scale.23 Given the high correlation between race an socioeconomic status, we did not include markers of socioeconomic status in the model used for mediation analyses. All analyses were performed with Stata software (Stata Corp., version 11, Texas, USA). A p-value <0.05 was considered statistically significant. Study data are available from the authors upon request for the purposes of replicating the study.
Results
Baseline characteristics and bivariate correlations for PM2.5 and BC
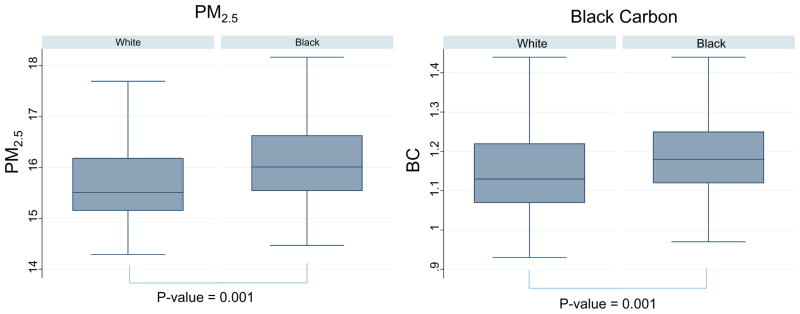
The analyses involved 1717 participants (66% female, 45% Blacks, 59±8 years) with available information on PM2.5 and BC. Baseline characteristics of participants are presented by tertiles of PM2.5 and BC in Table 1 and Supplemental Table I, respectively. The median estimated PM2.5 exposure was 15.7 μg/m3 (range: 14.3–19.1; inter-quartile range: 15.3–16.4). The median estimate of BC concentration was 1.16 abs (range: 0.93–1.92; inter-quartile range: 1.09–1.24). The mean (SD) estimates of PM2.5 and BC concentrations were 15.7±0.77 μg/m3 and 1.17±0.12 abs, respectively. Blacks had, on average, higher exposures to PM2.5 and BC than did Whites. Mean PM2.5 among Blacks was 16.1 (SD = 0.75) μg/m3 vs. 15.7 (0.73) μg/m3 among White. Mean BC exposure among Blacks was 1.19 (SD = 0.11) abs vs. 1.16 (0.13) abs among Whites (Figure 1) The baseline characteristics of the participants by race is shown in Supplemental Table II.
Table 1.
Baseline characteristics of participants by thirds PM2.5
| Variable | Overall summary statistics | Summary statistics by thirds of PM2.5 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of subjects | Mean (SD) or % | Bottom Third | Middle Third | Top Third | |||||
| n | Mean (SD) or % | n | Mean (SD) or % | n | Mean (SD) or % | ||||
| PM2.5 (ug/m3) | 1717 | 15.7(0.77) | 578 | 15.1 (0.27) | 567 | 15.8 (0.21) | 572 | 16.8 (0.49) | |
| Age (years) | 1717 | 59 (8) | 578 | 59 (8) | 567 | 59 (8) | 572 | 59 (8) | 0.84 |
| Female | 1717 | 1129 (66%) | 578 | 359 (62%) | 567 | 386 (68%) | 572 | 384 (67%) | 0.07 |
| Race - Black | 1717 | 773 (45%) | 578 | 150 (26%) | 567 | 294 (52%) | 572 | 329 (58%) | <0.0001 |
| Race - White | 1717 | 902 (53%) | 578 | 411 (71%) | 567 | 261 (46%) | 572 | 230 (40%) | |
| Smoker | 1713 | 192 (11%) | 578 | 55 (10%) | 566 | 67 (12%) | 569 | 70 (12%) | 0.007 |
| Diabetes | 1710 | 177 (10%) | 575 | 55 (10%) | 566 | 57 (10%) | 569 | 65 (11%) | 0.32 |
| HTN | 1715 | 757 (44%) | 578 | 215 (37%) | 566 | 261 (46%) | 571 | 281 (49%) | <0.0001 |
| Systolic BP | 1715 | 137 (20) | 577 | 136 (18) | 567 | 137 (20) | 571 | 138 (21) | 0.05 |
| Diastolic BP | 1714 | 81 (10) | 576 | 81 (10) | 567 | 81 (10) | 571 | 82 (10) | 0.016 |
| Glucose (mg/dl) | 1711 | 99 (26) | 576 | 97 (22) | 565 | 99 (25) | 570 | 102 (31) | <0.0001 |
| BMI (Kg/M2) | 1701 | 30 (6) | 571 | 30 (6) | 562 | 30 (6) | 568 | 31 (7) | <0.0001 |
| WHR | 1584 | 0.89 (0.09) | 546 | 0.89 (0.08) | 518 | 0.89 (0.09) | 520 | 0.89 (0.09) | 0.67 |
| fRHI | 1232 | 0.74 (0.46) | 429 | 0.78 (0.46) | 425 | 0.76 (0.48) | 378 | 0.68 (0.43) | 0.0013 |
| Log-hscrp (log-mg/l) | 1611 | 0.37 (1.24) | 547 | 0.29 (1.17) | 532 | 0.35 (1.28) | 532 | 0.46 (1.25) | 0.016 |
| Log-il6 ((log-pg/ml) | 1585 | 0.53 (0.75) | 538 | 0.42 (0.76) | 527 | 0.54 (0.75) | 520 | 0.63 (0.72) | <0.0001 |
| TC (mg/dl) | 1705 | 213 (42) | 573 | 217 (42) | 563 | 213 (42) | 569 | 209 (43) | 0.005 |
| HDL-c (mg/dL) | 1705 | 58 (15) | 573 | 56 (15) | 563 | 59 (15) | 569 | 58 (15) | 1.6 |
| Log-TG (mg/dL) | 1704 | 4.67 (0.49) | 573 | 4.76 (0.51) | 562 | 4.63 (0.48) | 569 | 4.62 (0.48) | <0.0001 |
| Income < $10K | 1554 | 93 (6.0) | 527 | 20 (3.8) | 506 | 30 (5.9) | 521 | 43 (8.3) | <0.0001 |
| Income - $10K–20K | 1554 | 201 (12.9) | 527 | 36 (6.8) | 506 | 70 (13.8) | 521 | 95 (18.2) | |
| Income - $20K–40K | 1554 | 451 (29.0) | 527 | 145 (27.5) | 506 | 161 (31.8) | 521 | 145 (27.8) | |
| Income - $40K–80K | 1554 | 515 (33.1) | 527 | 184 (34.1) | 506 | 165 (32.1) | 521 | 166 (31.9) | |
| Income > $80K | 1554 | 294 (18.9) | 527 | 142 (26.9) | 506 | 80 (15.8) | 521 | 72 (13.8) | |
| Education < HS | 1713 | 42 (2.5) | 578 | 11 (1.9) | 567 | 15 (2.7) | 568 | 16 (2.8) | 0.009 |
| Education- HS+ | 1713 | 844 (49.3) | 578 | 259 (44.8) | 567 | 295 (52.0) | 568 | 290 (51.1) | |
| Education- Bachelor+ | 1713 | 827 (48.3) | 578 | 308 (53.3) | 567 | 257 (45.3) | 568 | 262 (46.1) | |
PM2.5 – particulate matter with median aerodynamic diameter < 2.5 um, HTN- hypertension, BP – blood pressure, BMI – body mass index, WHR – waist-hip ratio, fRHI - Framingham reactive hyperemia index, Hx – history, BP – blood pressure, hsCRP –high sensitivity C-reactive protein, IL6 – interleukin-6, TC – total cholesterol, HDL-c – high-density lipoprotein cholesterol, TG – triglycerides.
Figure 1.
Distribution of environmental pollutants by race
*Association was significant after adjusting for age, sex, smoking, income and education
In univariate models, PM2.5 was correlated with a broad spectrum of factors. For example, PM2.5 exposures decreased with increasing income. There was also linear increase in mean blood glucose, BMI, and IL-6 concentrations and decrease in arterial endothelial function measured by fRHI across tertiles of PM2.5. (Table 1) There were similar but weaker patterns of associations for BC. (Supplemental Table I)
Multivariable correlates of PM2.5 and BC
The Black-White participant difference in exposure to PM2.5 and BC remained statistically significant after adjustment for age, sex, smoking status, income, and education. Furthermore, higher PM2.5 exposures were associated with higher systolic blood pressure, body mass index, blood glucose and IL-6, lower fRHI (i.e., worse endothelial function) in age- and sex-adjusted models. (Table 2) The associations of PM2.5 with glucose and fRHI remained statistically significant after further adjusting for smoking, race, income and education. Each 1.5-μg/m3 higher concentration of PM2.5 was associated with a 3.7-mg/dl (95% CI: 1.0 – 6.4) increase in blood glucose levels and a 0.06-unit (95% CI: 0.00 – 0.11) decrease in fRHI in the fully-adjusted model (Table 2). The associations between PM2.5 and CVD risk factors did not vary significantly by sex or race (p-value for interaction >0.05 for all). There were similar patterns but statistically nonsignificant associations observed for BC. (Supplemental Table III)
Table 2.
Association of environmental exposure to PM2.5 (per 1.5 ug/m3 higher concentration) with continuous variables
| Outcome | Adjustment | N | Beta (95% CI) | p-value |
|---|---|---|---|---|
| SBP | Unadjusted | 1710 | 1.73(−0.07,3.54) | 0.06 |
| Age & sex | 1710 | 1.86(0.10,3.62) | 0.04 | |
| Above + smoking | 1707 | 1.82(0.06,3.59) | 0.04 | |
| Above + race | 1665 | −0.50(−2.33,1.32) | 0.59 | |
| Above + income | 1505 | −1.10(−3.01,0.81) | 0.26 | |
| Above + education | 1505 | −0.98(−2.89,0.93) | 0.32 | |
|
| ||||
| Glucose | Unadjusted | 1706 | 4.79(2.38,7.20) | <0.001 |
| Age & sex | 1706 | 4.96(2.56,7.37) | <0.001 | |
| Above + smoking | 1702 | 5.01(2.60,7.43) | <0.001 | |
| Above + race | 1660 | 3.72(1.14,6.29) | <0.001 | |
| Above + income | 1499 | 3.63(0.92,6.35) | 0.01 | |
| Above + education | 1499 | 3.71(0.99,6.42) | 0.01 | |
|
| ||||
| BMI | Unadjusted | 1696 | 1.08(0.53,1.63) | <0.001 |
| Age & sex | 1696 | 1.06(0.51,1.61) | <0.001 | |
| Above + smoking | 1694 | 1.10(0.55,1.65) | <0.001 | |
| Above + race | 1652 | 0.16(−0.41,0.72) | 0.59 | |
| Above + income | 1495 | 0.16(−0.44,0.76) | 0.60 | |
| Above + education | 1495 | 0.19(−0.42,0.79) | 0.54 | |
|
| ||||
| fRHI | Unadjusted | 1229 | −0.09(−0.14,−0.03) | <0.001 |
| Age & sex | 1229 | −0.09(−0.14,−0.04) | <0.001 | |
| Above + smoking | 1228 | −0.09(−0.14,−0.04) | <0.001 | |
| Above + race | 1196 | −0.05(−0.10,0.00) | 0.06 | |
| Above + income | 1076 | −0.06(−0.11,−0.00) | 0.05 | |
| Above + education | 1076 | −0.06(−0.11,−0.00) | 0.05 | |
|
| ||||
| Log-hsCRP | Unadjusted | 1608 | 0.14(0.02,0.26) | 0.02 |
| Age & sex | 1608 | 0.12(0.01,0.24) | 0.04 | |
| Above + smoking | 1605 | 0.11(−0.01,0.22) | 0.07 | |
| Above + race | 1566 | −0.03(−0.15,0.09) | 0.61 | |
| Above + income | 1413 | −0.04(−0.17,0.08) | 0.51 | |
| Above + education | 1413 | −0.04(−0.17,0.09) | 0.55 | |
|
| ||||
| Log-IL6 | Unadjusted | 1582 | 0.18(0.11,0.25) | <0.001 |
| Age & sex | 1582 | 0.18(0.11,0.25) | <0.001 | |
| Above + smoking | 1578 | 0.17(0.10,0.24) | <0.001 | |
| Above + race | 1538 | 0.06(−0.01,0.14) | 0.09 | |
| Above + income | 1386 | 0.05(−0.02,0.13) | 0.18 | |
| Above + education | 1386 | 0.06(−0.02,0.13) | 0.15 | |
PM2.5 – particulate matter with median aerodynamic diameter < 2.5 um, SBP – systolic blood pressure, BMI – body mass index, fRHI - Framingham reactive hyperemia index, hsCRP –high sensitivity CRP, IL6 – interleukin-6
Environmental pollutants, race, and incident CVD and mortality outcome
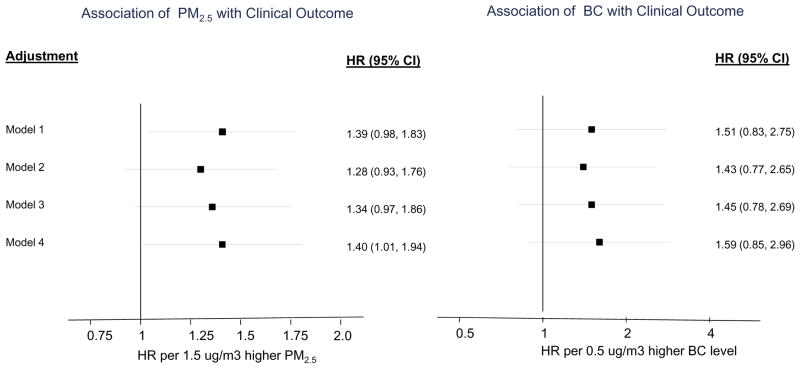
Over a median follow-up period of 8.3 years (12,888 person-years of follow-up), 140 incident events (70 deaths and 70 nonfatal CVD events) were observed. Each 1.5-μg/m3 higher concentration of PM2.5 was associated with 1.39 (95% CI 0.96 – 1.83) increase in hazard ratio of combined all-cause mortality and CVD events, after adjusting for age and sex. The association was similar after further adjustment for race and CVD risk factors. Black carbon was not significantly associated with events. (Figure 2)
Figure 2.
Association of environmental pollutants with clinical outcomes
Model 1 - Age + sex
Model 2 – Model 1 + smoking + race
Model 3 - Model 3 + SBP + diabetes + BMI
Model 4 – Model 3 + TC + HDL-c + TG
Blacks had 1.45 (95% CI, 1.00,2.09) higher risk of combined incident CVD events and all-cause mortality than Whites in models adjusted for traditional CVD risk factors. This association was modestly attenuated to 1.34 [0.91, 1.96] with adjustment for PM2.5 (Table 3) Mediation analyses showed that 24% of the association between race and combined clinical outcome is mediated by exposure to PM2.5. The association between race and clinical outcome was no longer significant with adjustment for income and education. (Table 3)
Table 3.
Effect of adjusting for PM2.5 or BC on the association between race and combined CVD events and all-cause mortality outcomes
| Adjustment | N | Cases | HR 95% (CI) | P-value | HR 95% (CI) | P-value |
|---|---|---|---|---|---|---|
| Adjusted for model on the left | Further adjusted for PM2.5 | |||||
|
| ||||||
| Model 1 | 1616 | 139 | 1.78(1.27,2.49) | 0.00 | 1.69(1.19,2.40) | 0.00 |
| Model 2 | 1596 | 139 | 1.42(0.99,2.03) | 0.06 | 1.32(0.91,1.92) | 0.14 |
| Model 3 | 1586 | 136 | 1.45(1.00,2.09) | 0.05 | 1.34(0.91,1.96) | 0.14 |
| Model 4 | 1437 | 124 | 1.29(0.86,1.93) | 0.22 | 1.23(0.81,1.87) | 0.33 |
|
| ||||||
| Adjusted for model on the left | Further adjusted for BC | |||||
|
| ||||||
| Model 1 | 1616 | 139 | 1.78(1.27,2.49) | 0.00 | 1.75(1.25,2.46) | 0.00 |
| Model 2 | 1596 | 139 | 1.42(0.99,2.03) | 0.06 | 1.39(0.97,1.99) | 0.08 |
| Model 3 | 1586 | 136 | 1.45(1.00,2.09) | 0.05 | 1.41(0.97,2.04) | 0.07 |
| Model 4 | 1437 | 124 | 1.29(0.86,1.93) | 0.22 | 1.26(0.84,1.90) | 0.27 |
Model 1: Age & sex
Model 2: Age, sex, smoking, SBP, diabetes, BMI
Model 3: Age, sex, smoking, SBP, diabetes, BMI, TC & HDL-c
Model 4: Age, sex, smoking, SBP, diabetes, BMI, TC, HDL-c, income & education
Discussion
We found that Blacks had significantly higher exposures to air pollutants (PM2.5, BC) and an increased risk of the combined endpoint of CVD events and death in a community-based cohort of adults in Western Pennsylvania. Particulate matter air pollution measured by PM2.5 was also independently associated with increased risk of combined CVD events and all-cause mortality as well as with elevated blood glucose and worse endothelial function after accounting for potential confounders, including race. The increased risk of clinical events in Blacks was partly mediated by exposure to PM2.5.There was no significant association of BC with clinical outcomes.
This study contributes towards a better understanding of the mechanism of racial differences in CVD events and mortality. Our findings suggest that higher exposures to PM2.5 may contribute to the racial differences in CVD outcomes observed in the Heart SCORE cohort, and is consistent with previous studies reporting associations between airborne fine particulate matter and CVD.5–8, 24 Of note, the association of Black race with higher risk of combined CVD events and all-cause mortality in our study was attenuated with adjustment for PM2.5. Indeed, mediation analyses showed that approximately 24% of the association observed between race and CVD events and all-cause mortality may be explained by exposure to PM2.5. However, this association was no longer statistically significant in models adjusting for markers of socioeconomic status (i.e., income and education), suggesting that socioeconomic status, race and exposure to environmental pollutants, have complex and interdependent relationships with CVD events and mortality. Given the high correlation between race an socioeconomic status, we did not include markers of socioeconomic status in the model used for mediation analyses.
Our findings also suggest potential mechanisms for the associations of PM2.5 with CVD and mortality, which may include hyperglycemia and endothelial dysfunction. These findings complement prior epidemiological and basic science studies of the mechanistic pathways that relate environmental pollution and CVD.5, 25–29 By contrast, the association of PM2.5 with IL-6, body mass index and blood pressure was attenuated and no longer significant after adjusting for race, income and education in the present study, although prior studies have indicated significant associations, in particular with inflammatory variables.9, 30, 31 The attenuation of the association observed in this study may be due to the intricate relationships that likely exist between race, socioeconomic status, exposure to environmental pollutants and inflammatory mileu, including confounding, effect modification and/ or effect mediation.
We observed a similar pattern of associations between BC and the various CVD outcomes as was observed for PM2.5, although effects were not statistically significant. Reported data supporting associations between BC and CVD outcomes are limited. BC is often interpreted as marker for diesel-related emissions, and observed associations with CVD events have been inconsistent, particularly in individuals without pre-existing atherosclerotic disease.12, 13 The current findings suggest that other sources or components of air pollution such as PM2.5 may be more important in the association of air pollution with CVD.
The present study has a number of strengths that merit consideration. First, we studied a racially diverse, community-based cohort of individuals not selected based on preexisting disease, such as diabetes or CVD. Hence, the findings are applicable to understanding associations between air pollution exposures, race and CVD among broad populations. Second, we were able to estimate residence-specific exposures for each participant for the year prior to clinical assessment using a spatial model for air pollution concentrations derived from a large number of concentration measures collected across the region. Third, the stability of the population in Western Pennsylvania was associated with a long residence of this cohort in their current homes, which provided a reliable and complete measure of pollution exposure over time.
Our study has a number of limitations. First, it is a single-center study and the range of air pollution concentrations across the study participants is somewhat smaller than that observed in multi-center studies such as MESA.8, 32 The smaller range of exposures may limit our ability to detect how differences in pollution affect risk. Second, we did not have information on duration of residence of participants in each location prior to entry into the study; hence, there may be misclassification of long-term exposure status depending on how long participants lived in a certain location. Third, the significant correlation between race, socioeconomic status and exposure to air pollutants makes identifying the individual effects of these variables challenging in mutually adjusted, multivariable models in this medium-sized study. Race may be more reflective of the social construct of ethnicity rather than underlying biological differences, and hence has more likelihood of being confounded by social factors, such as education and income.
Of note, we did not assess indoor sources of PM2.5 in the present study. Indoor air pollution is a serious concern. However, an important portion of indoor pollution is derived from outdoors, and these are importantly correlated.33 Residence-based outdoor pollution exposure estimates, which we used in this study, are repeatedly shown to significantly predict a wide range of health outcomes in studies worldwide.5–8 These exposure estimates do not represent the entirety of each individual’s pollution exposure, but rather reflect the persistent contrast in exposures across urban cohorts.
Regarding measurement of dependent variables, we used single measurement of the CVD risk factor correlates of the environmental pollutants presented in this study. Single measurement of exposure or outcome (compared to repeat measurement) is more likely to lead to random misclassification. Such non-differential misclassification is not likely to cause a systematic bias; instead it weakens any observed association between exposure and outcome (regression dilution). Therefore, any observed association would be considered valid, although, it may be weaker than the actual underlying relationship. Prior studies of environmental exposures have estimated air pollution over long periods of time (chronic exposures), even where a given CVD risk factor is measured at only one or a few points in time.34, 35 This is because pollution is a minor burden that accumulates daily over many years and many years of exposure can often precede any apparent physiologic alteration
In conclusion, we found significant racial differences in exposures to urban air pollutants and outcomes in a community-based cohort in Western Pennsylvania. Exposures to PM2.5 were associated with elevated blood glucose, worse endothelial function, and incident CVD events and all-cause mortality. Compared to Whites, Blacks had higher rate of CVD events and all-cause mortality that was partly explained by higher exposure to PM2.5. Further larger-sized, multicenter studies can help to better understand the role and mechanisms of environmental pollution exposures in racial differences in cardiovascular risk and outcomes.
Supplementary Material
Highlights.
We found Black individuals had significantly higher exposure to ambient fine particulate (PM2.5) compared to Whites.
Exposure to PM2.5, was independently associated with elevated blood glucose and worse endothelial function.
PM2.5 was associated with a higher risk of incident CVD events and all-cause mortality combined
Black participants, compared to Whites, had higher risk of combined incident CVD events and all-cause mortality, which was in part explained by higher concentration of PM2.5 in Blacks.
Acknowledgments
We thank the participants of the study.
Funding Sources: Pennsylvania Department of Health (ME-02-384), Harrisburg, PA, USA; National Institutes of Health (R01HL089292), Bethesda, MD, USA; Doris Duke Charitable Foundation (2015084), New York, NY, USA.
Abbreviations
- BC
Black carbon
- BMI
Body mass index
- CI
Confidence Interval
- CVD
Cardiovascular disease
- fRHI
Framingham reactive hyperemia index
- HeartSCORE
Heart Strategies Concentrating on Risk Evaluation
- HR
Hazard ratio
- IL-6
Interleukin-6
- PM2.5
Particles with median aerodynamic diameter < 2.5 μm
- SD
standard deviation
Footnotes
Disclosures: None
References
- 1.Stewart JA, Dundas R, Howard RS, Rudd AG, Wolfe CD. Ethnic differences in incidence of stroke: prospective study with stroke register. BMJ. 1999;318:967–71. doi: 10.1136/bmj.318.7189.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch GF, Gorelick PB. Stroke in African Americans. Neurol Clin. 2000;18:273–90. doi: 10.1016/s0733-8619(05)70192-4. [DOI] [PubMed] [Google Scholar]
- 3.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 4.AHA. Heart Disease and Stroke Statistics: 2006 Update. 2006. [Google Scholar]
- 5.Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, van Donkelaar A, Villeneuve PJ, Brion O, Jerrett M, Martin RV, Rajagopalan S, Goldberg MS, Pope CA, 3rd, Burnett RT. Long-term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36:3313–20. doi: 10.2337/dc12-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–7. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 7.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–58. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 8.Dockery DW. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environ Health Perspect. 2001;109(Suppl 4):483–6. doi: 10.1289/ehp.01109s4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope CA, Bhatnagar A, McCracken J, Abplanalp WT, Conklin DJ, O’Toole TE. Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.309279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Kunzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S, Storey RF Esc Working Group on Thrombosis EAfCP, Rehabilitation and Association ESCHF. Expert position paper on air pollution and cardiovascular disease. Eur Heart J. 2015;36:83–93b. doi: 10.1093/eurheartj/ehu458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills NL, Donaldson K, Hadoke PW, Boon NA, MacNee W, Cassee FR, Sandstrom T, Blomberg A, Newby DE. Adverse cardiovascular effects of air pollution. Nat Clin Pract Cardiovasc Med. 2009;6:36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- 12.Nichols JL, Owens EO, Dutton SJ, Luben TJ. Systematic review of the effects of black carbon on cardiovascular disease among individuals with pre-existing disease. Int J Public Health. 2013;58:707–24. doi: 10.1007/s00038-013-0492-z. [DOI] [PubMed] [Google Scholar]
- 13.Morfeld P, Mundt KA, Dell LD, Sorahan T, McCunney RJ. Meta-Analysis of Cardiac Mortality in Three Cohorts of Carbon Black Production Workers. Int J Environ Res Public Health. 2016:13. doi: 10.3390/ijerph13030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MR, Diez-Roux AV, Hajat A, Kershaw KN, O’Neill MS, Guallar E, Post WS, Kaufman JD, Navas-Acien A. Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Public Health. 2014;104:2130–7. doi: 10.2105/AJPH.2014.302135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su JG, Jerrett M, de Nazelle A, Wolch J. Does exposure to air pollution in urban parks have socioeconomic, racial or ethnic gradients? Environ Res. 2011;111:319–28. doi: 10.1016/j.envres.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty J, Zandbergen PA. Children at risk: measuring racial/ethnic disparities in potential exposure to air pollution at school and home. J Epidemiol Community Health. 2007;61:1074–9. doi: 10.1136/jech.2006.054130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohai P, Lantz PM, Morenoff J, House JS, Mero RP. Racial and socioeconomic disparities in residential proximity to polluting industrial facilities: evidence from the Americans’ Changing Lives Study. Am J Public Health. 2009;99(Suppl 3):S649–56. doi: 10.2105/AJPH.2007.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erqou S, Kip KE, Mulukutla SR, Aiyer AN, Reis SE. Endothelial Dysfunction and Racial Disparities in Mortality and Adverse Cardiovascular Disease Outcomes. Clin Cardiol. 2016;39:338–44. doi: 10.1002/clc.22534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulukutla SR, Venkitachalam L, Bambs C, Kip KE, Aiyer A, Marroquin OC, Reis SE. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J. 2010;31:2808–15. doi: 10.1093/eurheartj/ehq295. [DOI] [PubMed] [Google Scholar]
- 20.Tunno BJ, Michanowicz DR, Shmool JL, Kinnee E, Cambal L, Tripathy S, Gillooly S, Roper C, Chubb L, Clougherty JE. Spatial variation in inversion-focused vs 24-h integrated samples of PM2.5 and black carbon across Pittsburgh, PA. J Expo Sci Environ Epidemiol. 2016;26:365–76. doi: 10.1038/jes.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michanowicz DR, Shmool JLC, Tunno BJ, Tripathy S, Gilloly S, Kinnee E, Clougherty JE. A hybrid land use regression / AERMOD model for predicting intra-urban variation in PM2.5. Atmospheric Environment. 2016:131. [Google Scholar]
- 22.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ananth CV, VanderWeele TJ. Placental abruption and perinatal mortality with preterm delivery as a mediator: disentangling direct and indirect effects. Am J Epidemiol. 2011;174:99–108. doi: 10.1093/aje/kwr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Neill MS, Veves A, Zanobetti A, Sarnat JA, Gold DR, Economides PA, Horton ES, Schwartz J. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–20. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O’Neill MS, Herrington DM, Polak JF, Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution) J Am Coll Cardiol. 2012;60:2158–66. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–72. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. Diabetes Care. 2010;33:2196–201. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–46. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajat A, Allison M, Diez-Roux AV, Jenny NS, Jorgensen NW, Szpiro AA, Vedal S, Kaufman JD. Long-term exposure to air pollution and markers of inflammation, coagulation, and endothelial activation: a repeat-measures analysis in the Multi-Ethnic Study of Atherosclerosis (MESA) Epidemiology. 2015;26:310–20. doi: 10.1097/EDE.0000000000000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siponen T, Yli-Tuomi T, Aurela M, Dufva H, Hillamo R, Hirvonen MR, Huttunen K, Pekkanen J, Pennanen A, Salonen I, Tiittanen P, Salonen RO, Lanki T. Source-specific fine particulate air pollution and systemic inflammation in ischaemic heart disease patients. Occup Environ Med. 2015;72:277–83. doi: 10.1136/oemed-2014-102240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Diez Roux AV, Gassett AJ, Jacobs DR, Jr, Kronmal R, Larson TV, Navas-Acien A, Olives C, Sampson PD, Sheppard L, Siscovick DS, Stein JH, Szpiro AA, Watson KE. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): a longitudinal cohort study. Lancet. 2016;388:696–704. doi: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunno BDR, Cambal L, Holguin F, Lioy P, Jane E, Clougherty JE. Indoor source apportionment in urban communities near industrial sites. Atmospheric Environment. 2016;139:30–36. [Google Scholar]
- 34.Hennig FFK, Moebus S, Weinmayr G, Memmesheimer M, Jakobs H, Brocker-Preuss M, Fuhrer-Sakel D, Mohlenkamp S, Erbel R, Jockel KH, Hoffmann B Heinz Nixdorf Recall Study Investigative, Group. Association between source-specific particulate matter air pollution and hs-CRP: local traffic and industrial emissions. Environ Health Perspect. 2014;122:703–10. doi: 10.1289/ehp.1307081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yitshak Sade MKI, Liberty IF, Schwartz J, Novack V. The Association Between Air Pollution Exposure and Glucose and Lipids Levels. J Clin Endocrinol Metab. 2016;101:2460–7. doi: 10.1210/jc.2016-1378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.